Neurotensin Is a Lipid-Induced Gastrointestinal Peptide Associated with Visceral Adipose Tissue Inflammation in Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Clinical and Laboratory Assessments
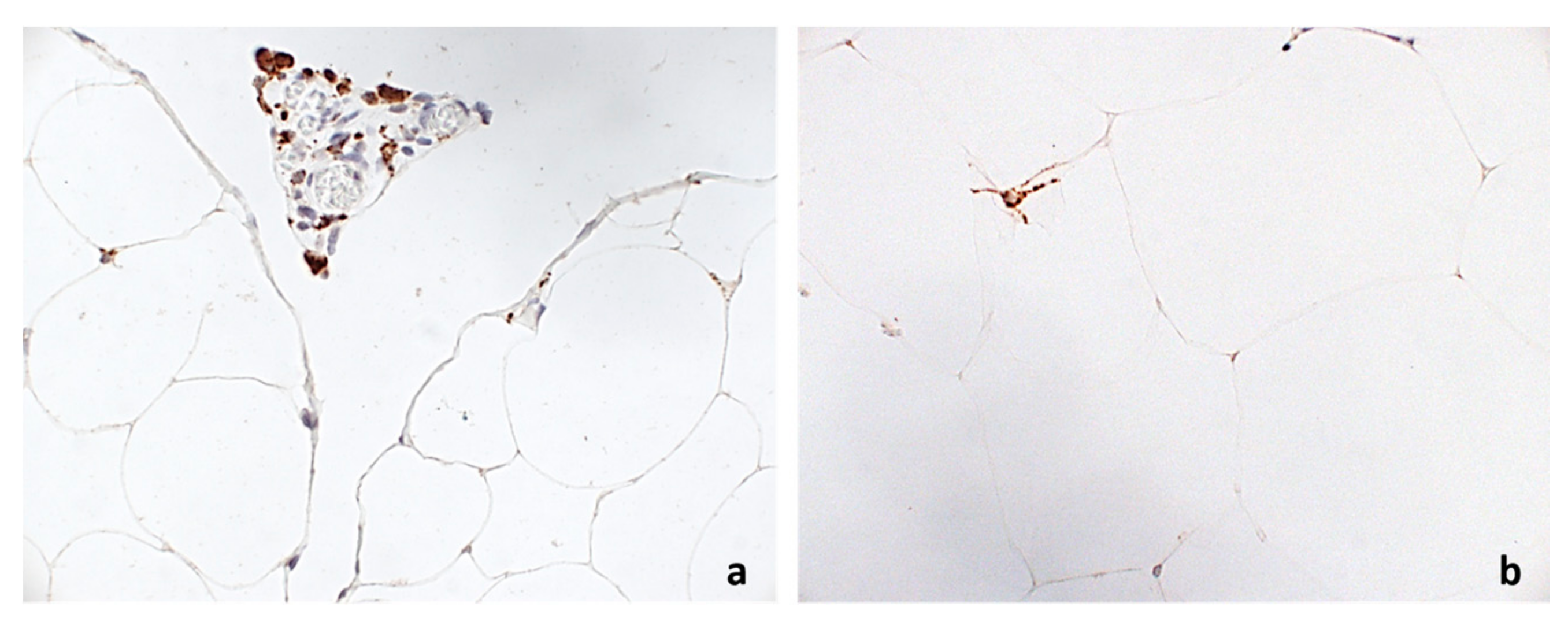
2.3. Histological and Immunohistochemical Analysis
2.4. Gene Expression Analysis—RealTime PCR
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
Funding
References
- Goedert, M.; Emson, P.C. The regional distribution of neurotensin-like immunoreactivity in central and peripheral tissues of the cat. Brain Res. 1983, 272, 291–297. [Google Scholar] [CrossRef]
- Ferris, C.F.; Hammer, R.A.; Leeman, S.E. Elevation of plasma neurotensin during lipid perfusion of rat small intestine. Peptides 1981, 2 (Suppl. S2), 263–266. [Google Scholar] [CrossRef]
- Cui, H.; Cai, F.; Belsham, D.D. Leptin signaling in neurotensin neurons involves STAT, MAP kinases ERK1/2, and p38 through c-Fos and ATF1. FASEB J. 2006, 20, 2654–2656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, J.A.; Bugescu, R.; Mayer, T.; Gata-Garcia, A.; Kurt, G.; Woodworth, H.L.; Leinninger, G.M. Loss of Action via Neurotensin-Leptin Receptor Neurons Disrupts Leptin and Ghrelin-Mediated Control of Energy Balance. Endocrinology 2017, 158, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Ciccarelli, G.; Cimini, F.A.; Ceccarelli, V.; Orho-Melander, M.; Melander, O.; Cavallo, M.G. Association between systemic leptin and neurotensin concentration in adult individuals with and without type 2 diabetes mellitus. J. Endocrinol. Investig. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Leeman, S.E.; Carraway, R.E. Neurotensin: Discovery, isolation, characterization, synthesis and possible physiological roles. Ann. N. Y. Acad. Sci. 1982, 400, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Carraway, R.E. Enhancement of jejunal absorption of conjugated bile acid by neurotensin in rats. Gastroenterology 2001, 120, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, J.; Zaytseva, Y.Y.; Liu, Y.; Rychahou, P.; Jiang, K.; Starr, M.E.; Kim, J.T.; Harris, J.W.; Yiannikouris, F.B.; et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature 2016, 533, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Melander, O.; Maisel, A.S.; Almgren, P.; Manjer, J.; Belting, M.; Hedblad, B.; Engström, G.; Kilger, U.; Nilsson, P.; Bergmann, A.; et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA 2012, 308, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Lyass, A.; Liu, Y.; Gaggin, H.; Trebnick, A.; Maisel, A.S.; D’Agostino, R.B.; Wang, T.J.; Massaro, J.; Vasan, R.S. Circulating Proneurotensin Concentrations and Cardiovascular Disease Events in the Community: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Mayas, D.; Moreno-Navarrete, J.M.; Catalán, V.; Gómez-Ambrosi, J.; Esteve, E.; Rodriguez-Hermosa, J.I.; Ruiz, B.; Ricart, W.; Peral, B.; et al. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity 2016, 18, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A. How obesity causes diabetes: Not a tall tale. Science 2005, 307, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Adipose tissue inflammation: A cause or consequence of obesity-related insulin resistance? Clin. Sci. (Lond.) 2016, 130, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Angelico, F.; Del Ben, M.; Di Martino, M.; Cimini, F.A.; Bertoccini, L.; Polimeni, L.; Catalano, C.; Fraioli, A.; Del Vescovo, R.; et al. Phenotypical heterogeneity linked to adipose tissue dysfunction in patients with Type 2 diabetes. Clin. Sci. (Lond.) 2016, 130, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Koon, H.W.; Kim, Y.S.; Xu, H.; Kumar, A.; Zhao, D.; Karagiannides, I.; Dobner, P.R.; Pothoulakis, C. Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis. Proc. Natl. Acad. Sci. USA 2009, 106, 8766–8771. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zuo, L.; Zhu, W.; Gong, J.; Zhang, W.; Guo, Z.; Gu, L.; Li, N.; Li, J. Telmisartan attenuates the inflamed mesenteric adipose tissue in spontaneous colitis by mechanisms involving regulation of neurotensin/microRNA-155 pathway. Biochem. Pharmacol. 2015, 93, 461–469. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009, 32, S13–S61. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement: Executive Summary. Circulation 2005, 112, 2735–27522. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Hellmich, S.; Bergmann, A. Proneurotensin 1–117, a stable neurotensin precursor fragment identified in human circulation. Peptides 2006, 27, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Leonetti, F.; Capoccia, D.; Di Cristofano, C.; Silecchia, G.; Orho-Melander, M.; Melander, O.; Cavallo, M.G. Increased plasma proneurotensin levels identify NAFLD in adults with and without type 2 diabetes. J. Clin. Endocrinol. Metab. 2018. [Google Scholar] [CrossRef] [PubMed]
- Piatek, J.; Witmanowski, H.; Paluszak, J.; Krauss, H.; Krawczyk, J. The effects of neurotensin on selected parameters of lipid metabolism in rats. Peptides 2005, 26, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G. Bariatric and metabolic surgery: A shift in eligibility and success criteria. Nat. Rev. Endocrinol. 2015, 11, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001, 15, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Mean ± Standard Deviation Rate (%) | Median (25°–75° Percentile) |
|---|---|---|
| Age (years) | 43.7 ± 9.6 | 42 (38–49) |
| Gender (M%) | 24% | - |
| BMI (kg/m2) | 43.6 ± 5.6 | 42.2 (40–47.4) |
| Waist circumference (cm) | 128 ± 11.4 | 126 (126–135.5) |
| SBP (mmHg) | 128.3 ± 16.1 | 130 (120–136) |
| DBP (mmHg) | 84.8 ± 18.2 | 80 (80–85) |
| Total Cholesterol (mg/dL) | 196 ± 32.4 | 194.5 (176.2–207) |
| HDL (mg/dL) | 48.2 ± 10.4 | 48 (39–55) |
| LDL (mg/dL) | 120.3 ± 29.7 | 120 (120–137) |
| Triglycerides (mg/dL) | 129.5 ± 44.9 | 127 (127–160) |
| FBG (mg/dL) | 104 ± 22.4 | 95 (90–120) |
| HbA1c (%—mmol/mol) | 5.9 ± 1 | 5.3 (5.1–5.8) |
| FBI (µU/L) | 16.8 ± 15.1 | 11.5 (9.7–16.6) |
| HOMA-IR | 4.5 ± 4.7 | 2.8 (2.2–4) |
| HOMA-β% | 164 ± 120 | 133.5 (67.8–212) |
| Pro-NT (pmol/L) | 178.6 ± 85 | 159.4 (118–216.9) |
| T2D (%) | 15% | - |
| MS (%) | 80% | - |
| NAFLD (%) | 50% | - |
| NASH (%) | 25% | - |
| Therapy with antidiabetic agents | 15% | - |
| Therapy with statins | 92% | - |
| Therapy with anti-hypertensive medications | 73% | - |
| Parameter | Correlation Coefficient | p-Value |
|---|---|---|
| Age | 0.43 | 0.004 |
| Gender (M/F) | 0.02 * | 0.89 |
| BMI | 0.31 | −0.16 |
| Waist circumference | −0.16 | 0.31 |
| FBG | 0.07 | 0.67 |
| FBI | 0.38 | 0.02 |
| HbA1c | 0.40 | 0.012 |
| Total Cholesterol | 0.02 | 0.89 |
| HDL | −0.04 | 0.78 |
| LDL | 0.02 | 0.88 |
| Triglycerides | 0.39 | 0.012 |
| AST | −0.08 | 0.61 |
| ALT | 0.04 | 0.80 |
| T2D yes/no | 0.33 * | 0.039 |
| NAFLD yes/no | 0.41 * | 0.01 |
| NAS score | 0.36 * | 0.023 |
| Gene | Correlation Coefficient | p-Value |
|---|---|---|
| NTN1 | −0.11 | 0.50 |
| UNC5B | 0.42 | 0.009 |
| CAV1 | 0.11 | 0.50 |
| IL8 | −0.08 | 0.61 |
| MIP1A | 0.08 | 0.61 |
| MIP2 | 0.11 | 0.50 |
| TIMP1 | 0.15 | 0.38 |
| GZMB | −0.13 | 0.43 |
| CASP3 | −0.02 | 0.9 |
| CASP7 | 0.09 | 0.58 |
| PARP1 | 0.16 | 0.43 |
| HIF-1α | 0.41 | 0.011 |
| WISP1 | 0.37 | 0.022 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barchetta, I.; Cimini, F.A.; Capoccia, D.; Bertoccini, L.; Ceccarelli, V.; Chiappetta, C.; Leonetti, F.; Di Cristofano, C.; Silecchia, G.; Orho-Melander, M.; et al. Neurotensin Is a Lipid-Induced Gastrointestinal Peptide Associated with Visceral Adipose Tissue Inflammation in Obesity. Nutrients 2018, 10, 526. https://doi.org/10.3390/nu10040526
Barchetta I, Cimini FA, Capoccia D, Bertoccini L, Ceccarelli V, Chiappetta C, Leonetti F, Di Cristofano C, Silecchia G, Orho-Melander M, et al. Neurotensin Is a Lipid-Induced Gastrointestinal Peptide Associated with Visceral Adipose Tissue Inflammation in Obesity. Nutrients. 2018; 10(4):526. https://doi.org/10.3390/nu10040526
Chicago/Turabian StyleBarchetta, Ilaria, Flavia Agata Cimini, Danila Capoccia, Laura Bertoccini, Valentina Ceccarelli, Caterina Chiappetta, Frida Leonetti, Claudio Di Cristofano, Gianfranco Silecchia, Marju Orho-Melander, and et al. 2018. "Neurotensin Is a Lipid-Induced Gastrointestinal Peptide Associated with Visceral Adipose Tissue Inflammation in Obesity" Nutrients 10, no. 4: 526. https://doi.org/10.3390/nu10040526
APA StyleBarchetta, I., Cimini, F. A., Capoccia, D., Bertoccini, L., Ceccarelli, V., Chiappetta, C., Leonetti, F., Di Cristofano, C., Silecchia, G., Orho-Melander, M., Melander, O., & Cavallo, M. G. (2018). Neurotensin Is a Lipid-Induced Gastrointestinal Peptide Associated with Visceral Adipose Tissue Inflammation in Obesity. Nutrients, 10(4), 526. https://doi.org/10.3390/nu10040526







