Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study
Abstract
:1. Introduction
2. Materials and Methods
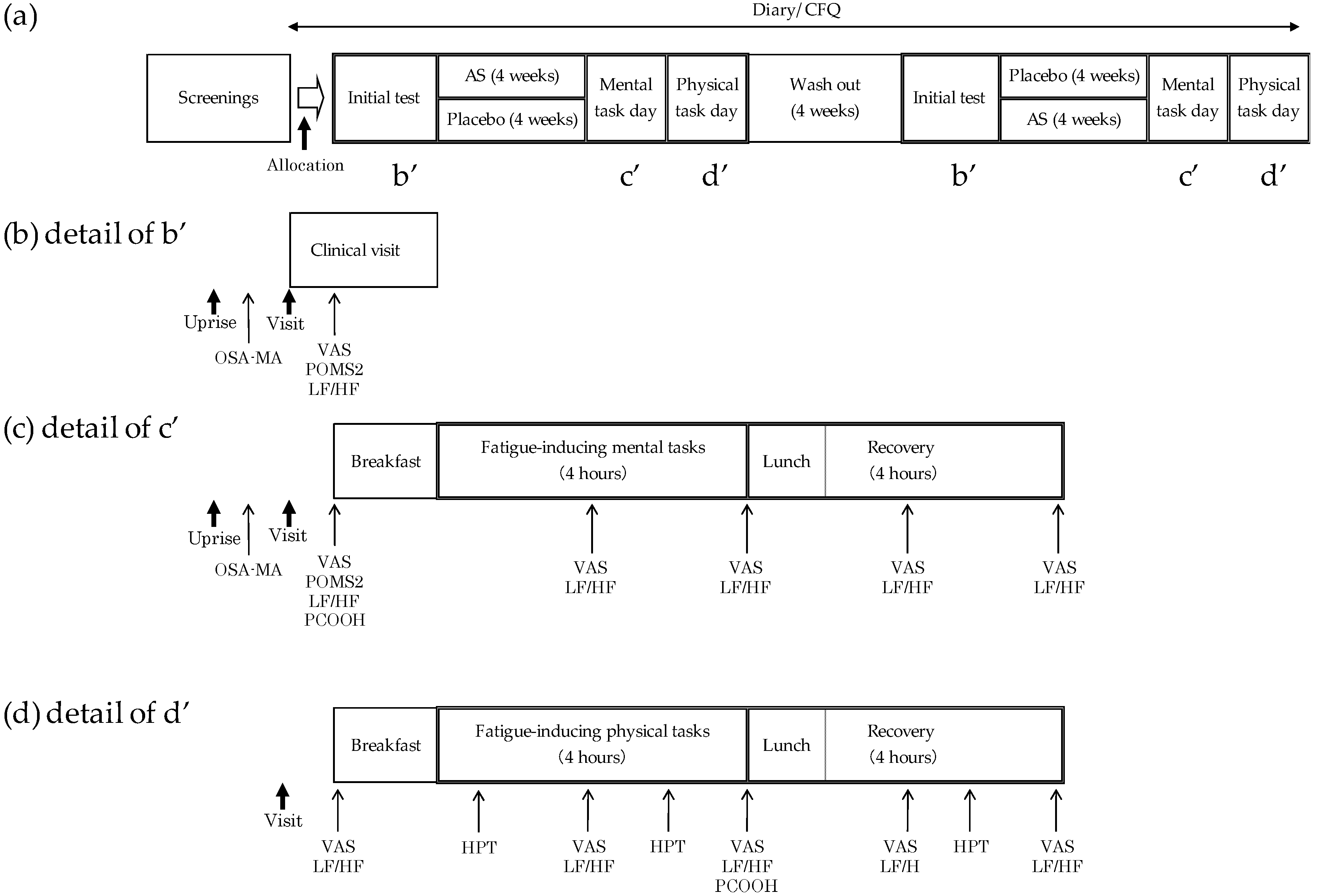
2.1. Study Design
2.2. Participants
2.3. Screenings and Initial Test
2.4. Fatigue-Inducing Tasks
2.5. Supplements
2.6. Primary and Secondary Outcomes
2.7. Statistical Analysis
3. Results
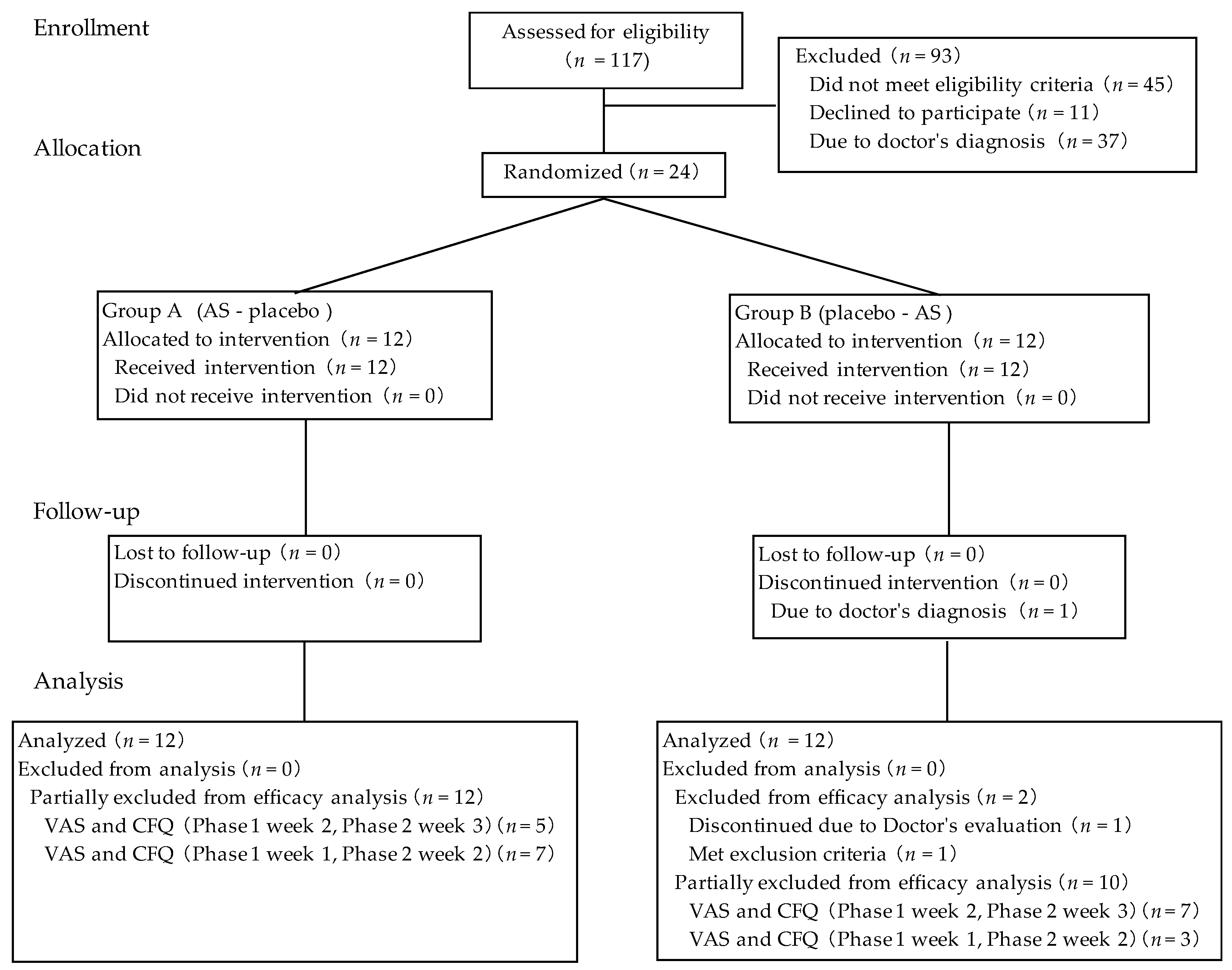
3.1. Participants
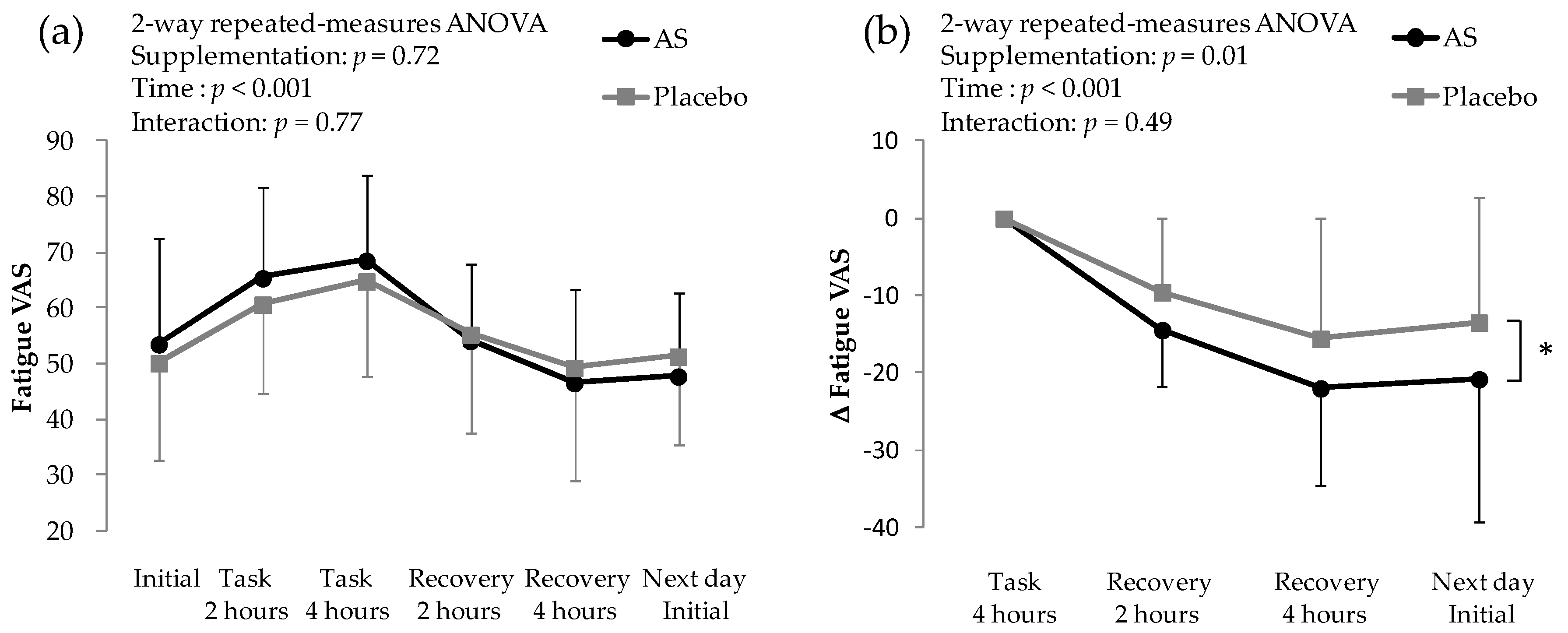
3.2. Subjective Fatigue Score on Mental and Physical Task Days
3.3. Fatigue Questionnaires Using CFQ
3.4. Secondary Outcomes
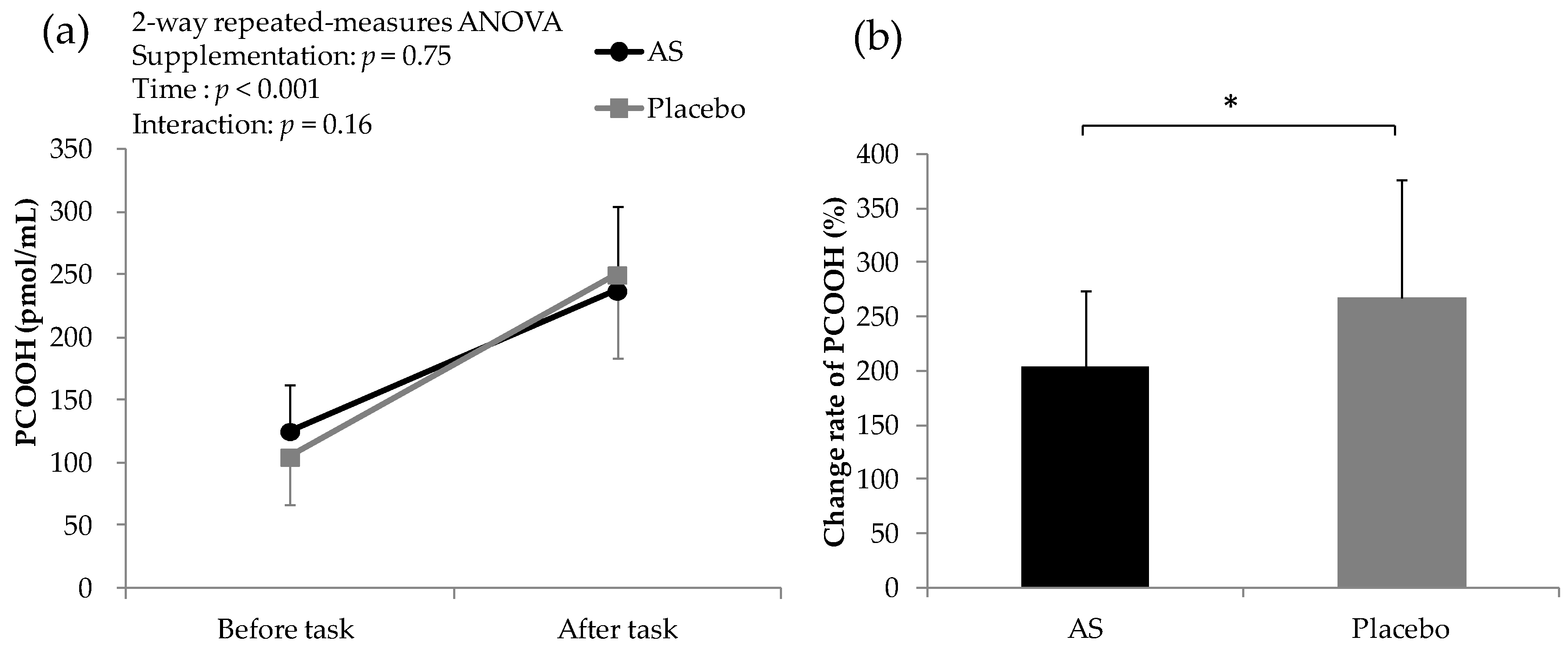
3.5. Blood Test
3.6. Safety Evaluation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barclay, J.K.; Hansel, M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can. J. Physiol. Pharmacol. 1991, 69, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sivoňová, M.; Žitňanová, I.; Hlinčíková, L.; Škodáček, I.; Trebatická, J.; Ďuračková, Z. Oxidative stress in university students during examinations. Stress 2004, 7, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Wong, C. Chronic fatigue syndrome: Oxidative stress and dietary modifications. Altern. Med. Rev. 2001, 6, 450–459. [Google Scholar] [PubMed]
- Manuel y Keenoy, B.; Moorkens, G.; Vertommen, J.; De Leeuw, I. Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sci. 2001, 68, 2037–2049. [Google Scholar] [CrossRef]
- Vecchiet, J.; Cipollone, F.; Falasca, K.; Mezzetti, A.; Pizzigallo, E.; Bucciarelli, T.; De Laurentis, S.; Affaitati, G.; De Cesare, D.; Giamberardino, M.A. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci. Lett. 2003, 335, 151–154. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Carpentero Burdeos, G.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.C. Lipid-peroxidation and antioxidants as biomarkers of tissue-damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [PubMed]
- Mori, J.; Yokoyama, H.; Sawada, T.; Miyashita, Y.; Nagata, K. Anti-oxidative properties of astaxanthin and related compounds. Mol. Cryst. Liq. Cryst. 2013, 580, 52–57. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Jaswir, I.; Monsur, H.A. Anti-inflammatory compounds of macro algae origin: A review. J. Med. Plants Res. 2011, 5, 7146–7154. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Shoji, N.; Nakagawa, K.; Asai, A.; Fujita, I.; Hashiura, A.; Nakajima, Y.; Oikawa, S.; Miyazawa, T. LC-MS/MS analysis of carboxymethylated and carboxyethylated phosphatidylethanolamines in human erythrocytes and blood plasma. J. Lipid Res. 2010, 51, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.H.R.; Galvão, E.L.; Barros, J.Â.C.; Conceição, M.M.; Sousa, E.M.B.D. Extraction, fatty acid profile and antioxidant activity of sesame extract (Sesamum Indicum L.). Braz. J. Chem. Eng. 2012, 29, 409–420. [Google Scholar] [CrossRef]
- Hemalatha, S. Ghafoorunissa Sesame lignans enhance the thermal stability of edible vegetable oils. Food Chem. 2007, 105, 1076–1085. [Google Scholar] [CrossRef]
- Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y.; Takahashi, J.; Tominaga, K.; Miura, N. Daily fatigue-reducing effect of astaxathin—A randomized, placebo-controlled, double-blind, parallel-group study. Jpn. Pharmacol. Ther. 2017, 45, 61–72. [Google Scholar]
- Takemoto, D.; Yasutake, Y.; Tomimori, N.; Ono, Y.; Shibata, H.; Hayashi, J. Sesame lignans and vitamin E supplementation improve subjective statuses and anti-oxidative capacity in healthy humans with feelings of daily fatigue. Glob. J. Health Sci. 2015, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Odebarg, J.M. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Osterlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef]
- Tomimori, N.; Rogi, T.; Shibata, H. Absorption, distribution, metabolism, and excretion of [14 C]sesamin in rats. Mol. Nutr. Food Res. 2017, 61, 1600844. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y. Development of guideline of clinical evaluation of anti-fatigue. J. Clin. Exp. Med. 2009, 228, 733–736. (In Japanese) [Google Scholar]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Yokoyama, K.; Watanabe, K. Japanese Translation of POMS 2: Profile of Mood States Second Edition; Kaneko Shobo: Tokyo, Japan, 2015. [Google Scholar]
- Yamamoto, Y.; Tanaka, H.; Takase, M.; Shirakawa, S. Standardization of revised version of OSA sleep inventory for middle age and aged. Brain Sci. Ment. Disord. 1999, 10, 401–409. [Google Scholar]
- Tanaka, M.; Shigihara, Y.; Fujii, H.; Hirayama, Y.; Watanabe, Y. Effect of CBEX-Dr-containing drink on physical fatigue in healthy volunteers. Jpn. Pharmacol. Ther. 2008, 36, 199–212. [Google Scholar]
- Ataka, S.; Tanaka, M.; Nozaki, S.; Mizuma, H.; Mizuno, K.; Tahara, T.; Sugino, T.; Shirai, T.; Kajimoto, Y.; Kuratsune, H.; et al. Effects of oral administration of caffeine and D-ribose on mental fatigue. Nutrition 2008, 24, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lorish, C.D.; Maisiak, R. The face scale: A brief, nonverbal method for assessing patient mood. Arthritis Rheumatol. 1986, 29, 906–909. [Google Scholar] [CrossRef]
- Takada, M.; Ebara, T.; Sakai, Y. The acceleration plethysmography system as a new physiological technology for evaluating autonomic modulations. Health Eval. Promot. 2008, 35, 373–377. [Google Scholar] [CrossRef]
- Kirchner, W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958, 55, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Watanabe, Y. Utility of an advanced trail making test as a neuropsychological tool for an objective evaluation of work efficiency during mental fatigue. In Fatigue Science for Human Health; Springer: New York, NY, USA, 2008; pp. 47–54. [Google Scholar]
- Nakamura, Y.; Mutoh, Y.; Miyashita, M. Determination of the peak power output during maximal brief pedalling bouts. J. Sports Sci. 1985, 3, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Takada, H.; Okino, K.; Niwa, Y. An evaluation method for heart rate variability, by using acceleration plethysmography. Health Eval. Promot. 2004, 31, 547–555. [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Nakagawa, K.; Suzuki, Y.; Asai, A.; Nagao, M.; Nagashima, K.; Oikawa, S.; Miyazawa, T. Liquid chromatography-tandem mass spectrometry determination of human plasma 1-palmitoyl-2-hydroperoxyoctadecadienoyl-phosphatidylcholine isomers via promotion of sodium adduct formation. Anal. Biochem. 2015, 471, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Conway, F.T.; Karsh, B.-T. Occupational stress in human computer interaction. Ind. Health 1999, 37, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kuo, C.-C.; Chou, J.; Delvolve, A.; Jackson, S.N.; Post, J.; Woods, A.S.; Hoffer, B.J.; Wang, Y.; Harvey, B.K. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009, 23, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-S.; Zhang, X.; Wu, Q.; Li, W.; Wang, C.-X.; Xie, G.-B.; Zhou, X.-M.; Shi, J.-X.; Zhou, M.-L. Astaxanthin offers neuroprotection and reduces neuroinflammation in experimental subarachnoid hemorrhage. J. Surg. Res. 2014, 192, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-S.; Zhang, X.; Zhou, M.-L.; Zhou, X.-M.; Li, N.; Li, W.; Cong, Z.-X.; Sun, Q.; Zhuang, Z.; Wang, C.-X.; et al. Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. J. Neurosurg. 2014, 121, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kim, C.-S.; Lee, Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011, 49, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/carnosine supplementation suppresses the expression of the inflammatory chemokine CCL24 in peripheral blood mononuclear cells from elderly people. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Yamano, E.; Tanaka, M.; Ishii, A.; Tsuruoka, N.; Abe, K.; Watanabe, Y. Effects of chicken essence on recovery from mental fatigue in healthy males. Med. Sci. Monit. 2013, 19, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Klimas, N.G.; Koneru, A.O.B. Chronic fatigue syndrome: Inflammation, immune function, and neuroendocrine interactions. Curr. Rheumatol. Rep. 2007, 9, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol. Pharm. Bull. 2006, 29, 2106–2110. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem. Biophys. Res. Commun. 2007, 366, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Nishijima, Y.; Shibata, H.; Kiso, Y.; Ohnuki, K.; Fushiki, T.; Moritani, T. Protective effect of sesamin administration on exercise-induced lipid peroxidation. Int. J. Sports Med. 2003, 24, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Kiso, Y. Antioxidative roles of sesamin, a functional lignan in sesame seed, and it’s effect on lipid- and alcohol-metabolism in the liver: A DNA microarray study. Biofactors 2004, 21, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Harada, M.; Nakahara, K.; Akimoto, K.; Shibata, H.; Miki, W.; Kiso, Y. Novel antioxidative metabolites in rat liver with ingested sesamin. J. Agric. Food Chem. 2003, 51, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Chavali, S.R.; Zhong, W.W.; Forse, R.A. Dietary α-linolenic acid increases TNF-α, and decreases IL-6, IL-10 in response to LPS: Effects of sesamin on the Δ-5 desaturation of ω6 and ω3 fatty acids in mice. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 185–191. [Google Scholar] [CrossRef]
- Hou, R.; Chen, H.; Tzen, J.; Jeng, K. Effect of sesame antioxidants on LPS-induced NO production by BV2 microglial cells. Neuroreport 2003, 14, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.C.; Jinn, T.R.; Hou, R.C.; Tzen, J.T. Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int. J. Biomed. Sci. 2006, 2, 284–288. [Google Scholar] [PubMed]
| VAS Scores on Screening Day | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | n | Male | Female | Age | BMI (kg/m2) | CFQ Score | Initial | After Physical Task (4 h) | After Recovery (4 h) | |
| Group A | AS | Placebo | 12 | 6 | 6 | 44.8 ± 7.2 | 21.8 ± 2.4 | 25.1 ± 2.4 | 36.3 ± 16.2 | 63.0 ± 16.2 | 50.3 ± 19.1 |
| Group B | Placebo | AS | 10 | 5 | 5 | 43.0 ± 8.6 | 21.7 ± 2.4 | 25.4 ± 4.6 | 36.1 ± 20.0 | 66.1 ± 10.0 | 51.6 ± 14.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients 2018, 10, 281. https://doi.org/10.3390/nu10030281
Imai A, Oda Y, Ito N, Seki S, Nakagawa K, Miyazawa T, Ueda F. Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients. 2018; 10(3):281. https://doi.org/10.3390/nu10030281
Chicago/Turabian StyleImai, Ayano, Yuriko Oda, Naoki Ito, Shinobu Seki, Kiyotaka Nakagawa, Teruo Miyazawa, and Fumitaka Ueda. 2018. "Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study" Nutrients 10, no. 3: 281. https://doi.org/10.3390/nu10030281
APA StyleImai, A., Oda, Y., Ito, N., Seki, S., Nakagawa, K., Miyazawa, T., & Ueda, F. (2018). Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients, 10(3), 281. https://doi.org/10.3390/nu10030281





