Understanding the Effect of Particle Size and Processing on Almond Lipid Bioaccessibility through Microstructural Analysis: From Mastication to Faecal Collection
Abstract
1. Introduction
2. Materials and Methods
2.1. Almond and Faecal Samples
2.2. Simulated Oral Digestion
2.3. Particle Size Distribution (PSD)
2.4. Lipid Release after Oral Processing and Mathematical Model
2.5. Results of Lipid Content Were Expressed as a Percentage of Dry Weight
2.6. Microstructural Analysis
2.7. Statistical Analysis
3. Results
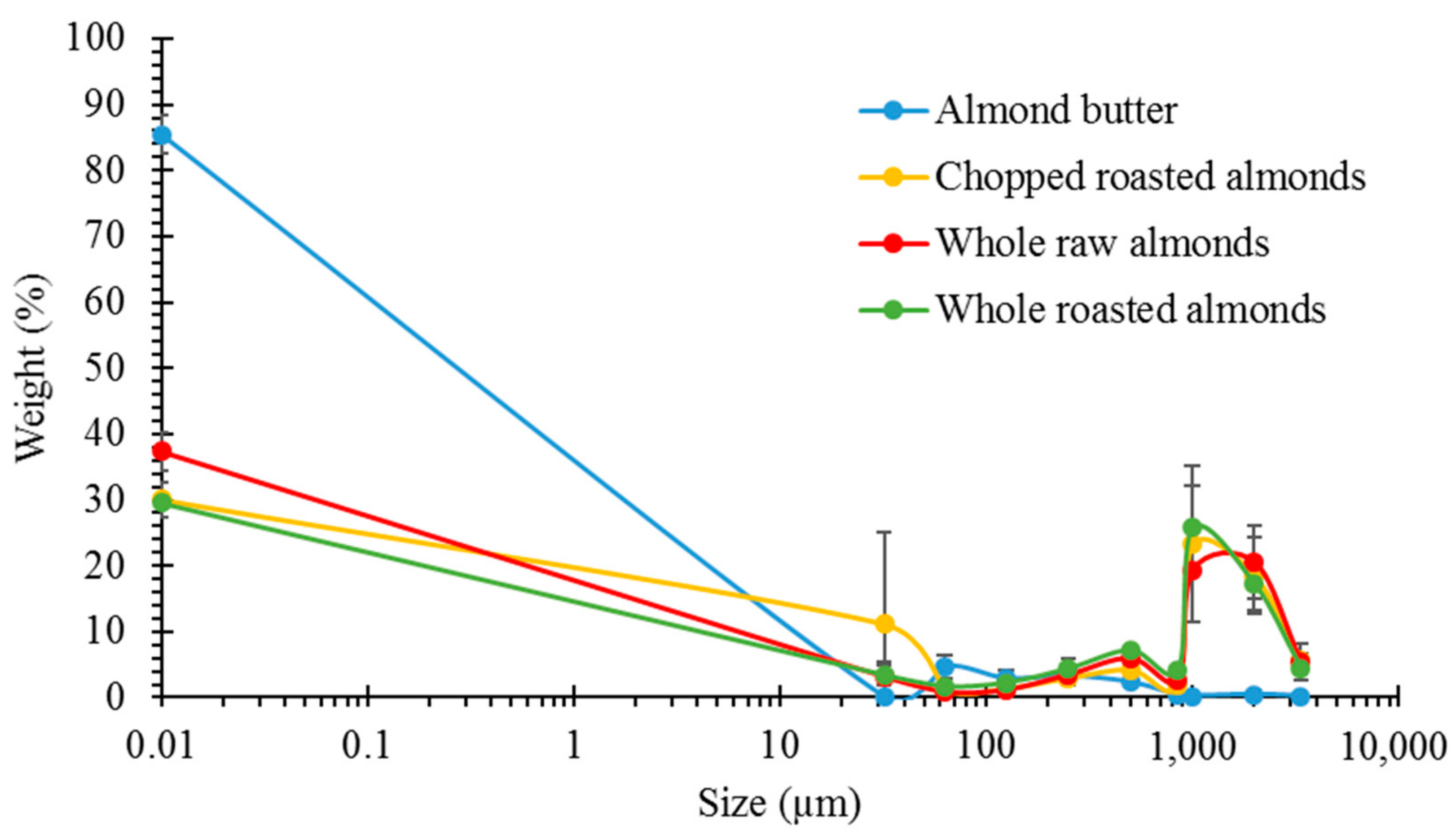
3.1. Particle Size Analysis
3.2. Lipid Release after Simulated Mastication and Predicted Lipid Release
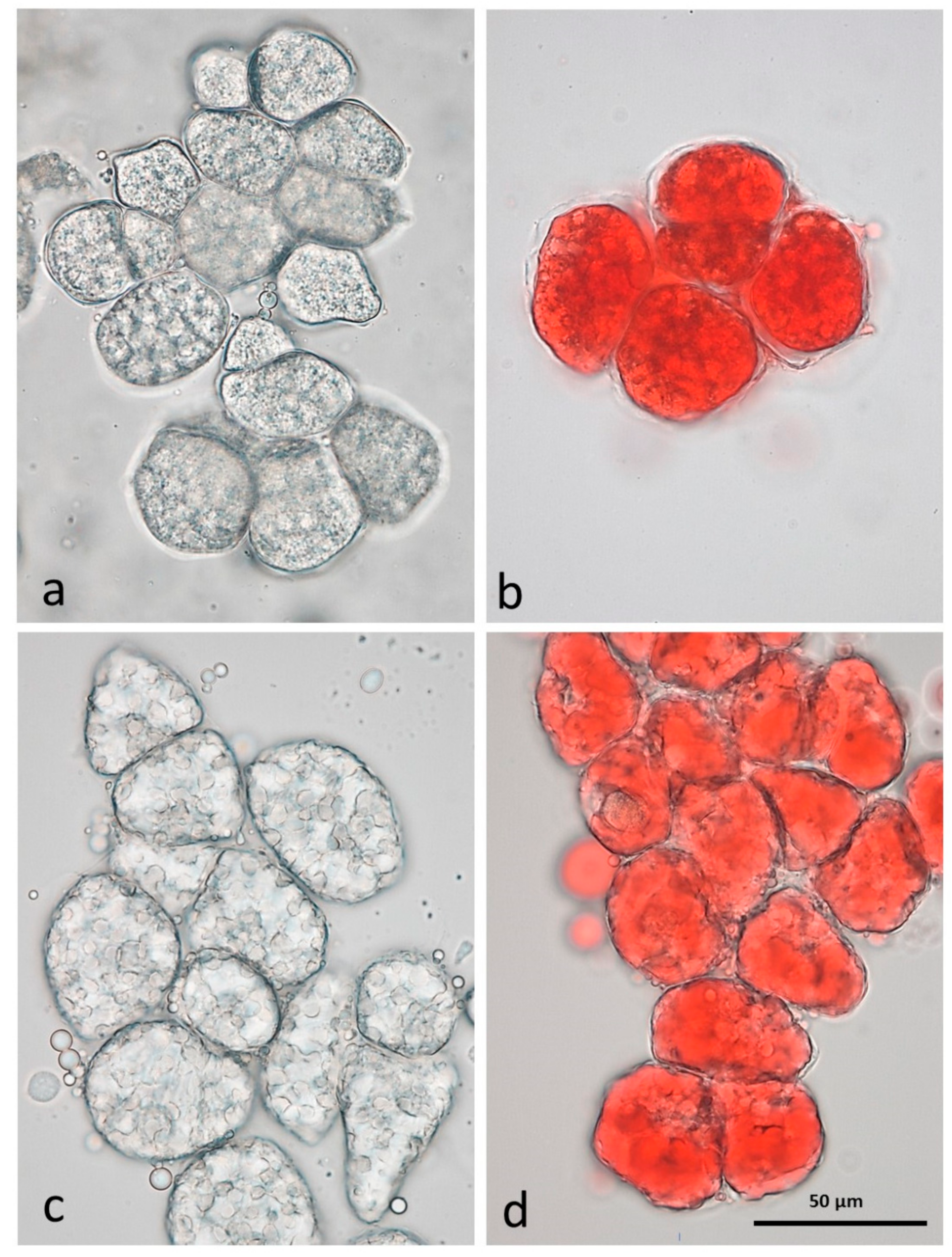
3.3. Microscopy Examination on Almond Baseline Samples
3.3.1. NA
3.3.2. RA
3.3.3. AB
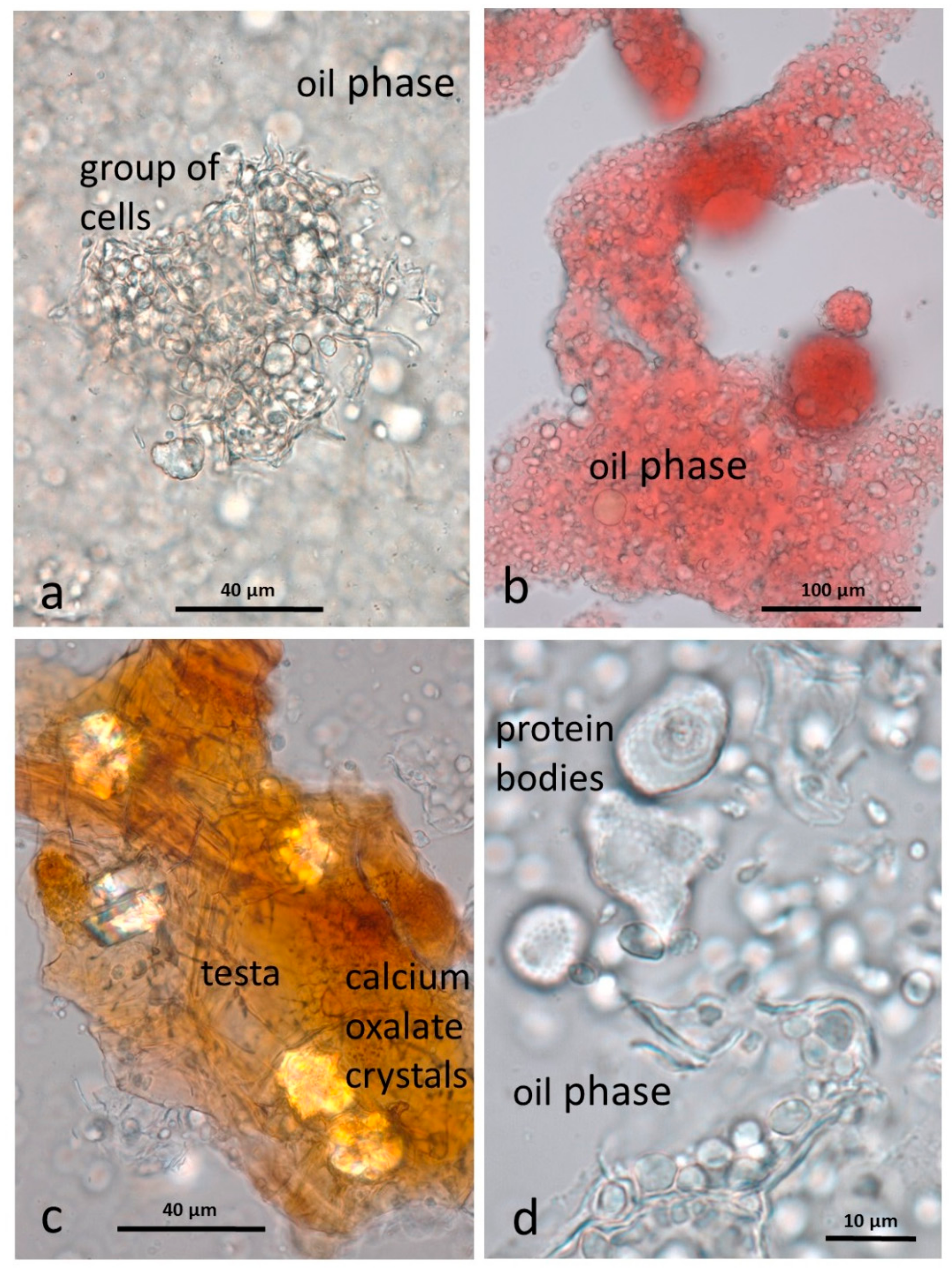
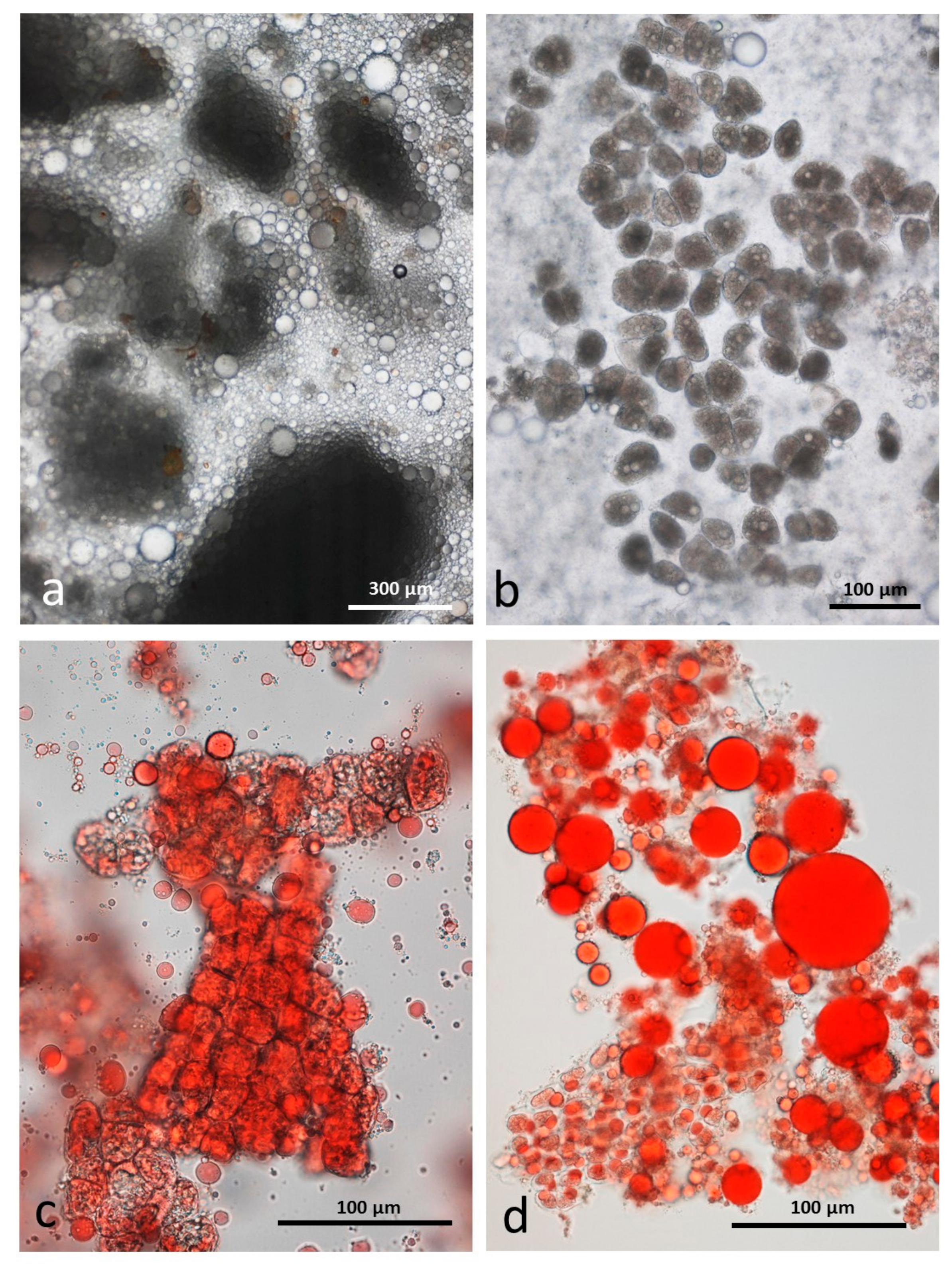
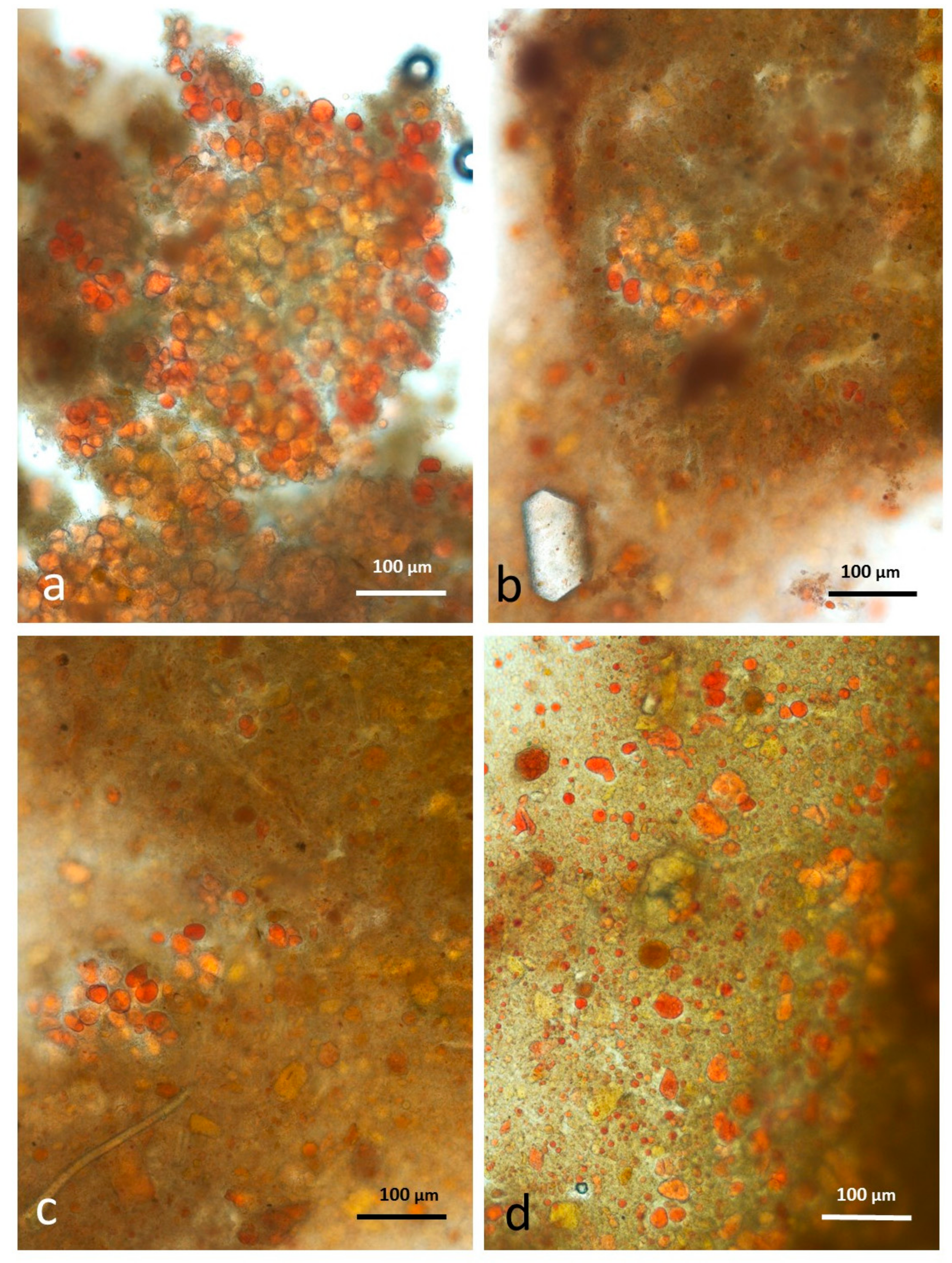
3.4. Microscopy Examination after Simulated Oral Digestion
3.4.1. Chewed NA
3.4.2. Chewed RA
3.4.3. Chewed DA
3.4.4. Chewed AB
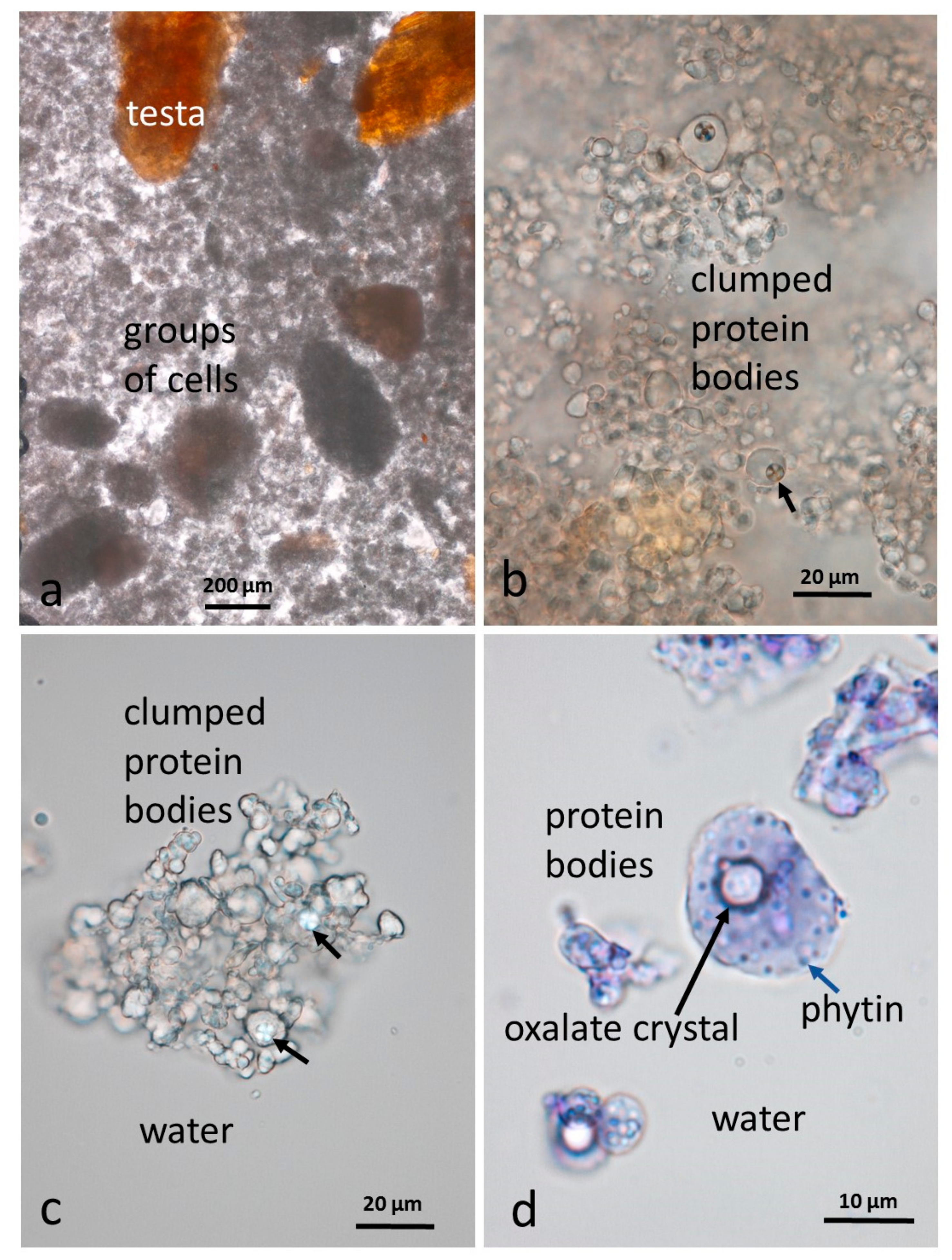
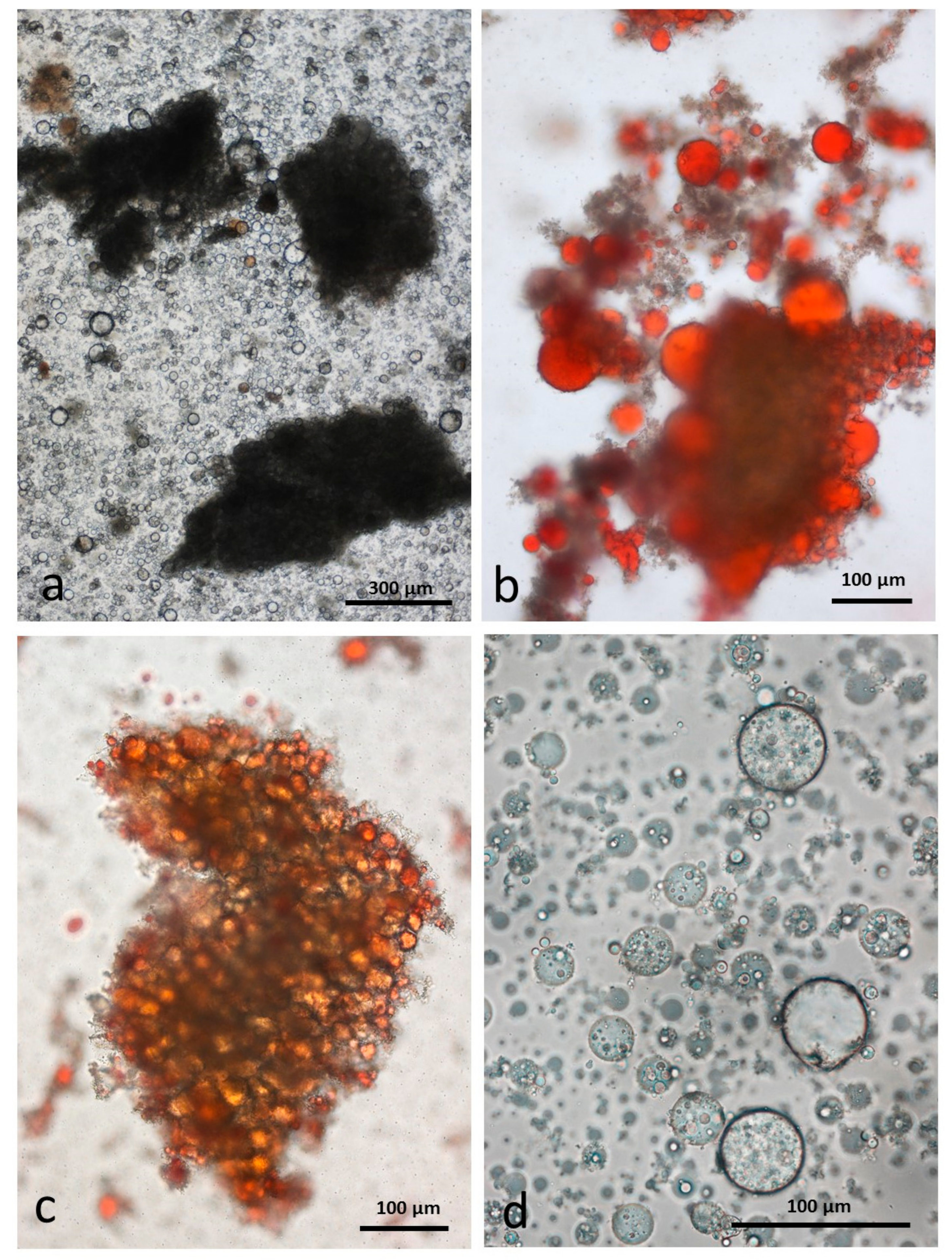
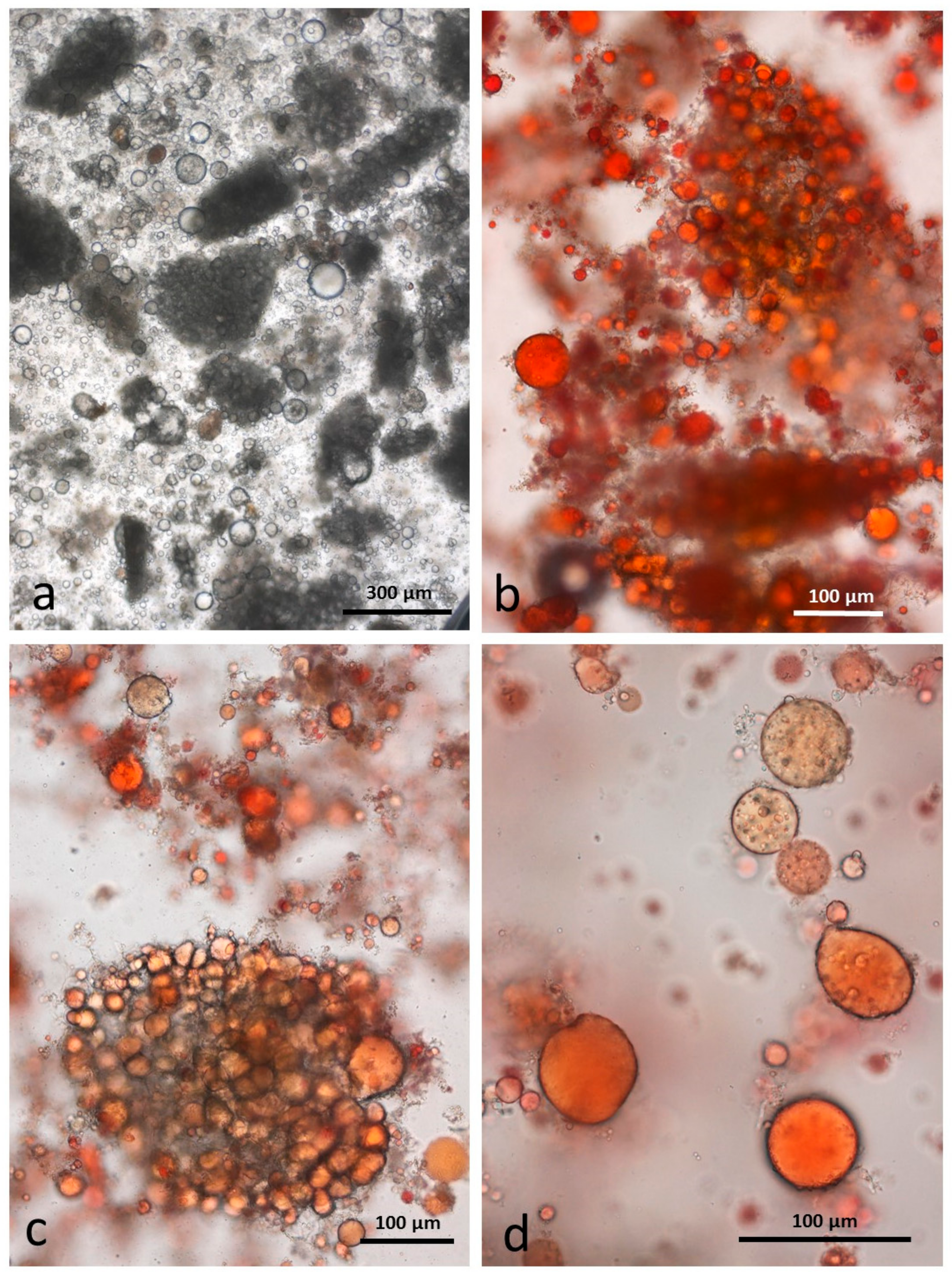
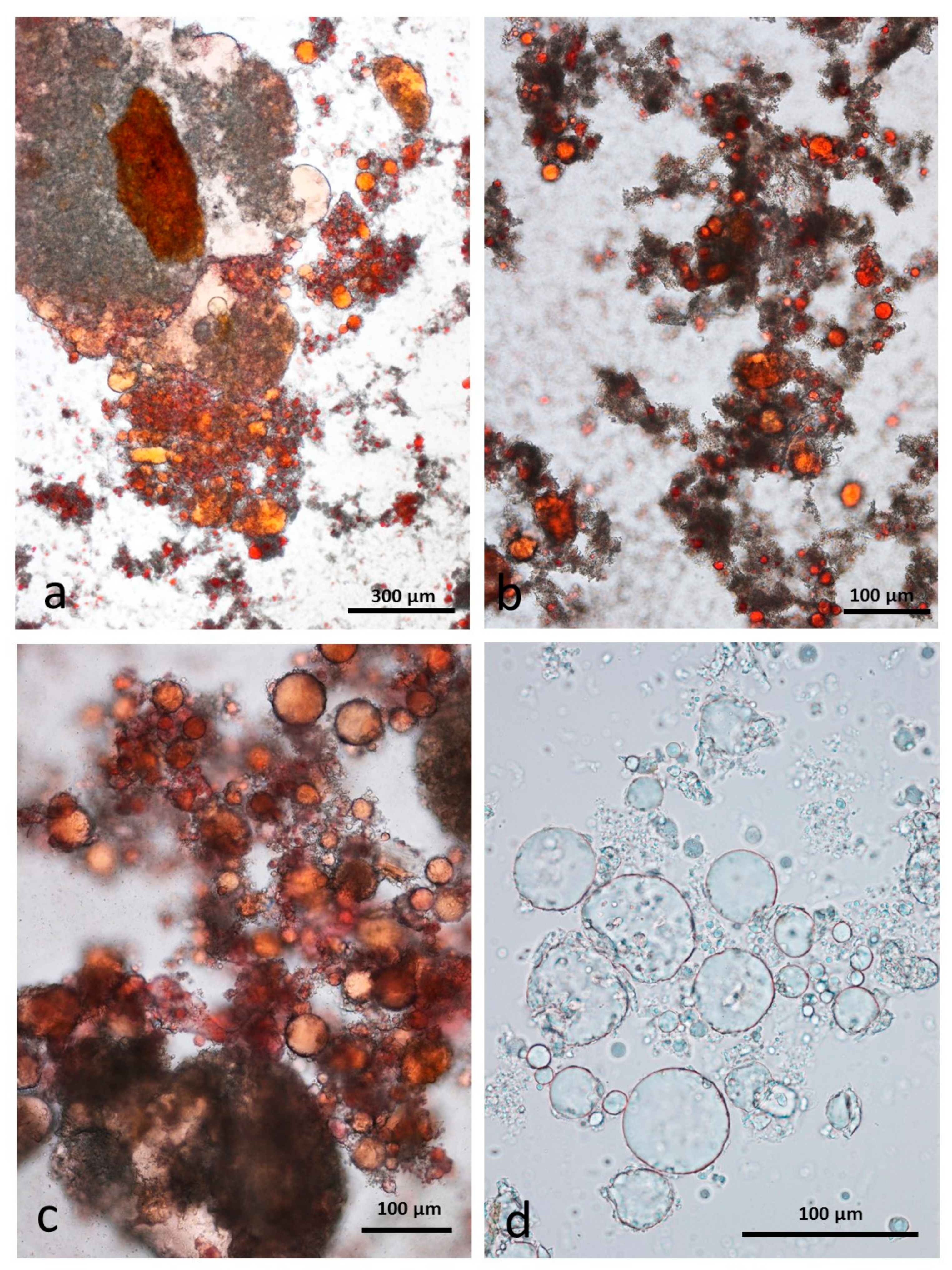
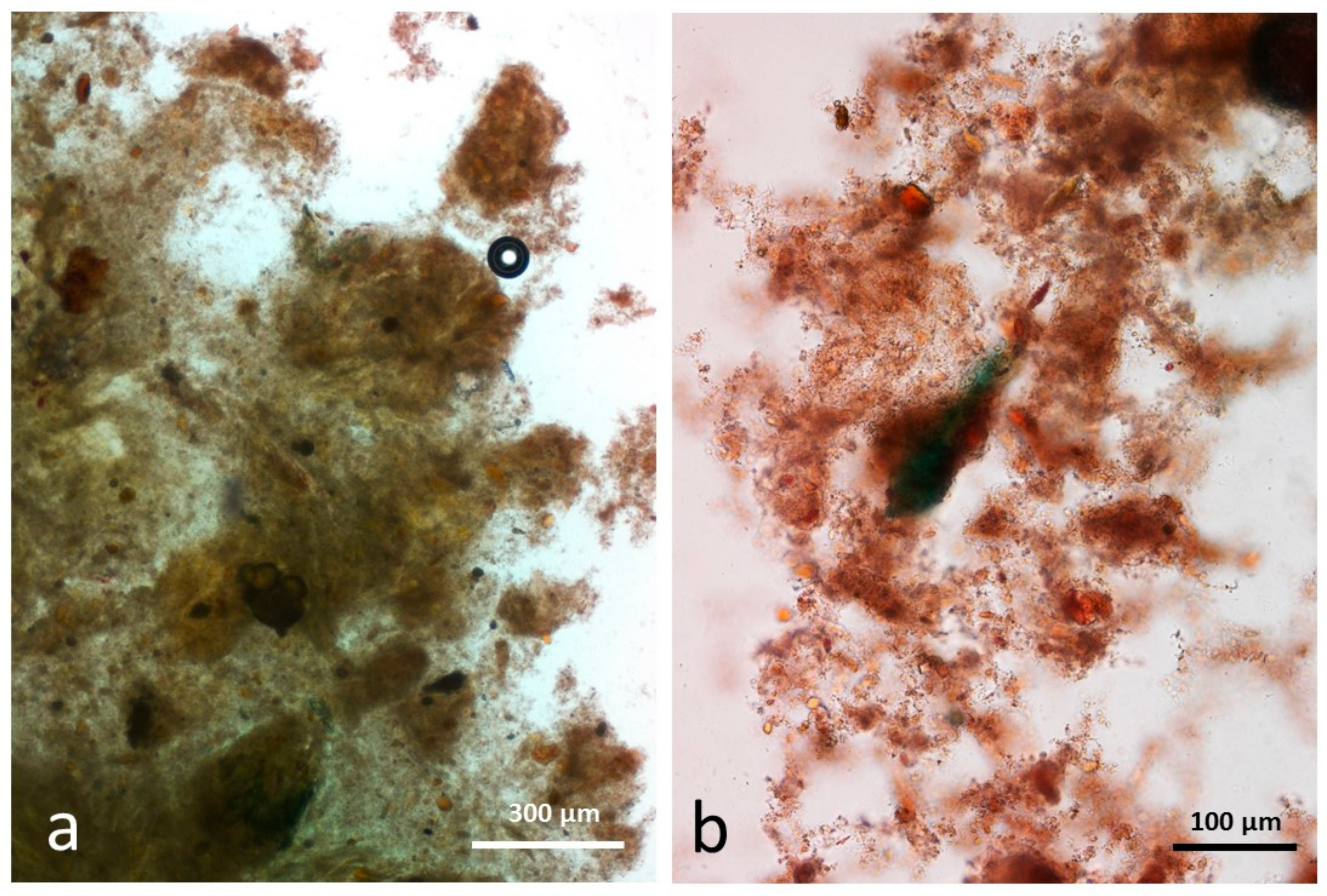
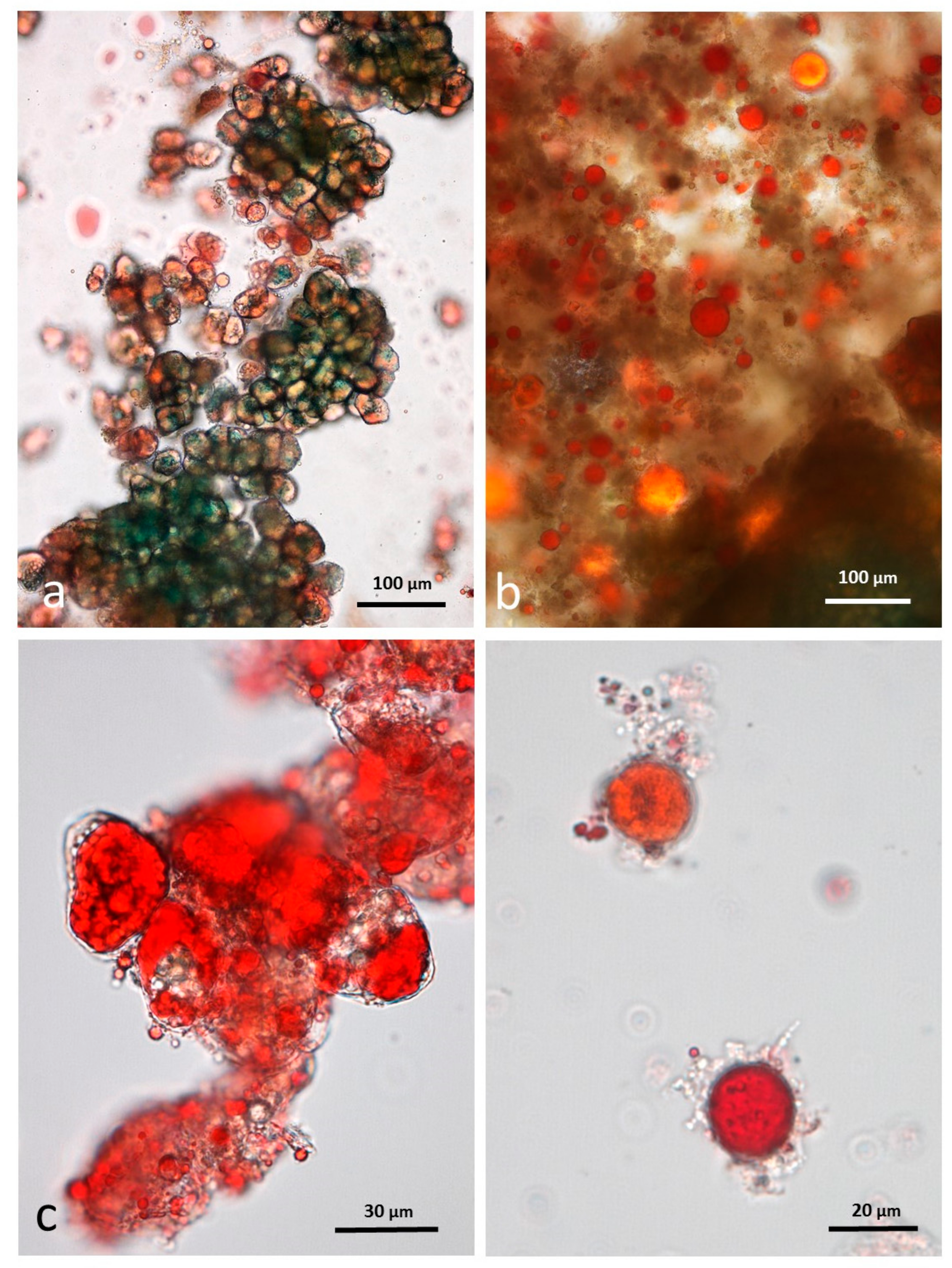
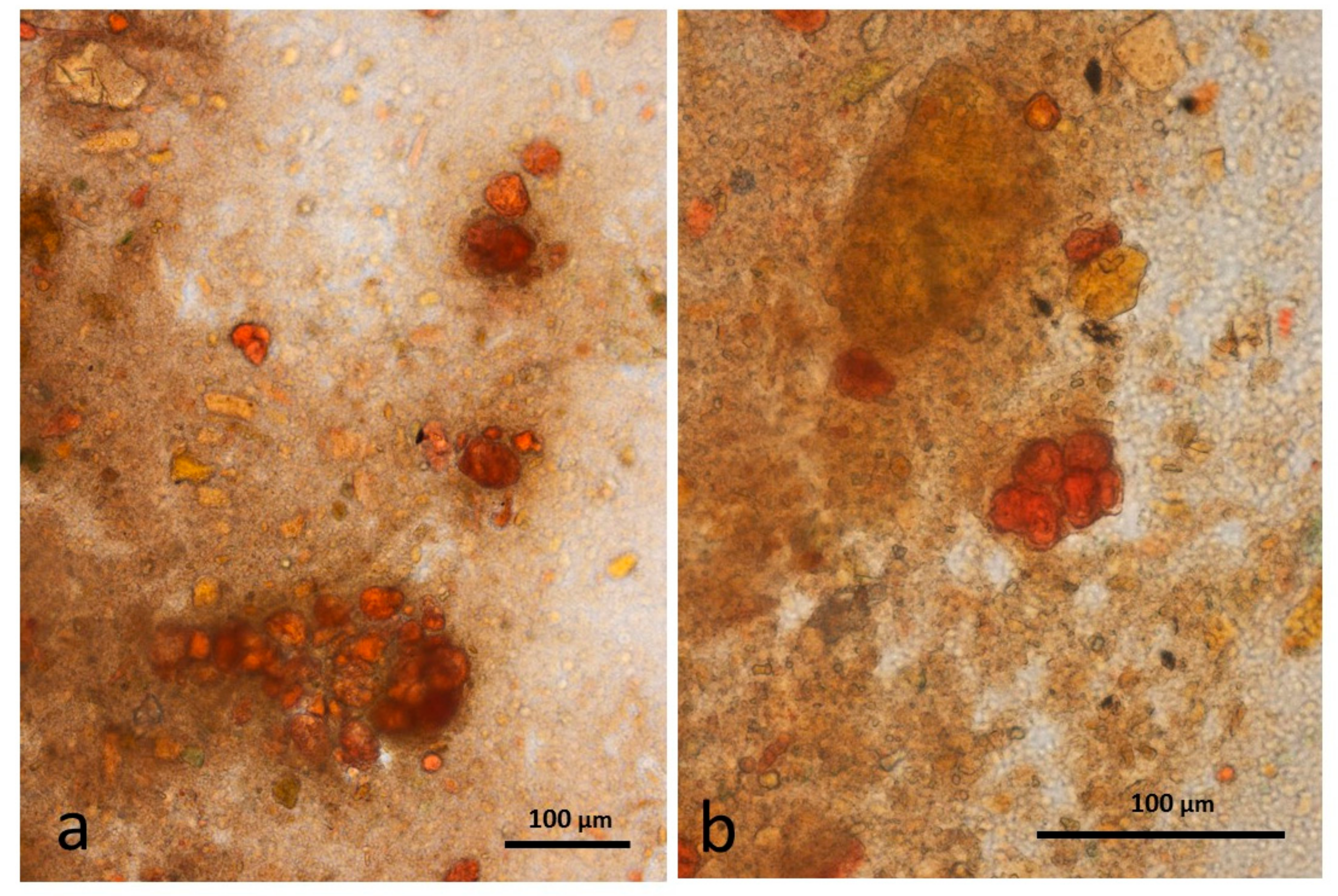
3.5. Microscopy Examination of Faecal Samples
3.6. Control Faeces from Test Diet (No Almonds Consumed) Stained with Sudan IV
3.7. Faeces from Test Diet with NA Stained with Sudan IV
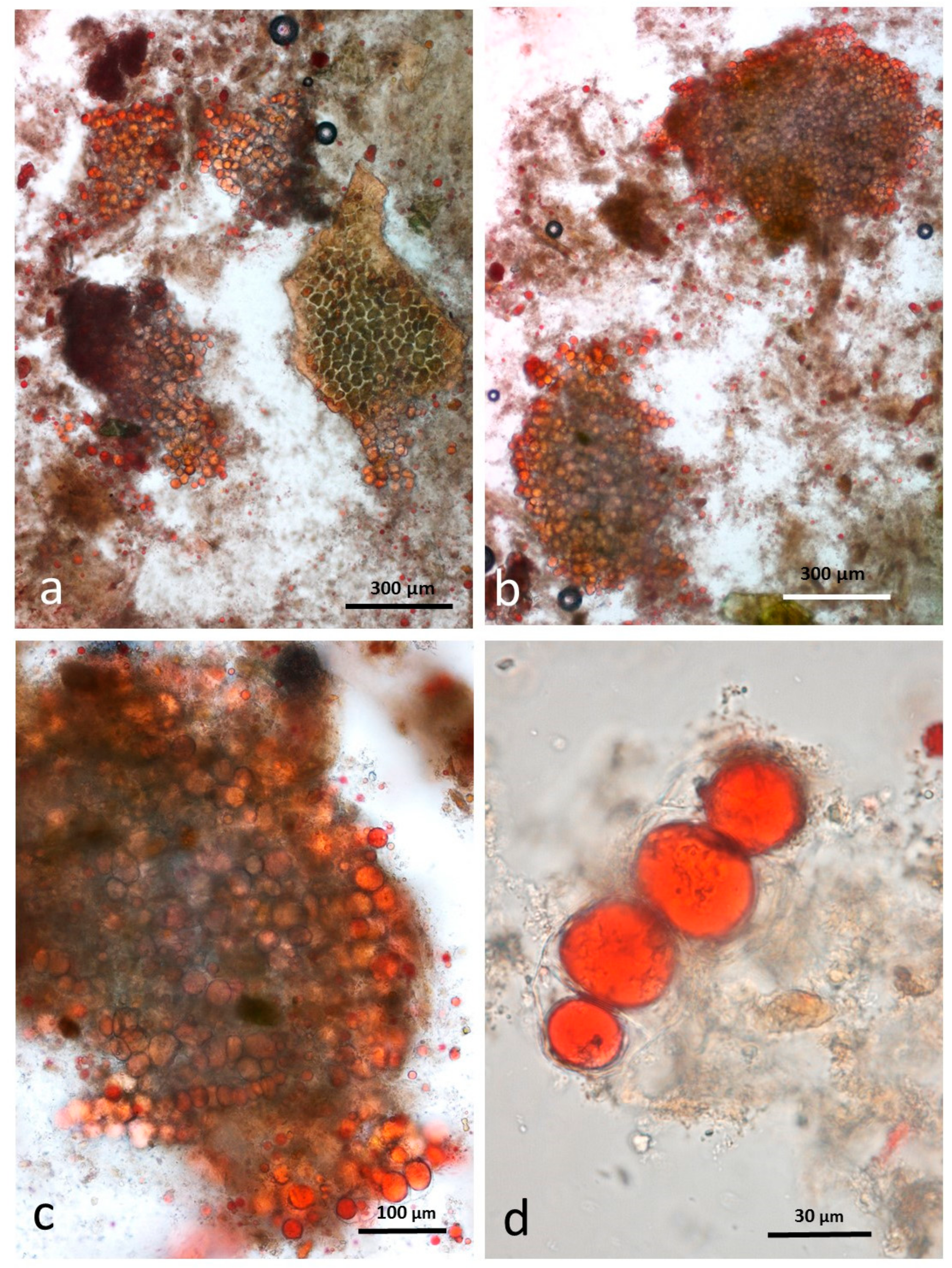
3.8. Faeces from Test Diet with RA Stained with Sudan IV
3.9. Faeces from Test Diet with DA Stained with Sudan IV
3.10. Faeces from Test Diet with AB Stained with Sudan IV
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NA | Natural Almonds |
| RA | Roasted Almonds |
| DA | Diced Almonds |
| AB | Almond Butter |
| GIT | Gastrointestinal Tract |
| ME | Metabolisable Energy |
| HAS | Human Salivary Amylase |
| PSD | Particle Size Distribution |
References
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Yang, J.; Marventano, S.; Micek, A.; Galvano, F.; Kales, S.N. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: A systematic review and meta-analysis of epidemiologic studies. Am. J. Clin. Nutr. 2015, 101, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Falasca, M.; Casari, I.; Maffucci, T. Cancer chemoprevention with nuts. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.R.; Kendall, C.W.; Ren, Y.; Parker, C.; Pacy, J.F.; Waldron, K.W.; Jenkins, D.J. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 2004, 80, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Faulks, R.M.; Rich, G.T.; Lo Turco, V.; Picout, D.R.; Lo Curto, R.B.; Bisignano, G.; Dugo, P.; Dugo, G.; Waldron, K.W.; et al. Release of protein, lipid, and vitamin E from almond seeds during digestion. J. Agric. Food Chem. 2008, 56, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Grassby, T.; Mandalari, G.; Grundy, M.M.; Edwards, C.H.; Bisignano, C.; Trombetta, D.; Smeriglio, A.; Chessa, S.; Ray, S.; Sanderson, J.; et al. In vitro and in vivo modeling of lipid bioaccessibility and digestion from almond muffins: The importance of the cell-wall barrier mechanism. J. Funct. Foods. 2017, 37, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.M.; Grassby, T.; Mandalari, G.; Waldron, K.W.; Butterworth, P.J.; Berry, S.E.; Ellis, P.R. Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am. J. Clin. Nutr. 2015, 101, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Grassby, T.; Picout, D.R.; Mandalari, G.; Faulks, R.M.; Kendall, C.W.; Rich, G.T.; Wickham, M.S.; Lapsley, K.; Ellis, P.R. Modelling of nutrient bioaccessibility in almond seeds based on the fracture properties of their cell walls. Food Funct. 2014, 5, 3096–3106. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.M.; Wilde, P.J.; Butterworth, P.J.; Gray, R.; Ellis, P.R. Impact of cell wall encapsulation of almonds on in vitro duodenal lipolysis. Food Chem. 2015, 185, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.A.; Gebauer, S.K.; Baer, D.J. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am. J. Clin. Nutr. 2012, 96, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Novotny, J.A.; Bornhorst, G.M.; Baer, D.J. Food processing and structure impact the metabolizable energy of almonds. Food Funct. 2016, 7, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Sarò, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Grundy, M.M.; Grassby, T.; Parker, M.L.; Cross, K.L.; Chessa, S.; Bisignano, C.; Barreca, D.; Bellocco, E.; Laganà, G.; et al. The effects of processing and mastication on almond lipid bioaccessibility using novel methods of in vitro digestion modelling and micro-structural analysis. Br. J. Nutr. 2014, 12, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Lott, J.N.A.; Buttrose, M.S. Location of reserves of mineral elements in seed protein bodies: Macadamia nut, walnut, and hazel nut. Can. J. Bot. 1978, 56, 2072–2082. [Google Scholar] [CrossRef]
- Grundy, M.M.; Carrière, F.; Mackie, A.R.; Gray, D.A.; Butterworth, P.J.; Ellis, P.R. The role of plant cell wall encapsulation and porosity in regulating lipolysis during the digestion of almond seeds. Food Funct. 2016, 7, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.E.; Tydeman, E.A.; Lewis, H.B.; Phalora, R.; Rosborough, J.; Picout, D.R.; Ellis, P.R. Manipulation of lipid bioaccessibility of almond seeds influences postprandial lipemia in healthy human subjects. Am. J. Clin. Nutr. 2008, 88, 922–999. [Google Scholar] [PubMed]
- McKiernan, F.; Mattes, R.D. Effects of Peanut Processing on Masticatory Performance during Variable Appetitive States. J. Nutr. Metab. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Cassady, B.A.; Hollis, J.H.; Fulford, A.D.; Considine, R.V.; Mattes, R.D. Mastication of almonds: Effects of lipid bioaccessibility, appetite, and hormone response. Am. J. Clin. Nutr. 2009, 89, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J. Nut consumption and body weight. Am. J. Clin. Nutr. 2003, 78 (Suppl. S3), 647S–650S. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Tey, S.L.; Gray, A.R.; Chisholm, A.; Smith, C.; Fleming, E.; Parnell, W. Association of Nut Consumption with Cardiometabolic Risk Factors in the 2008/2009 New Zealand Adult Nutrition Survey. Nutrients 2015, 7, 7523–7542. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Bes-Rastrollo, M.; Wedick, N.M.; Martinez-Gonzalez, M.A.; Li, T.Y.; Sampson, L.; Hu, F.B. Prospective study of nut consumption, long-term weight change, and obesity risk in women. Am. J. Clin. Nutr. 2009, 89, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
| Almond Meal | Lipid Released Due to Mastication (%) | Predicted Lipid Release (%) * | Total Lipid Potentially Available for Digestion (%) ** |
|---|---|---|---|
| NA | 8.9 ± 0.7 | 9.6 | 8.9 ± 0.7 b |
| RA | 11.8 ± 1.1 a | 12.6 | 11.8 ± 1.1 b |
| DA | 12.4 ± 0.8 a | 9.6 | 12.4 ± 0.8 b |
| AB | 6.2 ± 0.4 | 6.4 | 94.0 ± 4.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandalari, G.; Parker, M.L.; Grundy, M.M.-L.; Grassby, T.; Smeriglio, A.; Bisignano, C.; Raciti, R.; Trombetta, D.; Baer, D.J.; Wilde, P.J. Understanding the Effect of Particle Size and Processing on Almond Lipid Bioaccessibility through Microstructural Analysis: From Mastication to Faecal Collection. Nutrients 2018, 10, 213. https://doi.org/10.3390/nu10020213
Mandalari G, Parker ML, Grundy MM-L, Grassby T, Smeriglio A, Bisignano C, Raciti R, Trombetta D, Baer DJ, Wilde PJ. Understanding the Effect of Particle Size and Processing on Almond Lipid Bioaccessibility through Microstructural Analysis: From Mastication to Faecal Collection. Nutrients. 2018; 10(2):213. https://doi.org/10.3390/nu10020213
Chicago/Turabian StyleMandalari, Giuseppina, Mary L. Parker, Myriam M.-L. Grundy, Terri Grassby, Antonella Smeriglio, Carlo Bisignano, Roberto Raciti, Domenico Trombetta, David J. Baer, and Peter J. Wilde. 2018. "Understanding the Effect of Particle Size and Processing on Almond Lipid Bioaccessibility through Microstructural Analysis: From Mastication to Faecal Collection" Nutrients 10, no. 2: 213. https://doi.org/10.3390/nu10020213
APA StyleMandalari, G., Parker, M. L., Grundy, M. M.-L., Grassby, T., Smeriglio, A., Bisignano, C., Raciti, R., Trombetta, D., Baer, D. J., & Wilde, P. J. (2018). Understanding the Effect of Particle Size and Processing on Almond Lipid Bioaccessibility through Microstructural Analysis: From Mastication to Faecal Collection. Nutrients, 10(2), 213. https://doi.org/10.3390/nu10020213









