A Prenatal DHA Test to Help Identify Women at Increased Risk for Early Preterm Birth: A Proposal
Abstract
1. Introduction
2. Relationship between Maternal DHA Levels and Preterm Birth
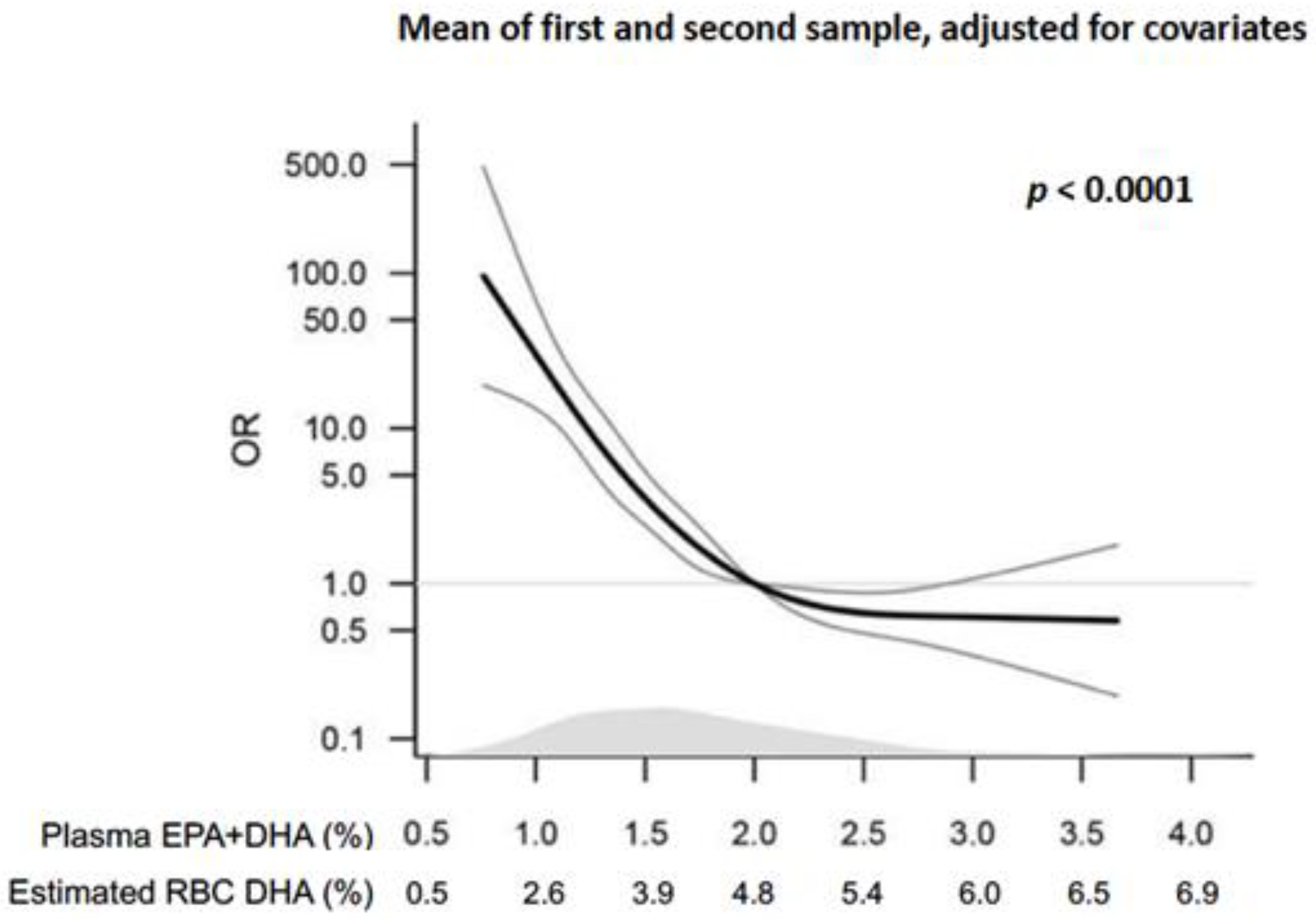
2.1. Epidemiology
2.2. Randomized Controlled Trials: Low-Risk Populations
2.3. Randomized Controlled Trials: High Risk Pregnancies
2.4. Upcoming Trials
2.5. Other Maternal and Child Outcomes Related to DHA
3. Is an RBC DHA of >5% a Reasonable Target?
4. Are There Risks Associated with an RBC DHA >5% in Pregnancy?
5. How Might a Target RBC DHA Level Be Used in Obstetric Practice?
6. Why Not Just Recommend Higher DHA Intake to Everyone and Not Test?
7. Possible Mechanisms for a DHA Effect on Early Preterm Birth
8. Conclusions
Author Contributions
Conflicts of Interest
References
- March of Dimes. Born Too Soon: Estimated Rates of Preterm Birth per 100 Live Births. 2010. Available online: https://www.marchofdimes.org/mission/global-preterm.aspx (accessed on 8 October 2018).
- Harris, W.S.; Baack, M.L. Beyond building better brains: Bridging the docosahexaenoic acid (DHA) gap of prematurity. J Perinatol. 2015, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Makrides, M.; Sim, N.; McPhee, A.; Quinlivan, J.; Gibson, R.; Umberger, W. Analysis of hospital cost outcome of DHA-rich fish-oil supplementation in pregnancy: Evidence from a randomized controlled trial. Prostaglandins Leukot. Essent. Fatty Acids 2015, 102, 5–11. [Google Scholar] [CrossRef]
- March of Dimes; The Partnership for Maternal Newborn; Child Health; Save the Children; World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Lauterbach, R. EPA + DHA in prevention of early preterm birth—Do we know how to apply it? EBioMedicine 2018, 35, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Halldorsson, T.I.; Thorne-Lyman, A.L.; Strom, M.; Gortz, S.; Granstrom, C.; Nielsen, P.H.; Wohlfahrt, J.; Lykke, J.A.; Langhoff-Roos, J.; et al. Plasma Concentrations of Long Chain N-3 Fatty Acids in Early and Mid-Pregnancy and Risk of Early Preterm Birth. EBioMedicine 2018. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Innis, S. Position of the American Dietetic Association and Dietitians of Canada: Dietary Fatty Acids. J. Am. Diet Assoc. 2007, 107, 1599–1611. [Google Scholar] [PubMed]
- March of Dimes. Vitamins and Other Nutrients during Pregnancy. Available online: https://www.marchofdimes.org/pregnancy/vitamins-and-other-nutrients-during-pregnancy.aspx (accessed on 8 October 2018).
- Food and Agriculture Organization of the United Nations. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FOA Food and Nutrition Paper: Geneva, Switzerland, 2010. [Google Scholar]
- Koletzko, B.; Cetin, I.; Brenna, J.T.; Perinatal Lipid Intake Working Group; Child Health Fundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Society for Clinical Nutrition and Metabolism; European Society for Paediatric Gastroenterology; Hepatology and Nutrition, Committee on Nutrition; et al. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lien, E.; Agostoni, C.; Bohles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Perinat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, T.M.; Lyden, E.; Anderson-Berry, A.; Hanson, C. Omega-3 Fatty Acid Intake of Pregnant Women and Women of Childbearing Age in the United States: Potential for Deficiency? Nutrients 2017, 9, 197. [Google Scholar] [CrossRef]
- Brantsaeter, A.L.; Englund-Ogge, L.; Haugen, M.; Birgisdottir, B.E.; Knutsen, H.K.; Sengpiel, V.; Myhre, R.; Alexander, J.; Nilsen, R.M.; Jacobsson, B.; et al. Maternal intake of seafood and supplementary long chain n-3 poly-unsaturated fatty acids and preterm delivery. BMC Pregnancy Childbirth 2017, 17, 41. [Google Scholar] [CrossRef]
- Leventakou, V.; Roumeliotaki, T.; Martinez, D.; Barros, H.; Brantsaeter, A.L.; Casas, M.; Charles, M.A.; Cordier, S.; Eggesbo, M.; van Eijsden, M.; et al. Fish intake during pregnancy, fetal growth, and gestational length in 19 European birth cohort studies. Am. J. Clin. Nutr. 2014, 99, 506–516. [Google Scholar] [CrossRef]
- Olsen, S.F.; Secher, N.J. Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: Prospective cohort study. BMJ 2002, 324, 447. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Kleinman, K.P.; Olsen, S.F.; Rich-Edwards, J.W.; Gillman, M.W. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: Results from a US pregnancy cohort. Am. J. Epidemiol. 2004, 160, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Colombo, J.; Gajewski, B.J.; Gustafson, K.M.; Mundy, D.; Yeast, J.; Georgieff, M.K.; Markley, L.A.; Kerling, E.H.; Shaddy, D.J. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 2013, 97, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy (review). Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ji, X.; Zhang, L.; Hou, Z.; Li, C.; Tong, Y. Fish oil supplementation improves pregnancy outcomes and size of the newborn: A meta-analysis of 21 randomized controlled trials. J. Matern. Fetal Neonatal. Med. 2016, 29, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Wong, M.; Rogozinska, E.; Thangaratinam, S. Effects of omega-3 fatty acids in prevention of early preterm delivery: A systematic review and meta-analysis of randomized studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Imhoff-Kunsch, B.; Briggs, V.; Goldenberg, T.; Ramakrishnan, U. Effect of n-3 long-chain polyunsaturated fatty acid intake during pregnancy on maternal, infant, and child health outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. 1), 91–107. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P.; DOMInO Investigative Team. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef]
- Horvath, A.; Koletzko, B.; Szajewska, H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Br. J. Nutr. 2007, 98, 253–259. [Google Scholar] [CrossRef]
- Olsen, S.F.; Secher, N.J.; Tabor, A.; Weber, T.; Walker, J.J.; Gluud, C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. Fish Oil Trials in Pregnancy (FOTIP) Team. BJOG 2000, 107, 382–395. [Google Scholar] [CrossRef]
- Olsen, S.F.; Osterdal, M.L.; Salvig, J.D.; Weber, T.; Tabor, A.; Secher, N.J. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: A randomised clinical trial with fish oil. Eur. J. Clin. Nutr. 2007, 61, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.; Thom, E.; Klebanoff, M.A.; Thorp, J., Jr.; Sorokin, Y.; Varner, M.W.; Wapner, R.J.; Caritis, S.N.; Iams, J.D.; Carpenter, M.W.; et al. Omega-3 fatty acid supplementation to prevent recurrent preterm birth: A randomized controlled trial. Obstet. Gynecol. 2010, 115, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, M.A.; Harper, M.; Lai, Y.; Thorp, J., Jr.; Sorokin, Y.; Varner, M.W.; Wapner, R.J.; Caritis, S.N.; Iams, J.D.; Carpenter, M.W.; et al. Fish consumption, erythrocyte fatty acids, and preterm birth. Obstet. Gynecol. 2011, 117, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Best, K.; Makrides, M. Omega-3 Fats to Reduce the Incidence of Prematurity: The ORIP Trial. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363566 (accessed on 10 October 2018).
- Carlson, S.E.; Gajewski, B.J.; Valentine, C.J.; Rogers, L.K.; Weiner, C.P.; DeFranco, E.A.; Buhimschi, C.S. Assessment of DHA on reducing early preterm birth: The ADORE randomized controlled trial protocol. BMC Pregnancy Childbirth 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Smuts, C.M.; Huang, M.; Mundy, D.; Plasse, T.; Major, S.; Carlson, S.E. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet. Gynecol. 2003, 101, 469–479. [Google Scholar] [PubMed]
- Ramakrishnan, U.; Stein, A.D.; Parra-Cabrera, S.; Wang, M.; Imhoff-Kunsch, B.; Juarez-Marquez, S.; Rivera, J.; Martorell, R. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: Randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr. Bull. 2010, 31, S108–S116. [Google Scholar] [CrossRef] [PubMed]
- Cheruku, S.R.; Montgomery-Downs, H.E.; Farkas, S.L.; Thoman, E.B.; Lammi-Keefe, C.J. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am.J.Clin.Nutr. 2002, 76, 608–613. [Google Scholar] [CrossRef]
- Gustafson, K.M.; Carlson, S.E.; Colombo, J.; Yeh, H.W.; Shaddy, D.J.; Li, S.; Kerling, E.H. Effects of docosahexaenoic acid supplementation during pregnancy on fetal heart rate and variability: A randomized clinical trial. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 331–338. [Google Scholar] [CrossRef]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef]
- Helland, I.B.; Saugstad, O.D.; Smith, L.; Saarem, K.; Solvoll, K.; Ganes, T.; Drevon, C.A. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics 2001, 108, E82. [Google Scholar] [CrossRef]
- Braarud, H.C.; Markhus, M.W.; Skotheim, S.; Stormark, K.M.; Froyland, L.; Graff, I.E.; Kjellevold, M. Maternal DHA Status during Pregnancy Has a Positive Impact on Infant Problem Solving: A Norwegian Prospective Observation Study. Nutrients 2018, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Kannass, K.N.; Shaddy, D.J.; Kundurthi, S.; Maikranz, J.M.; Anderson, C.J.; Blaga, O.M.; Carlson, S.E. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004, 75, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadottir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdottir, S.; et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Vinding, R.K.; Stokholm, J.; Sevelsted, A.; Sejersen, T.; Chawes, B.L.; Bonnelykke, K.; Thorsen, J.; Howe, L.D.; Krakauer, M.; Bisgaard, H. Effect of fish oil supplementation in pregnancy on bone, lean, and fat mass at six years: Randomised clinical trial. BMJ 2018, 362, k3312. [Google Scholar] [CrossRef] [PubMed]
- Markhus, M.W.; Skotheim, S.; Graff, I.E.; Froyland, L.; Braarud, H.C.; Stormark, K.M.; Malde, M.K. Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS ONE 2013, 8, e67617. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Simmer, K.; Neumann, M.; Gibson, R. Changes in the polyunsaturated fatty acids of breast milk from mothers of full-term infants over 30 wk of lactation. Am. J. Clin. Nutr. 1995, 61, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Kagawa, Y.; Kimura, F.; Miyazawa, T.; Saito, S.; Arima, T.; Nakai, K.; Yaegashi, N. Polyunsaturated Fatty Acid Levels in Maternal Erythrocytes of Japanese Women during Pregnancy and after Childbirth. Nutrients 2017, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Markhus, M.W.; Rasinger, J.D.; Malde, M.K.; Froyland, L.; Skotheim, S.; Braarud, H.C.; Stormark, K.M.; Graff, I.E. Docosahexaenoic Acid Status in Pregnancy Determines the Maternal Docosahexaenoic Acid Status 3-, 6- and 12 Months Postpartum. Results from a Longitudinal Observational Study. PLoS ONE 2015, 10, e0136409. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Holt, P.G.; Calder, P.C.; Taylor, A.L.; Prescott, S.L. Effects of n-3 polyunsaturated fatty acid supplementation in pregnancy on maternal and fetal erythrocyte fatty acid composition. Eur. J. Clin. Nutr. 2004, 58, 429–437. [Google Scholar] [CrossRef]
- Harris, W.S.; Pottala, J.V.; Varvel, S.A.; Borowski, J.J.; Ward, J.N.; McConnell, J.P. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: Observations from 160,000 patients. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 257–263. [Google Scholar] [CrossRef]
- Oken, E.; Kleinman, K.P.; Berland, W.E.; Simon, S.R.; Rich-Edwards, J.W.; Gillman, M.W. Decline in fish consumption among pregnant women after a national mercury advisory. Obstet. Gynecol. 2003, 102, 346–351. [Google Scholar] [PubMed]
- Food and Drug Administration. Advice about Eating Fish, from the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice, Availability; Health and Human Services, Ed.; Food and Drug Administration: Washington, DC, USA, 2017; pp. 6571–6574. [Google Scholar]
- Harris, W.S.; Polreis, J. Measurement of the Omega-3 Index in Dried Blood Spots. Ann. Clin. Lab. Res. 2016, 4, e1–e7. [Google Scholar] [CrossRef]
- Finer, L.B.; Zolna, M.R. Declines in Unintended Pregnancy in the United States, 2008–2011. N. Engl. J. Med. 2016, 374, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Asselin, G.; Merette, C.; Poulin, M.J.; Dodin, S. Validation of an FFQ for evaluation of EPA and DHA intake. Public Health Nutr. 2009, 12, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.M.; Rifas-Shiman, S.L.; Olsen, S.F.; Gold, D.R.; Gillman, M.W.; Oken, E. Associations of maternal prenatal dietary intake of n-3 and n-6 fatty acids with maternal and umbilical cord blood levels. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Al, M.; van Houwelingen, A.C.; Hornstra, G. Long-chain polyunsaturated fatty acids, pregnancy, and pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, S285–S291. [Google Scholar] [CrossRef] [PubMed]
- Shireman, T.I.; Kerling, E.H.; Gajewski, B.J.; Colombo, J.; Carlson, S.E. Docosahexaenoic acid supplementation (DHA) and the return on investment for pregnancy outcomes. Prostaglandins Leukot. Essent. Fatty Acids 2016, 111, 8–10. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Robinson, J.N.; Challis, J.R. The control of labor. N. Engl. J. Med. 1999, 341, 660–666. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Kinnun, J.J.; Leng, X.; Williams, J.A.; Wassall, S.R. How polyunsaturated fatty acid modify molecular organization in membranes: Insight from NMR studies of model sysytems. Biochim. Biophys. Acta 2015, 1848, 211–219. [Google Scholar] [CrossRef]
- Radosinska, J.; Kurahara, L.H.; Hiraishi, K.; Viczenczova, C.; Egan Benova, T.; Szeiffova Bacova, B.; Dosenko, V.; Navarova, J.; Obsitnik, B.; Imanaga, I.; et al. Modulation of cardiac connexin-43 by omega-3 fatty acid ethyl-ester supplementation demonstrated in spontaneously diabetic rats. Physiol. Res. 2015, 64, 795–806. [Google Scholar]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, K.H.; Harris, W.S. A Prenatal DHA Test to Help Identify Women at Increased Risk for Early Preterm Birth: A Proposal. Nutrients 2018, 10, 1933. https://doi.org/10.3390/nu10121933
Jackson KH, Harris WS. A Prenatal DHA Test to Help Identify Women at Increased Risk for Early Preterm Birth: A Proposal. Nutrients. 2018; 10(12):1933. https://doi.org/10.3390/nu10121933
Chicago/Turabian StyleJackson, Kristina H., and William S. Harris. 2018. "A Prenatal DHA Test to Help Identify Women at Increased Risk for Early Preterm Birth: A Proposal" Nutrients 10, no. 12: 1933. https://doi.org/10.3390/nu10121933
APA StyleJackson, K. H., & Harris, W. S. (2018). A Prenatal DHA Test to Help Identify Women at Increased Risk for Early Preterm Birth: A Proposal. Nutrients, 10(12), 1933. https://doi.org/10.3390/nu10121933




