Sourdough Fermentation Favorably Influences Selenium Biotransformation and the Biological Effects of Flatbread
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Piadina Preparation: Formulation, Fermentation, and Cooking
2.3. In Vitro Digestion
2.4. Determination of Se Content and Se Species
2.5. Total Antioxidant Capacity of Gastro-Intestinal Hydrolysates
2.6. Cell Cultures, L3 Supplementation, and Oxidative Stress
2.7. Cell Viability
2.8. Lactate Dehydrogenase Activity
2.9. Thiobarbituric Acid Reactive Substances Level
2.10. Cytosolic Total Antioxidant Capacity
2.11. Glutathione Peroxidase Activity
2.12. Thioredoxine Reductase Activity
2.13. Cell Protein Content
2.14. Statistical Analysis
3. Results
3.1. Se Concentration, Bioaccessibility, Speciation, and Total Antioxidant Capacity of Gastro-Intestinal Hydrolysates
3.2. Effects in Cultured Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 2, 609. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Selenium in human health and disease: An overview. In Selenium; Michalke, B., Ed.; Springer: Cham, Switzerland, 2018; pp. 3–26. ISBN 978-3-319-95389-2. [Google Scholar]
- Steinbrenner, H.; Speckmann, B.; Sies, H. Toward understanding success and failures in the use of selenium for cancer prevention. Antioxid. Redox. Signal. 2013, 19, 181–191. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The epidemiology of selenium and human cancer. Adv. Cancer Res. 2017, 136, 1–48. [Google Scholar] [CrossRef]
- Jabłońska, E.; Reszka, E. Selenium and epigenetics in cancer: Focus on DNA methylation. Adv. Cancer Res. 2017, 136, 193–234. [Google Scholar] [CrossRef]
- Chen, Y.C.; Prabhu, K.S.; Mastro, A.M. Is selenium a potential treatment for cancer metastasis? Nutrients 2013, 5, 1149–1168. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of sodium selenite in the prevention and treatment of cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef]
- Jain, R.B.; Choi, Y.S. Normal reference ranges for and variability in the levels of blood manganese and selenium by gender, age, and race/ethnicity for general U.S. population. J. Trace Elem. Med. Biol. 2015, 30, 142–152. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. In vivo and in vitro testing for selenium and selenium compounds bioavailability assessment in foodstuff. Crit. Rev. Food Sci. Nutr. 2017, 57, 805–833. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Thiry, C.; Ruttens, A.; Pussemier, L.; Schneider, Y.J. An in vitro investigation of species-dependent intestinal transport of selenium and the impact of this process on selenium bioavailability. Br. J. Nutr. 2013, 109, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Calasso, M.; Campanella, D.; De Angelis, M.; Gobbetti, M. Use of sourdough fermentation and mixture of wheat, chickpea, lentil and bean flours for enhancing the nutritional, texture and sensory characteristics of white bread. Int. J. Food Microbiol. 2014, 180, 78–87. [Google Scholar] [CrossRef]
- Guerzoni, M.E.; Gianotti, A.; Serrazanetti, D.I. Fermentation as a tool to improve healthy properties of bread. In Flour and Breads and Their Fortification in Health and Disease Prevention, 1st ed.; Preedy, V., Watson, R., Patel, V., Eds.; Elsevier: London, UK, 2011; pp. 385–393. ISBN 9780123808868. [Google Scholar]
- Galle, S.; Arendt, E.K. Exopolysaccharides from sourdough lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef]
- Saa, D.T.; Di Silvestro, R.; Dinelli, G.; Gianotti, A. Effect of sourdough fermentation and baking process severity on dietary fiber and phenolic compounds of immature wheat flour bread. LWT-Food Sci. Technol. 2017, 83, 26–32. [Google Scholar] [CrossRef]
- Nionelli, L.; Rizzello, C.G. Sourdough-based biotechnologies for the production of gluten-free foods. Foods 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.; Ripari, V. Composition and function of sourdough microbiota: From ecological theory to bread quality. Int. J. Food Microbiol. 2016, 239, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cevoli, C.; Gianotti, A.; Troncoso, R.; Fabbri, A. Quality evaluation by physical tests of a traditional Italian flat bread piadina during storage and shelf-life improvement with sourdough and enzymes. Eur. Food Res. Technol. 2015, 240, 1081–1089. [Google Scholar] [CrossRef]
- Bhatia, P.; Aureli, F.; D’Amato, M.; Prakash, R.; Cameotra, S.S.; Prakash Nagaraja, T.; Cubadda, F. Selenium bioaccessibility and speciation in biofortified Pleurotus mushrooms grown on selenium-rich agricultural residues. Food Chem. 2013, 140, 225–230. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento da Silva, E.; Aureli, F.; D’Amato, M.; Raggi, A.; Cadore, S.; Cubadda, F. Selenium bioaccessibility and speciation in selenium-enriched lettuce: Investigation of the selenocompounds liberated after in vitro simulated human digestion using two-dimensional HPLC-ICP-MS. J. Agric. Food Chem. 2017, 65, 3031–3038. [Google Scholar] [CrossRef] [PubMed]
- Marcolini, E.; Babini, E.; Bordoni, A.; Di Nunzio, M.; Laghi, L.; Maczó, A.; Picone, G.; Szerdahelyi, E.; Valli, V.; Capozzi, F. Bioaccessibility of the bioactive peptide carnosine during in vitro digestion of cured beef meat. J. Agric. Food Chem. 2015, 63, 4973–4978. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Res. Int. 2018, 113, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ghini, V.; Di Nunzio, M.; Tenori, L.; Valli, V.; Danesi, F.; Capozzi, F.; Luchinat, C.; Bordoni, A. Evidence of a DHA signature in the lipidome and metabolome of human hepatocytes. Int. J. Mol. Sci. 2017, 18, 359. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Valli, V.; Tomás-Cobos, L.; Tomás-Chisbert, T.; Murgui-Bosch, L.; Danesi, F.; Bordoni, A. Is cytotoxicity a determinant of the different in vitro and in vivo effects of bioactives? BMC Complement. Altern. Med. 2017, 17, 453. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Valli, V.; Bordoni, A. PUFA and oxidative stress. Differential modulation of the cell response by DHA. Int. J. Food Sci. Nutr. 2016, 67, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.; Danesi, F.; Gianotti, A.; Di Nunzio, M.; Saa, D.T.; Bordoni, A. Antioxidative and anti-inflammatory effect of in vitro digested cookies baked using different types of flours and fermentation methods. Food Res. Int. 2016, 88, 256–262. [Google Scholar] [CrossRef]
- Valli, V.; Taccari, A.; Di Nunzio, M.; Danesi, F.; Bordoni, A. Health benefits of ancient grains. Comparison among bread made with ancient, heritage and modern grain flours in human cultured cells. Food Res. Int. 2018, 107, 206–215. [Google Scholar] [CrossRef]
- Cubadda, F.; Aureli, F.; Ciardullo, S.; D’Amato, M.; Raggi, A.; Acharya, R.; Reddy, R.A.V.; Prakash, N.T. Changes in selenium speciation associated with increasing tissue concentrations of selenium in wheat grain. J. Agric. Food Chem. 2010, 58, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.J.; Fairweather-Tait, S.J.; Broadley, M.R.; Dickinson, S.J.; Foot, I.; Knott, P.; McGrath, S.P.; Mowat, H.; Norman, K.; Scott, P.R.; et al. Selenium concentration and speciation in biofortified flour and bread: Retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem. 2011, 126, 1771–1778. [Google Scholar] [CrossRef]
- Aureli, F.; Ouerdane, L.; Bierla, K.; Szpunar, J.; Prakash, N.T.; Cubadda, F. Identification of selenosugars and other low-molecular weight selenium metabolites in high-selenium cereal crops. Metallomics 2012, 4, 968–978. [Google Scholar] [CrossRef]
- Bierla, K.; Godin, S.; Lobinski, R.; Szpunar, J. Advances in electrospray mass spectrometry for the selenium speciation: Focus on Se-rich yeast. TrAC Trends Anal. Chem. 2018, 104, 87–94. [Google Scholar] [CrossRef]
- Landete, J.M.; Rodríguez, H.; De las Rivas, B.; Muñoz, R. High-added-value antioxidants obtained from the degradation of wine phenolics by Lactobacillus plantarum. J. Food. Prot. 2007, 70, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl. Environ. Microbiol. 2012, 78, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Peran, L.; Camuesco, D.; Comalada, M.; Nieto, A.; Concha, A.; Adrio, J.L.; Olivares, M.; Xaus, J.; Zarzuelo, A.; Galvez, J. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int. J. Colorectal. Dis. 2006, 21, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.H.; Byun, J.R. Occurrence of glutathione sulphydryl (GSH) and antioxidant activities in probiotic lactobacillus spp. Asian-Aust. J. Anim. Sci. 2004, 17, 1582–1585. [Google Scholar] [CrossRef]
- Ferri, M.; Serrazanetti, D.I.; Tassoni, A.; Baldissarri, M.; Gianotti, A. Improving the functional and sensorial profile of cereal-based fermented foods by selecting Lactobacillus plantarum strains via a metabolomics approach. Food Res. Int. 2016, 89, 1095–1105. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Selenium and redox signaling. Arch. Biochem. Biophys. 2017, 617, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Mustacich, D.; Powis, G. Thioredoxin reductase. Biochem. J. 2000, 346, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Monsefi, N.; Moritz, A.; Beiras-Fernandez, A. Selenium and cardiovascular surgery: An overview. Curr. Drug. Saf. 2012, 7, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Banning, A. Sulforaphane and selenium, partners in adaptive response and prevention of cancer. Free Radic. Res. 2006, 40, 775–787. [Google Scholar] [CrossRef]
- Hrdina, J.; Banning, A.; Kipp, A.; Loh, G.; Blaut, M.; Brigelius-Flohé, R. The gastrointestinal microbiota affects the selenium status and selenoprotein expression in mice. J. Nutr. Biochem. 2009, 20, 638–648. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Hesketh, J.E.; Sinclair, B.R.; Koolaard, J.P.; Roy, N.C. Selenium-enriched foods are more effective at increasing glutathione peroxidase (GPx) activity compared with selenomethionine: A meta-analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef]
- Cozza, G.; Rossetto, M.; Bosello-Travain, V.; Maiorino, M.; Roveri, A.; Toppo, S.; Zaccarin, M.; Zennaro, L.; Ursini, F. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: The polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Radic. Biol. Med. 2017, 112, 1–11. [Google Scholar] [CrossRef]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef]
- Miller, S.; Walker, S.W.; Arthur, J.R.; Nicol, F.; Pickard, K.; Lewin, M.H.; Howie, A.F.; Beckett, G.J. Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase. Clin. Sci. (Lond.) 2001, 100, 543–550. [Google Scholar] [CrossRef]
- Erkekoğlu, P.; Rachidi, W.; De Rosa, V.; Giray, B.; Favier, A.; Hincal, F. Protective effect of selenium supplementation on the genotoxicity of di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate treatment in LNCaP cells. Free Radic. Biol. Med. 2010, 49, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dacosta, C.; Wang, W.; Zhou, Z.; Liu, M.; Bao, Y. Synergy between sulforaphane and selenium in protection against oxidative damage in colonic CCD841 cells. Nutr. Res. 2015, 35, 610–617. [Google Scholar] [CrossRef] [PubMed]
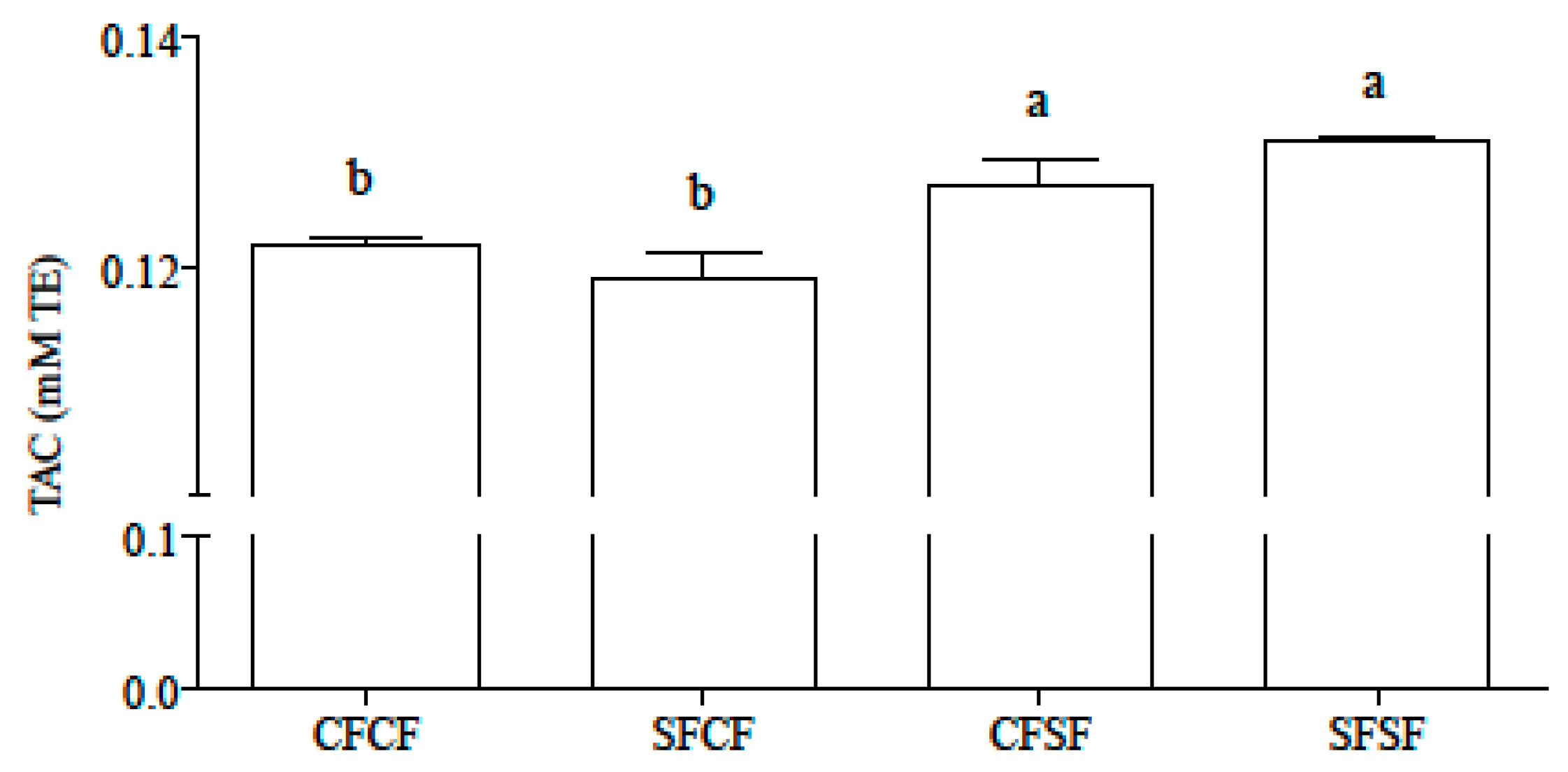
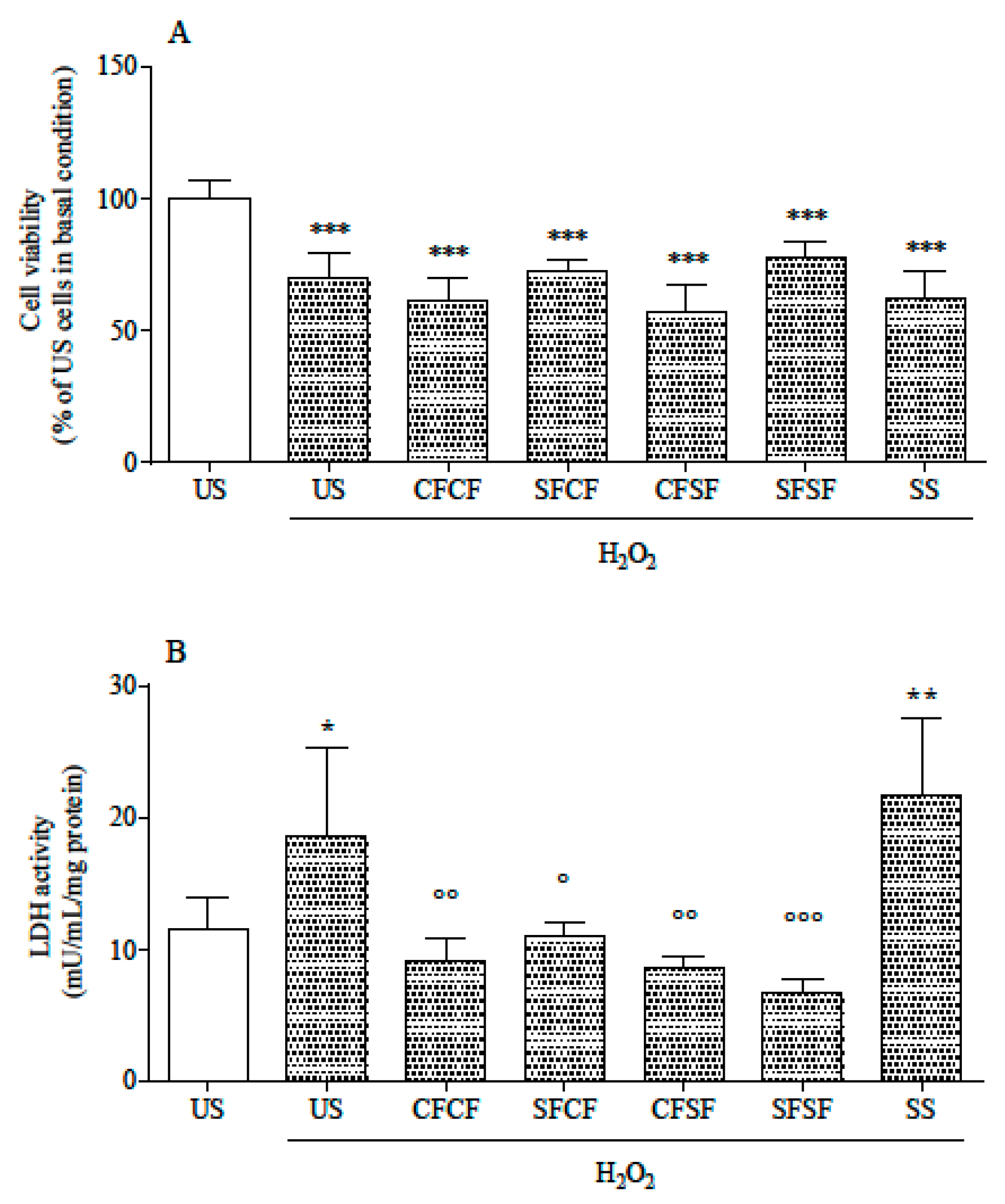
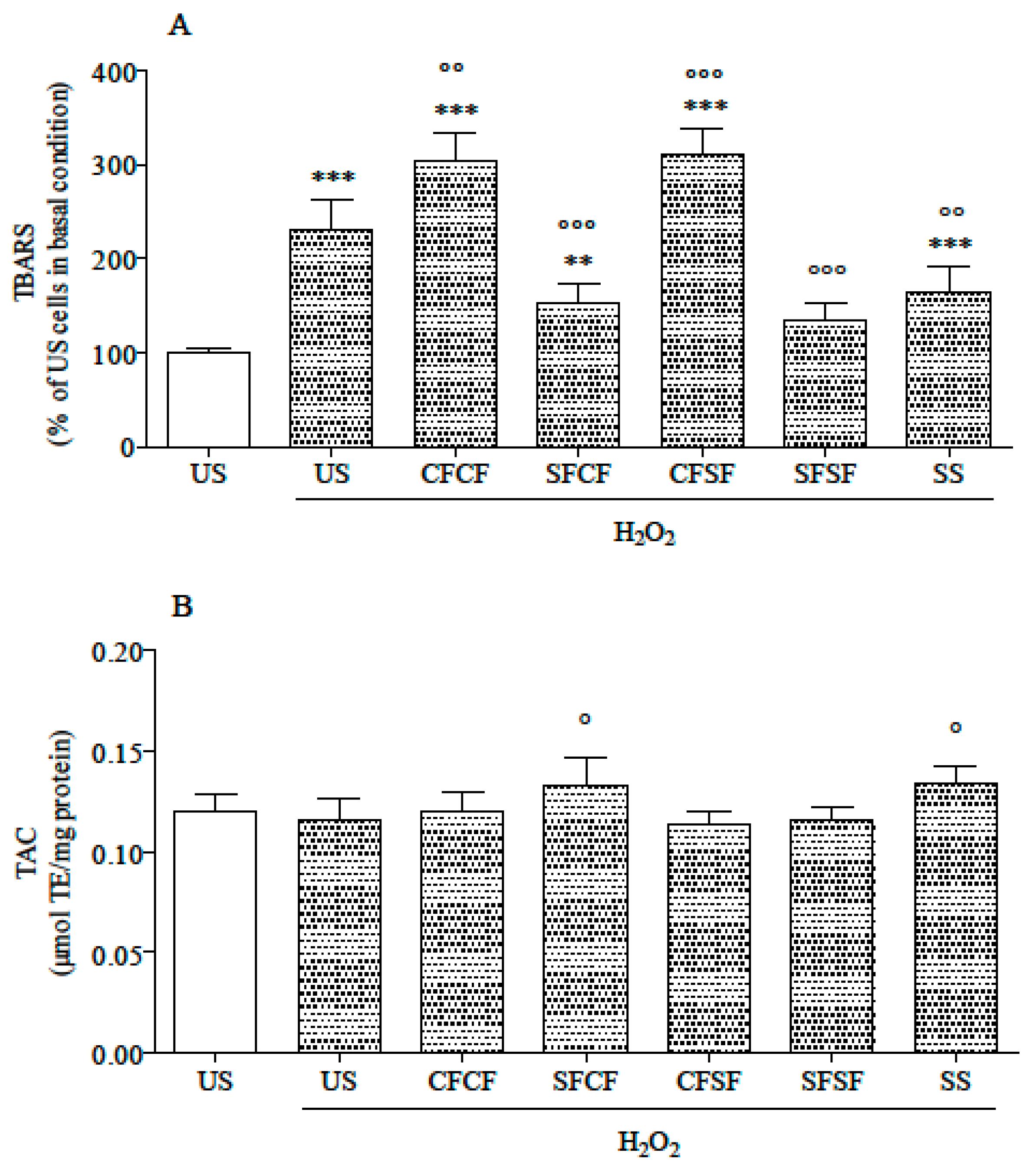
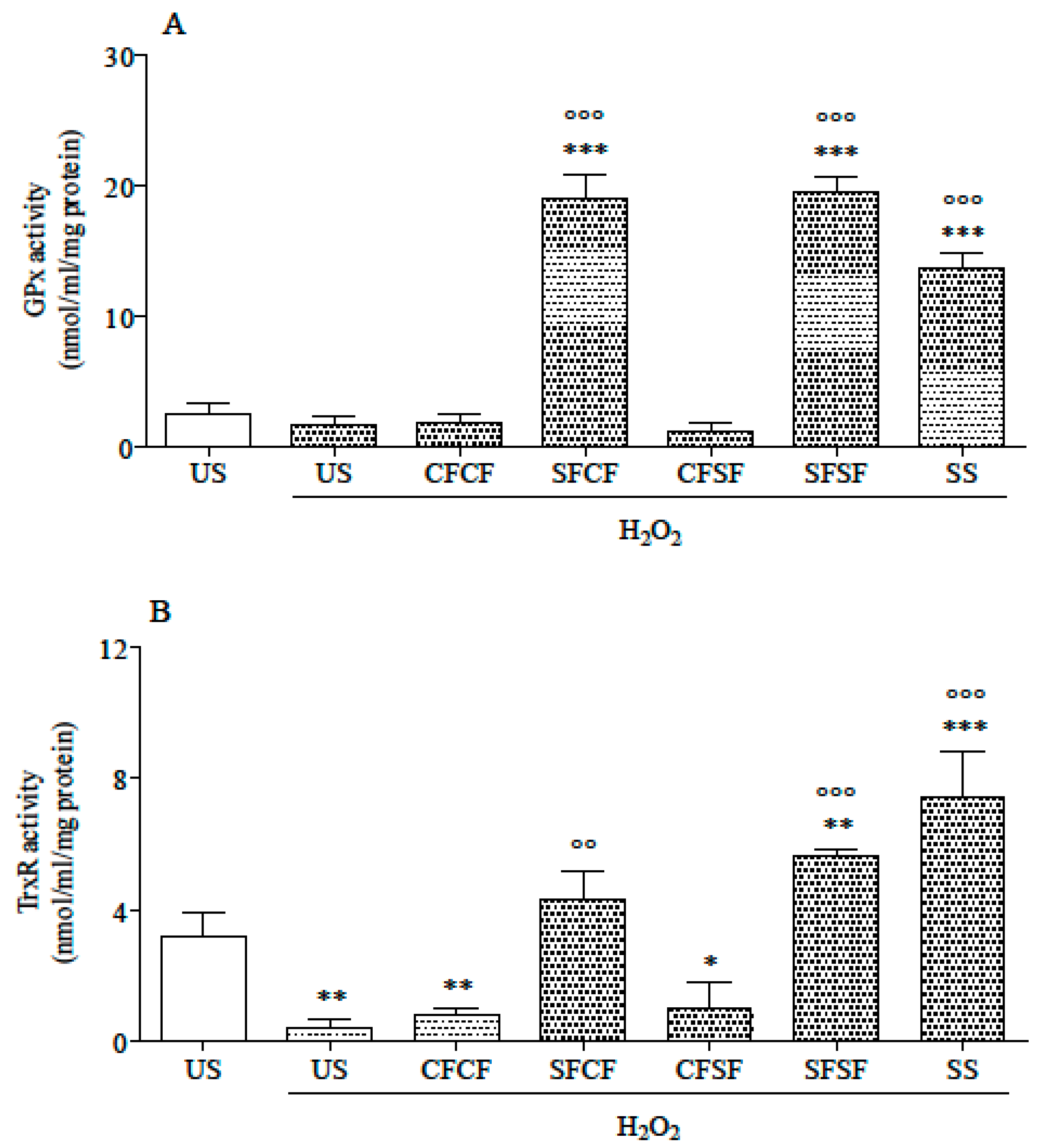
| Ingredients | CFCF | SFCF | CFSF | SFSF |
|---|---|---|---|---|
| Flour (g) | 624.5 | 624.5 | 490.8 | 490.8 |
| Clarified pork fat (g) | 62.5 | 62.5 | 49 | 49 |
| Water (mL) | 281.7 | 281.7 | 4.5 | 4.5 |
| NaCl (g) | 12.9 | 12.9 | 10.1 | 10.1 |
| Starch (g) | 18.4 | 18.4 | 14.4 | 14.4 |
| Na2SeO3 (mg) | - | 5.66 | - | 5.66 |
| Sourdough starter (g) | - | - | 431.2 | 431.2 |
| CFCF | SFCF | CFSF | SFSF | |||||
|---|---|---|---|---|---|---|---|---|
| TQ | L3 | TQ | L3 | TQ | L3 | TQ | L3 | |
| Total Se (µg/mL) | 0.022 ± 0.001 | 0.006 ± 0.000 | 0.264 ± 0.004 | 0.127 ± 0.003 | 0.012 ± 0.001 | 0.007 ± 0.000 | 0.265 ± 0.003 | 0.109 ± 0.004 |
| Void | MeSeCys (% Total Se) | SeMet (% Total Se) | SeIV (% Total Se) | SeVI (% Total Se) | |
|---|---|---|---|---|---|
| TQ fraction | |||||
| CFCF—TQ | 17 | 5 | 56 | - | 21 |
| SFCF—TQ | 3 | - | 14 | 84 | - |
| CFSF—TQ | 13 | - | 66 | - | 20 |
| SFSF—TQ | 10 | 2 | 53 | 35 | - |
| L3 fraction | |||||
| CFCF—L3 | 10 | - | 63 | - | 27 |
| SFCF—L3 | 2 | 1 | 12 | 85 | - |
| CFSF—L3 | 8 | - | 59 | - | 33 |
| SFSF—L3 | 9 | 5 | 46 | 40 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nunzio, M.; Bordoni, A.; Aureli, F.; Cubadda, F.; Gianotti, A. Sourdough Fermentation Favorably Influences Selenium Biotransformation and the Biological Effects of Flatbread. Nutrients 2018, 10, 1898. https://doi.org/10.3390/nu10121898
Di Nunzio M, Bordoni A, Aureli F, Cubadda F, Gianotti A. Sourdough Fermentation Favorably Influences Selenium Biotransformation and the Biological Effects of Flatbread. Nutrients. 2018; 10(12):1898. https://doi.org/10.3390/nu10121898
Chicago/Turabian StyleDi Nunzio, Mattia, Alessandra Bordoni, Federica Aureli, Francesco Cubadda, and Andrea Gianotti. 2018. "Sourdough Fermentation Favorably Influences Selenium Biotransformation and the Biological Effects of Flatbread" Nutrients 10, no. 12: 1898. https://doi.org/10.3390/nu10121898
APA StyleDi Nunzio, M., Bordoni, A., Aureli, F., Cubadda, F., & Gianotti, A. (2018). Sourdough Fermentation Favorably Influences Selenium Biotransformation and the Biological Effects of Flatbread. Nutrients, 10(12), 1898. https://doi.org/10.3390/nu10121898









