New Protocol for Production of Reduced-Gluten Wheat Bread and Pasta and Clinical Effect in Patients with Irritable Bowel Syndrome: A randomised, Double-Blind, Cross-Over Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sourdough Fermentation
2.2. Microbiological and Immunological Analyses of Fermented Wheat Flours
2.3. Wheat Bread and Pasta Making
2.4. Proximate Composition
2.5. Texture and Image Analysis
2.6. Color Measurement
2.7. Hydration Test
2.8. Cooking Loss and Water Absorption
2.9. Sensory Analysis
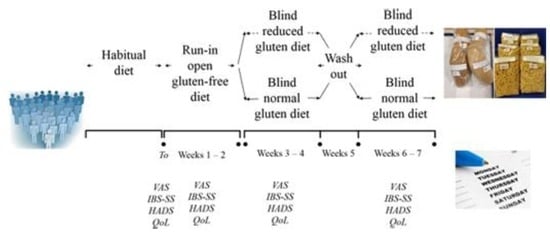
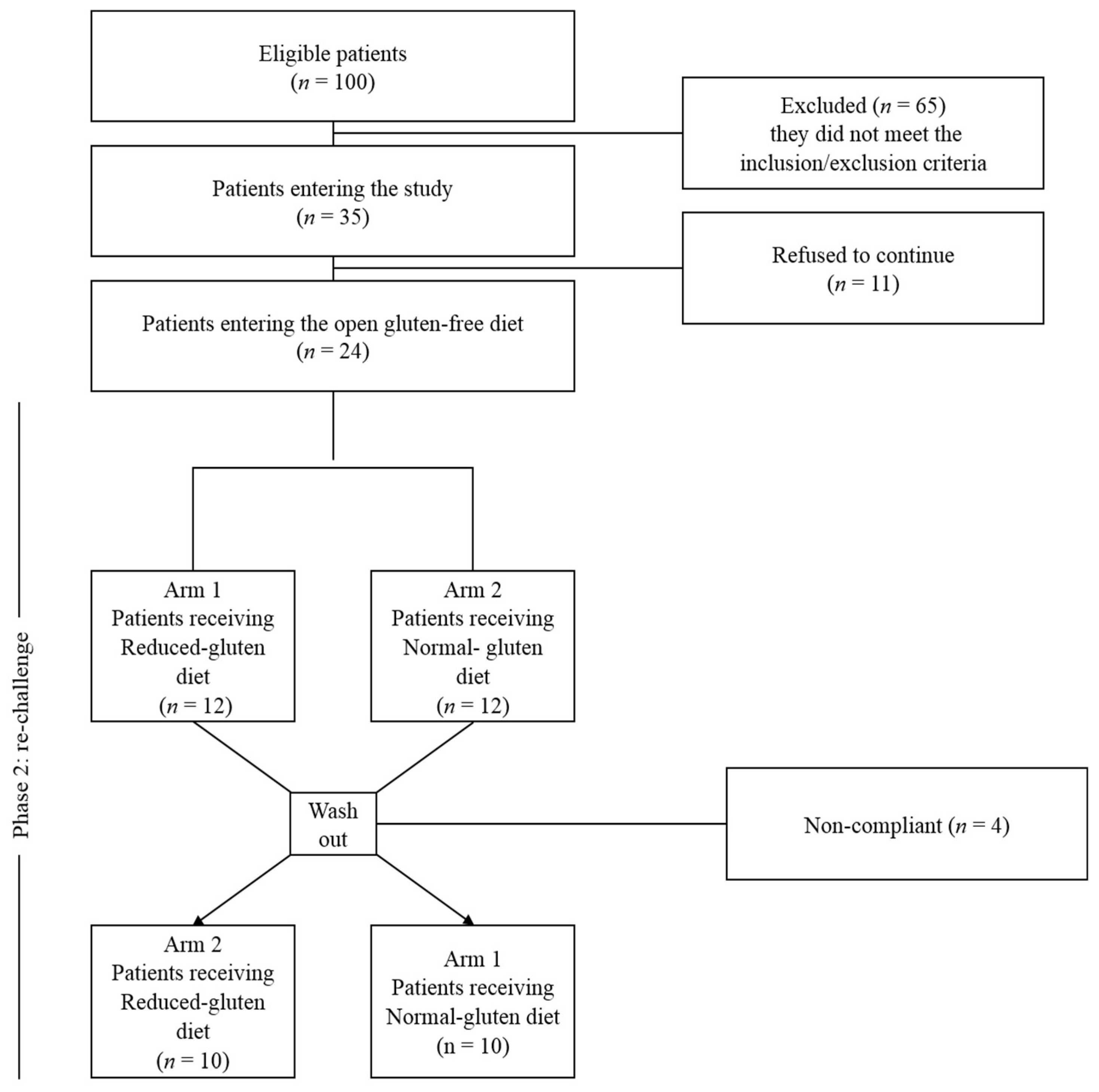
2.10. Patients and Study Design
2.11. Randomization and Blinding
2.12. Double Blind Cross Over Challenge
2.13. Outcomes
2.14. Statistical Analysis
3. Results
3.1. Characterization of Sourdoughs
3.2. Chemical and Nutritional Characteristics of Wheat Bread and Pasta with Reduced Content of Gluten
3.3. Texture and Color Characteristics
3.4. Hydration Test, Cooking Loss and Water Absorption
3.5. Sensory Characteristics
3.6. In vivo Gluten Challenge
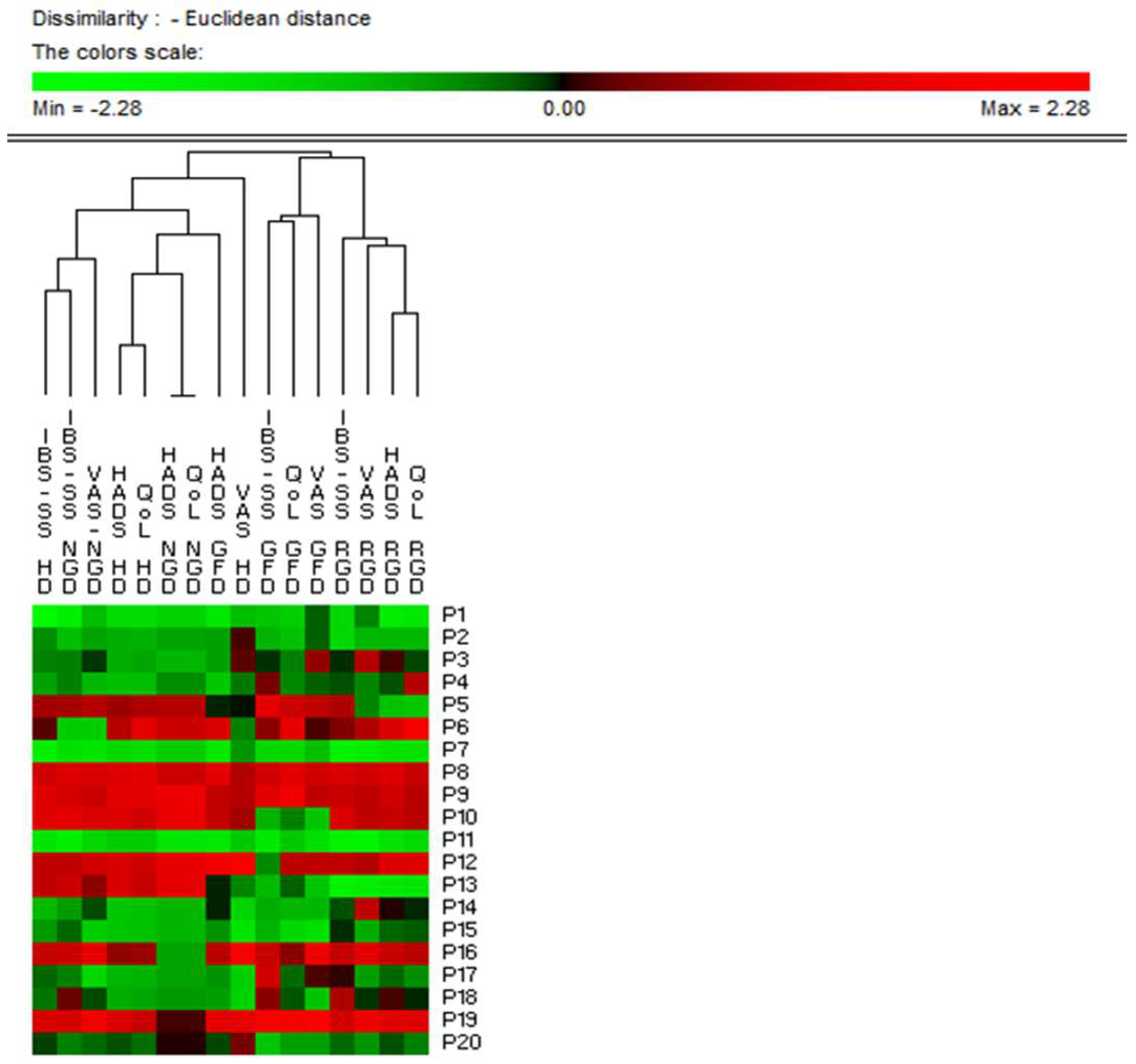
3.7. Clinical Scores
3.7.1. Open Gluten-Free Diet
3.7.2. Double Blind Cross over challenge
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Giorgio, R.; Volta, P.; Gibson, R. Sensitivity to wheat, gluten and FODMAPs in IBS: Fact or fiction? Gut 2016, 65, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sayuk, G.S.; Gyawali, C.P. Irritable bowel syndrome: Modern concepts and management options. Am. J. Med. 2015, 128, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Canavan, C.; West, J.; Card, T. Review article: The economic impact of the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2014, 40, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Yeo, W.S. A Meta-Analysis of the Clinical Use of Curcumin for Irritable Bowel Syndrome (IBS). J. Clin. Med. 2018, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Beyder, A.; Mazzone, A.; Strege, P.R. Loss of function of the voltage-gated sodium channel NaV1.5 (Channelopathies) in patiens with irritable bowel syndrome. Gastroenterology 2014, 146, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Lasch, K.; Zhou, W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology: The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Chang, L. The role of stress on physiological responses and clinical symptoms in irritable bowel syndrome. Gastroenterology 2011, 140, 761–765. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J. Gastroenterol. 2014, 20, 14105–14125. [Google Scholar] [CrossRef]
- Liebregts, T.; Adam, B.; Bredack, C.; Röth, A.; Heinzel, S.; Lester, S.; Downie-Doyle, S.; Smith, R.; Drew, P.; Talley, N.J.; Holtmann, G. Immune activation in patiens with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, K.S. Irritable bowel syndrome: Emerging paradigm in pathophysiology. World. J. Gastroenterol. 2014, 20, 2456–2469. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; De Bari, O.; Lembo, A.; Ballou, S. Irritable bowel syndrome and diet. Gastroenterol. Rep. 2017, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Arendt, E.K. Low FODMAPs and gluten-free foods for irritable bowel syndrome treatment: Lights and shadows. Food Res. Int. 2017, 110, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Aleadini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The overlapping area of non-celiac gluten sensitivity (NCGS) and wheat-sensitive irritable bowel syndrome (IBS): An update. Nutrients 2017, 9, 1268–1284. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology 2018, 154, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Turco, R.; Salvatore, S.; Miele, E.; Romano, C.; Marseglia, G.L.; Staiano, A. Does a low FODMAPs diet reduce symptoms of functional abdominal pain disorders? A systematic review in adult and paediatric population, on behalf of Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Martin, L.D.; Staudacher, H.M.; Lomer, M.C.E. The low FODMAP diet in the management of irritable bowel syndrome: An evidence-based review of FODMAP restriction, reintroduction and personalization in clinical practice. J. Hum. Nutr. Diet. 2018, 31, 239–255. [Google Scholar] [CrossRef]
- Zahedi, M.J.; Behrouz, V.; Azimi, M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patiens with diarrhea predominant irritable bowel syndrome: A randomized controlled trial. J. Gastroenterol. Hepatol. 2018, 33, 1192–1199. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barret, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten causes gastrointestinal symptoms in subjects without celiac disease: Adouble-blind randomized placebo-controlled trial. Am. J. Gastroenterol. 2011, 6, 508–514. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P.A. A Systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef]
- Pourmand, H.; Keshteli, A.H.; Saneei, P.; Daghaghzadeh, H.; Esmaillzadeh, A.; Adibi, P. Adherence to a low FODMAP diet in relation to symptoms of irritable bowel syndrome in iranian adults. Dig. Dis. Sci. 2018, 63, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Del Negro, V.; Angeletti, P.M.; Latella, G. Low-FODMAP diet improves irritable bowel syndrome symptoms: A meta-analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, R.; Koskenpato, J.; Hongisto, S.M.; Loponen, J.; Poussa, T.; Huang, X.; Sontag-Strohm, T.; Salmenkari, H.; Korpela, R. Pilot Study: Comparison of sourdough wheat bread and yeast-fermented wheat bread in individuals with wheat sensitivity and irritable bowel syndrome. Nutrients 2017, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-di-monosaccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC. Med. 2012, 10, 13. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P. Gluten and wheat intolerance today: Are modern wheat strains involved? Int. J. Food. Sci. Nutr. 2014, 65, 577–581. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Curiel, J.A.; Nionelli, L.; Vincentini, O.; Di Cagno, R.; Silano, M.; Gobbetti, M.; Coda, R. Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol. 2014, 37, 59–68. [Google Scholar] [CrossRef]
- Valdés, I.; Garcìa, E.; Lorente, M.; Mèndez, E. Innovative approach to low-level gluten determination in foods using a novel sandwich enzyme-linked immunosorbent assay protocol. Eur. J. Gastroenterol. Hepatol. 2003, 15, 465–474. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemistry, 10th ed.; AACC: St. Paul, MN, USA, 2003. [Google Scholar]
- AOCS. Preparation of methyl esters of fatty acids in Official methods and Recommended practices of the American Oil Chemists' Society, 6th ed.; AOCS Press: Champaign, IL, USA, 2009. [Google Scholar]
- AOAC. Official methods of analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- ISO. Food and feed products-General guidelines for the determination of nitrogen by the Kjeldahl method; International Organization for Standardization: Geneve, Switzerland, 2009. [Google Scholar]
- Zevallos, C.V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Pesoti, A.L.; De Oliveira, B.M.; De Oliveira, A.C.; Pompeu, D.G.; Goncalves, D.B.; Marangoni, S.; Da Silva, J.A.; Granieiro, P.A. Extraction, purification and characterization of inhibitor of trypsin from Chenopodium quinoa seeds. Food Sci. Technol. 2015, 35, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Gomez, L.; Sanchez-Monge, R.; Garcia-Olmedo, F.; Salcedo, G. Wheat tetrameric inhibitors of insect-amylases: Alloploidheterosis at the molecular level. Proc. Natl. Acad. Sci. USA 1989, 86, 3242–3246. [Google Scholar] [CrossRef] [PubMed]
- Curiel, A.; Coda, R.; Limitone, A.; Katina, K.; Raulio, M.; Giuliani, G.; Rizzello, C.G.; Gobbetti, M. Manufacture and characterization of pasta made with wheat flour rendered gluten-free using fungal proteases and selected sourdough lactic acid bacteria. J. Cereal. Sci. 2014, 59, 79–85. [Google Scholar] [CrossRef]
- Schoenlechner, R.; Drausinger, J.; Ottenschlaeger, V.; Jurackova, K.; Berghofer, E. Functional properties of gluten-free pasta produced from amaranth, quinoa and buckwheat. Plant. Foods Hum. Nutr. 2010, 65, 339–349. [Google Scholar] [CrossRef]
- D’egidio, M.G.; Mariani, B.M.; Nardi, S.; Novaro, P.; Cubadda, R. Chemical and technological variables and their relationship: A predictiveequativo for pasta cooking quality. Cereal Chem. 1990, 67, 275–281. [Google Scholar] [CrossRef]
- ISO. Sensory Analysis: Methodology, General Guidance for Establishing a Sensory Profile. Guidelines 13299; International Organization for Standardization: Geneve, Switzerland; p. 2003.
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whowerll, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lissner, S.; Koch, G.; Talley, N.J.; Drossman, D.; Rueegg, P.; Dunger-Baldauf, C.; Lefkowitz, M. Subject’s Global Assessment of Relief: An appropriate method to assess the impact of treatment on irritable bowel syndrome-related symptoms in clinical trials. J. Clin Epidemiol. 2003, 56, 310–316. [Google Scholar] [CrossRef]
- Andrae, D.A.; Patrick, D.L.; Drossman, D.A.; Covington, P.S. Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual. Life Outcomes 2013, 11, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Caraux, G.; Pinloche, S. PermutMatrix: A graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 2005, 21, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Foschia, M.; Horstmann, S.; Arendt, E.K.; Zannini, E. Nutritional therapy–facing the gap between celiac disease and gluten-free food. Int. J. Food Microbiol. 2016, 19, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fabiani, E.; Iacono, G.; D’Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; De Vitis, I. A prospective, double-blind, placebo controller trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr. 2007, 85, 160–166. [Google Scholar] [CrossRef]
- Rizzello, C.G.; De Angelis, M.; Di Cagno, R.; Camarca, A.; Silano, M.; Losito, I.; De Vincenzi, M.; De Bari, D.; Palmisano, F.; Maurano, F.; et al. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: New perspectives for celiac disease. Appl. Environ. Microbiol. 2007, 73, 4499–4507. [Google Scholar] [CrossRef]
- Brandt, M.J. Sourdough products for convenient use in baking. Food Microbiol. 2007, 24, 161–164. [Google Scholar] [CrossRef]
- Greco, L.; Gobbetti, M.; Auricchio, R.; Di Mase, R.; Landolfi, F.; Paparo, F.; Di Cagno, R.; De Angelis, M.; Rizzello, C.G.; Cassone, A. Safety for celiac patients of baked goods made of wheat flour hydrolyzed during food processing. Clin. Gastroenterol. Hepatol. 2011, 9, 24–29. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Armero, E.; Collar, C. Texture properties of formulated wheat doughs: Relationships with dough and bread technological quality. Z. Lebensm. Unters. Forsch. 1997, 204, 136–145. [Google Scholar] [CrossRef]
- Sicignano, A.; Di Monaco, R.; Masi, P.; Cavella, S. From raw material to dish: Past quality step by step. J. Sci. Food. Agric. 2015, 95, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Resmini, P.; Dalbon, G. Influence of the extrusion process on characteristics and structure of pasta. Food Struct. 1989, 8, 173–182. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef]
- Verbeke, K. Non celiac gluten sensitivity: What is the culprit? Gastroenterology 2018, 154, 471–473. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Steinera, D.; Longin, C.F.H.; Würschum, T.; Schweiggert, R.M.; Carle, R. Wheat and the irritable bowel syndrome–FODMAP levels of modern and ancient species and their retention during bread making. J. Funct. Foods 2016, 25, 257–266. [Google Scholar] [CrossRef]
- Singh, R.; Salem, A.; Nanavati, J.; Mullin, G.E. The role of diet in the treatment of irritable bowel syndrome: A systematic review. Gastroenterol. Clin. N. Am. 2018, 47, 107–137. [Google Scholar] [CrossRef]
- Biesiekierski, R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15–73. [Google Scholar] [CrossRef]

| NG Bread 1 | RG Bread | p Value NG vs. RG Bread | NG Pasta | RG Pasta | p Value NG vs. RG Pasta | |
|---|---|---|---|---|---|---|
| Energetic value (Kcal/100 g) | 258 ± 5.67 | 262 ± 2.88 | p = 0.112 | 360 ± 15.48 | 367 ± 8.07 | p = 0.204 |
| Moisture (%) | 29.0 ± 0.2 | 29.9 ± 0.3 | p = 0.104 | 10.2 ± 0.4B | 10.5 ± 0.8 | p = 0.361 |
| Fat (%) | 1.23 ± 0.01 | 1.21 ± 0.05 | p = 0.288 | 0.44 ± 0.01 | 0.45 ± 0.02 | p = 0.098 |
| Fatty acid methyl esters (% of fat) | ||||||
| C16:0 | 17.1 ± 0.3 | 18.3 ± 0.26 | p = 0.187 | 16.7 ± 0.22 | 17.3 ± 0.13 | p = 0.305 |
| C16:1 | 0.6 ± 0.03 | 1 ± 0.05 | p = 0.081 | 0.5± 0.02 | 0.4± 0.03 | p = 0.355 |
| C18:0 | 2.2 ± 0.08 | 2.3 ± 0.03 | p = 0.222 | 2.0± 0.04 | 2.1 ± 0.04 | p = 0.155 |
| C18:1 | 37.1 ± 1.64 | 37.2 ± 1.12 | p = 0.093 | 35.0 ± 0.84 | 33.3 ± 0.49 | p = 0.111 |
| C18:2 | 40.4 ± 1.73 | 38.9 ± 0.24 | p = 0.174 | 41.9 ± 1.64 | 42.5 ± 1.91 | p = 0.161 |
| Saturated fatty acids (% of DM) | 19.2 ± 0.34 | 20.6 ± 0.37 | p = 0.442 | 19.6 ± 0.84 | 19.4 ± 0.21 | p = 0.313 |
| Monounsaturated fatty acids (% of DM) | 38.3 ± 1.37 | 38.2 ± 1.64 | p = 0.177 | 35.7 ± 0.69 | 33.5 ± 1.13 | p = 0.657 |
| Polyunsaturated fatty acids (% of DM) | 42.5 ± 0.83 | 41.2 ± 0.91 | p = 0.405 | 44.7 ± 1.61 | 49.1 ± 2.11 | p = 0.318 |
| Total carbohydrate (% of DM) | 55.11 ± 0.32 | 53.54 ± 0.96 | p = 0.222 | 76.11 ± 0.83 | 81.4 ± 1.79 | p = 0.189 |
| FODMAPs (g/100 g) | 0.96 ± 0.015 | 0.90 ± 0.018 | p = 0.182 | 0.74 ± 0.007 | 0.70 ± 0.005 | p = 0.099 |
| Protein (N x 6.25) (g/100 g) | 7.98 ± 0.34 | 7.76 ± 0.17 | p = 0.242 | 11.32 ± 0.48 | 11.64 ± 0.50 | p = 0.163 |
| Crude fiber content (% of DM) | 2.2 ± 0.02 | 2.86 ± 0.12 | p = 0.114 | 3.17 ± 0.03 | 3.54 ± 0.15 | p = 0.106 |
| Na (% of DM) | 0.85 ± 0.07 | 0.81 ± 0.03 | p = 0.204 | 0.43 ± 0.06 | 0.45± 0.05 | p = 0.101 |
| Cl (% of DM) | 0.87 ± 0.00 | 0.77 ± 0.00 | p = 0.078 | 0.46 ± 0.01 | 0.44 ± 0.00 | p = 0.085 |
| K (mg/Kg) | 1216 ± 23.83 | 1171 ± 50.35 | p = 0.063 | 2953 ± 64.96 | 3003 ± 66.06 | p = 0.072 |
| Ca (mg/Kg) | 364 ± 15.65 | 354 ± 12.74 | p = 0.065 | 605 ± 6.65 | 401 ± 9.62 | p = 0.042 |
| Mg (mg/Kg) | 142 ± 6.11 | 138 ± 2.71 | p = 0.088 | 422 ± 9.28 | 480 ± 20.64 | p = 0.074 |
| P (mg/Kg) | 982 ± 10.81 | 908 ± 21.79 | p = 0.219 | 2615 ± 51.25 | 2670 ± 48.06 | p = 0.252 |
| Fe (mg/Kg) | 5.3 ± 0.21 | 5.1 ± 0.11 | p = 0.547 | 11.4 ± 0.12 | 10.1 ± 0.36 | p = 0.463 |
| NG Bread 1 | RG Bread | p Value | |
|---|---|---|---|
| Structural Characteristics | |||
| Hardness (g) | 4970 ± 256 | 5304 ± 204 | p = 0.291 |
| Resilience | 1.01 ± 0.01 | 0.91 ± 0.03 | p = 0.014 |
| Fracturability (g) | 3526 ± 166 | 2940 ± 87 | p = 0.022 |
| Specific volume (cm3/g) | 2.3 ± 0.01 | 2.2 ± 0.02 | p = 0.047 |
| Image Analysis | |||
| Black pixel area (%) | 40.3 ± 0.2 | 38.9 ± 0.3 | p = 0.031 |
| Color Analysis | |||
| L | 58 ± 2.08 | 42 ± 4.16 | p = 0.043 |
| a | 7.29 ± 0.52 | 12 ± 1.18 | p = 0.022 |
| b | 29.45 ± 0.18 | 28.88±0.25 | p = 0.111 |
| dE | 44.30 ± 1.72 | 58.58 ± 1.76 | p = 0.037 |
| NG Pasta | RG Pasta | ||
| Structural Characteristics | |||
| Hardness (N) | 5.10 ± 0.05 | 4.89 ± 0.02 | p = 0.034 |
| Cohesiveness | 0.81 ± 0.02 | 0.75 ± 0.01 | p = 0.094 |
| Springiness | 0.62 ± 0.01 | 0.60 ± 0.01 | p = 0.103 |
| Resilience | 0.55 ± 0.01 | 0.52 ± 0.01 | p = 0.157 |
| Gumminess (N) | 4.13 ± 0.04 | 4.11 ± 0.07 | p = 0.146 |
| Chewiness (N) | 2.56 ± 0.03 | 2.45 ± 0.04 | p = 0.254 |
| Color Analysis | |||
| L | 65 ± 1.01 | 59 ± 1.15 | p = 0.026 |
| a | 6.92 ± 0.08 | 6.45 ± 0.12 | p = 0.013 |
| b | 35.8 ± 0.14 | 34.9 ± 0.23 | p = 0.011 |
| dE | 43.22 ± 1.38 | 46.71 ± 1.48 | p = 0.008 |
| Clinical Scores | Diet | Average Value ± SD | p Value | ||||
|---|---|---|---|---|---|---|---|
| Habitual Diet vs. Run-in Open Gluten-Free Diet | Run-in Open Gluten-Free Diet vs. Reduced-Gluten Diet | Run-in Open-Gluten-Free diet vs. Normal-Gluten Diet | Reduced-Gluten Diet vs. Normal-Gluten Diet | ||||
| IBS-SS | Habitual diet | 237.3 ± 70.25 | IBS-SS | 0.000 | 0.002 | 0.000 | 0.166 |
| Run-in Open gluten-free diet | 164.8 ± 79.15 | ||||||
| Reduced-gluten diet | 214.4 ± 97.59 | ||||||
| Normal-gluten diet | 229.5 ± 79.82 | ||||||
| VAS | Habitual diet | 4.0 ± 1.54 | VAS | 0.000 | 0.000 | 0.000 | 0.042 |
| Run-in Open gluten-free diet | 2.8 ± 1.40 | ||||||
| Reduced-gluten diet | 3.7 ± 1.77 | ||||||
| Normal-gluten diet | 4.3 ± 1.67 | ||||||
| HADS | Habitual diet | 22.9 ± 12.70 | HADS | 0.027 | 0.314 | 0.423 | 0.357 |
| Run-in Open gluten-free diet | 20.3 ± 10.65 | ||||||
| Reduced-gluten diet | 19.7 ± 10.82 | ||||||
| Normal-gluten diet | 20.7 ± 12.35 | ||||||
| QoL | Habitual diet | 50.8 ± 27.94 | QoL | 0.000 | 0.000 | 0.046 | 0.357 |
| Run-in Open gluten-free diet | 39.5 ± 24.92 | ||||||
| Reduced-gluten diet | 45.2 ± 26.43 | ||||||
| Normal-gluten diet | 47.3 ± 24.69 | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calasso, M.; Francavilla, R.; Cristofori, F.; De Angelis, M.; Gobbetti, M. New Protocol for Production of Reduced-Gluten Wheat Bread and Pasta and Clinical Effect in Patients with Irritable Bowel Syndrome: A randomised, Double-Blind, Cross-Over Study. Nutrients 2018, 10, 1873. https://doi.org/10.3390/nu10121873
Calasso M, Francavilla R, Cristofori F, De Angelis M, Gobbetti M. New Protocol for Production of Reduced-Gluten Wheat Bread and Pasta and Clinical Effect in Patients with Irritable Bowel Syndrome: A randomised, Double-Blind, Cross-Over Study. Nutrients. 2018; 10(12):1873. https://doi.org/10.3390/nu10121873
Chicago/Turabian StyleCalasso, Maria, Ruggiero Francavilla, Fernanda Cristofori, Maria De Angelis, and Marco Gobbetti. 2018. "New Protocol for Production of Reduced-Gluten Wheat Bread and Pasta and Clinical Effect in Patients with Irritable Bowel Syndrome: A randomised, Double-Blind, Cross-Over Study" Nutrients 10, no. 12: 1873. https://doi.org/10.3390/nu10121873
APA StyleCalasso, M., Francavilla, R., Cristofori, F., De Angelis, M., & Gobbetti, M. (2018). New Protocol for Production of Reduced-Gluten Wheat Bread and Pasta and Clinical Effect in Patients with Irritable Bowel Syndrome: A randomised, Double-Blind, Cross-Over Study. Nutrients, 10(12), 1873. https://doi.org/10.3390/nu10121873