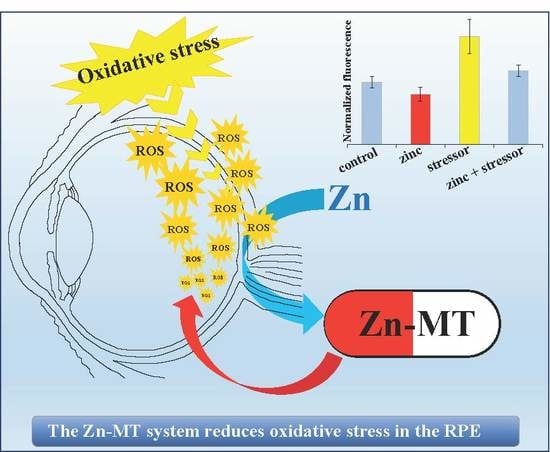
The Zinc-Metallothionein Redox System Reduces Oxidative Stress in Retinal Pigment Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents and Materials
2.3. Experimental Procedures
2.3.1. Human RPE Cell Line and Cultured Conditions
2.3.2. Gene Expression of MT Isoforms Following Zinc Supplementation
2.3.3. Induction of Oxidative Stress in Cultured HRPEsv Cells
2.3.4. Intracellular Localization of MTs by Conventional Immunocytochemistry
2.3.5. Quantification of Zn-MTs in HRPEsv Cells
2.4. Statistical Analysis
3. Results and Discussion
3.1. Optimizacion of HRPEsv Cell Culture Conditions
3.2. MT Gene Expression in HRPEsv Following Zinc Supplementation
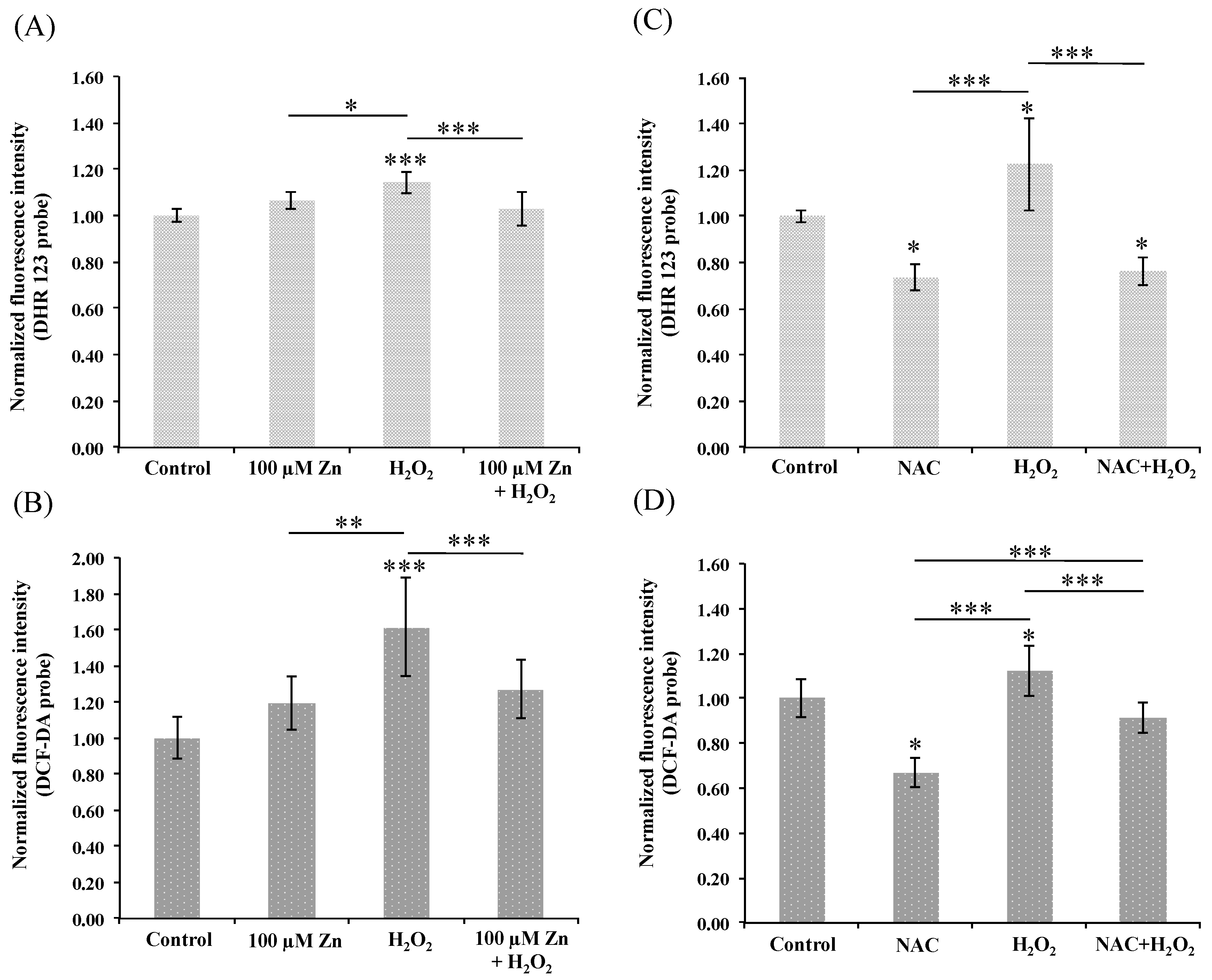
3.3. Induction of Zn-MT System Reduces Oxidative Stress in Cultured HRPEsv Cells
3.4. Intracellular Distribution of MTs
3.5. Induction of the Zn-MT System in HRPEsv Cells
3.5.1. HRPEsv Cultured Cells Treated with Zinc
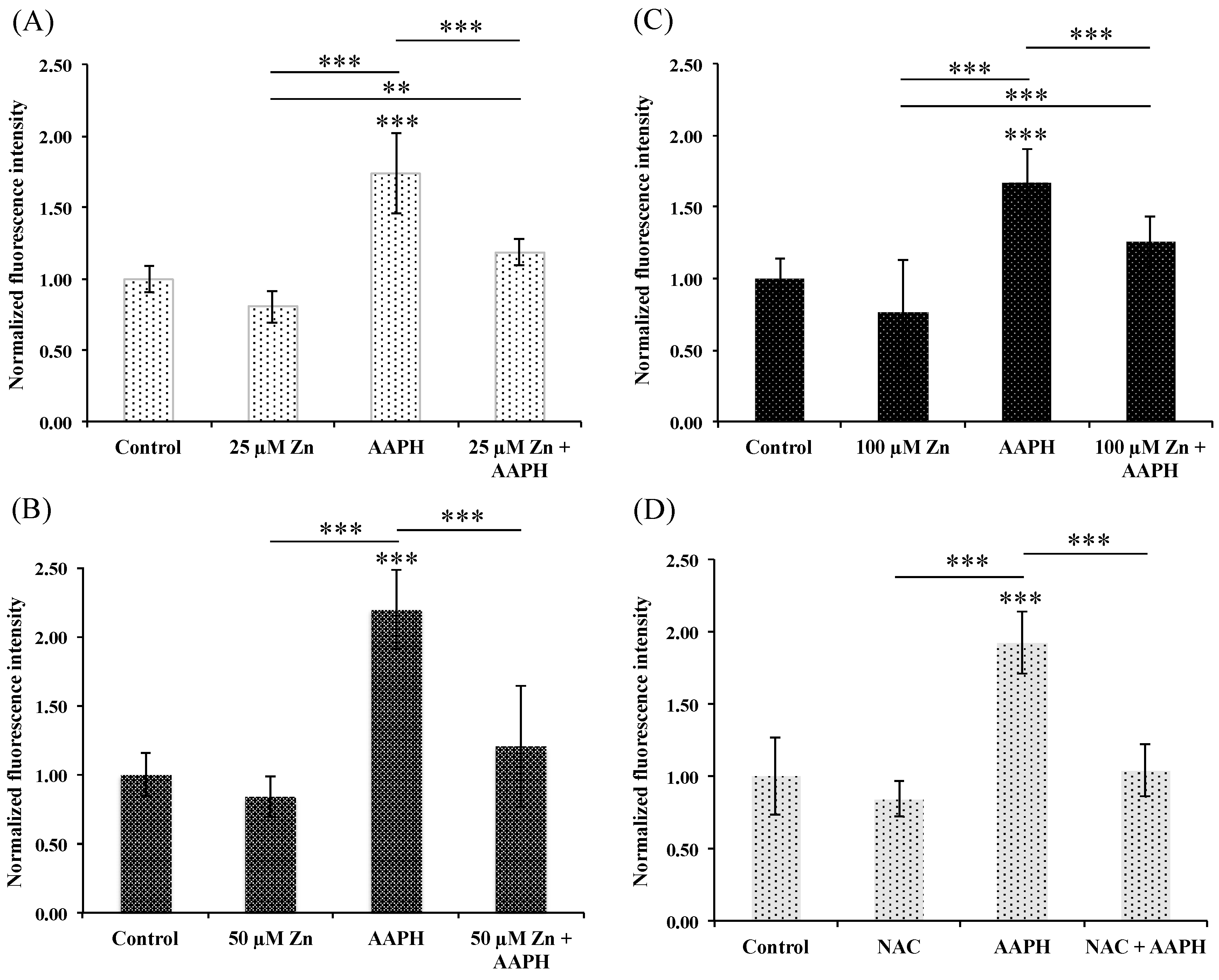
3.5.2. HRPEsv Cultured Cells Treated with Zinc and Subsequent Oxidative-Stress Induction by AAPH
3.6. Does the Zn-MTs Redox System Reduce Oxidative Stress in HRPEsv Cultured Cells?
3.7. Biological Implications of the Zn-MTs Antioxidant System in the RPE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F. The Eye, Basic Sciences in Practice, 3rd ed.; Saunders Elsevier: Edinburgh, UK, 2008; Volume 540, ISBN 978-0702028410. [Google Scholar]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, G.M.; Kijlstra, A.; Peek, R.; de Vos, A.F. Retinal pigment epithelium-immune system interactions: Cytokine production and cytokine-induced changes. Prog. Retin. Eye Res. 2001, 20, 29–48. [Google Scholar] [CrossRef]
- Hageman, G.S.; Luthert, P.J.; Victor Chong, N.H.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernadez, E., Nelson, R., Eds.; University of Utha Health Sciences Center: Salt Lake, UT, USA, 2011. [Google Scholar]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci. Rep. 2016, 6, 25792. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Strauss, R.W.; Lu, L.; Hafiz, G.; Wolfson, Y.; Shah, S.M.; Sophie, R.; Mir, T.A.; Scholl, H.P. Is there excess oxidative stress and damage in eyes of patients with retinitis pigmentosa? Antioxid. Redox Signal. 2015, 23, 643–648. [Google Scholar] [CrossRef]
- Boulton, M.; Dayhaw-Barker, P. The role of the retinal pigment epithelium: Topographical variation and ageing changes. Eye 2001, 15 Pt 3, 384–389. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M. The role of melanin in the RPE. In The Retinal Pigment Epithelium; Marmor, M.F., Wolfensberger, T.J., Eds.; Oxford Univ: Oxford, UK, 1998. [Google Scholar]
- Pacifici, R.E.; Davies, K.J. Protein, lipid and DNA repair systems in oxidative stress: The free-radical theory of aging revisited. Gerontology 1991, 37, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Iglesias, H.; Alvarez, L.; García, M.; Petrash, C.; Sanz-Medel, A.; Coca-Prados, M. Metallothioneins (MTs) in the human eye: A perspective article on the zinc–MT redox cycle. Metallomics 2014, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1079–1086. [Google Scholar] [CrossRef]
- Chung, R.S.; Hidalgo, J.; West, A.K. New insight into the molecular pathways of metallothionein-mediated neuroprotection and regeneration. J. Neurochem. 2008, 104, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Drozd, A.; Wojewska, D.; Peris-Díaz, M.D.; Jakimowicz, P.; Krężel, A. Crosstalk of the structural and zinc buffering properties of mammalian metallothionein-2. Metallomics 2018, 10, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.M.; Zweifach, A.; Lynes, M.A. Metallothionein regulates intracellular zinc signaling during CD4(+) T cell activation. BMC Immunol. 2016, 17, 13. [Google Scholar] [CrossRef]
- Bell, S.G.; Vallee, B.L. The metallothionein/thionein system: An oxidoreductive metabolic zinc link. Chembiochem 2009, 10, 55–62. [Google Scholar] [CrossRef]
- Metallothioneins in Normal and Cancer Cells. Available online: https://link.springer.com/book/10.1007%2F978-3-319-27472-0 (accessed on 29 November 2018).
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics. 2010, 2, 306–317. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef]
- Maret, W.; Vallee, B.L. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc. Natl. Acad. Sci. USA 1998, 95, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J. Metallothionein redox cycle and function. Exp. Biol. Med. 2006, 231, 1459–1467. [Google Scholar] [CrossRef]
- Alvarez, L.; Gonzalez-Iglesias, H.; Garcia, M.; Ghosh, S.; Sanz-Medel, A.; Coca-Prados, M. The stoichiometric transition from Zn6Cu1-metallothionein to Zn7-metallothionein underlies the up-regulation of metallothionein (MT) expression: Quantitative analysis of MT-metal load in eye cells. J. Biol. Chem. 2012, 287, 28456–28469. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Iglesias, H.; Petrash, C.; Rodríguez-Menéndez, S.M.; Garcia, M.; Alvarez, L.; Fernández-Vega Cueto, L.; Fernández, B.; Pereiro, R.; Sanz-Medel, A.; Coca-Prados, M. Quantitative distribution of Zn, Fe and Cu in the human lens and study of the Zn-metallothionein redox system in cultured lens epithelial cells by elemental MS. J. Anal. At. Spectrom. 2017, 32, 1746–1756. [Google Scholar] [CrossRef]
- Rodríguez-Menéndez, S.; Fernández, B.; García, M.; Álvarez, L.; Fernández, M.L.; Sanz-Medel, A.; Coca-Prados, M.; Pereiro, R.; González-Iglesias, H. Quantitative study of zinc and metallothioneins in the human retina and RPE cells by mass spectrometry-based methodologies. Talanta 2018, 178, 222–230. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Tan, J.S.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Ophthalmology 2008, 115, 334–341. [Google Scholar] [CrossRef]
- Van Leeuwen, R.; Boekhoorn, S.; Vingerling, J.R.; Witteman, J.C.; Klaver, C.C.; Hofman, A.; de Jong, P.T. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 2005, 294, 3101–3107. [Google Scholar] [CrossRef]
- Lu, H.; Hunt, D.M.; Ganti, R.; Davis, A.; Dutt, K.; Alam, J.; Hunt, R.C. Metallothionein protects retinal pigment epithelial cells against apoptosis and oxidative stress. Exp. Eye Res. 2002, 74, 83–92. [Google Scholar] [CrossRef]
- Barde, M.P.; Barde, P.J. What to use to express the variability of data: Standard deviation or standard error of mean? Perspect Clin Res. 2012, 3, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Pao, P.J.; Emri, E.; Abdirahman, S.B.; Soorma, T.; Zeng, H.H.; Hauck, S.M.; Thompson, R.B.; Lengyel, I. The effects of zinc supplementation on primary human retinal pigment epithelium. J. Trace Elem. Med. Biol. 2018, 49, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.R.; Keen, C. Calculations of the distribution of zinc in a computer model of human serum. J. Nutr. 1989, 119, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Gasull, T.; Giralt, M.; Hernandez, J.; Martinez, P.; Bremner, I.; Hidalgo, J. Regulation of metallothionein concentrations in rat brain: Effect of glucocorticoids, zinc, copper, and endotoxin. Am. J. Physiol. 1994, 266, E760–E767. [Google Scholar] [CrossRef] [PubMed]
- Haq, F.; Mahoney, M.; Koropatnick, J. Signaling events for metallothionein induction. Mutat. Res. 2003, 533, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Giedroc, D.P.; Chen, X.; Apuy, J.L. Metal response element (MRE)-binding transcription factor-1 (MTF-1): Structure, function, and regulation. Antioxid. Redox Signal. 2001, 3, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Daniels, P.J.; Bittel, D.; Smirnova, I.V.; Winge, D.R.; Andrews, G.K. Mammalian metal response element-binding transcription factor-1 functions as a zinc sensor in yeast, but not as a sensor of cadmium or oxidative stress. Nucleic Acids Res. 2002, 30, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Sun, S. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol. Ther. 2010, 9, 109–110. [Google Scholar] [CrossRef]
- Ezeriņa, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.; Wang, Y.J.; Milligan, M.; Li, X.; Ji, J.A. Analysis of 2,2’-azobis (2-amidinopropane) dihydrochloride degradation and hydrolysis in aqueous solutions. J. Pharm. Sci. 2011, 100, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.J., Jr.; Miceli, M.V.; Newsome, D.A. Zinc protects against oxidative damage in cultured human retinal pigment epithelial cells. Free Radic. Biol. Med. 1999, 26, 704–713. [Google Scholar] [CrossRef]
- Tate, D.J.; Newsome, D.A. A novel zinc compound (zinc monocysteine) enhances the antioxidant capacity of human retinal pigment epithelial cells. Curr. Eye Res. 2006, 31, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.P.; Osborne, N.N. Zinc and energy requirements in induction of oxidative stress to retinal pigmented epithelial cells. Neurochem. Res. 2003, 28, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, D.; Curtis, T.; Chen, M.; Xu, H. Zinc protects oxidative stress-induced RPE death by reducing mitochondrial damage and preventing lysosome rupture. Oxid. Med. Cell Longev. 2017, 6926485. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Pérez, M.J.; Cederbaum, A.I. Metallothionein 2A induction by zinc protects HEPG2 cells against CYP2E1-dependent toxicity. Free Radic. Biol. Med. 2003, 34, 443–455. [Google Scholar] [CrossRef]
- Suemori, S.; Shimazawa, M.; Kawase, K.; Satoh, M.; Nagase, H.; Yamamoto, T.; Hara, H. Metallothionein, an endogenous antioxidant, protects against retinal neuron damage in mice. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3975–3982. [Google Scholar] [CrossRef]
- Lindeque, J.Z.; Levanets, O.; Louw, R.; van der Westhuizen, F.H. The involvement of metallothioneins in mitochondrial function and disease. Curr. Protein Pept. Sci. 2010, 11, 292–309. [Google Scholar] [CrossRef]
- Krezel, A.; Hao, Q.; Maret, W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Vasák, M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta. 1985, 827, 36–44. [Google Scholar] [CrossRef]
- Nagano, T.; Itoh, N.; Ebisutani, C.; Takatani, T.; Miyoshi, T.; Nakanishi, T.; Tanaka, K. The transport mechanism of metallothionein is different from that of classical NLS-bearing protein. J. Cell. Physiol. 2000, 185, 440–446. [Google Scholar] [CrossRef]
- Cherian, M.G.; Apostolova, M.D. Nuclear localization of metallothionein during cell proliferation and differentiation. Cell. Mol. Biol. 2000, 46, 347–356. [Google Scholar] [PubMed]
- Li, Y.; Maret, W. Human metallothionein metallomics. J. Anal. At. Spectrom. 2008, 23, 1055–1062. [Google Scholar] [CrossRef]
- Romero-Isart, N.; Vašák, M. Advances in the structure and chemistry of metallothioneins. J. Inorg. Biochem. 2002, 88, 388–396. [Google Scholar] [CrossRef]
- Tate, D.J., Jr.; Newsome, D.A. Zinc induces catalase expression in cultured fetal human retinal pigment epithelial cells. Curr. Eye Res. 1997, 16, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.E.R.; Stillman, M.J. Metal-dependent protein folding: Metallation of metallothionein. J. Inorg. Biochem. 2006, 100, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Zatta, P. Metallothioneins in Biochemistry and Pahotology; World Scientific Publishing Co Pte Ltd.: Singapore, 2008; ISBN 978-981-277-893-2. [Google Scholar]
- Irvine, G.W.; Korkola, N.; Stillman, M.J. Isolated domains of recombinant human apo-metallothionein 1A are folded at neutral pH: A denaturant and heat-induced unfolding study using ESI-MS. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M. Advances in metallothionein structure and functions. J. Trace Elem. Med. Biol. 2005, 19, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Gedamu, L. Molecular analyses of metallothionein gene regulation. Prog. Nucleic Acid Res. Mol. Biol. 1998, 59, 257–288. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Asanuma, M.; Miyazaki, I.; Tachibana, H.; Wada, J.; Sogawa, N.; Sugaya, T.; Kitamura, S.; Maeshima, Y.; Shikata, K.; et al. High glucose increases metallothionein expression in renal proximal tubular epithelial cells. Exp. Diabetes Res. 2011, 11, 534872. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Kubasik-Kladna, K.; Aboul-Enein, H.Y. The role oxidative stress in the pathogenesis of eye diseases: Current status and a dual role of physical activity. Mini Rev. Med. Chem. 2015, 16, 241–257. [Google Scholar] [CrossRef]
- Fronk, A.H.; Vargis, E. Methods for culturing retinal pigment epithelial cells: A review of current protocols and future recommendations. J. Tissue Eng. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.K.; Ravi, K.; Balaiya, S.; Brar, V.S.; Chalam, K.V. Lutein protects retinal pigment epithelium from cytotoxic oxidative stress. Cutan. Ocul. Toxicol. 2014, 33, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Fidilio, A.; Lazzara, F.; Piazza, C.; Geraci, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Retinal protection and distribution of curcumin in vitro and in vivo. Front. Pharmacol. 2018, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Krezel, A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol. Med. 2007, 13, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Varin, A.; Larbi, A.; Dedoussis, G.V.; Kanoni, S.; Jajte, J.; Rink, L.; Monti, D.; Malavolta, M.; Marcellini, F.; Mocchegiani, E.; et al. In vitro and in vivo effects of zinc on cytokine signalling in human T cells. Exp. Gerontol. 2008, 43, 472–482. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
| Treatment | Zn-Binding Proteins | natZn | 68Zn | Total Zn (natZn + 68Zn) |
|---|---|---|---|---|
| (μg·g−1 Protein) | (μg·g−1 Protein) | (μg·g−1 Protein) | ||
| Control | Zn-MTs | 8.9 ± 0.2 | 0.00 ± 0.00 | 8.9 ± 0.2 |
| Other Zn-binding proteins | 11.4 ± 0.4 | 0.00 ± 0.00 | 11.4 ± 0.4 | |
| All Zn binding proteins/molecules | 20.3 ± 0.6 | 0.00 ± 0.00 | 20.3 ± 0.6 | |
| 25 µM 68Zn | Zn-MTs | 5.7 ± 0.3 | 28.2 ± 8.4 | 33.9 ± 8.7 |
| Other Zn-binding proteins | 6.6 ± 0.1 | 9.03 ± 0.03 | 15.6 ± 0.1 | |
| All Zn binding proteins/molecules | 12.3 ± 0.4 | 37.2 ± 8.4 | 49.6 ± 8.8 | |
| 50 µM 68Zn | Zn-MTs | 10.4 ± 5.0 | 104.0 ± 13.5 | 114.4 ± 18.5 |
| Other Zn-binding proteins | 8.3 ± 0.9 | 14.8 ± 3.5 | 23.1 ±4.4 | |
| All Zn binding proteins/molecules | 18.6 ± 5.9 | 118.8 ± 17.0 | 137.5 ± 22.9 | |
| 100 µM 68Zn | Zn-MTs | 10.2 ± 0.4 | 441.8 ± 2.1 | 452.0 ± 2.5 |
| Other Zn-binding proteins | 5.3 ± 0.3 | 29.7 ± 1.4 | 35.0 ± 1.6 | |
| All Zn binding proteins/molecules | 15.5 ± 0.7 | 471.5 ± 3.5 | 487.0 ± 4.1 |
| Treatment | natMTs (mg·g−1) | 68MTs | TotalMTs (mg·g−1) | Zn:Cu:MT Stoichiometry |
|---|---|---|---|---|
| (Fold-Change) a | (mg·g−1) | (Fold-Change) a | ||
| Control | 0.61 ± 0.02 (1) | 0.00 ± 0.00 | 0.61 ± 0.02 (1) | 1.4 ± 0.1:0.15 ± 0.02:1 |
| 25 µM 68Zn | 0.169 ± 0.001 (0.27) | 0.84 ± 0.28 | 1.01 ± 0.28 (1.66) | 3.1 ± 0.1:0.16 ± 0.03:1 |
| 50 µM 68Zn | 0.20 ± 0.10 (0.33) | 2.01 ± 0.21 | 2.21 ± 0.31 (3.62) | 4.8 ± 0.1:0.09 ± 0.02:1 |
| 100 µM 68Zn | 0.16 ± 0.01 (0.26) | 7.13 ± 0.17 | 7.30 ± 0.18 (11.97) | 7 ± 1:0.07 ± 0.02:1 |
| Treatment | Zn-Binding Proteins | natZn | 68Zn | Total Zn (natZn + 68Zn) |
|---|---|---|---|---|
| (μg·g−1 Protein) | (μg·g−1 Protein) | (μg·g−1 Protein) | ||
| Control | Zn-MTs | 12.0 ± 2.8 | 0.00 ± 0.00 | 12.0 ± 2.8 |
| Other Zn-binding proteins | 28.3 ± 9.5 | 0.00 ± 0.00 | 28.3 ± 9.5 | |
| All Zn binding proteins/molecules | 40.3 ± 12.3 | 0.00 ± 0.00 | 40.3 ± 12.3 | |
| AAPH | Zn-MTs | 11.2 ± 2.4 | 0.00 ± 0.00 | 11.2 ± 2.4 |
| Other Zn-binding proteins | 13.5 ± 0.1 | 0.00 ± 0.00 | 13.5 ± 0.1 | |
| All Zn binding proteins/molecules | 24.7 ± 2.5 | 0.00 ± 0.00 | 24.7 ± 2.5 | |
| 25 µM 68Zn + AAPH | Zn-MTs | 6.9 ± 0.8 | 34.5 ± 5.6 | 41.4 ± 6.4 |
| Other Zn-binding proteins | 9.0 ± 3.2 | 18.2 ± 11.6 | 27.2 ± 14.8 | |
| All Zn binding proteins/molecules | 15.9 ± 4.0 | 52.6 ± 17.2 | 68.5 ± 21.2 | |
| 50 µM 68Zn + AAPH | Zn-MTs | 17.5 ± 0.5 | 131.9 ± 4.0 | 149.4 ± 4.5 |
| Other Zn-binding proteins | 7.9 ± 0.3 | 17.6 ± 0.8 | 25.5 ± 1.2 | |
| All Zn binding proteins/molecules | 25.4 ± 0.9 | 149.5 ± 4.8 | 174.9 ± 5.7 | |
| 100 µM 68Zn + AAPH | Zn-MTs | 16.2 ± 0.4 | 532.3 ± 16.3 | 548.5 ± 15.9 |
| Other Zn-binding proteins | 6.9 ± 0.9 | 50.4 ± 33.2 | 57.4 ± 34.2 | |
| All Zn binding proteins/molecules | 23.1 ± 1.3 | 582.7 ± 49.5 | 605.9 ± 50.8 |
| Treatment | natMTs (mg·g−1) | 68MTs | TotalMTs (mg·g−1) | Zn:Cu:MT Stoichiometry |
|---|---|---|---|---|
| (Fold-Change) a | (mg·g−1) | (Fold-Change) a | ||
| Control | 1.1 ± 0.4 (1) | 0.00 ± 0.00 | 1.1 ± 0.4 (1) | 1.4 ± 0.1:0.11 ± 0.042:1 |
| AAPH | 0.5 ± 0.1 (0.45) | 0.00 ± 0.00 | 0.5 ± 0.1 (0.45) | 2.07 ± 0.05:0.17 ± 0.04:1 |
| 25 µM 68Zn + AAPH | 0.16 ± 0.02 (0.14) | 0.8 ± 0.4 | 0.9 ± 0.4 (0.82) | 4.2 ± 1.1:0.13 ± 0.03:1 |
| 50 µM 68Zn + AAPH | 0.27 ± 0.02 (0.24) | 2.1 ± 0.2 | 2.3 ± 0.2 (2.09) | 5.9 ± 0.6:0.15 ± 0.01:1 |
| 100 µM 68Zn + AAPH | 0.15 ± 0.08 (0.14) | 6.6 ± 0.4 | 6.7 ± 0.5 (6.09) | 7.4 ± 0.2:0.03 ± 0.01:1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Menéndez, S.; García, M.; Fernández, B.; Álvarez, L.; Fernández-Vega-Cueto, A.; Coca-Prados, M.; Pereiro, R.; González-Iglesias, H. The Zinc-Metallothionein Redox System Reduces Oxidative Stress in Retinal Pigment Epithelial Cells. Nutrients 2018, 10, 1874. https://doi.org/10.3390/nu10121874
Rodríguez-Menéndez S, García M, Fernández B, Álvarez L, Fernández-Vega-Cueto A, Coca-Prados M, Pereiro R, González-Iglesias H. The Zinc-Metallothionein Redox System Reduces Oxidative Stress in Retinal Pigment Epithelial Cells. Nutrients. 2018; 10(12):1874. https://doi.org/10.3390/nu10121874
Chicago/Turabian StyleRodríguez-Menéndez, Sara, Montserrat García, Beatriz Fernández, Lydia Álvarez, Andrés Fernández-Vega-Cueto, Miguel Coca-Prados, Rosario Pereiro, and Héctor González-Iglesias. 2018. "The Zinc-Metallothionein Redox System Reduces Oxidative Stress in Retinal Pigment Epithelial Cells" Nutrients 10, no. 12: 1874. https://doi.org/10.3390/nu10121874
APA StyleRodríguez-Menéndez, S., García, M., Fernández, B., Álvarez, L., Fernández-Vega-Cueto, A., Coca-Prados, M., Pereiro, R., & González-Iglesias, H. (2018). The Zinc-Metallothionein Redox System Reduces Oxidative Stress in Retinal Pigment Epithelial Cells. Nutrients, 10(12), 1874. https://doi.org/10.3390/nu10121874