Relationship between Dietary n-6 Fatty Acid Intake and Hypertension: Effect of Glycated Hemoglobin Levels
Abstract
1. Introduction
2. Materials and Methods
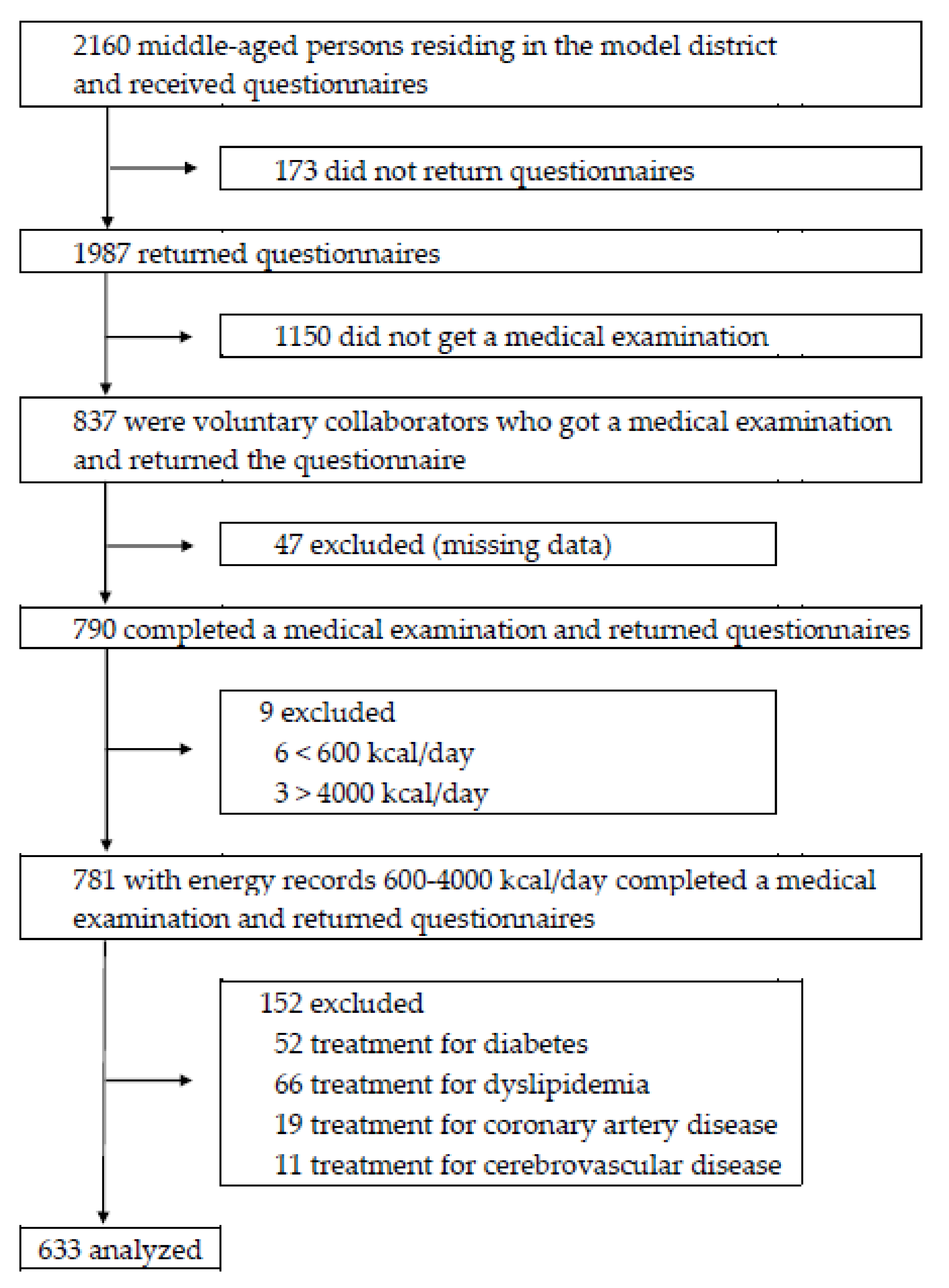
2.1. Study Design and Participants
2.2. Blood Pressure Measurements
2.3. Nutritional Assessment
2.4. Other Variables
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results
3.1. Participant Characteristics in Different Blood Pressure Groups
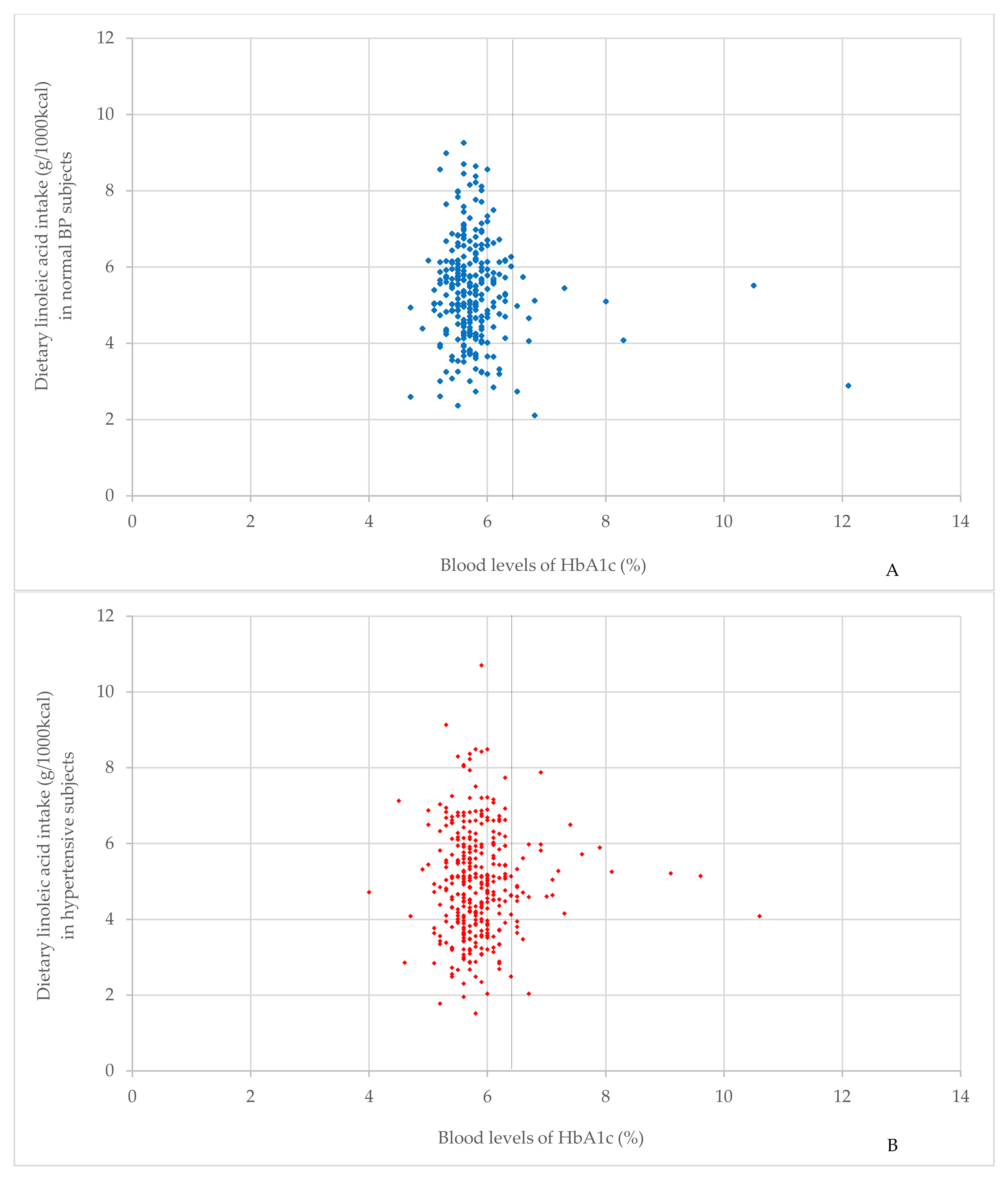
3.2. Interaction of HbA1c Level
3.3. Characteristics of the Study Population in Different Blood Pressure Groups according to HbA1c Level
3.4. Relationship between n-6 Fatty Acid Intake and Hypertension according to HbA1c level.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Goodarz, D.; Shibuya, K.; Adair-Rohani, H.; AAlMazroa, M.; Amann, M.; Anderson, H.R.; GAndrews, K.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Lawes, C.M.; Rodgers, A.; Bennett, D.A.; Parag, V.; Suh, I.; Ueshima, H.; MacMahon, S. Blood pressure and cardiovascular disease in the Asia Pacific region. J. Hypertens. 2003, 21, 707–716. [Google Scholar] [PubMed]
- Midgley, J.P.; Matthew, A.G.; Greenwood, C.M.; Logan, A.G. Effect of reduced dietary sodium on blood pressure: A meta-analysis of randomized controlled trials. JAMA 1996, 275, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure: The Trials of Hypertension Prevention, phase II. Arch. Intern. Med. 1997, 157, 657–667. [Google Scholar] [CrossRef]
- Whelton, S.P.; Chin, A.; Xin, X.; He, J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2002, 136, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; He, J.; Frontini, M.G.; Ogden, L.G.; Motsamai, O.I.; Whelton, P.K. Effects of alcohol reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2001, 38, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.J.; Folsom, A.R.; Ma, J.; Arnett, D.K.; McGovern, P.G.; Eckfeldt, J.H. Plasma fatty acid composition and 6-year incidence of hypertension in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol. 1999, 150, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, H.E.; Koeller, E.; Greer, N.; MacDonald, R.; Kane, R.; Wilt, T.J. Effects on Health Outcomes of a Mediterranean Diet With No Restriction on Fat Intake: A Systematic Review and Meta-analysis. Ann. Int. Med. 2016, 165, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R.; Colin, P.R.; Erlinger, T.P.; Rosner, B.A.; Laranjo, N.M.; et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Stamler, J.; Nakagawa, H.; Elliott, P.; Ueshima, H.; Chan, Q.; Brown, I.J.; Tzoulaki, I.; Saitoh, I.; Dyer, I.; et al. For the INTERMAP Research Group. Relationship of dietary linoleic acid to blood pressure: The International Study of Macro- Micronutrients and Blood Pressure. Hypertension 2008, 52, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Oster, P.; Arab, L.; Schellenberg, B.; Kohlmeier, M.; Schlierf, G. Linoleic acid and blood pressure. Prog. Food Nutr. Sci. 1980, 4, 39–40. [Google Scholar] [PubMed]
- Wood, D.A.; Butler, S.; Riemersma, R.A.; Thompson, M.; Oliver, M.F. Adipose tissue and platelet fatty acids and coronary heart disease in Scottish men. Lancet 1984, 2, 117–121. [Google Scholar] [CrossRef]
- Riemersma, R.A.; Wood, D.A.; Butler, S.; Elton, R.A.; Oliver, M.; Salo, M.; Nikkari, T.; Vartiainen, E.; Puska, P.; Gey, F.; et al. Linoleic acid content in adipose tissue and coronary heart disease. BMJ 1986, 292, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A.; Naukkarinen, V.; Huttunen, J.K.; Mattila, S.; Kumlin, T. Fatty acid composition of serum lipids predicts myocardial infarction. BMJ 1982, 285, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manson, J.E.; Forman, J.P.; Gaziano, J.M.; Buring, J.E.; Sesso, H.D. Dietary Fatty Acids and the Risk of Hypertension in Middle-Aged and Older Women. Hypertension 2010, 56, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Pala, V.; Russo, P.; Risé, P.; Moreno, L.A.; De Henauw, S.; Mehlig, K.; Veidebaum, T.; Molnár, D.; Tornaritis, M.; et al. Associations of Whole Blood n-3 and n-6 Polyunsaturated Fatty Acids with Blood Pressure in Children and Adolescents—Results from the IDEFICS/I.Family Cohort. PLoS ONE 2016, 2, e0165981. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, W.M.; Galli, C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: A background review paper. Ann. Nutr. Metab. 2009, 55. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Aoyagi, T.; Yang, T. mPGES-1 protects against DOCA-salt hypertension via inhibition of oxidative stress or stimulation of NO/cGMP. Hypertension 2010, 55, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Koba, S.; Pakala, R.; Watanabe, T.; Katagiri, T.; Benedict, C.R. Synergistic interaction between thromboxane A2 and mildly oxidized low density lipoproteins on vascular smooth muscle cell proliferation. Prostaglandins Leukot. Essent. Fatty Acids 2000, 63, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Butkus, A.; Skrinska, V.A.; Schumacher, O.P. Thromboxane production and platelet aggregation in diabetic subjects with clinical complications. Thrombo Res. 1980, 19, 211–223. [Google Scholar] [CrossRef]
- Brunner, D.; Klinger, J.; Weisbort, J.; Tuval, M.; Nakash, J.; Rosenberg, C.H.; Nissim, S. Thromboxane, prostacyclin, beta-thrombo-globin, and diabetes mellitus. Clin. Ther. 1984, 6, 636–642. [Google Scholar] [PubMed]
- Kuang, S.J.; Qian, J.S.; Yang, H.; Rao, F.; Chen, X.Y.; Zhang, M.Z.; Shan, Z.X.; Lin, Q.X.; Xue, Y.M.; Wu, S.L.; et al. The enhancement of TXA2 receptors-mediated contractile response in intrarenal artery dysfunction in type 2 diabetic mice. Eur. J. Pharmacol. 2017, 805, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.A.; Ogungbade, G.O.; Hercule, H.C.; Oyekan, A.O.; Mutembei, L. Alteration in endothelin receptor sub-type responsiveness and in the endothelin-TXA(2) mimetic U46619 interaction, in type-2 hypertensive diabetic Zucker rats. Diabetes Res. Clin. Pract. 2004, 63, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Shikatown Population. Available online: http://www.town.shika.ishikawa.jp/jyuumin/shika_town_pop/shika_population.html (accessed on 5 March 2017).
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self- administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Schakel, S.F.; Dennis, B.H.; Wold, A.C.; Conway, R.; Zhao, L.; Okuda, N.; Moag-Stahlberg, A.; Robertson, C.; Van Heel, N.; Buzzard, I.M.; et al. Enhancing data on nutrient composition of foods eaten by participants in the INTERMAP Study in China, Japan, the United Kingdom and the United States. J. Food Compost. Anal. 2003, 16, 395–408. [Google Scholar] [CrossRef]
- Stamler, J.; Elliott, P.; Appel, L.; Chan, Q.; Buzzard, M.; Dennis, B.; Dyer, A.R.; Elmer, P.; Greenland, P.; Jones, D.; et al. Higher blood pressure in middle-aged American adults with less education–role of multiple dietary factors: The INTERMAP Study. J. Hum. Hypertens. 2003, 17, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 334, 757–761. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Yokoyama, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; Kita, T.; et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ. J. 2009, 73, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.R.; Suchindran, C.M.; Ringel, A.; Davis, J.M.; Hibbeln, J.R. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013, 346, e7007. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All Subjects | Male | Female | p Value |
|---|---|---|---|---|
| No. of subjects | 633 | 299 | 334 | |
| Age | 61.0 (11.5) | 60.8 (11.1) | 61.2 (11.8) | 0.733 |
| Smoking status, n (%) | <0.001 | |||
| Never or Ex-smoker | 503 (79) | 196 (66) | 307 (92) | |
| Current | 130 (21) | 103 (34) | 27 (8) | |
| Drinking habit, n (%) | <0.001 | |||
| yes | 268 (42) | 206 (79) | 62 (20) | |
| no | 365 (58) | 93 (31) | 268 (80) | |
| Exercise habit, n (%) | 0.526 | |||
| yes | 345 (55) | 159 (53) | 186 (57) | |
| no | 288 (45) | 140 (47) | 148 (43) | |
| Height (cm) | 160 (9.65) | 168 (6.92) | 154 (6.74) | <0.001 |
| Weight (kg) | 59.9 (11.8) | 67.3 (10.4) | 53.2 (8.39) | <0.001 |
| Waist circumference (cm) | 83.8 (9.26) | 85.8 (8.70) | 81.9 (9.37) | <0.001 |
| BMI (kg/m2) | 23.2 (3.24) | 23.9 (2.98) | 22.5 (3.32) | <0.001 |
| SBP (mmHg) | 139 (20.4) | 143 (20.3) | 135 (19.6) | <0.001 |
| DBP (mmHg) | 80.7 (11.8) | 83.5 (12.0) | 78.3 (11.0) | <0.001 |
| HbA1c (NSGP) (%) | 5.82 (0.605) | 5.81 (0.549) | 5.83 (0.383) | 0.686 |
| Energy and Nutrients | ||||
| Energy (kcal) | 1863 (616) | 2088 (630) | 1611 (529) | <0.001 |
| Nutrients (g/1000 kcal) | ||||
| Protein | 38.1 (8.18) | 14.4 (3.13) | 38.3 (7.58) | 0.637 |
| Carbohydrate | 134.1 (21.1) | 134 (22.2) | 134 (19.6) | <0.001 |
| Sodium | 2.45 (0.540) | 2.40 (0.515) | 2.49 (0.559) | 0.036 |
| Potassium | 1.39 (0.430) | 1.22 (0.349) | 1.55 (0.467) | <0.001 |
| Calcium | 0.294 (0.113) | 0.258 (0.103) | 0.326 (0.112) | <0.001 |
| Total dietary fiber | 6.54 (2.19) | 5.65 (1.75) | 7.33 (2.25) | <0.001 |
| SFA | 7.27 (2.17) | 6.48 (1.97) | 7.97 (2.11) | <0.001 |
| MUFA | 9.76 (2.65) | 8.95 (2.60) | 10.5 (2.49) | <0.001 |
| PUFA | 6.82 (1.74) | 6.33 (1.69) | 7.26 (1.69) | <0.001 |
| n-3 fatty acid | 1.48 (0.503) | 1.40 (0.474) | 1.57 (0.516) | <0.001 |
| n-6 fatty acid | 5.30 (1.41) | 4.91 (1.36) | 5.67 (1.35) | <0.001 |
| LA | 5.15 (1.38) | 4.76 (1.33) | 5.50 (1.33) | <0.001 |
| GLA (mg/1000 kcal) | 4.09 (2.68) | 3.82 (2.44) | 4.32 (2.87) | 0.019 |
| EA (mg/1000 kcal) | 24.6 (8.54) | 23.1 (8.33) | 25.9 (8.52) | <0.001 |
| DGLA (mg/1000 kcal) | 16.0 (5.10) | 14.8 (4.98) | 17.0 (4.98) | <0.001 |
| AA (mg/1000 kcal) | 92.7 (30.7) | 87.9 (31.6) | 100 (29.4) | <0.001 |
| DPA (mg/1000 kcal) | 5.25 (3.26) | 5.07 (3.09) | 5.42 (3.40) | 0.175 |
| Characteristic | Hypertension | Normal BP | p Value |
|---|---|---|---|
| No. of subjects | 350 | 283 | |
| Men, n (%) | 196 (56) | 103 (36) | <0.001 |
| Age | 64.5 (10.7) | 56.7 (11.0) | <0.001 |
| Smoking status, n (%) | 0.311 | ||
| Never or Ex-smoker | 273 (78) | 230 (81) | |
| Current | 77 (22) | 53 (19) | |
| Drinking habit, n (%) | <0.001 | ||
| yes | 178 (51) | 90 (32) | |
| no | 172 (49) | 193 (68) | |
| Exercise habit, n (%) | 0.496 | ||
| yes | 195 (56) | 150 (53) | |
| no | 155 (44) | 133 (47) | |
| Height (cm) | 160 (9.79) | 160 (9.48) | 0.233 |
| Weight (kg) | 61.4 (11.5) | 58.0 (11.9) | 0.419 |
| Waist circumference (cm) | 85.5 (9.02) | 81.6 (9.13) | 0.755 |
| BMI (kg/m2) | 23.8 (3.17) | 22.4 (3.18) | 0.755 |
| SBP (mmHg) | 151 (18.3) | 124 (9.10) | <0.001 |
| DBP (mmHg) | 85.7 (12.2) | 74.5 (7.44) | <0.001 |
| HbA1c (NSGP) (%) | 5.85 (0.593) | 5.78 (0.617) | 0.134 |
| Energy and Nutrients | |||
| Energy (kcal) | 1895 (625) | 1822 (604) | 0.142 |
| Nutrients (g/1000 kcal) | |||
| Protein | 38.0 (8.64) | 38.3 (7.58) | 0.637 |
| Carbohydrate | 134 (22.2) | 134 (19.6) | 0.933 |
| Sodium | 2.49 (0.540) | 2.39 (0.536) | 0.024 |
| Potassium | 1.39 (0.454) | 1.39 (0.400) | 0.934 |
| Calcium | 0.294 (0.114) | 0.294 (0.111) | 0.955 |
| Total dietary fiber | 6.59 (2.29) | 6.48 (2.07) | 0.528 |
| SFA | 6.87 (2.06) | 7.76 (2.21) | <0.001 |
| MUFA | 9.33 (2.69) | 10.3 (2.51) | <0.001 |
| PUFA | 6.62 (1.76) | 7.06 (1.69) | 0.002 |
| n-3 fatty acid | 1.48 (0.504) | 1.49 (0.503) | 0.728 |
| n-6 fatty acid | 5.12 (1.43) | 5.54 (1.34) | <0.001 |
| LA | 4.96 (1.40) | 5.38 (1.32) | <0.001 |
| GLA (mg/1000 kcal) | 3.94 (2.41) | 4.26 (2.98) | 0.151 |
| EA (mg/1000 kcal) | 23.7 (8.60) | 25.6 (8.36) | 0.005 |
| DGLA (mg/1000 kcal) | 15.4 (5.22) | 16.6 (4.88) | 0.003 |
| AA (mg/1000 kcal) | 90.4 (31.8) | 95.5 (29.1) | 0.037 |
| DPA (mg/1000 kcal) | 5.35 (3.20) | 5.14 (3.34) | 0.437 |
| n-6 Fatty Acid | HbA1c Level | Hypertension | Normal BP | p Value for Interaction |
|---|---|---|---|---|
| Average (SD) | Average (SD) | |||
| n-6 fatty acid (g/1000 kcal) | Normal | 5.12 (1.31) | 5.59 (1.33) | 0.035 |
| High | 5.11 (1.10) | 4.54 (1.22) | ||
| LA (g/1000 kcal) | Normal | 4.96 (1.43) | 5.42 (1.31) | 0.033 |
| High | 4.94 (1.09) | 4.37 (1.21) | ||
| GLA (mg/1000 kcal) | Normal | 3.87 (2.41) | 4.21 (3.00) | 0.682 |
| High | 4.78 (2.25) | 5.50 (2.52) | ||
| EA (mg/1000 kcal) | Normal | 23.6 (8.74) | 25.6 (8.43) | 0.949 |
| High | 24.7 (6.95) | 26.9 (6.90) | ||
| DGLA (mg/1000 kcal) | Normal | 15.3 (5.22) | 16.6 (4.94) | 0.585 |
| High | 16.3 (5.27) | 16.6 (3.57) | ||
| AA (mg/1000 kcal) | Normal | 89.9 (31.5) | 95.4 (29.3) | 0.629 |
| High | 96.6 (34.9) | 96.9 (27.5) | ||
| DPA (mg/1000 kcal) | Normal | 5.30 (3.14) | 5.05 (3.33) | 0.147 |
| High | 5.87 (3.82) | 7.29 (2.82) |
| Characteristic | HbA1c < 6.5 | p | HbA1c ≥ 6.5 | p | ||
|---|---|---|---|---|---|---|
| HTN | NBP | HTN | NBP | |||
| No. of subjects | 321 | 271 | 29 | 12 | ||
| Men, n (%) | 178 (55) | 96 (35) | <0.001 | 18 (62) | 7 (58) | 0.823 |
| Age | 64.2 (10.6) | 56.5 (11.0) | <0.001 | 67.6 (11.6) | 62.2 (9.47) | 0.164 |
| Smoking status, n (%) | 0.533 | 0.254 | ||||
| Never or Ex-smoker | 254 (79) | 220 (81) | 19 (66) | 10 (83) | ||
| Current | 67 (21) | 51 (19) | 10 (34) | 2 (17) | ||
| Drinking habit, n (%) | <0.001 | 0.558 | ||||
| yes | 163 (51) | 85 (31) | 15 (52) | 5 (42) | ||
| no | 158 (49) | 186 (69) | 14 (48) | 7 (58) | ||
| Exercise habit, n (%) | 0.261 | 0.141 | ||||
| yes | 183 (57) | 142 (52) | 17 (59) | 4 (33) | ||
| no | 138 (43) | 129 (48) | 12 (41) | 8 (67) | ||
| Height (cm) | 160 (9.76) | 160 (9.48) | 0.951 | 161 (10.3) | 162 (9.73) | 0.661 |
| Weight (kg) | 61.1 (11.2) | 57.6 (11.6) | <0.001 | 64.9 (14.0) | 65.5 (15.8) | 0.907 |
| Waist circumference (cm) | 85.1 (8.87) | 81.4 (8.93) | <0.001 | 89.4 (9.84) | 86.4 (12.5) | 0.421 |
| BMI (kg/m2) | 23.7 (3.13) | 22.3 (3.01) | <0.001 | 24.8 (3.50) | 24.8 (5.43) | 0.979 |
| SBP (mmHg) | 152 (18.5) | 124 (9.23) | <0.001 | 148 (16.1) | 124 (5.45) | <0.001 |
| DBP (mmHg) | 86.1 (12.2) | 74.5 (7.46) | <0.001 | 81.8 (12.0) | 74.0 (7.44) | 0.016 |
| HbA1c (NSGP) (%) | 5.73 (0.342) | 5.69 (0.299) | 0.157 | 7.19 (1.01) | 7.73 (1.80) | 0.227 |
| Energy and Nutrients | ||||||
| Energy (kcal) | 1905 (632) | 1808 (590) | 0.055 | 1788 (541) | 2170 (823) | 0.159 |
| Nutrients (g/1000 kcal) | ||||||
| Protein | 37.9 (8.65) | 38.3 (7.63) | 0.578 | 39.2 (8.56) | 39.2 (6.64) | 0.992 |
| Carbohydrate | 134 (22.6) | 134 (19.6) | 0.947 | 134 (18.1) | 135 (21.7) | 0.904 |
| Sodium | 2.49 (0.545) | 2.39 (0.535) | 0.030 | 2.49 (0.496) | 2.38 (0.576) | 0.544 |
| Potassium | 1.39 (0.461) | 1.39 (0.402) | 0.852 | 1.43 (0.374) | 1.38 (0.346) | 0.733 |
| Calcium | 0.293 (0.116) | 0.294 (0.116) | 0.875 | 0.302 (0.096) | 0.290 (0.098) | 0.714 |
| Total dietary fiber | 6.57 (2.30) | 6.49 (2.08) | 0.638 | 6.74 (2.21) | 6.21 (2.00) | 0.482 |
| SFA | 6.84 (2.07) | 7.79 (2.25) | <0.001 | 7.11 (1.99) | 7.29 (0.867) | 0.695 |
| MUFA | 9.33 (2.74) | 10.3 (2.52) | <0.001 | 9.36 (2.12) | 9.30 (1.33) | 0.931 |
| PUFA | 6.62 (1.80) | 7.11 (1.68) | 0.001 | 6.70 (1.33) | 6.11 (1.51) | 0.220 |
| n-3 fatty acid | 1.47 (0.504) | 1.49 (0.506) | 0.632 | 1.57 (0.497) | 1.54 (0.439) | 0.852 |
| n-6 fatty acid | 5.12 (1.46) | 5.59 (1.33) | <0.001 | 5.11 (1.10) | 4.54 (1.22) | 0.155 |
| LA | 4.96 (1.43) | 5.42 (1.31) | <0.001 | 4.94 (1.09) | 4.37 (1.21) | 0.148 |
| GLA (mg/1000kcal) | 3.87 (2.41) | 4.21 (3.00) | 0.139 | 4.78 (2.25) | 5.50 (2.52) | 0.400 |
| EA (mg/1000kcal) | 23.6 (8.74) | 25.6 (8.43) | 0.006 | 24.7 (6.95) | 26.9 (6.90) | 0.372 |
| DGLA (mg/1000kcal) | 15.3 (5.22) | 16.6 (4.94) | 0.002 | 16.3 (5.27) | 16.6 (3.57) | 0.825 |
| AA (mg/1000kcal) | 89.9 (31.5) | 95.4 (29.3) | 0.027 | 96.6 (34.9) | 96.9 (27.5) | 0.975 |
| DPA (mg/1000kcal) | 5.30 (3.14) | 5.05 (3.33) | 0.349 | 5.87 (3.82) | 7.29 (2.82) | 0.201 |
| HbA1c Level | Model 1 | Model 2 | |
|---|---|---|---|
| OR (95% CI, p Value) | OR (95% CI, p Value) | ||
| All subjects | n-6 | 0.898 (0.788 to 1.022, 0.104) | 0.884 (0.771 to 1.013, 0.077) |
| LA | 0.899 (0.787 to 1.026, 0.114) | 0.886 (0.771 to 1.019, 0.089) | |
| High HbA1c | n-6 | 3.676 (1.060 to 12.76, 0.040) | 3.618 (1.019 to 12.84, 0.047) |
| LA | 3.993 (1.090 to 14.63, 0.037) | 3.986 (1.050 to 15.13, 0.042) | |
| Normal HbA1c | n-6 | 0.870 (0.761 to 0.995, 0.041) | 0.857 (0.744 to 0.987, 0.032) |
| LA | 0.885 (0.759 to 0.997, 0.045) | 0.858 (0.744 to 0.991, 0.037) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, H.; Hara, A.; Tsujiguchi, H.; Thi Thu Nguyen, T.; Kambayashi, Y.; Miyagi, S.; Yamada, Y.; Suzuki, K.; Shimizu, Y.; Nakamura, H. Relationship between Dietary n-6 Fatty Acid Intake and Hypertension: Effect of Glycated Hemoglobin Levels. Nutrients 2018, 10, 1825. https://doi.org/10.3390/nu10121825
Nakamura H, Hara A, Tsujiguchi H, Thi Thu Nguyen T, Kambayashi Y, Miyagi S, Yamada Y, Suzuki K, Shimizu Y, Nakamura H. Relationship between Dietary n-6 Fatty Acid Intake and Hypertension: Effect of Glycated Hemoglobin Levels. Nutrients. 2018; 10(12):1825. https://doi.org/10.3390/nu10121825
Chicago/Turabian StyleNakamura, Haruki, Akinori Hara, Hiromasa Tsujiguchi, Thao Thi Thu Nguyen, Yasuhiro Kambayashi, Sakae Miyagi, Yohei Yamada, Keita Suzuki, Yukari Shimizu, and Hiroyuki Nakamura. 2018. "Relationship between Dietary n-6 Fatty Acid Intake and Hypertension: Effect of Glycated Hemoglobin Levels" Nutrients 10, no. 12: 1825. https://doi.org/10.3390/nu10121825
APA StyleNakamura, H., Hara, A., Tsujiguchi, H., Thi Thu Nguyen, T., Kambayashi, Y., Miyagi, S., Yamada, Y., Suzuki, K., Shimizu, Y., & Nakamura, H. (2018). Relationship between Dietary n-6 Fatty Acid Intake and Hypertension: Effect of Glycated Hemoglobin Levels. Nutrients, 10(12), 1825. https://doi.org/10.3390/nu10121825





