Testosterone-Associated Dietary Pattern Predicts Low Testosterone Levels and Hypogonadism
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Definitions
2.3. Questionnaires
2.4. Anthropometric Measurements
2.5. Laboratory Measurements
2.6. Statistical Analysis
3. Results
3.1. Total T Levels Are Positively Associated with TS and SMM and Negatively Associated with Age, Obesity-Related Cardiometabolic Diseases, and RBC Rheology
3.2. Relationship between Serum Total T and Potential Variables
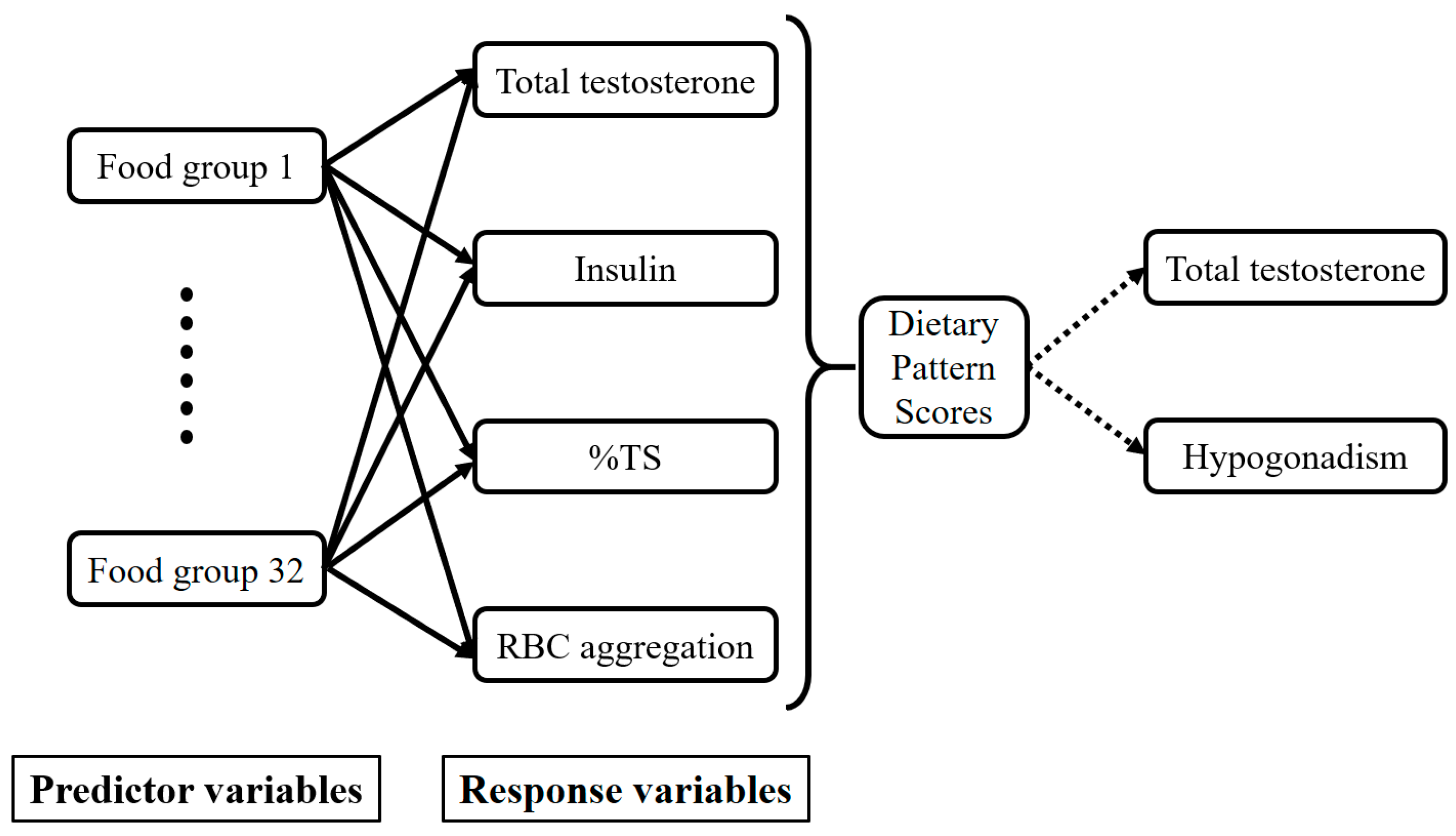
3.3. T-Associated Dietary Pattern Scores by the Reduced Rank Regression (RRR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, N.E.; Key, T.J. The effects of diet on circulating sex hormone levels in men. Nutr. Res. Rev. 2000, 13, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Morley, J.E.; Korenman, S.G. Biological actions of androgens. Endocr. Rev. 1987, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Dhindsa, S.; Abuaysheh, S.; Batra, M.; Kuhadiya, N.D.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Diminished androgen and estrogen receptors and aromatase levels in hypogonadal diabetic men: Reversal with testosterone. Eur. J. Endocrinol. 2018, 178, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.S.; van der Schouw, Y.T. Testosterone, shbg and cardiovascular health in postmenopausal women. Int J. Impot. Res. 2010, 22, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Appleby, P.N.; Davey, G.K.; Key, T.J. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002, 13, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Fui, M.N.; Dupuis, P.; Grossmann, M. Lowered testosterone in male obesity: Mechanisms, morbidity and management. Asian J. Androl. 2014, 16, 223–231. [Google Scholar] [PubMed]
- Gapstur, S.M.; Gann, P.H.; Kopp, P.; Colangelo, L.; Longcope, C.; Liu, K. Serum androgen concentrations in young men: A longitudinal analysis of associations with age, obesity, and race. The cardia male hormone study. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1041–1047. [Google Scholar]
- Jensen, T.K.; Andersson, A.M.; Jorgensen, N.; Andersen, A.G.; Carlsen, E.; Petersen, J.H.; Skakkebaek, N.E. Body mass index in relation to semen quality and reproductive hormones among 1,558 danish men. Fertil. Steril. 2004, 82, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Pitteloud, N.; Mootha, V.K.; Dwyer, A.A.; Hardin, M.; Lee, H.; Eriksson, K.F.; Tripathy, D.; Yialamas, M.; Groop, L.; Elahi, D.; et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005, 28, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Hagymasi, K.; Reismann, P.; Racz, K.; Tulassay, Z. Role of the endocrine system in the pathogenesis of non-alcoholic fatty liver disease. Orv. Hetil. 2009, 150, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Shaten, J.; Stern, M.P.; Smith, G.D.; Kuller, L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. Mrfit research group. Multiple risk factor intervention trial. Am. J. Epidemiol. 1996, 143, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Feinleib, M.; Zhang, L.; Rohrmann, S.; Rifai, N.; Nelson, W.G.; Dobs, A.; Basaria, S.; Golden, S.H.; Platz, E.A. Androgens and diabetes in men: Results from the third national health and nutrition examination survey (nhanes iii). Diabetes Care 2007, 30, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Saad, F.; Guay, A. The dark side of testosterone deficiency: Ii. Type 2 diabetes and insulin resistance. J. Androl. 2009, 30, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.B.; Dixon, J.M.; Suarez, E.A.; Murad, M.H.; Guey, L.T.; Wittert, G.A. Clinical review: Endogenous testosterone and mortality in men: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 3007–3019. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M. Hypogonadism and male obesity: Focus on unresolved questions. Clin. Endocrinol. (Oxf.) 2018, 89, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M. Testosterone and glucose metabolism in men: Current concepts and controversies. J. Endocrinol. 2014, 220, R37–55. [Google Scholar] [CrossRef] [PubMed]
- Bjorntorp, P. Hormonal control of regional fat distribution. Hum. Reprod. 1997, 12 (Suppl. 1), 21–25. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.L.; Song, Y.; Manson, J.E.; Hunter, D.J.; Lee, C.C.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 2009, 361, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Locatelli, V. Testosterone a key factor in gender related metabolic syndrome. Obes. Rev. 2018, 19, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Aversa, A.; Isidori, A.M.; Gooren, L.J. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: A review. Curr. Diabetes Rev. 2012, 8, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Casu, C.; Nemeth, E.; Rivella, S. Hepcidin agonists as therapeutic tools. Blood 2018, 131, 1790–1794. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.C.; Chang, C.C.; Chiou, H.Y.; Chang, J.S. Serum ferritin is inversely correlated with testosterone in boys and young male adolescents: A cross-sectional study in taiwan. PLoS ONE 2015, 10, e0144238. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.; Laine, F.; Massart, C.; Sandret, L.; Piguel, X.; Brissot, P.; Balkau, B.; Deugnier, Y.; Bonnet, F. Liver iron overload is associated with elevated shbg concentration and moderate hypogonadotrophic hypogonadism in dysmetabolic men without genetic haemochromatosis. Eur. J. Endocrinol. 2011, 165, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, F.; Zhang, H.; Gao, Y.; Tan, A.; Zhang, S.; Xiao, Q.; Zhang, B.; Huang, L.; Ye, B. The association between the levels of serum ferritin and sex hormones in a large scale of Chinese male population. PLoS ONE 2013, 8, e75908. [Google Scholar] [CrossRef] [PubMed]
- Kyung, N.H.; Barkan, A.; Klibanski, A.; Badger, T.M.; McArthur, J.W.; Axelrod, L.; Beitins, I.Z. Effect of carbohydrate supplementation on reproductive hormones during fasting in men. J. Clin. Endocrinol. Metab. 1985, 60, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Lado-Abeal, J.; Prieto, D.; Lorenzo, M.; Lojo, S.; Febrero, M.; Camarero, E.; Cabezas-Cerrato, J. Differences between men and women as regards the effects of protein-energy malnutrition on the hypothalamic-pituitary-gonadal axis. Nutrition 1999, 15, 351–358. [Google Scholar] [CrossRef]
- Santos, A.M.; Ferraz, M.R.; Teixeira, C.V.; Sampaio, F.J.; da Fonte Ramos, C. Effects of undernutrition on serum and testicular testosterone levels and sexual function in adult rats. Horm. Metab. Res. 2004, 36, 27–33. [Google Scholar] [PubMed]
- Nagata, C.; Takatsuka, N.; Kawakami, N.; Shimizu, H. Relationships between types of fat consumed and serum estrogen and androgen concentrations in japanese men. Nutr. Cancer 2000, 38, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Lu Cui, Q.-B.G. Regulation of lipid metabolism in rat leydig cells testosterone synthesis and proliferation. Int. J. Clin. Exp. Med. 2016, 9, 8224–8229. [Google Scholar]
- Mínguez-Alarcón, L.; Chavarro, J.E.; Mendiola, J.; Roca, M.; Tanrikut, C.; Vioque, J.; Jorgensen, N.; Torres-Cantero, A.M. Fatty acid intake in relation to reproductive hormones and testicular volume among young healthy men. Asian J. Androl. 2017, 19, 184–190. [Google Scholar]
- Giltay, E.J.; Geleijnse, J.M.; Heijboer, A.C.; de Goede, J.; Oude Griep, L.M.; Blankenstein, M.A.; Kromhout, D. No effects of n-3 fatty acid supplementation on serum total testosterone levels in older men: The alpha omega trial. Int. J. Androl. 2012, 35, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Langfort, J.L.; Zarzeczny, R.; Nazar, K.; Kaciuba-Uscilko, H. The effect of low-carbohydrate diet on the pattern of hormonal changes during incremental, graded exercise in young men. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Rosner, W.; Khan, M.S.; New, M.I.; Pang, S.Y.; Wissel, P.S.; Kappas, A. Diet-hormone interactions: Protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sci. 1987, 40, 1761–1768. [Google Scholar] [CrossRef]
- Mikulski, T.; Ziemba, A.; Nazar, K. Metabolic and hormonal responses to body carbohydrate store depletion followed by high or low carbohydrate meal in sedentary and physically active subjects. J. Physiol. Pharmacol. 2010, 61, 193–200. [Google Scholar] [PubMed]
- Huang, M.; Liu, J.; Lin, X.; Goto, A.; Song, Y.; Tinker, L.F.; Chan, K.K.; Liu, S. Relationship between dietary carbohydrates intake and circulating sex hormone-binding globulin levels in postmenopausal women. J. Diabetes 2018, 10, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Sjaarda, L.A.; Schisterman, E.F.; Schliep, K.C.; Plowden, T.; Zarek, S.M.; Yeung, E.; Wactawski-Wende, J.; Mumford, S.L. Dietary carbohydrate intake does not impact insulin resistance or androgens in healthy, eumenorrheic women. J. Clin. Endocrinol. Metab. 2015, 100, 2979–2986. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Lin, S.M.; Huang, T.C.; Chao, J.C.; Chen, Y.C.; Pan, W.H.; Bai, C.H. Serum ferritin and risk of the metabolic syndrome: A population-based study. Asia Pac. J. Clin. Nutr. 2013, 22, 400–407. [Google Scholar] [PubMed]
- World Health Organization. WHO Guidelines Approved by the guidelines Review Committee. In Use of Glycated Haemoglobin (hba1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a Who Consultation. World Health Organization. 2011. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26158184 (accessed on 1 October 2018).
- Tan, C.-E.; Ma, S.; Wai, D.; Chew, S.-K.; Tai, E.-S. Can we apply the national cholesterol education program adult treatment panel definition of the metabolic syndrome to Asians? Diabetes Care 2004, 27, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Paduch, D.A.; Brannigan, R.E.; Fuchs, E.F.; Kim, E.D.; Marmar, J.L.; Sandlow, J.I. The laboratory diagnosis of testosterone deficiency. Urology 2014, 83, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Pen, W.-H.; Kai-Li, L.; Mei-Shu, Y. Reproducibility and validity of a Chinese food frequency questionnaire used in Taiwan. Asia Pac. J. Clin. Nutr. 2006, 15, 161. [Google Scholar] [PubMed]
- Ling, C.H.; de Craen, A.J.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.; Westendorp, R.G.; Maier, A.B. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, D.J.; Matsumoto, A.M.; Araujo, A.B.; McKinlay, J.B. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J. Clin. Endocrinol. Metab. 2009, 94, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Priezzhev, A.; Shin, S.; Yaya, F.; Meglinski, I. Characterization of shear stress preventing red blood cells aggregation at the individual cell level: The temperature dependence. Clin. Hemorheol. Microcirc. 2016, 64, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Marwitz, S.E.; Woodie, L.N.; Blythe, S.N. Western-style diet induces insulin insensitivity and hyperactivity in adolescent male rats. Physiol. Behav. 2015, 151, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Haring, R.; Grarup, N.; Vandenput, L.; Wallaschofski, H.; Lorentzen, E.; Hansen, T.; Mellstrom, D.; Pedersen, O.; Nauck, M.; et al. Causal relationship between obesity and serum testosterone status in men: A bi-directional mendelian randomization analysis. PLoS ONE 2017, 12, e0176277. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O. Western-style diet, sex steroids and metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Haskell, J.; Vinson, N.; Terracio, L. Characterization of insulin and insulin-like growth factor i receptors of purified leydig cells and their role in steroidogenesis in primary culture: A comparative study. Endocrinology 1986, 119, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Pitteloud, N.; Hardin, M.; Dwyer, A.A.; Valassi, E.; Yialamas, M.; Elahi, D.; Hayes, F.J. Increasing insulin resistance is associated with a decrease in leydig cell testosterone secretion in men. J. Clin. Endocrinol. Metab. 2005, 90, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Tena-Sempere, M.; Pinilla, L.; Gonzalez, L.C.; Dieguez, C.; Casanueva, F.F.; Aguilar, E. Leptin inhibits testosterone secretion from adult rat testis in vitro. J. Endocrinol. 1999, 161, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chang, C.C.; Yuan, K.C.; Yeh, H.J.; Fang, S.U.; Cheng, T.; Teng, K.T.; Chao, K.C.; Tang, J.H.; Kao, W.Y.; et al. Red blood cell aggregation-associated dietary pattern predicts hyperlipidemia and metabolic syndrome. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Liu, K.L.; Chang, C.S.; Su, C.T.; Chen, S.H.; Lee, Y.C.; Chang, J.S. Ferric citrate supplementation reduces red-blood-cell aggregation and improves cd163+ macrophage-mediated hemoglobin metabolism in a rat model of high-fat-diet-induced obesity. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, O.; Guerci, B.; Muller, S.; Candiloros, H.; Mejean, L.; Donner, M.; Stoltz, J.F.; Drouin, P. Increased erythrocyte aggregation in insulin-dependent diabetes mellitus and its relationship to plasma factors: A multivariate analysis. Metabolism 1994, 43, 1182–1186. [Google Scholar] [CrossRef]
- Chong-Martinez, B.; Buchanan, T.A.; Wenby, R.B.; Meiselman, H.J. Decreased red blood cell aggregation subsequent to improved glycaemic control in type 2 diabetes mellitus. Diabet. Med. 2003, 20, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Bachman, E.; Travison, T.G.; Basaria, S.; Davda, M.N.; Guo, W.; Li, M.; Connor Westfall, J.; Bae, H.; Gordeuk, V.; Bhasin, S. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: Evidence for a new erythropoietin/hemoglobin set point. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Rotker, K.L.; Alavian, M.; Nelson, B.; Baird, G.L.; Miner, M.M.; Sigman, M.; Hwang, K. Association of subcutaneous testosterone pellet therapy with developing secondary polycythemia. Asian J. Androl. 2018, 20, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Kanias, T.; Sinchar, D.; Osei-Hwedieh, D.; Baust, J.J.; Jordan, A.; Zimring, J.C.; Waterman, H.R.; de Wolski, K.S.; Acker, J.P.; Gladwin, M.T. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion 2016, 56, 2571–2583. [Google Scholar] [CrossRef] [PubMed]
| Total Testosterone (ng/mL), Quartiles $ | p for Trend * | ||||
|---|---|---|---|---|---|
| Q1 (n = 32) | Q2 (n = 30) | Q3 (n = 32) | Q4 (n = 31) | ||
| Age (years) | 44.11 ± 9.89 | 42.85 ± 10.25 | 40.37 ± 12.79 | 37.27 ± 12.55 | 0.013 |
| Anemia (n, %) | 1 (3.1) | 1 (3.3) | 0 (0.0) | 1 (3.2) | 0.787 |
| TS <20% | 8 (25.0) | 6 (20.0) | 2 (6.3) | 1 (3.2) | 0.031 |
| Iron overload (n, %) | 8 (25.0) | 12 (40.0) | 7 (21.9) | 5 (16.1) | 0.174 |
| Central obesity (n, %) | 25 (78.1) | 15 (50.0) | 13 (40.6) | 8 (25.8) | <0.001 |
| Type 2 diabetes (n, %) | 4 (15.4) | 3 (13.6) | 2 (7.1) | 2 (6.9) | 0.653 |
| NAFLD (n, %) | 31 (96.9) | 29 (96.7) | 25 (78.1) | 16 (51.6) | <0.001 |
| MetS (n, %) | 12 (46.2) | 9 (42.9) | 1 (3.6) | 4 (13.8) | <0.001 |
| Hypogonadism (n, %) | 20 (62.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <0.001 |
| Anthropometry | |||||
| W/H ratio | 0.93 ± 0.07 | 0.91 ± 0.04 | 0.89 ± 0.06 | 0.87 ± 0.06 | <0.001 |
| BMI (kg/m2) | 28.01 ± 4.10 | 26.55 ± 4.79 | 25.36 ± 3.62 | 23.03 ± 3.20 | <0.001 |
| Total body fat mass (%) | 27.34 ± 5.04 | 26.60 ± 4.48 | 24.94 ± 4.61 | 22.19 ± 4.82 | <0.001 |
| Skeleton muscle mass (%) | 66.91 ± 4.90 | 67.66 ± 4.43 | 69.32 ± 4.56 | 72.02 ± 4.78 | <0.001 |
| Visceral fat mass (%) | 4.40 ± 1.22 | 4.16 ± 1.23 | 3.76 ± 1.14 | 3.12 ± 1.00 | <0.001 |
| Glucose Biomarkers | |||||
| HOMA-IR | 3.09 ± 1.85 | 2.82 ± 1.68 | 1.60 ± 0.74 | 1.51 ± 0.62 | <0.001 |
| Fasting glucose (mg/dL) | 88.78 ± 12.95 | 96.60 ± 24.17 | 87.47 ± 8.53 | 87.52 ± 8.81 | 0.278 |
| HbA1c (%) | 5.89 ± 0.60 | 5.99 ± 0.97 | 5.73 ± 0.63 | 5.61 ± 0.49 | 0.048 |
| Insulin (µIU/mL) | 13.78 ± 6.96 | 11.58 ± 6.60 | 7.32 ± 3.13 | 6.93 ± 2.82 | <0.001 |
| Lipid Biomarkers | |||||
| Total cholesterol (mg/dL) | 192.69 ± 40.92 | 208.13 ± 39.75 | 201.22 ± 42.58 | 184.94 ± 26.41 | 0.322 |
| TG (mg/dL) | 173.47 ± 89.90 | 141.87 ± 56.90 | 133.22 ± 147.17 | 96.29 ± 60.22 | 0.002 |
| HDL-C (mg/dL) | 42.90 ± 7.44 | 48.51 ± 11.32 | 51.48 ± 13.49 | 54.93 ± 14.57 | <0.001 |
| LDL-C (mg/dL) | 119.97 ± 34.55 | 130.40 ± 36.94 | 122.03 ± 34.17 | 106.42 ± 26.84 | 0.068 |
| Iron-Related Biomarkers | |||||
| Hb (gm/dL) | 15.29 ± 1.76 | 15.48 ± 2.18 | 16.51 ± 2.68 | 15.54 ± 1.09 | 0.277 |
| Free Hb (µg/mL) | 173.59 ± 55.04 | 144.69 ± 59.00 | 157.40 ± 45.81 | 146.03 ± 41.47 | 0.129 |
| Fe (µg/dL) | 99.69 ± 38.27 | 109.83 ± 40.59 | 116.31 ± 32.65 | 115.90 ± 31.98 | 0.057 |
| Ferritin (ng/mL) | 226.75 ± 121.70 | 260.45 ± 192.24 | 219.12 ± 141.86 | 206.27 ± 160.15 | 0.409 |
| TS (%) | 28.76 ± 11.12 | 32.81 ± 13.21 | 35.26 ± 12.20 | 37.06 ± 10.35 | 0.004 |
| sCD163 (ng/mL) | 1050.01 ± 491.15 | 786.83 ± 338.61 | 805.03 ± 532.38 | 791.08 ± 533.29 | 0.090 |
| Hepcidin (ng/mL) | 188.13 ± 102.72 | 196.78 ± 103.90 | 198.31 ± 93.59 | 158.54 ± 84.68 | 0.286 |
| RBC deformability SS1/2 (Pa) | 2.28 ± 0.17 | 2.33 ± 0.19 | 2.17 ± 0.17 | 2.20 ± 0.17 | 0.014 |
| RBC aggregation CSS (mPa) | 312.26 ± 64.77 | 306.70 ± 64.13 | 260.40 ± 55.98 | 240.62 ± 44.06 | <0.001 |
| Sex Hormone Biomarkers | |||||
| Total T (ng/mL) | 2.57 ± 0.55 | 3.53 ± 0.19 | 4.44 ± 0.33 | 5.80 ± 0.81 | <0.001 |
| SHBG (nmol/L) | 23.55 ± 12.25 | 26.87 ± 7.76 | 34.49 ± 13.21 | 40.03 ± 14.42 | <0.001 |
| Bio-T (ng/mL) | 1.52 ± 0.44 | 1.93 ± 0.31 | 2.24 ± 0.52 | 2.80 ± 0.63 | <0.001 |
| Free-T (pg/mL) | 59.24 ± 16.64 | 79.10 ± 13.33 | 88.07 ± 18.31 | 109.85 ± 23.46 | <0.001 |
| Parameters | Univariate | Model 1 * | Model 2 # | Model 3 † | ||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| Log Age (years) | −1.463 (−2.201~−0.724) | <0.001 | −1.463 (−2.201~−0.724) | <0.001 | −1.098 (−1.903~−0.293) | 0.008 | −1.159 (−1.876~−0.442) | 0.002 |
| W/H ratio | −9.115 (−12.698~−5.531) | <0.001 | −7.623 (−11.519~−3.727) | <0.001 | −1.610 (−7.150~3.930) | 0.566 | ||
| Log BMI (kg/m2) | −3.789 (−5.057~−2.521) | <0.001 | −3.557 (−4.768~−2.346) | <0.001 | −3.557 (−4.768~−2.346) | <0.001 | −1.426 (−3.104~0.252) | 0.095 |
| Blood Biomarkers | ||||||||
| HOMA-IR | −0.403 (−0.552~−0.253) | <0.001 | −0.366 (−0.511~−0.222) | <0.001 | −0.188 (−0.360~−0.016) | 0.033 | ||
| Fasting glucose (mg/dL) | −0.006 (−0.020~0.007) | 0.366 | ||||||
| Log Insulin (µIU/mL) | −1.204 (−1.614~−0.794) | <0.001 | −1.156 (−1.543~−0.768) | <0.001 | −0.723 (−1.210~−0.235) | 0.004 | −0.713 (−1.259~−0.166) | 0.011 |
| Total cholesterol (mg/dL) | −0.003 (−0.009~0.003) | 0.311 | ||||||
| Log TG (mg/dL) | −0.882 (−1.249~−0.516) | <0.001 | −0.708 (−1.091~−0.324) | <0.001 | −0.345 (−0.732~0.041) | 0.080 | ||
| Log HDL (mg/dL) | 1.908 (0.998~2.817) | <0.001 | 1.736 (0.865~2.607) | <0.001 | 0.789 (−0.130~1.707) | 0.092 | ||
| LDL-C (mg/dL) | −0.006 (−0.013~0.001) | 0.059 | ||||||
| RBC (MIL/mm3) | 0.051 (−0.255~0.357) | 0.741 | ||||||
| Log Hb (gm/dL) | 1.350 (−0.412~3.111) | 0.132 | ||||||
| Free Hb (µg/mL) | −0.002 (−0.007~0.003) | 0.350 | ||||||
| Serum Fe (µg/dL) | 0.004 (−0.002~0.010) | 0.204 | ||||||
| Log Ferritin (ng/mL) | −0.259 (−0.597~0.079) | 0.132 | ||||||
| Log TS (%) | 0.820 (0.227~1.414) | 0.007 | 0.718 (0.151~1.286) | 0.014 | 0.577 (0.066~1.087) | 0.027 | 0.675 (0.076~1.274) | 0.048 |
| sCD163 (ng/mL) | −0.001 (−0.001~−0.000025) | 0.062 | ||||||
| Hepcidin (ng/mL) | −0.002 (−0.005~0.001) | 0.175 | ||||||
| Log RBC Deformability SS1/2 (Pa) | −3.137 (−6.300~0.025) | 0.052 | ||||||
| Log RBC Aggregation CSS (mPa) | −2.447 (−3.483~−1.411) | <0.001 | −2.223 (−3.239~−1.207) | <0.001 | −1.650 (−2.635~−0.665) | 0.001 | −1.025 (−2.043~−0.008) | 0.048 |
| Food Group | Explained Variation (%) | Factor Loading * |
|---|---|---|
| Bread and pastries | 13.62 | 0.35 |
| Dairy products | 7.37 | 0.26 |
| Desserts | 6.57 | 0.24 |
| Eating out | 6.30 | 0.24 |
| Homemade foods | 31.17 | −0.53 |
| Noodles | 10.53 | −0.31 |
| Dark green vegetables | 7.27 | −0.25 |
| Total explained variation (%): | 59.53 |
| Dietary Pattern Scores, Quartile $ | p for Trend * | ||||
|---|---|---|---|---|---|
| Q1 (n = 52) | Q2 (n = 53) | Q3 (n = 53) | Q4 (n = 53) | ||
| Age (years) | 37.59 ± 11.81 | 40.23 ± 11.46 | 41.78 ± 12.24 | 45.63 ± 11.24 | 0.014 |
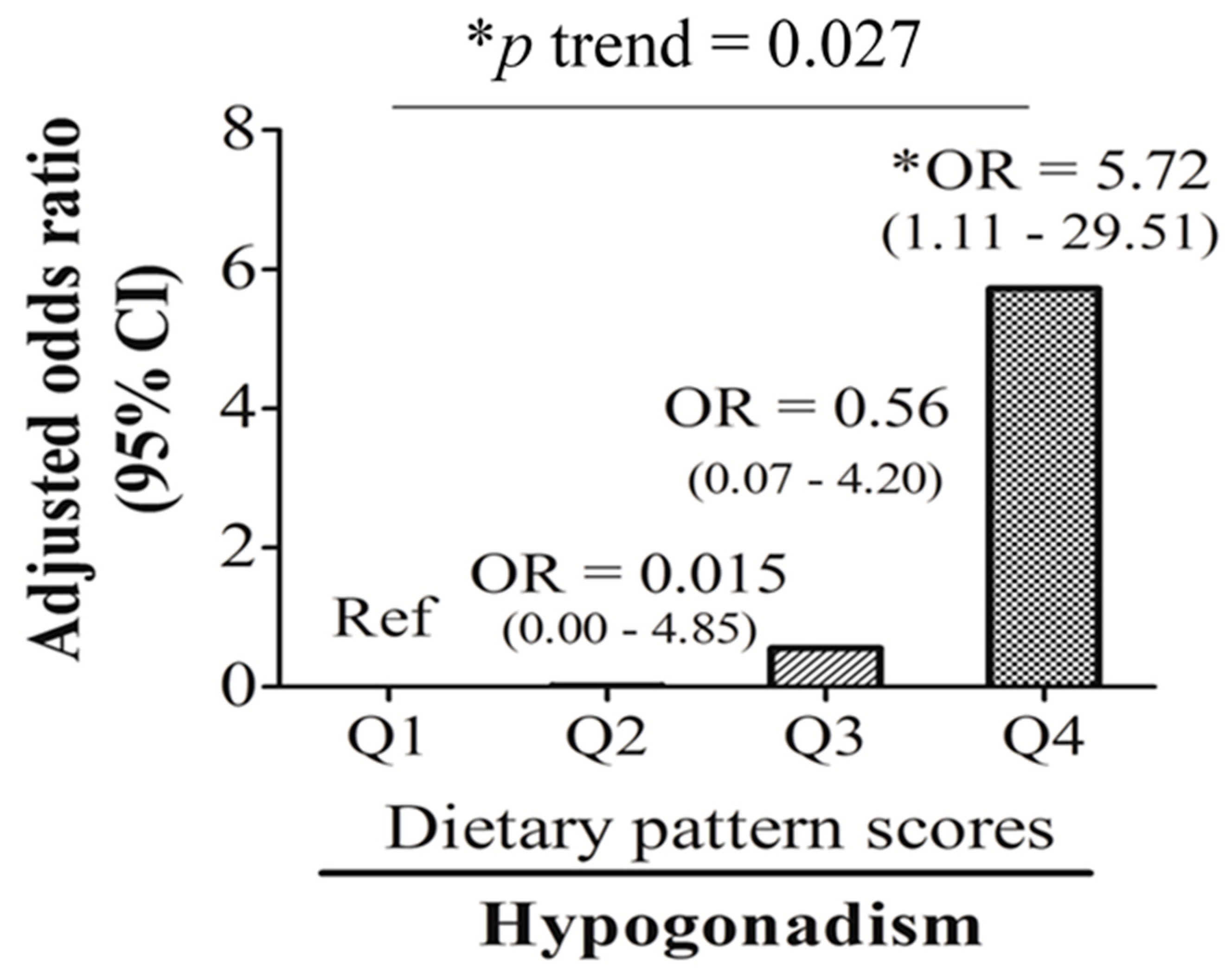
| Hypogonadism (n, %) | 3 (11.5) | 1 (3.7) | 4 (14.8) | 10 (38.5) | 0.006 |
| Iron overload (n, %) | 2 (8.0) | 7 (25.9) | 8 (29.6) | 7 (28.0) | 0.229 |
| NAFLD (n, %) | 17 (65.4) | 18 (66.7) | 26 (96.3) | 23 (88.5) | 0.008 |
| Type 2 diabetes (n, %) | 1 (3.8) | 1 (3.7) | 4 (14.8) | 4 (15.4) | 0.264 |
| MetS (n, %) | 2 (8.0) | 4 (14.8) | 9 (33.3) | 11 (44.0) | 0.011 |
| Anthropometry | |||||
| W/H ratio | 0.87 ± 0.05 | 0.90 ± 0.07 | 0.92 ± 0.05 | 0.91 ± 0.07 | 0.007 |
| BMI (kg/m2) | 23.41 ± 2.22 | 24.77 ± 4.58 | 26.65 ± 3.51 | 26.91 ± 5.22 | 0.001 |
| Total body fat mass (%) | 22.98 ± 4.13 | 23.72 ± 5.02 | 26.44 ± 4.28 | 26.95 ± 6.08 | 0.001 |
| Skeleton muscle mass (%) | 71.48 ± 4.40 | 70.51 ± 4.99 | 67.81 ± 4.13 | 67.32 ± 6.01 | 0.001 |
| Visceral fat mass (%) | 3.25 ± 0.88 | 3.52 ± 1.18 | 4.11 ± 0.99 | 4.35 ± 1.57 | <0.001 |
| Glucose Biomarkers | |||||
| Fasting glucose (mg/dL) | 86.64 ± 6.67 | 87.48 ± 6.71 | 94.93 ± 26.10 | 93.92 ± 15.58 | 0.043 |
| HbA1c (%) | 5.56 ± 0.44 | 5.69 ± 0.60 | 6.10 ± 1.03 | 5.98 ± 0.68 | 0.012 |
| Insulin (µIU/mL) | 7.28 ± 3.47 | 7.01 ± 2.84 | 11.12 ± 5.32 | 13.42 ± 7.86 | <0.001 |
| HOMA-IR | 1.54 ± 0.69 | 1.52 ± 0.65 | 2.53 ± 1.14 | 3.20 ± 2.16 | <0.001 |
| Lipid Biomarkers | |||||
| Total cholesterol (mg/dL) | 188.76 ± 26.96 | 203.96 ± 30.80 | 200.59 ± 48.19 | 192.88 ± 49.12 | 0.802 |
| TG (mg/dL) | 85.64 ± 40.81 | 113.33 ± 54.36 | 147.44 ± 59.33 | 160.84 ± 98.34 | <0.001 |
| HDL-C (mg/dL) | 52.60 ± 11.17 | 53.51 ± 12.62 | 47.40 ± 10.79 | 46.79 ± 15.43 | 0.038 |
| LDL-C (mg/dL) | 113.92 ± 29.42 | 127.07 ± 29.63 | 127.89 ± 41.56 | 115.20 ± 37.50 | 0.882 |
| Iron Biomarkers | |||||
| RBC (MIL/mm3) | 5.32 ± 0.80 | 5.25 ± 0.71 | 5.38 ± 0.80 | 5.23 ± 0.82 | 0.855 |
| Hb (gm/dL) | 15.76 ± 1.97 | 15.91 ± 2.22 | 16.04 ± 2.19 | 15.50 ± 2.33 | 0.736 |
| Free Hb (µg/mL) | 149.03 ± 47.10 | 147.82 ± 48.55 | 174.95 ± 53.21 | 161.42 ± 42.20 | 0.158 |
| Fe (µg/dL) | 108.92 ± 34.95 | 119.48 ± 29.80 | 109.00 ± 38.24 | 111.08 ± 33.85 | 0.896 |
| Ferritin (ng/mL) | 150.09 ± 81.65 | 248.26 ± 154.48 | 258.70 ± 191.44 | 237.62 ± 170.30 | 0.052 |
| TS (%) | 33.14 ± 10.75 | 36.46 ± 10.48 | 30.61 ± 12.41 | 32.64 ± 11.29 | 0.466 |
| sCD163 (ng/mL) | 822.54 ± 523.70 | 670.10 ± 239.59 | 940.25 ± 655.14 | 959.94 ± 437.00 | 0.144 |
| Hepcidin (ng/mL) | 154.22 ± 74.12 | 175.59 ± 84.87 | 218.43 ± 112.81 | 189.68 ± 102.76 | 0.081 |
| RBC deformability SS1/2 (Pa) | 2.19 ± 0.18 | 2.21 ± 0.16 | 2.25 ± 0.19 | 2.28 ± 0.19 | 0.079 |
| RBC aggregation CSS (mPa) | 246.59 ± 46.42 | 260.89 ± 52.52 | 298.89 ± 68.71 | 299.50 ± 70.07 | 0.001 |
| Sex Hormone Biomarkers | |||||
| Total T (ng/mL) | 4.83 ± 1.23 | 4.73 ± 1.20 | 3.56 ± 0.83 | 3.54 ± 1.36 | <0.001 |
| Free T (pg/mL) | 96.35 ± 21.27 | 91.31 ± 25.44 | 79.96 ± 20.97 | 67.13 ± 23.91 | <0.001 |
| SHBG (nmol/L) | 32.98 ± 12.64 | 36.68 ± 15.04 | 25.03 ± 6.73 | 35.22 ± 17.21 | 0.683 |
| Dietary Pattern Scores, Quartiles $ | p for Trend | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | p Value | Quartile 3 | p Value | Quartile 4 | p Value | ||
| Univariate | Ref | −0.105 (−0.784−0.573) | 0.756 | −1.276 (−1.857–−0.695) | <0.001 | −1.297 (−2.035–−0.558) | 0.001 | <0.001 |
| Model 1 * | Ref | −0.044 (−0.711−0.624) | 0.896 | −1.198 (−1.778–−0.619) | <0.001 | −1.192 (−1.979–−0.406) | 0.004 | <0.001 |
| Model 2 # | Ref | 0.155 (−0.464−0.773) | 0.618 | −0.852 (−1.500–−0.205) | 0.011 | −0.872 (−1.722–−0.023) | 0.044 | 0.004 |
| Dietary Pattern Scores $ | p for Trend | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | p Value | Q3 | p Value | Q4 | p Value | ||
| Univariate | ||||||||
| BFM (%) | Ref | 0.741 (−1.800~3.283) | 0.561 | 3.456 (1.136~5.776) | 0.004 | 3.969 (1.074~6.864) | 0.008 | 0.001 |
| SMM (%) | Ref | −0.973 (−3.573~1.626) | 0.456 | −3.673 (−6.027~−1.320) | 0.003 | −4.162 (−7.095~−1.228) | 0.006 | 0.001 |
| VFM (%) | Ref | 0.272 (−0.304~0.849) | 0.348 | 0.862 (0.339~1.384) | 0.002 | 1.098 (0.384~1.812) | 0.003 | <0.001 |
| SFM (%) | Ref | 0.477 (−1.496~2.450) | 0.630 | 2.685 (0.829~4.541) | 0.005 | 8.497 (−2.753~19.747) | 0.135 | 0.030 |
| Age adjusted | ||||||||
| BFM (%) | Ref | 0.540 (−1.996~3.077) | 0.671 | 3.475 (1.094~5.855) | 0.005 | 3.593 (0.504~6.681) | 0.024 | 0.002 |
| SMM (%) | Ref | −0.763 (−3.356~1.829) | 0.557 | −3.678 (−6.093~−1.262) | 0.004 | −3.756 (−6.883~−0.629) | 0.020 | 0.002 |
| VFM (%) | Ref | 0.211 (−0.356~0.778) | 0.457 | 0.841 (0.307~1.375) | 0.003 | 0.974 (0.214~1.734) | 0.013 | 0.001 |
| SFM (%) | Ref | 0.337 (−1.640~2.314) | 0.733 | 2.708 (0.810~4.607) | 0.006 | 8.637 (−3.453~20.727) | 0.157 | 0.035 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.-Y.; Chen, Y.C.; Lin, P.; Shih, C.-K.; Bai, C.-H.; Yuan, K.-C.; Lee, S.-Y.; Chang, J.-S. Testosterone-Associated Dietary Pattern Predicts Low Testosterone Levels and Hypogonadism. Nutrients 2018, 10, 1786. https://doi.org/10.3390/nu10111786
Hu T-Y, Chen YC, Lin P, Shih C-K, Bai C-H, Yuan K-C, Lee S-Y, Chang J-S. Testosterone-Associated Dietary Pattern Predicts Low Testosterone Levels and Hypogonadism. Nutrients. 2018; 10(11):1786. https://doi.org/10.3390/nu10111786
Chicago/Turabian StyleHu, Tzu-Yu, Yi Chun Chen, Pei Lin, Chun-Kuang Shih, Chyi-Huey Bai, Kuo-Ching Yuan, Shin-Yng Lee, and Jung-Su Chang. 2018. "Testosterone-Associated Dietary Pattern Predicts Low Testosterone Levels and Hypogonadism" Nutrients 10, no. 11: 1786. https://doi.org/10.3390/nu10111786
APA StyleHu, T.-Y., Chen, Y. C., Lin, P., Shih, C.-K., Bai, C.-H., Yuan, K.-C., Lee, S.-Y., & Chang, J.-S. (2018). Testosterone-Associated Dietary Pattern Predicts Low Testosterone Levels and Hypogonadism. Nutrients, 10(11), 1786. https://doi.org/10.3390/nu10111786






