A Double-Blind Controlled Study to Evaluate the Effects of Yogurt Enriched with Lactococcus lactis 11/19-B1 and Bifidobacterium lactis on Serum Low-Density Lipoprotein Level and Antigen-Specific Interferon-γ Releasing Ability
Abstract
:1. Introduction
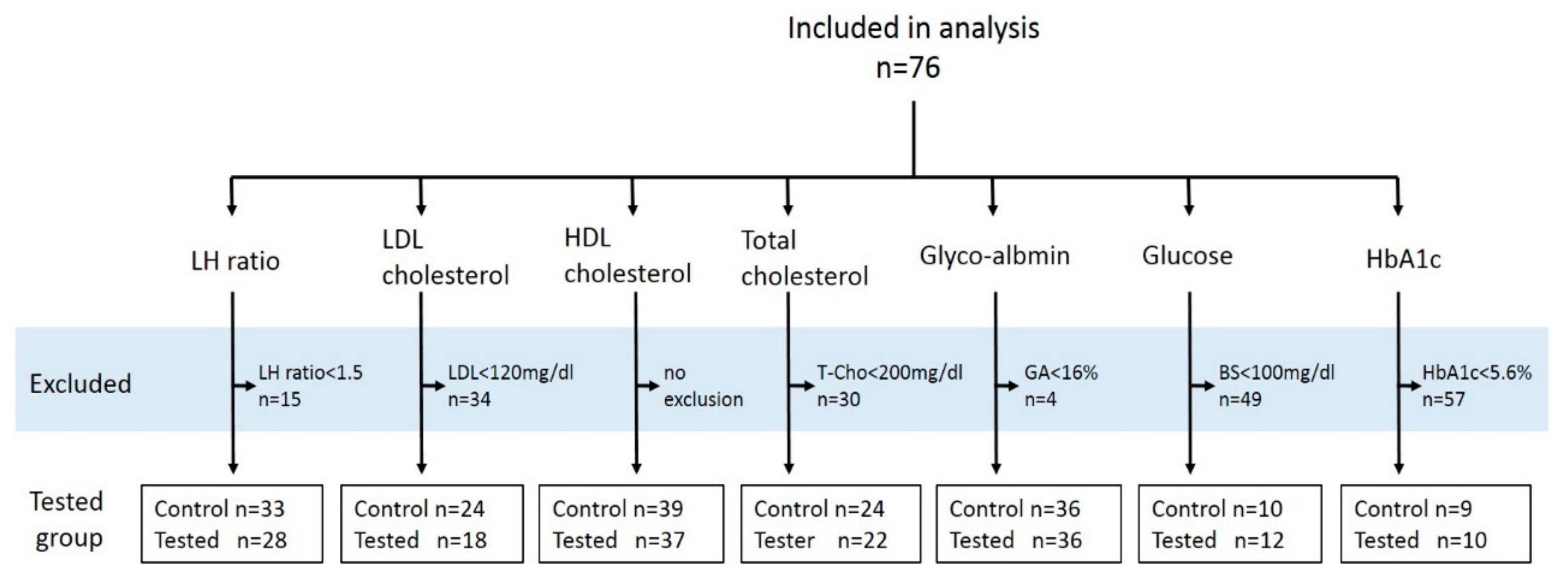
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Yogurts
2.4. Human Cytomegalovirus-Specific Interferon-γ Release Test
2.5. Statistical Analysis
3. Results
3.1. Effect of Yogurt Intake on Serum Parameters
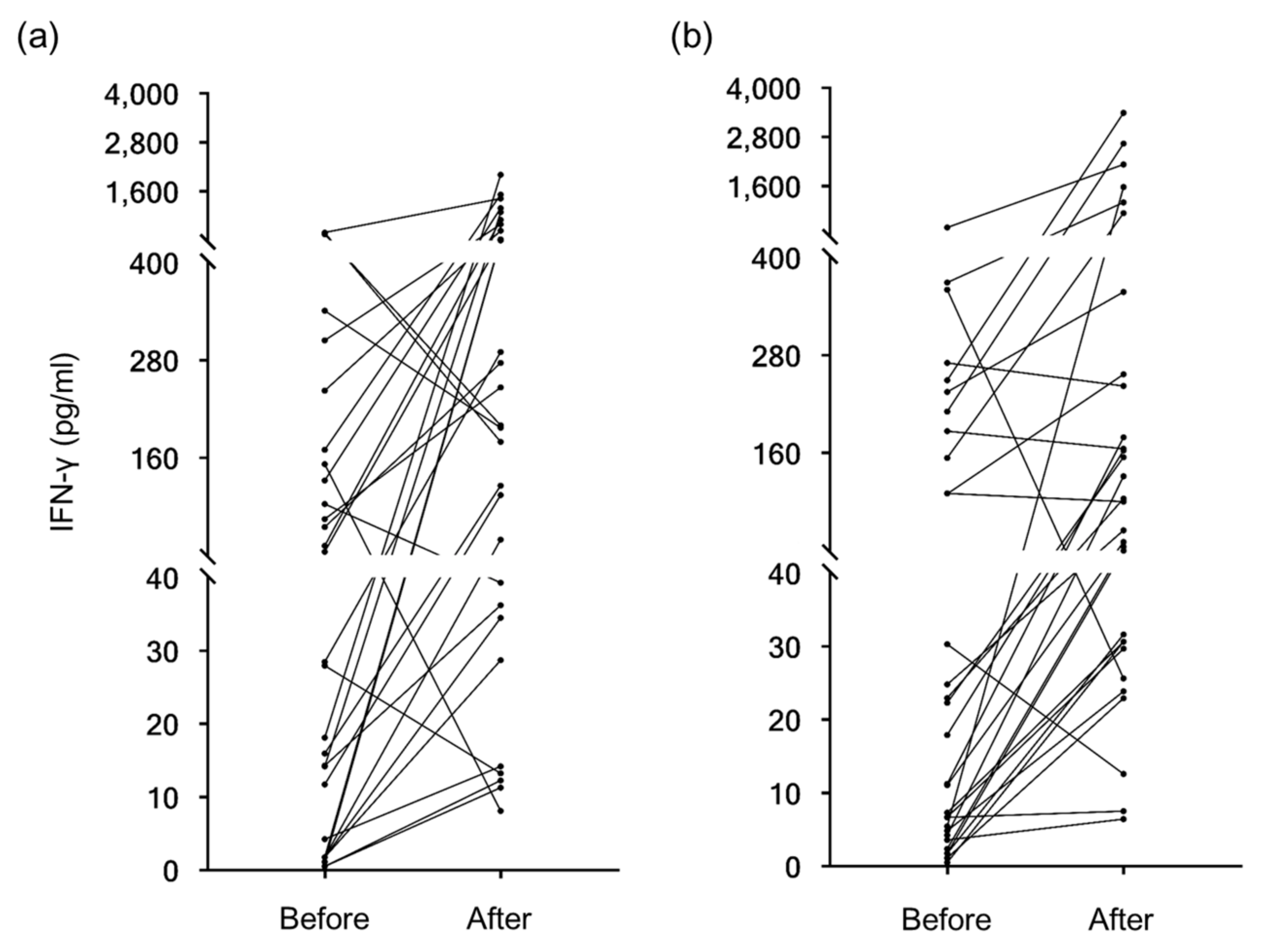
3.2. Effect of Yogurt Intake on Cellular Immunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salque, M.; Bogucki, P.I.; Pyzel, J.; Sobkowiak-Tabaka, I.; Grygiel, R.; Szmyt, M.; Evershed, R.P. Earliest evidence for cheese making in the sixth millennium BC in northern Europe. Nature 2013, 493, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. Chapter V. Lactic acid as inhibiting intestinal putrefaction. In The Prolongation of Life. Optimistic Studies; Mitchell, S.P.C., Ed.; Putnam: Cambridge, MA, USA, 1908; pp. 161–183. [Google Scholar]
- McFarland, L.V. From yaks to yogurt: The history, development, and current use of probiotics. Clin. Infect. Dis. 2015, 60, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, M.J.; Kim, P.; Kim, J.H. Cloning and production of a novel bacteriocin, lactococcin K, from Lactococcus lactis subsp. lactis MY23. Biotechnol. Lett. 2006, 28, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Lacroix, C.; Thuault, D.; Bourgeois, C.M.; Simard, R.E. Characterization of diacetin B, a bacteriocin from Lactococcus lactis subsp. lactis bv. diacetylactis UL720. Can. J. Microbiol. 1995, 41, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, S.H. Inhibitory effect of Lactococcus lactis HY449 on cariogenic biofilm. J. Microbiol. Biotechnol. 2016, 26, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Ono, Y.; Sekimizu, K. Lactic acid bacteria activating immunity improve survival in bacteria infection model of silkworm. Drug Discov. Ther. 2016, 10, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Hamamoto, H.; Kamimura, M.; Sekimizu, K. Activation of the silkworm cytokine by bacterial and fungal cell wall components via a reactive oxygen species-triggered mechanism. J. Biol. Chem. 2008, 238, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Food and Agriculture organization. Codex standard fermented milks (CODEX STAN 243-2003). In Milk and Milk Products, 2nd ed.; FAO/WHO: Rome, Italy, 2011. [Google Scholar]
- Furui, Y.; Satake, M.; Hoshi, Y.; Uchida, S.; Suzuki, K.; Tadokoro, K. Cytomegalovirus (CMV) seroprevalence in Japan blood donors and high detection frequency of CMV DNA in elderly donors. Transfusion 2013, 53, 2190–2197. [Google Scholar] [PubMed]
- Kobayashi, T.; Sato, J.; Ikuta, K.; Kanno, R.; Nishiyama, K.; Koshizuka, T.; Ishioka, K.; Suzutani, T. Modification of the HCMV-specific IFN-γ release test (QuantiFERON-CMV) and a novel proposal for its application. Fukushima J. Med. Sci. 2017, 63, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Akedo, I.; Otani, T.; Suzuki, T.; Nakamura, T.; Takeyama, I.; Ishiguro, S.; Miyaoka, E.; Sobue, T.; Kakizoe, T. Ramdomized trial of dietary fiber and Lactobaccillus casei administration for prevention of colorectal tumors. Int. J. Cancer 2005, 20, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Kumai, H.; Nakamichi, N.; Sugiyama, T.; Deguchi, R.; Takagi, A.; Koga, Y. Suppression of Helicobacter pylori-induced interleukin-8 production in vitro and within the gastric mucosa by a live Lactobacillus strain. J. Gastroenterol. Hepatol. 2006, 21, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.M.; Aiba, Y.; Takagi, A.; Kamiya, S.; Miwa, T.; Koga, Y. Prevention of Helicobacter pylori infection by lactobacillus in a gnotobiotic murine model. Gut 1997, 41, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.E.; Stuer-Lauridsen, B.; Eskesen, D. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12. Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef] [PubMed]
- De Vrese, M.; Winkler, P.; Rautenberg, P.; Harder, T.; Noah, C.; Laue, C.; Ott, S.; Hampe, J.; Schreiber, S.; Heller, K.; et al. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, control trial. Vaccine 2006, 24, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; Gibson, G.R. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 259–281. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.R.; Williams, C.M. Effects of probiotics and prebiotics on blood lipids. Br. J. Nutr. 1998, 80, S225–S230. [Google Scholar] [PubMed]
- Minami, J.; Kondo, S.; Yanagisawa, N.; Odamaki, T. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomized controlled trial. J. Nutr. Sci. 2015, 4, e4. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, M.; Pohjavuori, E.; Haahtela, T.; Korpela, R.; Kuitunen, M.; Sarnesto, A.; Vaarala, O.; Savilahti, E. Induction of inflammation as a possible mechanism of probiotic effect in atopic eczema-dermatitis syndrome. J. Allergy Clin. Immunol. 2005, 115, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Rigassio-Radler, D.; Denmark, R.; Haley, T.; Touger-Decker, R. Effect of Lactobacillus rhamnosus LGG and Bifidobacterium animalis ssp. lactis BB-12 on health-ralated quality of life in college students affected by upper respiratory infections. Br. J. Nutr. 2013, 109, 1999–2007. [Google Scholar] [PubMed]
- Pitkala, K.H.; Strandberg, T.E.; Finne Soveri, U.H.; Ouwehand, A.C.; Poussa, T.; Salminen, S. Fermented milk containing Bifidobacterium lactis BB-12 on stool frequency, defecation, fecal microbiota and safety of excessive ingestion in healty female students. J. Nutr. Food 2005, 8, 39–51. [Google Scholar]
- Lee, Y.; Ba, Z.; Roberts, R.F.; Rogers, C.J.; Fleming, J.A.; Meng, H.; Furumoto, E.J.; Kris-Etherton, P.M. Effects of Bifidobacterium animalis subsp. lactis BB-12 on the lipid/lipoprotein profile and short chain fatty acids in healthy young adults: A randomized controlled trial. Nutr. J. 2017, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Gilliland, S.E. Effect of fermented milk (yogurt) containing Lactobacillu acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J. Am. Coll. Nutr. 1999, 18, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Aoki-Yoshida, A.; Kimoto-Nira, H.; Yamagishi, N.; Tomita, S.; Sekiyama, Y.; Wakagi, M.; Sakurai, M.; Ippoushi, K.; Suzuki, C.; et al. Dietary intake of heat-killed Lactococcus lactis H61 delays age-related hearing loss in C57BL/6J mice. Sci. Rep. 2016, 6, 23556. [Google Scholar] [CrossRef] [PubMed]
- Kinoto-Nira, H.; Suzuki, C.; Kobayashi, M.; Sasaki, K.; Kurisaki, J.; Mizumachi, K. Anti-aging effect of a lactococcal strain: Analysis using senescence-accelerated mice. Br. J. Nutr. 2007, 98, 1178–1186. [Google Scholar]
- Fukushima, Y.; Kawata, Y.; Hara, H.; Terada, A.; Mitsuoka, T. Effect of a probiotic formula on intestinal immunoglobulin A. Food Microbiol 1998, 42, 39–44. [Google Scholar] [CrossRef]
- Macpherson, A.J.; Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004, 303, 1662–1665. [Google Scholar] [CrossRef]
- Yasui, H.; Kiyoshima, J.; Hori, T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin. Diagn. Lab. Immunl. 2004, 11, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, Y.; Izumo, T.; Izumi, F.; Maekawa, T.; Shibata, H.; Nakano, A.; Kishi, A.; Akatani, K.; Kiso, Y. Antiallergic effects of Lactobacillus pentosus strain S-PT84 mediated by modulation of Th1/Th2 immunobalance and induction of IL-10 production. Int. Arch. Allergy Immunol. 2007, 145, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T.; Takahashi, H.; Jounai, K.; Ohshio, K.; Kanayama, M.; Tazumi, K.; Tanihata, Y.; Miura, Y.; Fujiwara, D.; Yamamoto, N. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 2015, 114, 727–733. [Google Scholar] [CrossRef] [PubMed]
| Control Yogurt Group (n = 39) | Test Yogurt Group (n = 37) | p Value | |
|---|---|---|---|
| Age | 43.8 ± 11.7 | 40.9 ± 10.6 | 0.27 * |
| Sex (% of male) | 38.5 | 37.8 | 0.96 ** |
| Total cholesterol (mg/dL) | 214.3 ± 36.5 | 215.3 ± 34.2 | 0.90 * |
| LDL cholesterol (mg/dL) | 129.7 ± 35.0 | 133.0 ± 31.3 | 0.67 * |
| HDL cholesterol (mg/dL) | 61.9 ± 14.0 | 63.6 ± 16.4 | 0.67 * |
| LDL/HDL ratio | 1.97 (1.16–4.83) | 2.21 ± 0.92 | 0.65 *** |
| Glucose (mg/dL) | 94.5 (78–133) | 95.0 (80–140) | 0.85 *** |
| Glyco-albumin (%) | 13.9 ± 1.4 | 13.7 ± 1.1 | 0.43 *** |
| HbA1c (%) | 5.4 ± 0.3 | 5.3 (4.9–6.8) | 0.81 *** |
| IFN-γ (pg/mL) | 84.6 (0.5–8702.5) | 30.3 (0.5–5696.4) | 0.59 *** |
| Control Yogurt Group (n = 39) | p Value * | Test Yogurt Group (n = 37) | p Value * | |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 209.8 ± 34.8 | 0.06 | 198.0 (150–303) | <0.001 |
| LDL cholesterol (mg/dL) | 130.5 ± 31.3 | 0.26 | 123.2 ± 31.9 | 0.01 |
| HDL cholesterol (mg/dL) | 61.9 ± 14.4 | 0.79 | 62.7 ± 14.5 | 0.54 |
| LDL/HDL ratio | 2.21 ± 0.70 | 0.09 | 1.92 (0.90–4.34) | 0.02 |
| Glucose (mg/dL) | 94.0 (80–199) | 0.19 | 95.0 (68–140) | 0.17 |
| Glyco-albumin (%) | 13.7 ± 1.4 | 0.16 | 13.8 ± 1.0 | 0.85 |
| HbA1c (%) | 5.5 (4.9–6.2) | 0.01 | 5.4 (5.0–6.6) | 0.32 |
| IFN-γ | 276.9 (8.1–10255.7) | 0.42 | 154.9 (6.5–4592.5) | 0.46 |
| Group | Before | After | p Value * | |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | Control (n = 24) | 236.2 ± 24.9 | 228.0 ± 29.0 | 0.04 |
| Test (n = 22) | 236.7 ± 26.5 | 225.1 ± 30.6 | <0.001 | |
| LDL ** cholesterol (mg/dL) | Control (n = 24) | 151.8 ± 23.4 | 146.8 ± 24.7 | 0.14 |
| Test (n = 18) | 159.1 ± 25.7 | 149.3 ± 24.4 | 0.02 | |
| HDL ** cholesterol (mg/dL) | Control (n = 39) | 61.9 ± 14.0 | 61.8 ± 14.4 | 0.92 |
| Test (n = 37) | 63.6 ± 16.3 | 62.7 ± 14.5 | 0.52 | |
| LDL/HDL ratio | Control (n = 33) | 2.5 ± 0.8 | 2.4 ± 0.6 | 0.12 |
| Test (n = 28) | 2.5 ± 0.8 | 2.4 ± 0.8 | <0.001 | |
| Glucose (mg/dL) | Control (n = 10) | 112.4 ± 9.8 | 108.0 ± 9.8 | 0.17 |
| Test (n = 12) | 108.1 ± 11.6 | 106.4 ± 12.7 | 0.25 | |
| Glyco-albumin (%) | Control (n = 36) | 13.7 ± 1.2 | 13.7 ± 1.1 | 0.39 |
| Test (n = 36) | 13.6 ± 0.9 | 13.8 ± 1.0 | 0.03 | |
| HbA1c (%) | Control (n = 9) | 5.7 ± 0.2 | 5.7 ± 0.3 | 0.83 |
| Test (n = 10) | 5.9 ± 0.4 | 5.8 ± 0.5 | 0.14 | |
| IFN-γ ** | Control (n = 29) | 121.3 ± 177.5 | 765.6 ± 1837.9 | 0.037 |
| Test (n = 30) | 101.2 ± 144.8 | 472.1 ± 847.6 | 0.029 |
| Group | Number | Before | After | p Value * |
|---|---|---|---|---|
| Control yogurt | 15 | 102.9 ± 13.3 | 104.4 ± 21.6 | 0.93 |
| Test yogurt | 19 | 101.8 ± 11.7 | 98.6 ± 21.5 | 0.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, K.; Kobayashi, T.; Sato, Y.; Watanabe, Y.; Kikuchi, R.; Kanno, R.; Koshizuka, T.; Miyazaki, N.; Ishioka, K.; Suzutani, T. A Double-Blind Controlled Study to Evaluate the Effects of Yogurt Enriched with Lactococcus lactis 11/19-B1 and Bifidobacterium lactis on Serum Low-Density Lipoprotein Level and Antigen-Specific Interferon-γ Releasing Ability. Nutrients 2018, 10, 1778. https://doi.org/10.3390/nu10111778
Nishiyama K, Kobayashi T, Sato Y, Watanabe Y, Kikuchi R, Kanno R, Koshizuka T, Miyazaki N, Ishioka K, Suzutani T. A Double-Blind Controlled Study to Evaluate the Effects of Yogurt Enriched with Lactococcus lactis 11/19-B1 and Bifidobacterium lactis on Serum Low-Density Lipoprotein Level and Antigen-Specific Interferon-γ Releasing Ability. Nutrients. 2018; 10(11):1778. https://doi.org/10.3390/nu10111778
Chicago/Turabian StyleNishiyama, Kyoko, Takahiro Kobayashi, Yuko Sato, Yoshihisa Watanabe, Riki Kikuchi, Ryoko Kanno, Tetsuo Koshizuka, Nozomu Miyazaki, Ken Ishioka, and Tatsuo Suzutani. 2018. "A Double-Blind Controlled Study to Evaluate the Effects of Yogurt Enriched with Lactococcus lactis 11/19-B1 and Bifidobacterium lactis on Serum Low-Density Lipoprotein Level and Antigen-Specific Interferon-γ Releasing Ability" Nutrients 10, no. 11: 1778. https://doi.org/10.3390/nu10111778
APA StyleNishiyama, K., Kobayashi, T., Sato, Y., Watanabe, Y., Kikuchi, R., Kanno, R., Koshizuka, T., Miyazaki, N., Ishioka, K., & Suzutani, T. (2018). A Double-Blind Controlled Study to Evaluate the Effects of Yogurt Enriched with Lactococcus lactis 11/19-B1 and Bifidobacterium lactis on Serum Low-Density Lipoprotein Level and Antigen-Specific Interferon-γ Releasing Ability. Nutrients, 10(11), 1778. https://doi.org/10.3390/nu10111778






