Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cherry Samples
2.3. Phenolic Compounds
2.3.1. Extraction
2.3.2. HPLC-DAD Analysis
2.4. Biological Acitivity Evaluation
2.4.1. Antioxidant Capacity
DPPH
Nitric Oxide
Superoxide Radical
2.4.2. α-Glucosidase Inhibitory Activity
2.4.3. Protective Effect in Human Erythrocytes against Oxidative Damage
Inhibition of Hemoglobin Oxidation
Inhibition of Hemolysis
2.5. Cell Culture Conditions and Treatments
2.5.1. Cytoprotection Assay
2.5.2. MTT Cell Proliferation Assay
2.5.3. LDH Assay
2.5.4. Cell Cycle Distribution Analysis
2.6. Statistical Analysis of Results
3. Results and Discussion
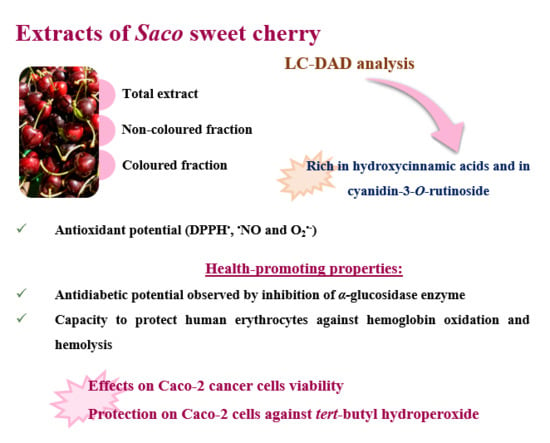
3.1. Phenolic Profile
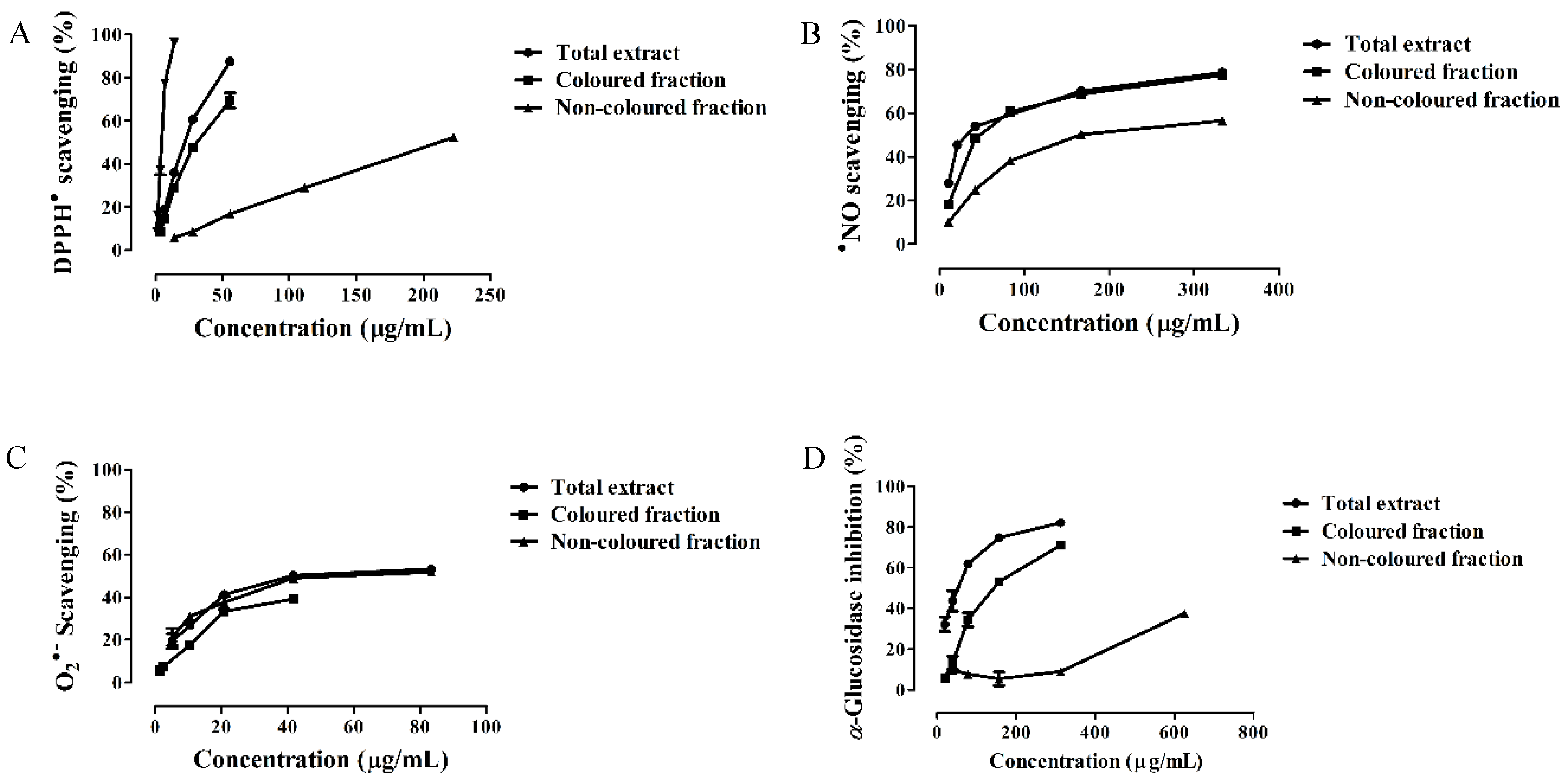
3.2. Antioxidant Capacity
3.3. α-Glucosidase Inhibitory Activity
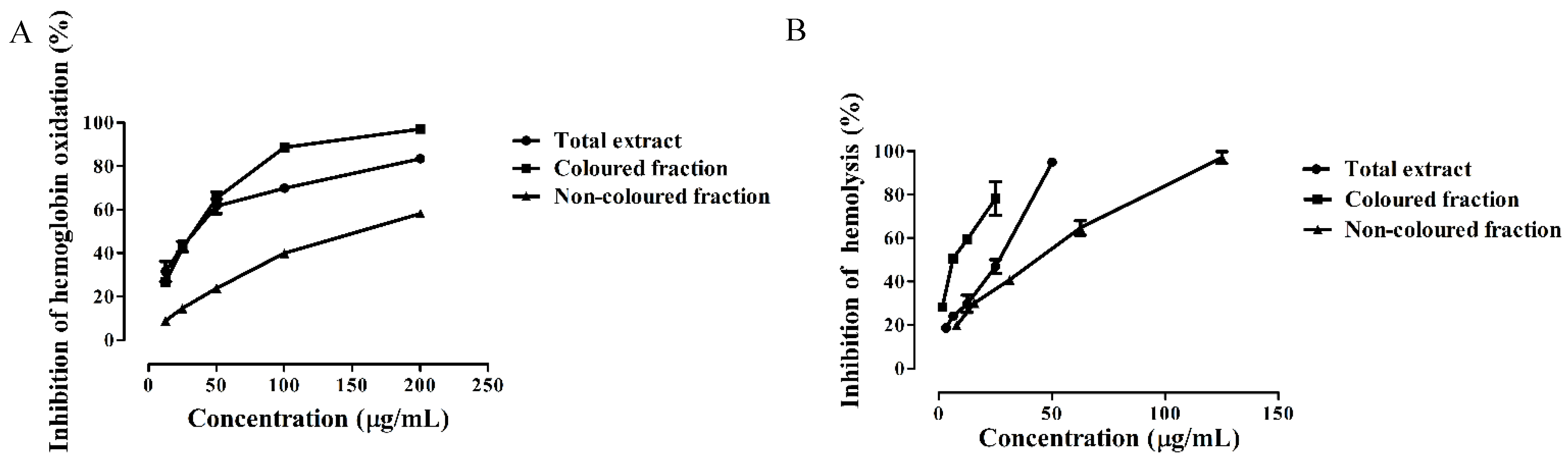
3.4. Protective Effects of Saco Extracts against ROO• in Human Blood Samples
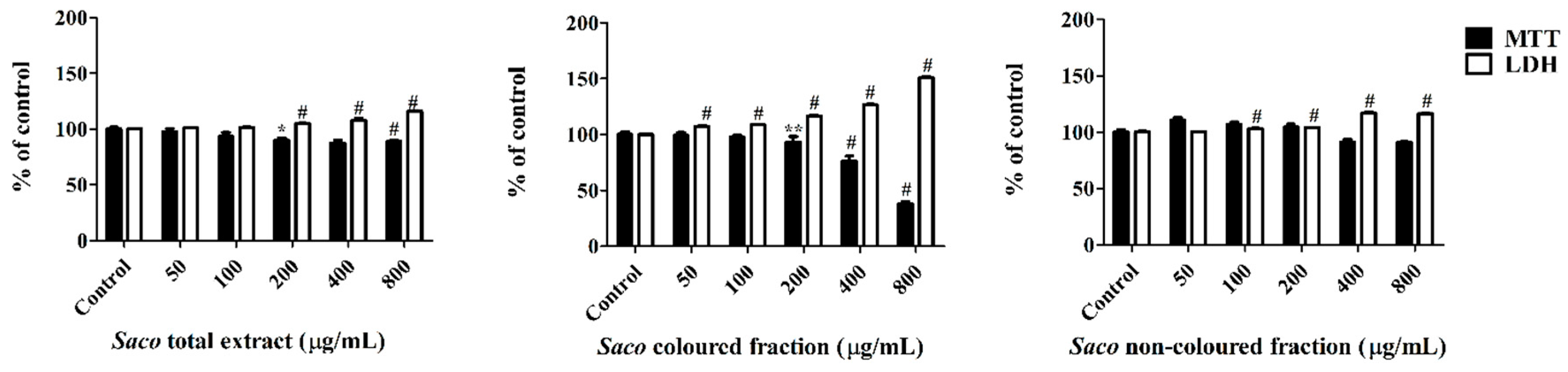
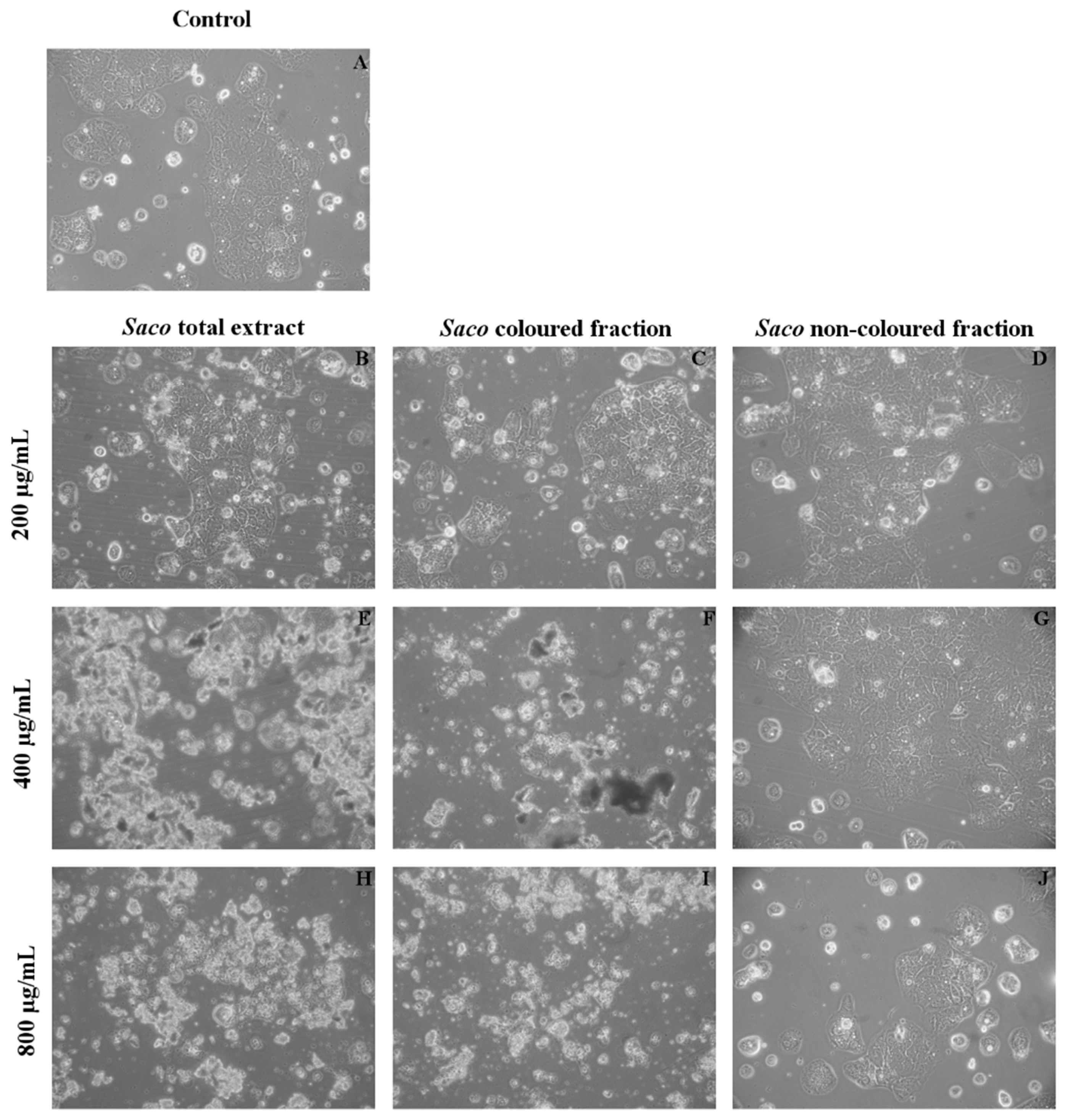
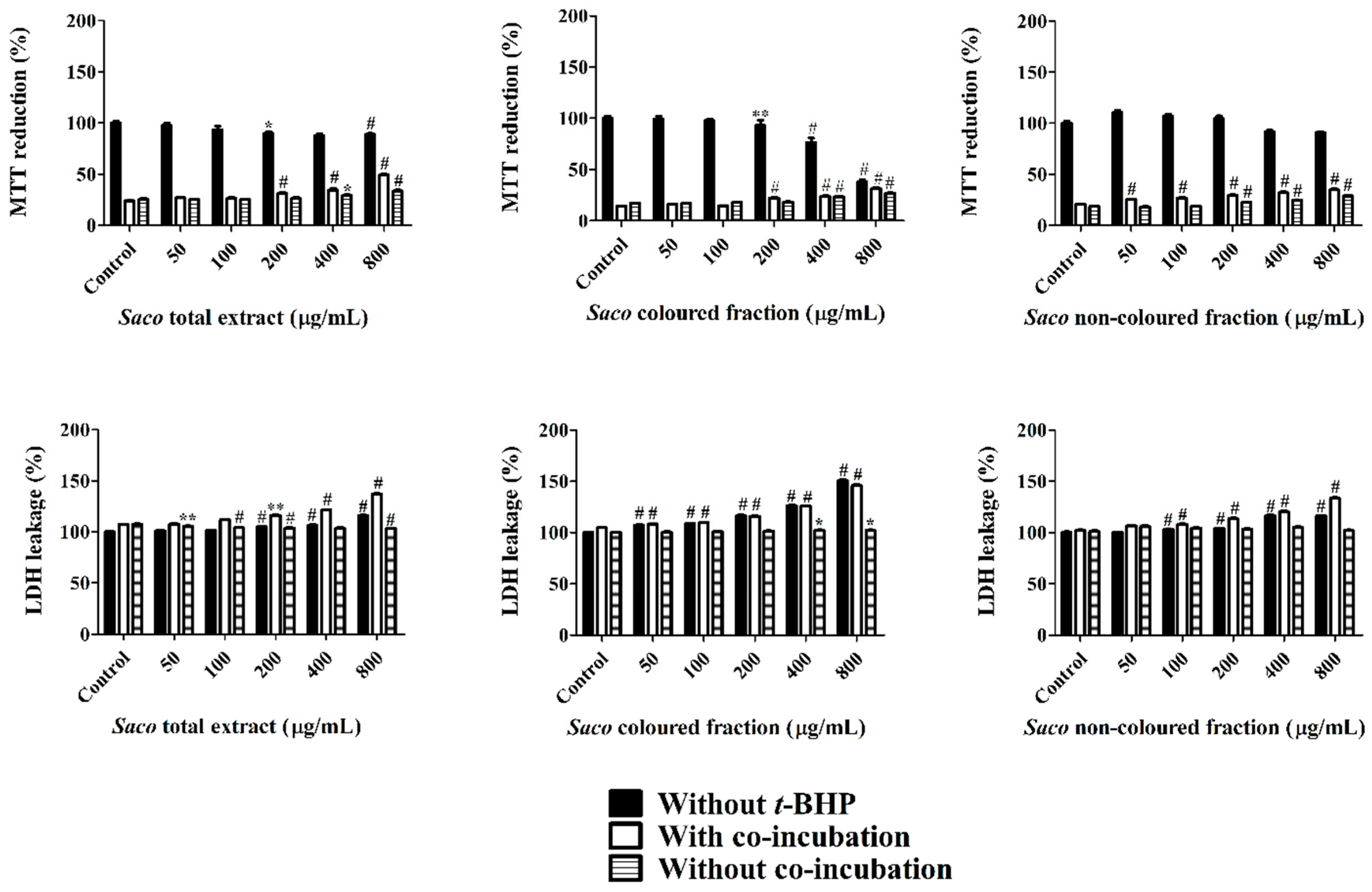
3.5. Effect of Sweet Cherry Extracts in Caco-2 Cell Viability
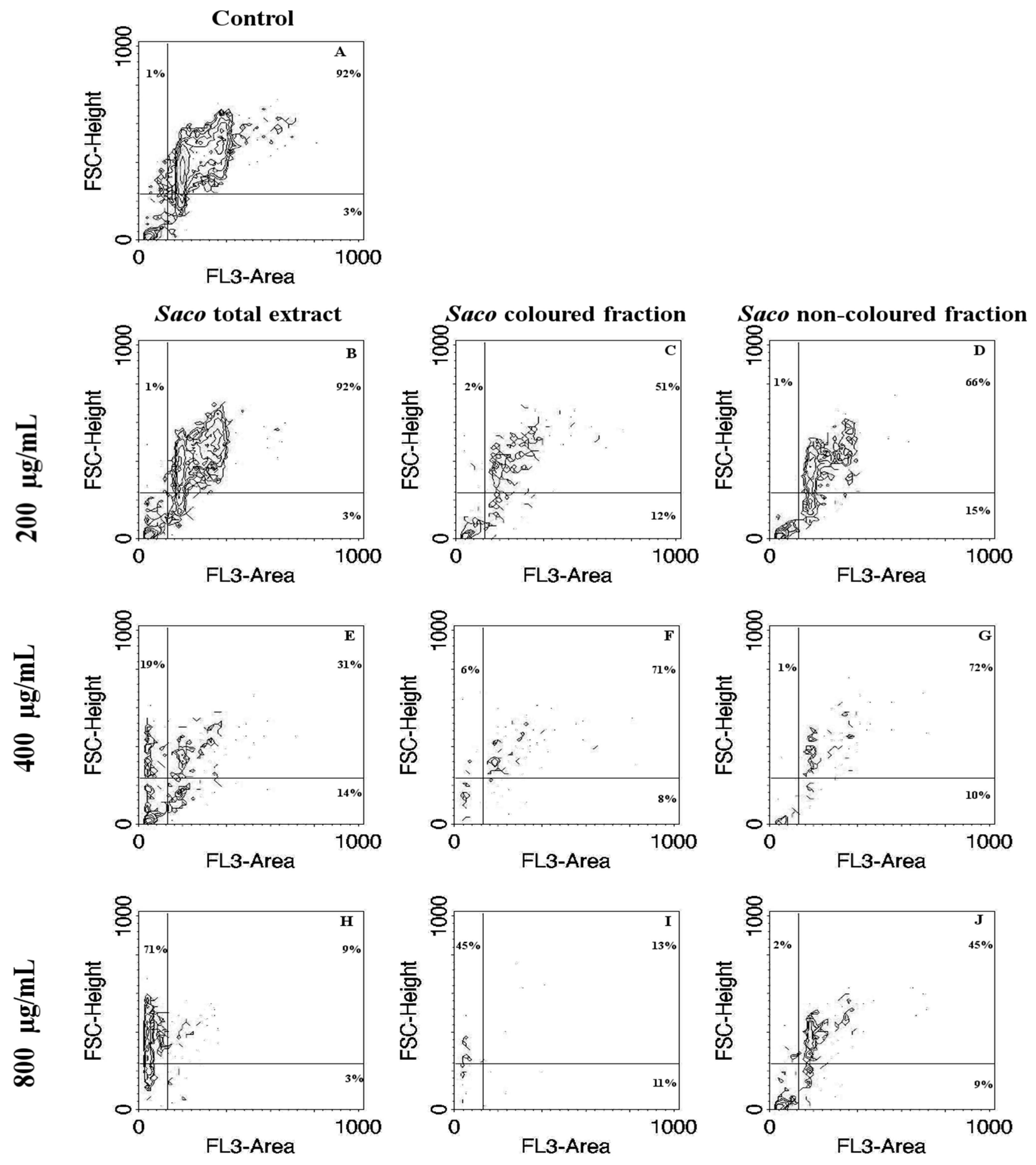
3.6. Cytoprotection Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Samad, N.B.; Debnath, T.; Ye, M.; Hasnat, M.A.; Lim, B.O. In vitro antioxidant and anti-inflammatory activities of Korean blueberry (Vaccinium corymbosum L.) extracts. Asian Pac. J. Trop. Biomed. 2014, 4, 807–815. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Silva, B.; Simões, M.; Silva, L.R. Nutrients, bioactive compounds and bioactivity: The health benefits of sweet cherries (Prunus avium L.). Curr. Nutr. Food Sci. 2017, 13, 1–20. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of a-glucosidase and a-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Melnyk, J.P.; Tsao, R.; Marcone, M.F. How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Res. Int. 2011, 44, 14–22. [Google Scholar] [CrossRef]
- Gengaihi, S.; Ella, F.M.A.; Emad, M.H.; Shalaby, E.; Doha, H. Antioxidant activity of phenolic compounds from different grape wastes. Food Process. Technol. 2014, 5. [Google Scholar] [CrossRef]
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Silva, B.M.; Silva, L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Matias, A.A.; Almeida, A.P.C.; Bronze, M.R.; Alves, P.M.; De Sousa, H.C.; Duarte, C.M.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 2. Evaluation of SCF extracts as promising natural chemotherapeutical agents. J. Supercrit. Fluids 2011, 55, 1007–1013. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bickford, R.; Valverde, C. Global Agricultural Information Network; USDA Foreign Agricultural Service: Madrid, Spain, 2015; pp. 1–23.
- Silva, L.R.; Teixeira, R. Phenolic profile and biological potential of Endopleura uchi extracts. Asian Pac. J. Trop. Med. 2015, 8, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Chisté, R.C.; Freitas, M.; Mercadante, A.Z.; Fernandes, E. Carotenoids inhibit lipid peroxidation and hemoglobin oxidation, but not the depletion of glutathione induced by ROS in human erythrocytes. Life Sci. 2014, 99, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.B.; Rodrigues, E.; Chisté, R.C.; Fernandes, E.; Mercadante, A.Z. The Amazonian fruit Byrsonima crassifolia effectively scavenges reactive oxygen and nitrogen species and protects human erythrocytes against oxidative damage. Food Res. Int. 2014, 64, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.; Campos, G.; Santos, A.O.; Falcão, A.; Silvestre, S.; Alves, G. Synthesis, in vitro evaluation and QSAR modelling of potential antitumoral 3,4-dihydropyrimidin-2-(1H)-thiones. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Gonçalves, B.; Landbo, A.K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Singer, A.A.M.; Kirakosyan, A.; Urcuyo-Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food 2008, 11, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; González-Paramás, A.M.; Santos-Buelga, C.; Mezzetti, B.; Quiles, J.L.; Battino, M.; Giampieri, F.; et al. Strawberry (cv. Romina) methanolic extract and anthocyanin-enriched fraction improve lipid profile and antioxidant status in HepG2 cells. Int. J. Mol. Sci. 2017, 18, 1149. [Google Scholar] [CrossRef]
- Moein, S.; Mein, M.; Farmani, F.; Sabahi, Z. Different methods evaluation of antioxidant properties of Myrtus communis extract and its fractions. Trends Pharm. Sci. 2015, 1, 153–158. [Google Scholar]
- Jacob, R.A.; Spinozzi, G.M.; Vicky, A.; Kelley, D.S.; Prior, R.L.; Ars, A.; Children, A. Consumption of cherries lowers plasma urate in healthy women. J. Nutr. 2003, 133, 1826–1829. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Maier, C. In vitro antioxidant activities and polyphenol contents of seven commercially available fruits. Pharmacogn. Res. 2016, 8, 97–105. [Google Scholar] [CrossRef]
- Prior, R.L.; Sintara, M.; Chang, T. Multi-radical (ORACMR5) antioxidant capacity of selected berries and effects of food processing. J. Berry Res. 2016, 6, 159–173. [Google Scholar] [CrossRef]
- Tumbas, V.; Čanadanović-Brunet, J.; Gille, L.; Dilas, S.; Ćetković, G. Superoxide anion radical scavenging activity of bilberry (Vaccinium myrtillus L.). J. Berry Res. 2010, 1, 13–23. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Valdez-Morales, M.; Avila, J.G.; El-Hafidi, M.; Alarcón, J. Phytochemical profile and the antioxidant activity of Chilean wild black-berry fruits, Aristotelia chilensis (Mol) Stuntz (Elaeocarpaceae). Food Chem. 2010, 119, 886–895. [Google Scholar] [CrossRef]
- Mendes, L.; De Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Juvekar, A.R.; Khatri, D.K. α-Glucosidase and α-amylase inhibitory activity of Indigofera cordifolia seeds and leaves extract. Int. J. Pharm. Pharm. Sci. 2014, 6, 152–155. [Google Scholar]
- Akkarachiyasit, S.; Yibchok-Anun, S.; Wacharasindhu, S.; Adisakwattana, S. In vitro inhibitory effects of cyandin-3-rutinoside on pancreatic α-amylase and its combined effect with acarbose. Molecules 2011, 16, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, X.; Liu, Y.; Leng, F.; Li, X.; Sun, C.; Chen, K. Bioassay-based isolation and identification of phenolics from sweet cherry that promote active glucose consumption by HepG2 cells. J. Food Sci. 2015, 80, C234–C240. [Google Scholar] [CrossRef] [PubMed]
- Lachin, T. Effect of antioxidant extract from cherries on diabetes. Recent. Pat. Endocr. Metab. Immune Drug. Discov. 2014, 8, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Olaokun, O.O.; Mcgaw, L.J.; Eloff, J.N.; Naidoo, V. Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes. BMC. Complement. Altern. Med. 2013, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Asgar, A. Anti-diabetic potential of phenolic compounds: A review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Varì, R.; Filesi, C.; Archivio, M.D.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Volti, G.L.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. (Lond.) 2015, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Yibchok-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-rutinoside alleviates postprandia hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.M.; Gu, W.; Costa, S.S.; Jeppesen, P.B. Phenolic substances from Ocimum species enhance glucose-stimulated insulin secretion and modulate the expression of key insulin regulatory genes in mice pancreatic islets. J. Nat. Prod. 2017, 80, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; González-Paramás, A.M.; Damiani, E.; Astolfi, P.; Martinez-Sanchez, G.; Bompadre, S.; Quiles, J.L.; Santos-Buelga, C.; Battino, M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem. Toxicol. 2012, 50, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.S.; Silva, B.M.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem. Toxicol. 2009, 47, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- García-Becerra, L.; Mitjans, M.; Rivas-Morales, C.; Verde-Star, J.; Oranday-Cárdenas, A.; Vinardell María, P. Antioxidant comparative effects of two grape pomace Mexican extracts from vineyards on erythrocytes. Food Chem. 2016, 194, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Piatti, E. Honey flavonoids as protection agents against oxidative damage to human red blood cells. Food Chem. 2007, 104, 1635–1640. [Google Scholar] [CrossRef]
- Kitagawa, S.; Sakamoto, H.; Tano, H. Inhibitory effects of flavonoids on free radical-induced hemolysis and their oxidative effects on hemoglobin. Chem. Pharm. Bull. (Tokyo) 2004, 52, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Kubow, S.; Iskandar, M.M.; Melgar-Bermudez, E.; Sleno, L.; Sabally, K.; Azadi, B.; How, E.; Prakash, S.; Burgos, G.; Zum Felde, T. Effects of simulated human gastrointestinal digestion of two purple-fleshed potato cultivars on anthocyanin composition and cytotoxicity in colonic cancer and non-tumorigenic cells. Nutrients 2017, 9, 953. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.B.; Comunello, L.N.; Maciel, É.S.; Giubel, S.R.; Bruno, A.N.; Chiela, E.C.F.; Lenz, G.; Gnoatto, S.C.B.; Buffon, A.; Gosmann, G. The inhibitory effects of phenolic and terpenoid compounds from Baccharis trimera in SiHa cells: Differences in their activity and mechanism of action. Molecules 2013, 18, 11022–11032. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and proapoptotic activities of anthocyanin and anthocyanidin extracts from blueberry fruits on B16-F10 melanoma cells. Food Nutr. Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Mutalib, M.A.; Ali, F.; Othman, F.; Ramasamy, R.; Rahmat, A. Phenolics profile and anti-proliferative activity of Cyphomandra Betacea fruit in breast and liver cancer cells. Springerplus 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Lazzè, M.C.; Savio, M.; Pizzala, R.; Cazzalini, O.; Perucca, P.; Scovassi, A.I.; Stivala, L.A.; Bianchi, L. Anthocyanins induce cell cycle perturbations and apoptosis in different human cell lines. Carcinogenesis 2004, 25, 1427–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, G.C.; Chen, C.S.; Chang, W.T.; Wu, M.F.; Cheng, F.T.; Shiau, D.K.; Hsu, C.L. Antioxidant activity and anticancer effect of ethanolic and aqueous extracts of the roots of Ficus beecheyana and their phenolic components. J. Food Drug. Anal. 2018, 26, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Moita, E.; Valentão, P.; Fernandes, F.; Monteiro, P.; Andrade, P.B. Effects of colored and noncolored phenolics of echium plantagineum L. bee pollen in caco-2 cells under oxidative stress induced by tert-butyl hydroperoxide. J. Agric. Food Chem. 2015, 63, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Bedoya-Ramírez, D.; Cilla, A.; Contreras-Calderón, J.; Alegría-Torán, A. Evaluation of the antioxidant capacity, furan compounds and cytoprotective/cytotoxic effects upon Caco-2 cells of commercial Colombian coffee. Food Chem. 2017, 219, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Aherne, S.A.; O’Brien, N.M. Mechanism of protection by the flavonoids, quercetin and rutin, against tert-butylhydroperoxide- and menadione-induced DNA single strand breaks in Caco-2 cells. Free Radic. Biol. Med. 2000, 29, 507–514. [Google Scholar] [CrossRef]
| Non-Colored Phenolics | Total Extract | Non-Colored Fraction |
|---|---|---|
| Hydroxybenzoic acid derivative 1 | 1337.85 ± 68.16 | 1839.54 ± 5.09 a |
| Hydroxycinnamic acid derivative 1 | 494.32 ± 51.66 | 679.69 ± 71.03 |
| Hydroxycinnamic acid derivative 2 | 143.45 ± 21.30 | 197.24 ± 29.28 |
| Hydroxybenzoic acid derivative 2 | 25.08 ± 0.92 | 34.48 ± 1.27 |
| 3-O-Caffeoylquinic acid | 1482.97 ± 54.15 | 2039.09 ± 74.45 a |
| ρ-Coumaric acid derivative 1 | 50.02 ± 0.55 | 68.78 ± 0.76 |
| ρ-Coumaroylquinic acid | nq | nq |
| Hydroxycinnamic acid derivative 3 | 372.26 ± 35.99 | 511.86 ± 49.48 |
| 5-O-Caffeoylquinic acid | 734.38 ± 44.86 | 1009.77 ± 61.68 a |
| Hydroxycinnamic acid derivative 4 | 2835.87 ± 143.08 | 3899.33 ± 196.73 a |
| Caffeic acid | 1263.49 ± 98.92 | 1737.30 ± 136.01 a |
| p-Coumaric acid derivative 2 | 528.74 ± 19.83 | 727.02 ± 27.26 |
| Hydroxycinnamic acid derivative 5 | 704.96 ± 97.52 | 969.31 ± 134.08 a |
| Hydroxycinnamic acid derivative 6 | 196.75 ± 16.19 | 270.53 ± 22.26 |
| p-Coumaric acid | 21.07 ± 1.64 | 28.96 ± 2.26 |
| Hydroxycinnamic acid derivative 7 | 666.97 ± 67.02 | 917.089 ± 92.15 a |
| Hydroxycinnamic acid derivative 8 | 175.97 ± 16.59 | 241.95 ± 22.80 |
| Quercetin-3-O-glucoside | nq | nq |
| Kaempferol-3-O-rutinoside | nq | nq |
| Quercetin | 35.58 ± 3.73 | 48.93 ± 5.13 |
| Σ | 11,069.73 | 15,220.88 |
| Anthocyanins | Spectra of Absorption (nm) | Total Extract | Coloured Fraction |
|---|---|---|---|
| Unknown 1 | 500 | 2.99 ± 0.24 | nd |
| Cyanidin-3-O-glucoside | 500 | 193.48 ± 0.54 | 3427.93 ± 4.39 a |
| Cyanidin-3-O-rutinoside | 500 | 3865.64 ± 2.95 | 15656.18 ± 25.71 a |
| Unknown 2 | 500 | 341.16 ± 2.82 | nq |
| Pelargonidin-3-O-rutinoside | 500 | 337.464 ± 20.19 | 130.39 ± 1.22 a |
| Peonidin-3-O-rutinoside | 500 | nq | nd |
| Σ | 4740.73 | 19,214.50 |
| Extract | DPPH• | •NO | O2•− | α-Glucosidase | Hemoglobin Oxidation | Hemolysis |
|---|---|---|---|---|---|---|
| Total extract | 21.88 ± 0.32 | 33.72 ± 0.89 | 41.68 ± 0.72 | 53.15 ± 1.32 | 34.29 ± 0.88 | 28.71 ± 0.73 |
| Colored fraction | 31.39 ± 0.60 a | 47.44 ± 0.67 a | 16.58 ± 0.27 (IC25) | 142.02 ± 1.17 a | 33.86 ± 0.70 | 9.44 ± 0.48 |
| Non-colored fraction | 210.86 ± 0.86 a,b | 167.96 ± 0.92 a,b | 69.40 ± 1.22 a,b | 456.19 ± 3.74 (IC25) | 155.13 ± 1.45 a,b | 48.31 ± 1.07 a,b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10, 1688. https://doi.org/10.3390/nu10111688
Gonçalves AC, Rodrigues M, Santos AO, Alves G, Silva LR. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients. 2018; 10(11):1688. https://doi.org/10.3390/nu10111688
Chicago/Turabian StyleGonçalves, Ana C., Márcio Rodrigues, Adriana O. Santos, Gilberto Alves, and Luís R. Silva. 2018. "Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits" Nutrients 10, no. 11: 1688. https://doi.org/10.3390/nu10111688
APA StyleGonçalves, A. C., Rodrigues, M., Santos, A. O., Alves, G., & Silva, L. R. (2018). Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients, 10(11), 1688. https://doi.org/10.3390/nu10111688