Preventive Effect of Garlic Oil and Its Organosulfur Component Diallyl-Disulfide on Cigarette Smoke-Induced Airway Inflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CS-Induced Airway Inflammation
2.3. Analysis of Bronchoalveolar Lavage Fluid (BALF)
2.4. Histopathology and Immunohistochemistry
2.5. Quantitative Analysis for Histopathology
2.6. Immunoblotting
2.7. Statistical Analysis
3. Results
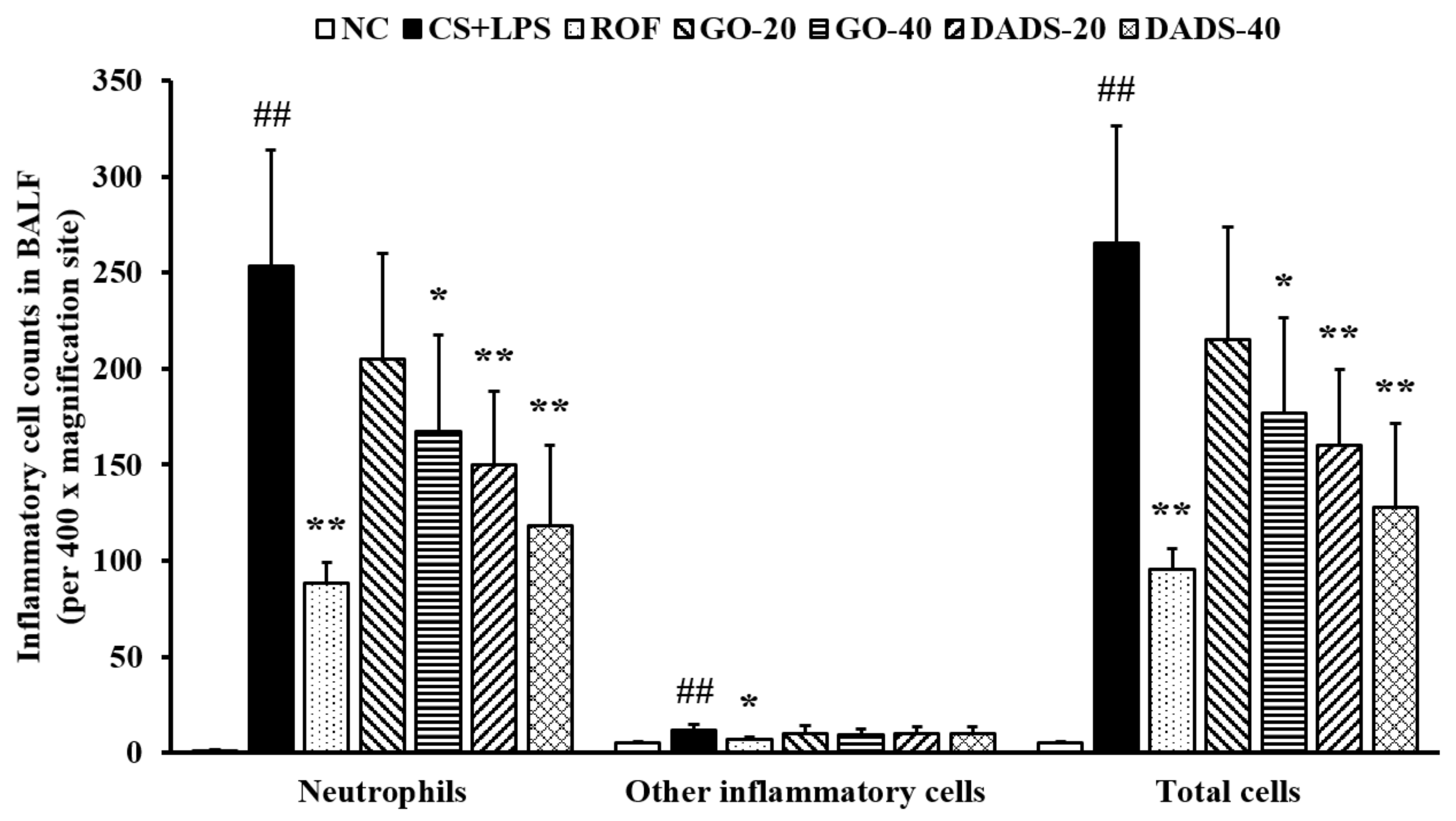
3.1. Effects of GO and DADS on Body Weight Gains and Inflammatory Cell Counts in BALF
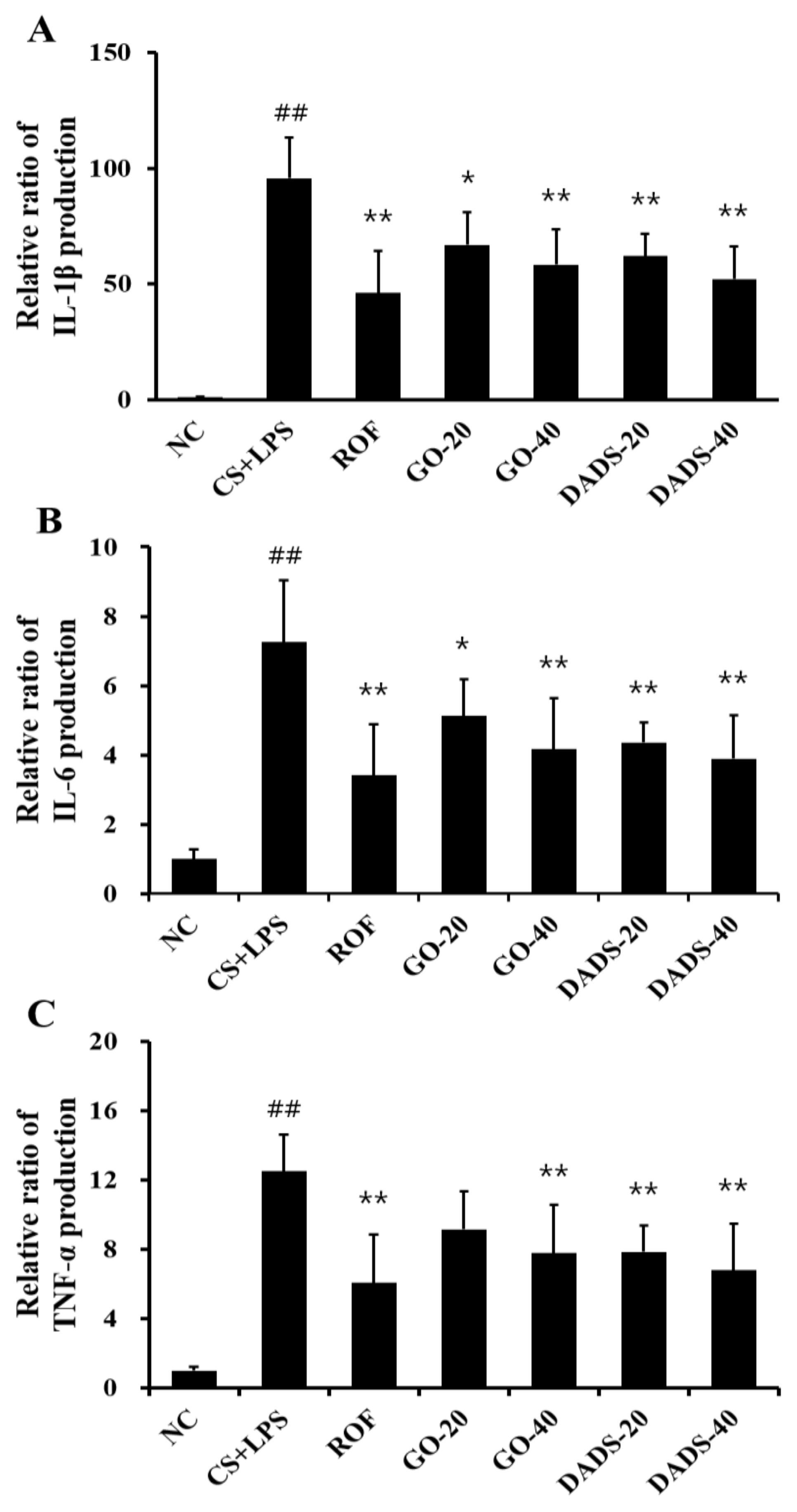
3.2. Effects of GO and DADS on the Production of Proinflammatory Cytokines
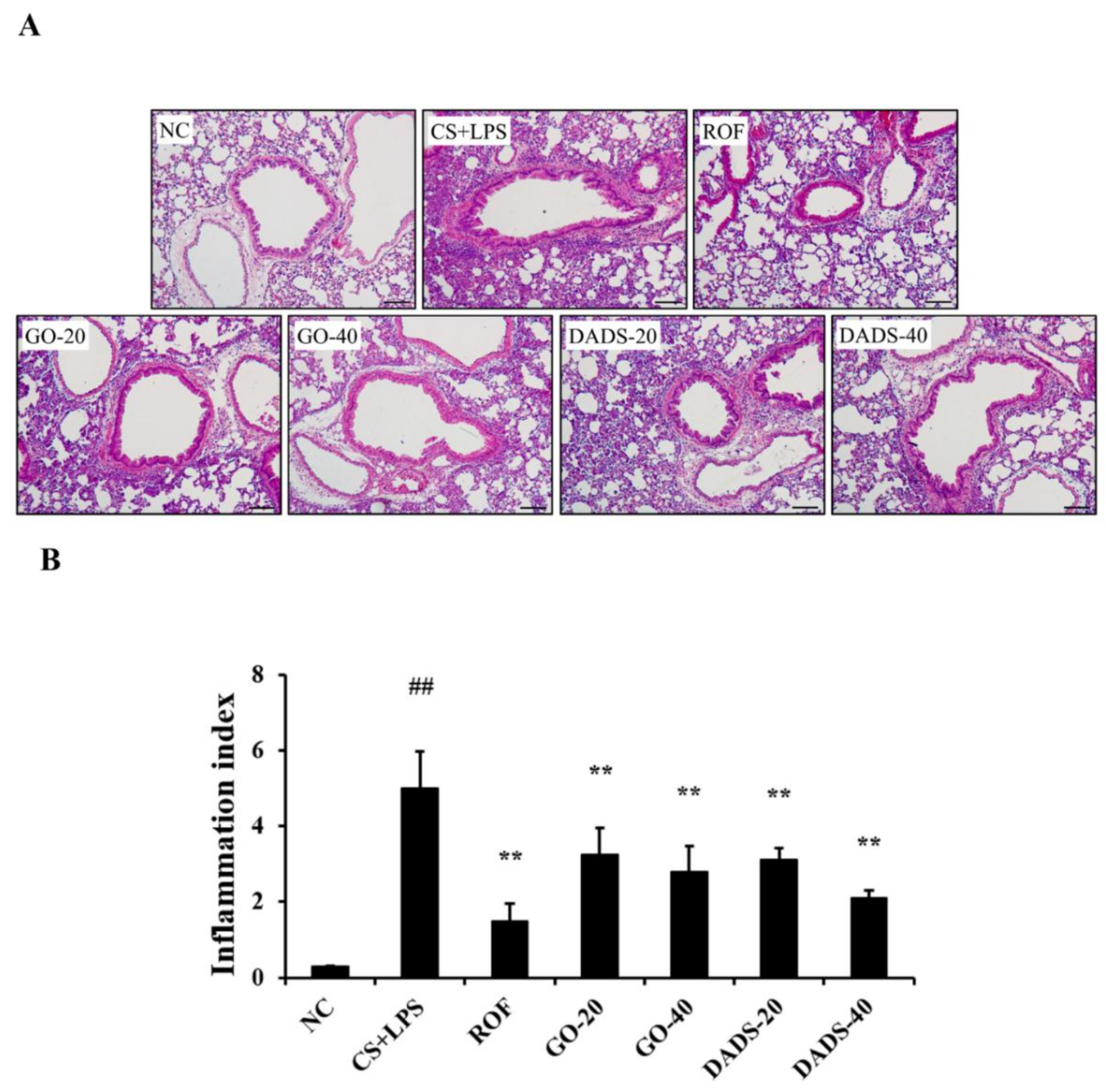
3.3. Effects of GO and DADS on the Infiltration of Inflammatory Cells into Lung Tissues
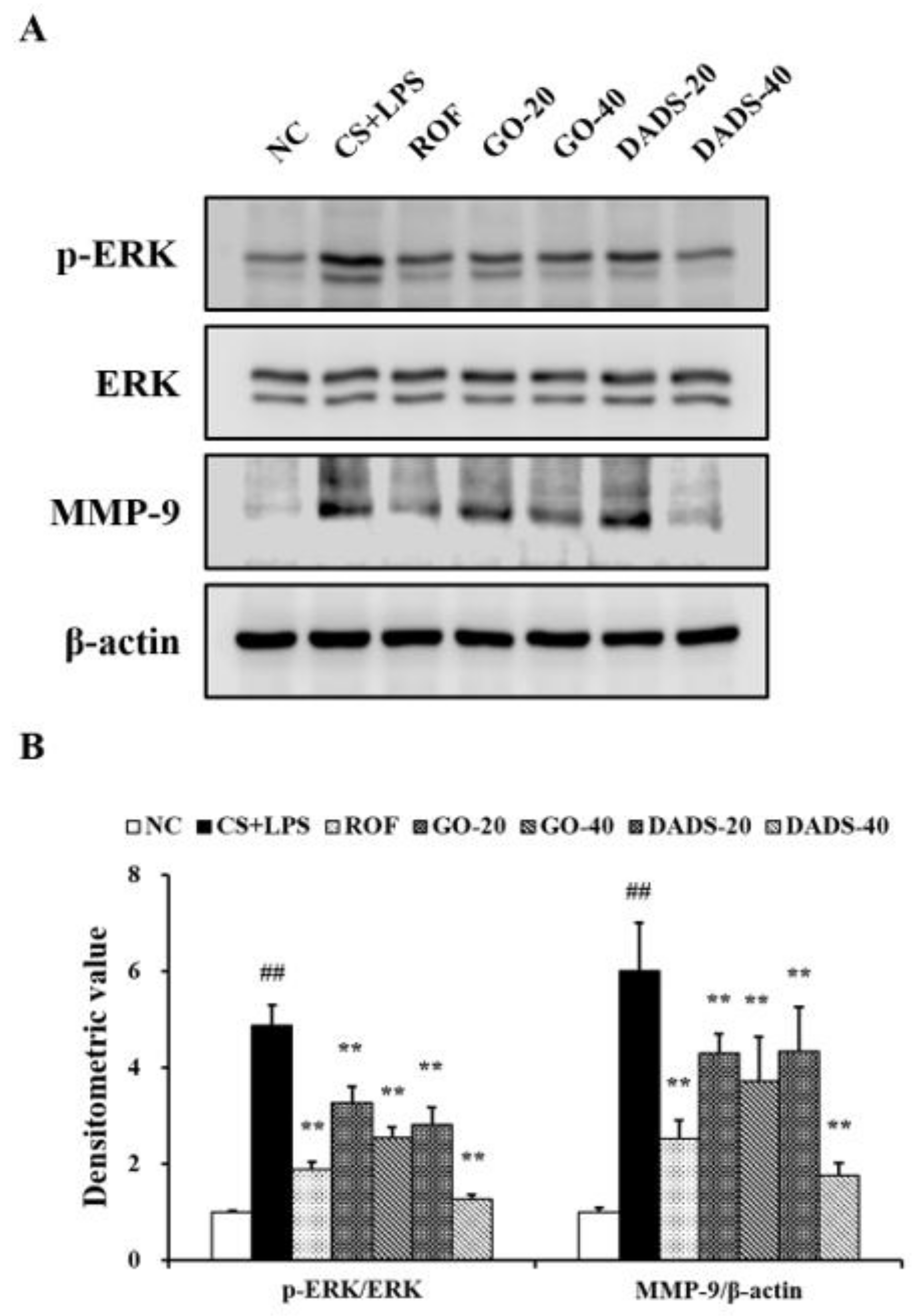
3.4. Effects of GO and DADS on ERK Phosphorylation and MMP-9 Expression
3.5. Effects of GO and DADS on MMP-9 Expression in the Lung Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Song, F.; Windsor, L.J. Cigarette smoke condensate affects the collagen-degrading ability of human gingival fibroblast. J. Periodontal Res. 2009, 44, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Kim, S.H.; Ko, J.W.; Park, S.H.; Lee, I.C.; Ryu, J.M.; Kim, J.C.; Shin, I.S. HemoHIM, a herbal preparation, alleviates airway inflammation caused by cigarette smoke and lipopolysaccharide. Lab. Anim. Res. 2017, 33, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bao, J.; Shi, Y.; Zhang, B.; Yuan, L.; Li, J.; Zhang, L.; Sun, M.; Zhang, L.; Sun, W. Effect of simvastatin on MMPs and TIMPs in cigarette smoke-induced rat COPD model. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Baek, H.; Park, S.; Shin, D.; Jung, J.; Park, S.; Kim, J.; Bae, H. The therapeutic effects of tuberostemonine against cigarette-induced acute lung inflammation in mice. Eur. J. Pharmacol. 2016, 774, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Shin, N.R.; Shin, I.S.; Kwon, O.K.; Kim, J.S.; Oh, S.R.; Kim, J.H.; Ahn, K.S. Silibinin inhibits neutrophilic inflammation and mucus secretion induced by cigarette smoke via suppression of ERK-SP1 pathway. Phytother. Res. 2016, 30, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Boccalini, G.; Lucarini, L.; Catarinicchia, S.; Guasti, D.; Masini, E.; Nistri, S. Protection from cigarette smoke-induced lung dysfunction and damage by H2 relaxin (Serelaxin). J. Pharmacol. Exp. Ther. 2016, 357, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Boehme, S.A.; Franz-Bacon, K.; Ludhk, J.; DiTirro, D.N.; Ly, T.W.; Bacon, K.B. MAP3K19 is overexpressed in COPD and is a central mediator of cigarette smoke-induced pulmonary inflammation and lower airway destruction. PLoS ONE. 2016, 11, e0167169. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Tran, H.B.; Hamon, R.; Roscioli, E.; Hodge, G.; Jersmann, H.; Wenn, M.; Reynolds, P.N.; Yeung, A.; Treiberg, J.; et al. Nonantibiotic macrolides restore airway macrophage phagocytic function with potential anti-inflammatory effects in chronic lung diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L678–L687. [Google Scholar] [CrossRef] [PubMed]
- Leus, N.G.; van den Bosch, T.; van der Wouden, P.E.; Krist, K.; Ourailidou, M.E.; Eleftheriadis, N.; Kistemaker, L.E.; Bos, S.; Gjaltema, R.A.; Mekonnen, S.A.; et al. HDAC1-3 inhibitor MS-275 enhances IL10 expression in RAW264.7 macrophages and reduces cigarette smoke-induced airway inflammation in mice. Sci. Rep. 2017, 7, e45047. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Shi, Q.S.; Liang, Q.; Huang, X.M.; Chen, Y.B. Antifungal effect and mechanism of garlic oil on penicillium funiculosum. Appl. Microbiol. Biotechnol. 2014, 98, 8337–8346. [Google Scholar] [CrossRef] [PubMed]
- Balaha, M.; Kandeel, S.; Elwan, W. Garlic oil inhibits dextran sodium sulfate-induced ulcerative colitis in rats. Life Sci. 2016, 146, 40–51. [Google Scholar] [CrossRef] [PubMed]
- El-Akabawy, G.; El-Sherif, N.M. Protective role of garlic oil against oxidative damage induced by furan exposure from weaning through adulthood in adult rat testis. Acta Histochem. 2016, 118, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.W.; Park, S.H.; Lee, I.C.; Lee, S.M.; Shin, I.S.; Kang, S.S.; Moon, C.; Kim, S.H.; Heo, J.D.; Kim, J.C. Protective effects of garlic oil against 1,3-dichloro-2-propanol-induced hepatotoxicity: Role of CYP2E1 and MAPKs. Mol. Cell. Toxicol. 2016, 12, 185–195. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zeng, T.; Zhao, X.L.; Xie, K.Q. Garlic oil suppressed nitrosodiethylamine-induced hepatocarcinoma in rats by inhibiting PI3K-AKT-NF-κB pathway. Int. J. Biol. Sci. 2015, 11, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, S.; Mohanam, L.; Raja, V.; Dev, A.; Prabhu, V. Antiobesity, antioxidant and hepatoprotective effects of diallyl trisulphide (DATS) alone or in combination with Orlistat on HFD induced obese rats. Biomed. Pharmacother. 2017, 93, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhu, X.; Huang, W.; Xu, H.; Zhao, Z.; Li, S.; Li, S.; Cai, J.; Cao, J. Garlic-derived organosulfur compound exerts antitumor efficacy via activation of MAPK pathway and modulation of cytokines in SGC-7901 tumor-bearing mice. Int. Immunopharmacol. 2017, 48, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Miltonparbu, S.; Sumedha, N.C.; Senthilraja, P. Diallyl trisulfide, a garlic polysulfide protects against As-induced renal oxidative nephrotoxicity, apoptosis and inflammation in rats by activating the Nrf2/ARE signaling pathway. Int. Immunopharmacol. 2017, 50, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Han, J.H.; Ko, J.W.; Park, S.H.; Shin, N.R.; Jung, T.Y.; Kim, H.A.; Kim, S.H.; Shin, I.S.; Kim, J.C. Diallyl disulfide attenuates acetaminophen-induced renal injury in rats. Lab. Anim. Res. 2016, 32, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Lu, C.C.; Weng, C.J.; Yen, G.C. Protective effects of diallyl sulfide on ovalbumin-induced pulmonary inflammation of allergic asthma mice by microRNA-144, -34a, and -34b/c-modulated Nrf2 activation. J. Agric. Food Chem. 2016, 64, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Hong, J.; Jeon, C.M.; Shin, N.R.; Kwon, O.K.; Kim, H.S.; Kim, J.C.; Oh, S.R.; Ahn, K.S. Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food Chem. Toxicol. 2013, 62, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Lee, S.M.; Ko, J.W.; Park, S.H.; Shin, I.S.; Moon, C.; Kim, S.H.; Kim, J.C. Role of mitogen-activated protein kinases and nuclear factor-kappa B in 1,3-dichloro-2-propanol-induced hepatic injury. Lab. Anim. Res. 2016, 32, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T.; Butt, M.S.; Iqbal, J. Garlic: Nature’s protection against physiological threats. Crit. Rev. Food. Sci. Nutr. 2009, 49, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K. Traditional herbs: A remedy for cardiovascular disorders. Phytomedicine 2016, 23, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Sobenin, I.A.; Andrianova, I.V.; Lakunin, K.Y.; Karagodin, V.P.; Bobryshev, Y.V.; Orekhov, A.N. Anti-atherosclerotic effects of garlic preparation in freeze injury model of atherosclerosis in cholesterol-fed rabbits. Phytomedicine 2016, 23, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Emerging anti-inflammatory strategies for COPD. Eur. Respir. J. 2012, 40, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.; Serdarevic, D.; Hanania, N.A. The effects of cigarette smoke on airway inflammation in asthma and COPD: Therapeutic implications. Respir. Med. 2012, 106, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, J.; Wang, T.; Zhang, X.; Liu, L.; Wang, H.; Wu, Y.; Xu, D.; Wen, F. Silymarin attenuates cigarette smoke extract-induced inflammation via simultaneous inhibition of autophagy and ERK/p38 MAPK pathway in human bronchial epithelial cells. Sci. Rep. 2016, 3, e37751. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Hsieh, W.Y.; Hsieh, J.S.; Liu, F.C.; Tsai, C.H.; Lu, L.C.; Huang, C.Y.; Wu, C.L.; Lin, C.S. Alternative roles of STAT3 and MAPK signaling pathways in the MMPs activation and progression of lung injury induced by cigarette smoke exposure in ACE2 knockout mice. Int. J. Biol. Sci. 2016, 12, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cobbs, A.; George, J.; Chima, A.; Tuyishime, F.; Zhao, X. Endocytosis of Albumin Induces Matrix Metalloproteinase-9 by Activating the ERK Signaling Pathway in Renal Tubule Epithelial Cells. Int. J. Mol. Sci. 2017, 18, 1758. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bao, C.; Ma, Z.; Xu, B.; Ying, X.; Liu, X.; Zhang, X. Perfluorooctanoic acid stimulates ovarian cancer cell migration, invasion via ERK/NF-κB/MMP-2/-9 pathway. Toxicol. Lett. 2018, 294, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liang, C.; Pan, Q.; Wang, Y. Eya2 overexpression promotes the invasion of human astrocytoma through the regulation of ERK/MMP9 signaling. Int. J. Mol. Med. 2017, 40, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Su, J.; He, H.; Wu, Y.; Xia, H.; Zeng, X.; Dai, W.; Ai, X.; Ling, H.; Jiang, H.; et al. Identification of potential targets for diallyl disulfide in human gastric cancer MGC-803 cells using proteomics approaches. Oncol. Rep. 2015, 33, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Redmon, N.; Mazzio, E.; Taka, E.; Reuben, J.S.; Day, A.; Sadrud-Din, S.; Flores-Rozas, H.; Soliman, K.F.; Darling-Reed, S. Diallyl disulfide inhibits TNFα induced CCL2 release through MAPK/ERK and NF-Kappa-B signaling. Cytokine 2015, 75, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, I.C.; Ko, J.W.; Moon, C.; Kim, S.H.; Shin, I.S.; Seo, Y.W.; Kim, H.C.; Kim, J.C. Diallyl disulfide prevents cyclophosphamide-induced hemorrhagic cystitis in Rats through the inhibition of oxidative damage, MAPKs, and NF-κB pathway. Biomol. Ther. (Seoul) 2015, 23, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Lee, S.H.; Chen, K.M.; Lii, C.K.; Liu, C.T. Effect of garlic oil on neutrophil infiltration in the small intestine of endotoxin-injected rats and its association with levels of soluble and cellular adhesion molecules. J. Agric. Food Chem. 2011, 59, 7717–7725. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.-W.; Jeong, S.-H.; Kwon, H.-J.; Shin, N.-R.; Seo, Y.-S.; Kim, J.-C.; Shin, I.-S.; Kim, J.-S. Preventive Effect of Garlic Oil and Its Organosulfur Component Diallyl-Disulfide on Cigarette Smoke-Induced Airway Inflammation in Mice. Nutrients 2018, 10, 1659. https://doi.org/10.3390/nu10111659
Ko J-W, Jeong S-H, Kwon H-J, Shin N-R, Seo Y-S, Kim J-C, Shin I-S, Kim J-S. Preventive Effect of Garlic Oil and Its Organosulfur Component Diallyl-Disulfide on Cigarette Smoke-Induced Airway Inflammation in Mice. Nutrients. 2018; 10(11):1659. https://doi.org/10.3390/nu10111659
Chicago/Turabian StyleKo, Je-Won, Seong-Hun Jeong, Hyung-Jun Kwon, Na-Rae Shin, Yun-Soo Seo, Jong-Choon Kim, In-Sik Shin, and Joong-Sun Kim. 2018. "Preventive Effect of Garlic Oil and Its Organosulfur Component Diallyl-Disulfide on Cigarette Smoke-Induced Airway Inflammation in Mice" Nutrients 10, no. 11: 1659. https://doi.org/10.3390/nu10111659
APA StyleKo, J.-W., Jeong, S.-H., Kwon, H.-J., Shin, N.-R., Seo, Y.-S., Kim, J.-C., Shin, I.-S., & Kim, J.-S. (2018). Preventive Effect of Garlic Oil and Its Organosulfur Component Diallyl-Disulfide on Cigarette Smoke-Induced Airway Inflammation in Mice. Nutrients, 10(11), 1659. https://doi.org/10.3390/nu10111659




