Cataract Preventive Role of Isolated Phytoconstituents: Findings from a Decade of Research
Abstract
1. Introduction
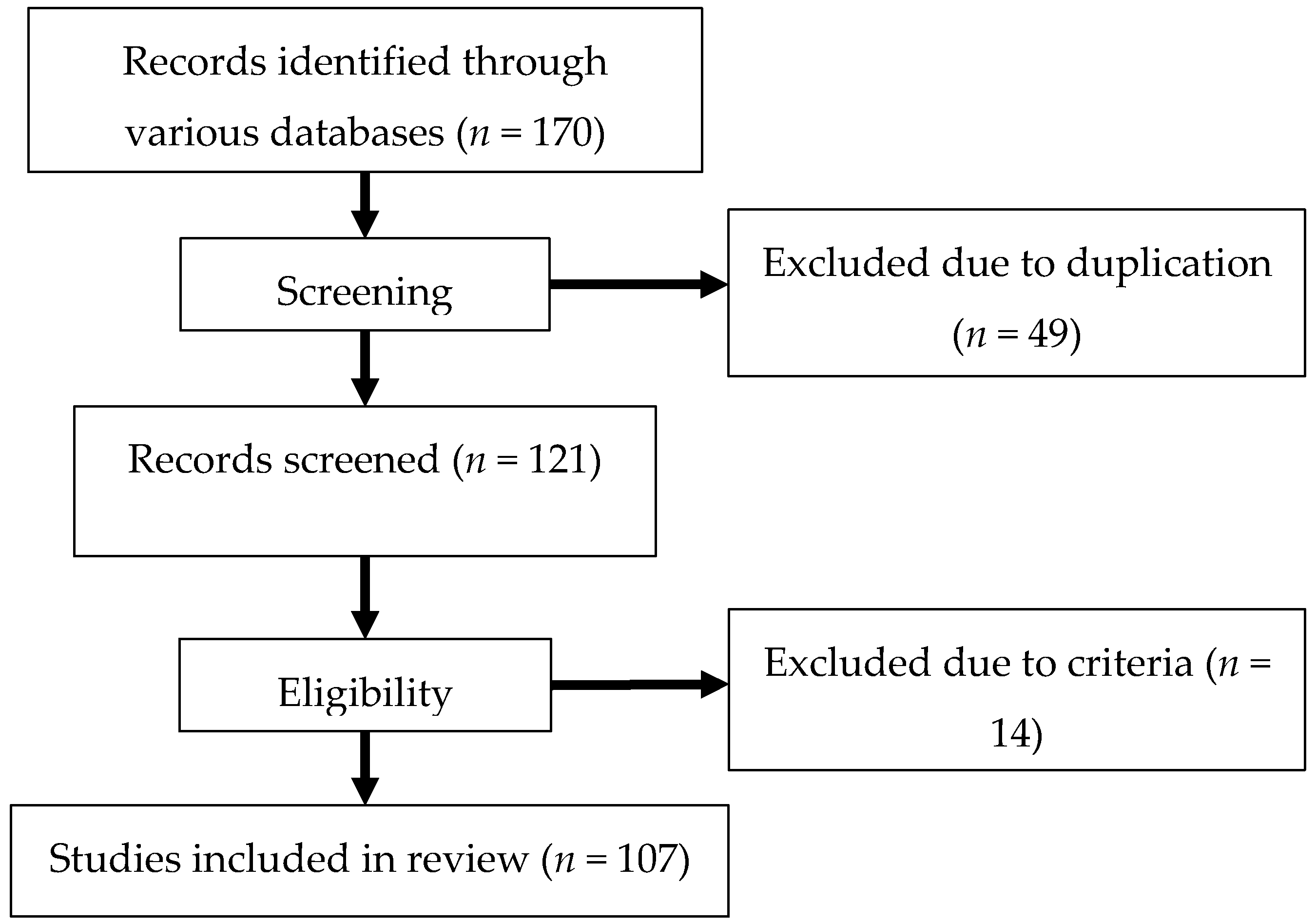
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
3. Results
3.1. Experimental Cataract Models
3.2. In Vitro Models
3.2.1. Oxidative Stress Model
H2O2-Induced Cataract
3.2.2. Diabetic Cataract
Aldose Reductase (AR) Activity
Xylose-Induced Lens Opacity
Galactose-Induced Lens Opacity
Formation of Advanced Glycation End (AGE) Products
3.3. In Vivo Models
3.3.1. Diabetic Cataract
3.3.2. Selenite-Induced Cataract
3.3.3. UV-Induced Cataract
3.3.4. Steroid-Induced Cataract
3.4. Anti-Cataract Phytoconstituents
3.4.1. 1-O-Galloyl-β-d-glucose (β-Glucogallin)
3.4.2. 1,3-Di-O-caffeoylquinic Acid
3.4.3. 1,5-Di-hydroxy-1,5-di-[(E)-3-(4-hydroxyphenyl)-2-propenoic]-3-pentanonyl Ester (DHDP)
3.4.4. 1,5-Di-O-caffeoylquinic Acid
3.4.5. 1,3,6-Trihydroxy-2-methoxymethylanthraquinone
3.4.6. 1,2,3,6-Tetra-O-galloyl-β-d-glucose
3.4.7. 1,3,5,8-Tetrahydroxyxanthone
3.4.8. 2″,4″-O-Diacetylquercitrin
3.4.9. 3-Isomangostin
3.4.10. 3′,4-Dihydroxy-3,5′-dimethoxy-bibenzyl (Gigantol)
3.4.11. 3′,5′-Dimethoxy-(1,1′-biphenyl)-3,4-diol 3-O-β-d-glucopyranoside
3.4.12. 3,5-Di-O-caffeoylquinic Acid
3.4.13. 4-O-Butylpaeoniflorin and Palbinone
3.4.14. 4,5-Di-O-trans-caffeoyl-d-quinic Acid
3.4.15. 5-O-Feruloly Quinic Acid
3.4.16. 5,7,4′ Trihydroxyisoflavone (Genistein)
3.4.17. 20(S)-Ginsenoside Rh2
3.4.18. Acteoside
3.4.19. Basilicumin [7-(3-hydroxypropyl)-3-methyl-8-β-O-d-glucoside-2H-chromen-2-one]
3.4.20. Caffeic Acid
3.4.21. Canangafruiticoside E
3.4.22. Capsofulvesin A [((2S)-l-O-(6Z,9Z,12Z,15Zoctadecatetraenoyl)-2-O-(4Z,10Z,13Zhexadecatetraenoyl)-3-O-β-d-galactopyranosyl Glycerol)]
3.4.23. Caryatin-3′ methyl ether-7-O-β-d-glucoside
3.4.24. C-Phycocyanin (C-PC)
3.4.25. Davallialactone
3.4.26. Delphinidin 3-O-β-galactopyranoside-3′-O-β-glucopyranoside
3.4.27. Desmethylanhydroicaritin
3.4.28. Ellagic Acid
3.4.29. Epiberberine
3.4.30. Geraniin
3.4.31. Hipolon
3.4.32. Hirsutrin
3.4.33. Hopeafuran
3.4.34. Hypolaetin 7-O-[6‴-O-acetyl-β-d-allopyranosyl-(1→2)]-6″-O-acetyl-β-d-glucopyranoside
3.4.35. Isocampneoside II
3.4.36. Isorhamnetin-3-glucoside
3.4.37. Kaempferol
3.4.38. Kakkalide
3.4.39. Lucidumol A [(24S)-24,25-Dihydroxylanost-8-ene-3,7-dione]
3.4.40. Lupeol
3.4.41. Luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromomenone)
3.4.42. Luteolin-7-O-β-d-glucopyranoside
3.4.43. Magnoflorine
3.4.44. Methyl-3,5-di-O-caffeoylquinate
3.4.45. Mumeic Acid-A
3.4.46. Puerariafuran
3.4.47. Quercetin-3-O-β-d-glucoside
3.4.48. Quercitrin (Quercetin 3-O-α-l-rhamnoside)
3.4.49. Rhetsinine
3.4.50. Rosmarinic Acid
3.4.51. Scopoletin
3.4.52. Semilicoisoflavone B
3.4.53. Sulfuretin and Butein
3.4.54. Syringic Acid
3.4.55. Swertisin
3.4.56. Valoneic Acid Dilactone
4. Discussion and Outlook
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation end-product |
| AR | Aldose reductase |
| ARI | Aldose reductase inhibition |
| BLAR | Bovine lens aldose reductase |
| GC | Glucocorticoid |
| GSH | Glutathione |
| HLAR | Human lens aldose reductase |
| LEC | Lens epithelial cell |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| RHAR | Recombinant human aldose reductase |
| RLAR | Rat lens aldose reductase |
| ROS | Reactive oxygen species |
| SDH | Sorbitol dehydrogenase |
| STZ | Streptozotocin |
| UV | Ultraviolet |
References
- Lou, M.F. Redox regulation in the lens. Prog. Retin. Eye Res. 2003, 22, 657–682. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Fischel, J.D.; Lipton, J.R. Cataract surgery and recent advances: A review. Nurs. Stand. 1996, 10, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Spector, A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995, 9, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.; Garner, W.H. Hydrogen peroxide and human cataract. Exp. Eye Res. 1981, 33, 673–681. [Google Scholar] [CrossRef]
- Cui, X.L.; Lou, M.F. The effect and recovery of long-term H2O2 exposure on lens morphology and biochemistry. Exp. Eye Res. 1993, 57, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zigler, J.S., Jr.; Jernigan, H.M., Jr.; Garland, D.; Reddy, V.N. The effects of “oxygen radicals” generated in the medium on lenses in organ culture: Inhibition of damage by chelated iron. Arch. Biochem. Biophys. 1985, 241, 163–172. [Google Scholar] [CrossRef]
- Caird, F.I.; Hutchinson, M.; Pirie, A. Cataract and Diabetes. Br. Med. J. 1964, 2, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Chodos, J.B.; Habegger-Chodos, H.E. Cataract formation in human and experimental diabetes. Part I. Surv. Ophthalmol. 1960, 5, 129–159. [Google Scholar] [PubMed]
- Snow, A.; Shieh, B.; Chang, K.C.; Pal, A.; Lenhart, P.; Ammar, D.; Ruzycki, P.; Palla, S.; Reddy, G.B.; Petrash, J.M. Aldose reductase expression as a risk factor for cataract. Chem. Biol. Interact. 2015, 234, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hayman, S.; Kinoshita, J.H. Isolation and Properties of Lens Aldose Reductase. J. Biol. Chem. 1965, 240, 877–882. [Google Scholar] [PubMed]
- Obazawa, H.; Merola, L.O.; Kinoshita, J.H. The effects of xylose on the isolated lens. Investig. Ophthalmol. 1974, 13, 204–209. [Google Scholar]
- Ai, Y.; Zheng, Z.; O’Brien-Jenkins, A.; Bernard, D.J.; Wynshaw-Boris, T.; Ning, C.; Reynolds, R.; Segal, S.; Huang, K.; Stambolian, D. A mouse model of galactose-induced cataracts. Hum. Mol. Genet. 2000, 9, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Franke, S.; Dawczynski, J.; Strobel, J.; Niwa, T.; Stahl, P.; Stein, G. Increased levels of advanced glycation end products in human cataractous lenses. J. Cataract Refract. Surg. 2003, 29, 998–1004. [Google Scholar] [CrossRef]
- Ramalho, J.S.; Marques, C.; Pereira, P.C.; Mota, M.C. Role of glycation in human lens protein structure change. Eur. J. Ophthalmol. 1996, 6, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Abiko, T.; Abiko, A.; Ishiko, S.; Takeda, M.; Horiuchi, S.; Yoshida, A. Relationship between autofluorescence and advanced glycation end products in diabetic lenses. Exp. Eye Res. 1999, 68, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Joon, T.L.; Foo, K.; Panagiotopoulos, S.; Jerums, G.; Taylor, H.R. In vivo assessment of an animal model of diabetic cataract: Medical intervention studies. Dev. Ophthalmol. 1994, 26, 57–62. [Google Scholar] [PubMed]
- Patil, M.A.; Suryanarayana, P.; Putcha, U.K.; Srinivas, M.; Reddy, G.B. Evaluation of neonatal streptozotocin induced diabetic rat model for the development of cataract. Oxid. Med. Cell Longev. 2014, 2014, 463264. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Chung, S.S. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999, 13, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shearer, T.R.; David, L.L.; Anderson, R.S. Selenite cataract: A review. Curr. Eye Res. 1987, 6, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Shearer, T.R.; David, L.L.; Anderson, R.S.; Azuma, M. Review of selenite cataract. Curr. Eye Res. 1992, 11, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Shearer, T.R.; Ma, H.; Fukiage, C.; Azuma, M. Selenite nuclear cataract: Review of the model. Mol. Vis. 1997, 3, 8. [Google Scholar] [PubMed]
- Dillon, J. UV-B as a pro-aging and pro-cataract factor. Doc. Ophthalmol. 1994, 88, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P. Cataract and UV radiation. Doc. Ophthalmol. 1994, 88, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Kronschlager, M.; Lofgren, S.; Yu, Z.; Talebizadeh, N.; Varma, S.D.; Soderberg, P. Caffeine eye drops protect against UV-B cataract. Exp. Eye Res. 2013, 113, 26–31. [Google Scholar] [CrossRef] [PubMed]
- James, E.R. The etiology of steroid cataract. J. Ocul. Pharmacol. Ther. 2007, 23, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Shui, Y.B.; Kojima, M.; Sasaki, K. A new steroid-induced cataract model in the rat: Long-term prednisolone applications with a minimum of X-irradiation. Ophthalmic Res. 1996, 28, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Shui, Y.B.; Vrensen, G.F.; Kojima, M. Experimentally induced steroid cataract in the rat: A scanning electron microscopic study. Surv. Ophthalmol. 1997, 42, S127–S132. [Google Scholar] [CrossRef]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 2016, 111, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Puppala, M.; Ponder, J.; Suryanarayana, P.; Reddy, G.B.; Petrash, M.; LaBarbera, D.V. The isolation and characterization of β-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PLoS ONE 2012, 7, e31399. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Laffin, B.; Ponder, J.; Énzsöly, A.; Németh, J.; Labarbera, D.V.; Petrash, J.M. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem. Biol. Interact. 2013, 202, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Hong, E.Y.; Whang, W.K. Inhibitory Effect of Chemical Constituents Isolated from Artemisia iwayomogi on Polyol Pathway and Simultaneous Quantification of Major Bioactive Compounds. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.N.; Lee, M.Y.; Kim, J.K.; Suh, H.W.; Lim, S.S. Aldose reductase inhibitory compounds from Xanthium strumarium. Arch. Pharm. Res. 2013, 36, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hwang, S.H.; Lim, S.S. Characterization of DHDP, a novel aldose reductase inhibitor isolated from Lysimachia christinae. J. Funct. Foods 2017, 37, 241–248. [Google Scholar] [CrossRef]
- Paek, J.H.; Lim, S.S. Preparative isolation of aldose reductase inhibitory compounds from Nardostachys chinensis by elution–extrusion counter-current chromatography. Arch. Pharm. Res. 2014, 37, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.H.; Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Cho, J.H.; Kim, J.H.; Kim, J.S. Anthraquinones from the Roots of Knoxia valerianoides inhibit the formation of advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharm. Res. 2010, 33, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Li, Z.; Luo, H.; Zhang, W.; Chen, L.; Xu, X. Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2004, 14, 6041–6044. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, D.S.; Kim, N.H.; Lee, Y.M.; Kim, J.; Kim, J.S. Galloyl glucoses from the seeds of Cornus officinalis with inhibitory activity against protein glycation, aldose reductase, and cataractogenesis ex vivo. Biol. Pharm. Bull. 2011, 34, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.H.; Luo, C.T.; Chen, H.; Lin, J.N.; Ye, C.L.; Mao, S.S.; Li, Y.L. Xanthones from Swertia mussotii as multitarget-directed antidiabetic agents. Chem. Med. Chem. 2014, 9, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kim, I.S.; Lee, Y.M.; Lee, Y.; Kim, J.H.; Kim, J.S. 2″,4″-O-diacetylquercitrin, a novel advanced glycation end-product formation and aldose reductase inhibitor from Melastoma sanguineum. Chem. Pharm. Bull. (Tokyo) 2013, 61, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Ersam, T.; Shimizu, K. The inhibitory activity of aldose reductase in vitro by constituents of Garcinia mangostana Linn. Phytomedicine 2015, 22, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Han, H.; He, C.; Yang, L.; Wang, Z. Identification of the metabolites of gigantol in rat urine by ultra-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Biomed. Chromatogr. 2014, 28, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Hu, X.; Wang, M.; Wan, W.; Yang, Q.; Sun, X.; Gu, Q.; Gao, X.X.; Wang, Z.T.; Gu, L.Q.; et al. Anti-osmotic and antioxidant activities of gigantol from Dendrobium aurantiacum var. denneanum against cataractogenesis in galactosemic rats. J. Ethnopharmacol. 2015, 172, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.; Xu, L. Phenols and a triterpene from Dendrobium aurantiacum var. denneanum (Orchidaceae). Biochem. Syst. Ecol. 2006, 34, 658–660. [Google Scholar] [CrossRef]
- Hu, J.; Fan, W.; Dong, F.; Miao, Z.; Zhou, J. Chemical components of Dendrobium chrysotoxum. Chin. J. Chem. 2012, 30, 1327–1330. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Wan, W.; Yang, Q.; Ma, W.; Chen, D.; Hu, J.M.; Chen, C.Y.O.; Wei, X.Y. Gigantol from Dendrobium chrysotoxum Lindl. binds and inhibits aldose reductase gene to exert its anti-cataract activity: An in vitro mechanistic study. J. Ethnopharmacol. 2017, 198, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Jung, S.H.; Lee, Y.M.; Choi, S.J.; Sun, H.; Kim, J.S. Phenolic Compounds from the Leaves and Twigs of Osteomeles schwerinae That Inhibit Rat Lens Aldose Reductase and Vessel Dilation in Zebrafish Larvae. J. Nat. Prod. 2015, 78, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Yoo, N.H.; Kim, N.H.; Lee, Y.M.; Kim, C.S.; Kim, J.; Kim, J.H.; Kim, J.S. 3,5-Di-O-caffeoyl-epi-quinic acid from the leaves and stems of Erigeron annuus inhibits protein glycation, aldose reductase, and cataractogenesis. Biol. Pharm. Bull. 2010, 33, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.F.; Poetsch, F.; Nahrstedt, A. Structure assignment of natural quinic acid derivatives using proton nuclear magnetic resonance techniques. Phytochem. Anal. 1998, 9, 177–185. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.S. Identification of new dicaffeoylquinic acids from Chrysanthemum morifolium and their antioxidant activities. Planta Med. 2005, 71, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.M.; Lee, B.W.; Kim, J.H.; Kim, J.S. Chemical constituents from the aerial parts of Aster koraiensis with protein glycation and aldose reductase inhibitory activities. J. Nat. Prod. 2012, 75, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Islam, M.D.N.; Kwon, Y.S.; Jin, S.E.; Son, Y.K.; Park, J.J.; Sohn, H.S.; Choi, J.S. Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem. Toxicol. 2011, 49, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.; Tran, M.N.; Lee, I.S.; Yun, M.L.; Jin, S.K.; Jung, H.J.; Lee, S.M.; Na, M.K.; Bae, K. Inhibitors of aldose reductase and formation of advanced glycation end-products in Moutan cortex (Paeonia suffruticosa). J. Nat. Prod. 2009, 72, 1465–1470. [Google Scholar]
- Kadota, S.; Terashima, S.; Basnet, P.; Kikuchi, T.; Namba, T.; Palbinone, A. Novel Terpenoid from Peaonia albiflora; Potent Inhibitory Activity on 3α-Hydroxysteroid Dehydrogenase. Chem. Pharm. Bull. 1993, 41, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nakamura, S.; Zhuang, Y.; Yoshikawa, M.; Mohamed, G.; Hussein, E.; Matsuo, K.; Matsuda, H. Medicinal Flowers. XXXX. 1) Structures of Dihydroisocoumarin Glycosides and Inhibitory Effects on Aldose Reducatase from the Flowers of Hydrangea macrophylla var. thunbergii. Chem. Pharm. Bull. 2013, 61, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Jung, H.A.; Kang, S.S.; Lee, J.H.; Cho, Y.S.; Moon, K.H.; Choi, J.S. Inhibitory activity of aralia continentalis roots on protein tyrosine phosphatase 1B and rat lens aldose reductase. Arch. Pharm. Res. 2012, 35, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Kim, J.M.; Lee, Y.M.; Kim, Y.S.; Kim, J.H.; Kim, J.S. Puerariafuran, a new inhibitor of advanced glycation end products (AGEs) isolated from the roots of Pueraria lobata. Chem. Pharm. Bull. 2006, 54, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, J.E.; Furusawa, J.I.; Baba, J.; Takeshita, T.; Yamasaki, M.; Nohara, T. Studies on the constituents of Pueraria lobata. III. Isoflavonoids and related compounds in the roots and the voluble stems. Chem. Pharm. Bull. (Tokyo) 1987, 35, 4846–4850. [Google Scholar] [CrossRef]
- Kim, S.B.; Hwang, S.H.; Wang, Z.; Yu, J.M.; Lim, S.S. Rapid identification and isolation of inhibitors of rat lens aldose reductase and antioxidant in Maackia amurensis. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, N.H.; Jung, D.H.; Jang, D.S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Genistein inhibits aldose reductase activity and high glucose-induced TGF-beta2 expression in human lens epithelial cells. Eur. J. Pharmacol. 2008, 594, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Ersam, T.; Yu, H.; Zhang, C.; Jin, F.; Shimizu, K. 20(S)-Ginsenoside Rh2 as aldose reductase inhibitor from Panax ginseng. Bioorg. Med. Chem. Lett. 2014, 24, 4407–4409. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Kim, J.K.; Jang, J.M.; Cui, C.B.; Lim, S.S. Analysis of the inhibitory activity of Abeliophyllum distichum leaf constituents against aldose reductase by using high-speed counter current chromatography. Arch. Pharm. Res. 2013, 36, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Lee, I.S.; Jung, S.H.; Lee, Y.M.; Lee, Y.R.; Kim, J.H.; Sun, H.; Kim, J.S. Caffeoylated phenylpropanoid glycosides from Brandisia hancei inhibit advanced glycation end product formation and aldose reductase in vitro and vessel dilation in larval zebrafish in vivo. Planta Med. 2013, 79, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Tehseen, Y.; Maryam, K.; Uroos, M.; Siddiqui, B.S.; Hameed, A.; Iqbal, J. Bioorganic Chemistry Identification of new potent inhibitor of aldose reductase from Ocimum basilicum. Bioorg. Chem. 2017, 75, 62–70. [Google Scholar]
- Koo, D.C.; Baek, S.Y.; Jung, S.H.; Shim, S.H. Aldose reductase inhibitory compounds from extracts of Dipsacus asper. Biotechnol. Bioprocess Eng. 2013, 18, 926–931. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, Y.S.; Kim, S.H.; Bae, Y.S.; Lim, S.S. Inhibition of aldose reductase by phenylethanoid glycoside isolated from the seeds of Paulownia coreana. Biol. Pharm. Bull. 2011, 34, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kang, Y.H.; Jung, J.Y.; Kang, I.J.; Han, S.N.; Chung, J.S.; Shin, H.K.; Lim, S.S. Inhibitory constituents of aldose reductase in the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008, 31, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Yoo, N.H.; Lee, Y.M.; Yoo, J.L.; Kim, Y.S.; Kim, J.S. Constituents of the flowers of Erigeron annuus with inhibitory activity on the formation of advanced glycation end products (AGEs) and aldose reductase. Arch. Pharm. Res. 2008, 31, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Paek, J.H.; Shin, K.H.; Kang, Y.; Lee, J.; Lim, S.S. Rapid Identification of Aldose Reductase Inhibitory Compounds from Perilla frutescens. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Kim, J.K.; Jang, J.M.; Kwon, S.O.; Cui, C.B.; Lim, S.S. The inhibitory effect of Prunella vulgaris L. on aldose reductase and protein glycation. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Nakamura, S.; Fujimoto, K.; Ohta, T.; Ogawa, K.; Yoshikawa, M.; Matsuda, H. Structure of constituents isolated from the flower buds of Cananga odorata and their inhibitory effects on aldose reductase. J. Nat. Med. 2014, 68, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Choi, S.H.; Moon, H.E.; Park, J.J.; Jung, H.A.; Woo, M.H.; Woo, H.C.; Choi, J.S. The inhibitory activities of the edible green alga Capsosiphon fulvescens on rat lens aldose reductase and advanced glycation end products formation. Eur. J. Nutr. 2014, 53, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Salama, M.M.; Abd-Elrahman, E.H.; El-Maraghy, S.A. Antidiabetic activity of phenolic compounds from Pecan bark in streptozotocin-induced diabetic rats. Phytochem. Lett. 2011, 4, 337–341. [Google Scholar] [CrossRef]
- Chethan, S.; Dharmesh, S.M.; Malleshi, N.G. Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusine coracana) polyphenols. Bioorg. Med. Chem. 2008, 16, 10085–10090. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem. Pharm. Bull. (Tokyo) 2002, 50, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.M.; Hetta, M.H.; Samhan, F.A.; El Din, R.A.S.; Ali, G.H. Phytochemical and antibacterial study of five freshwater algal species. Asian J. Plant Sci. Res. 2012, 11, 109–116. [Google Scholar]
- Kumari, R.P.; Anbarasu, K. Protective role of C-phycocyanin against secondary changes during sodium selenite mediated cataractogenesis. Nat. Products Bioprospect. 2014, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.P.; Sivakumar, J.; Thankappan, B.; Anbarasu, K. C-phycocyanin modulates selenite-induced cataractogenesis in rats. Biol. Trace Elem. Res. 2013, 151, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid.-Based Complement Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Kumari, R.P.; Ramkumar, S.; Thankappan, B.; Natarajaseenivasan, K.; Balaji, S.; Anbarasu, K. Transcriptional regulation of crystallin, redox, and apoptotic genes by C-Phycocyanin in the selenite-induced cataractogenic rat model. Mol. Vis. 2015, 21, 26. [Google Scholar] [PubMed]
- Kothadia, A.D.; Shenoy, A.M.; Shabaraya, A.R.; Rajan, M.S.; Viradia, U.M.; Patel, N.H. Evaluation of Cataract Preventive Action of Phycocyanin. Int. J. Pharm. Sci. Drug Res. 2011, 3, 42–44. [Google Scholar]
- Ou, Y.; Yuan, Z.; Li, K.; Yang, X. Phycocyanin may suppress d-galactose-induced human lens epithelial cell apoptosis through mitochondrial and unfolded protein response pathways. Toxicol. Lett. 2012, 215, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, W.H.; Park, S.D.; Moon, H.I. Aldose reductase inhibitors from Litchi chinensis Sonn. J. Enzyme Inhib. Med. Chem. 2009, 24, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Yoon, N.Y.; Kang, S.S.; Kim, Y.S.; Choi, J.S. Inhibitory activities of prenylated flavonoids from Sophora flavescens against aldose reductase and generation of advanced glycation endproducts. J. Pharm. Pharmacol. 2008, 60, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, J.K.; Jang, J.M.; Lim, S.S. Inhibitory Effect of the Phenolic Compounds from Geranium thunbergii on Rat Lens Aldose Reductase and Galactitol Formation. Korean J. Med. Crop. Sci. 2012, 20, 222–230. [Google Scholar] [CrossRef]
- Kim, J.M.; Jang, D.S.; Lee, Y.M.; Yoo, J.L.; Kim, Y.S.; Kim, J.H.; Kim, J.S. Aldose-reductase- and protein-glycation-inhibitory principles from the whole plant of Duchesnea chrysantha. Chem. Biodivers. 2008, 5, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Sawant, L.; Singh, V.K.; Dethe, S.; Bhaskar, A.; Balachandran, J.; Mundkinajeddu, D.; Agarwal, A. Aldose reductase and protein tyrosine phosphatase 1B inhibitory active compounds from Syzygium cumini seeds. Pharm. Biol. 2015, 53, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Yoon, N.Y.; Bae, H.J.; Min, B.S.; Choi, J.S. Inhibitory activities of the alkaloids from Coptidis Rhizoma against aldose reductase. Arch. Pharm. Res. 2008, 31, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, U.D.; Ling, L.T.; Manaharan, T.; Appleton, D. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011, 127, 21–27. [Google Scholar] [CrossRef]
- Huang, G.J.; Hsieh, W.T.; Chang, H.Y.; Huang, S.S.; Lin, Y.C.; Kuo, Y.H. α-glucosidase and aldose reductase inhibitory activities from the fruiting body of Phellinus merrillii. J. Agric. Food Chem. 2011, 59, 5702–5706. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, J.K.; Kang, Y.H.; Lee, J.Y.; Kang, I.J.; Lim, S.S. Aldose Reductase Inhibitory Activity of Compounds from Zea mays L. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar]
- Morikawa, T.; Chaipech, S.; Matsuda, H.; Hamao, M.; Umeda, Y.; Sato, H.; Tamura, H.; Kon’i, H.; Ninomiya, K.; Yoshikawa, M.; et al. Antidiabetogenic oligostilbenoids and 3-ethyl-4-phenyl-3,4-dihydroisocoumarins from the bark of Shorea roxburghii. Bioorg. Med. Chem. 2012, 20, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Güvenç, A.; Okada, Y.; Akkol, E.K.; Duman, H.; Okuyama, T.; Çaliş, I. Investigations of anti-inflammatory, antinociceptive, antioxidant and aldose reductase inhibitory activities of phenolic compounds from Sideritis brevibracteata. Food Chem. 2010, 118, 686–692. [Google Scholar] [CrossRef]
- Rodriguez-Lyon, M.L.; Diaz-Lanza, A.M.; Bernabé, M.; Villaescusa-castillo, L. Flavone glycosides containing acetylated sugars from Sideritis hyssopifolia. Magn. Reson. Chem. 2000, 38, 684–687. [Google Scholar] [CrossRef]
- Lenherr, A.; Mabry, T.J. Acetylated allose-containing flavonoid glucosides from Stachys anisochila. Phytochemistry 1987, 26, 1185–1188. [Google Scholar] [CrossRef]
- Patel, D.K.; Prasand, S.K.; Kumar, R.; Hemalatha, S. Cataract: A major secondary complication of diabetes, its epidemiology and an overview on major medicinal plants screened for anticataract activity. Asian Pac. J. Trop. Dis. 2011, 1, 323–329. [Google Scholar] [CrossRef]
- Devi, V.G.; Rooban, B.N.; Sasikala, V.; Sahasranamam, V.; Abraham, A. Isorhamnetin-3-glucoside alleviates oxidative stress and opacification in selenite cataract in vitro. Toxicol. In Vitro 2010, 24, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kim, Y.J.; Jung, S.H.; Kim, J.H.; Kim, J.S. Flavonoids from Litsea japonica Inhibit AGEs Formation and Rat Lense Aldose Reductase in Vitro and Vessel Dilation in Zebrafish. Planta Med. 2017, 83, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hwang, S.H.; Suh, H.W.; Lim, S.S. Phytochemical analysis of Agrimonia pilosa Ledeb, its antioxidant activity and aldose reductase inhibitory potential. Int. J. Mol. Sci. 2017, 18, 379. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Jung, D.H.; Lee, I.S.; Choi, S.J.; Yu, S.Y.; Ku, S.K.; Kim, M.H.; Kim, J.S. Effects of Allium victorialis leaf extracts and its single compounds on aldose reductase, advanced glycation end products and TGF-β1 expression in mesangial cells. BMC Complement Altern. Med. 2013, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, M.Y.; Park, W.H.; Moon, H.I. Aldose reductase inhibitors from Viola hondoensis W. Becker et H Boss. Am. J. Chin. Med. 2008, 36, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tian, J.; Zhang, J.; Wang, K.; Liu, L.; Yang, B.; Bao, L.; Liu, H. Triterpenes and meroterpenes from Ganoderma lucidum with inhibitory activity against HMGs reductase, aldose reductase and α-glucosidase. Fitoterapia 2017, 120, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, X.; Guan, S.; Xia, J.; Sun, J.; Guo, H.; Guo, D. Analysis of Triterpenoids in Ganoderma lucidum Using Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Ganoderic acid Df, a new triterpenoid with aldose reductase inhibitory activity from the fruiting body of Ganoderma lucidum. Fitoterapia 2010, 81, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Inhibition of aldose reductase in vitro by constituents of Ganoderma lucidum. Planta Med. 2010, 76, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Kondo, R.; Shimizu, K. Structure-activity relationships of lanostane-type triterpenoids from Ganoderma lingzhi as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5900–5903. [Google Scholar] [CrossRef] [PubMed]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Ranganatha, L.V.; Prasad, M.N.N. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constituents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multiple stages. S. Afr. J. Bot. 2014, 95, 54–63. [Google Scholar] [CrossRef]
- Asha, R.; Devi, V.G.; Abraham, A. Chemico-Biological Interactions Lupeol, a pentacyclic triterpenoid isolated from Vernonia cinerea attenuate selenite induced cataract formation in Sprague Dawley rat pups. Chem. Biol. Interact. 2016, 245, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Anticataractogenic Activity of Luteolin. Chem. Biodivers. 2016, 13, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Vit, P.; Jacob, T.J. Putative Anticataract Properties of Honey Studied by the Action of Flavonoids on a Lens Culture Model. J. Heal. Sci. 2008, 54, 196–202. [Google Scholar] [CrossRef]
- Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Kim, J.S. Constituents of the flowers of Platycodon grandiflorum with inhibitory activity on advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharm. Res. 2010, 33, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Rooban, B.N.; Sasikala, V.; Devi, V.G.; Sahasranamam, V.; Abraham, A. Prevention of selenite induced oxidative stress and cataractogenesis by luteolin isolated from Vitex negundo. Chem. Biol. Interact. 2012, 196, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Lee, J.H.; Lee, M.H.; Lee, B.W.; Kwon, H.S.; Park, C.H.; Shim, K.B.; Kim, H.T.; Baek, I.Y.; Jang, D.S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012, 135, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rodriguez, J.P.; Quilantang, N.G.; Lee, M.H.; Cho, E.J.; Jacinto, S.D.; Lee, S. Determination of flavonoids from Perilla frutescens var. japonica seeds and their inhibitory effect on aldose reductase. Appl. Biol. Chem. 2017, 60, 155–162. [Google Scholar] [CrossRef]
- Morikawa, T.; Xie, H.; Wang, T.; Matsuda, H.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXXII. Aminopeptodase N and aldose reductase inhibitors from Sinocrassula indica: Structures of Sinocrassosides B4, B5, C1 and D1-D3. Chem. Pharm. Bull. (Tokyo) 2008, 56, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Tao, J.; Ueda, K.; Yoshikawa, M. Bioactive constituents of Chinese natural medicines. VII. Inhibitors of degranulation in RBL-2H3 cells and absolute stereostructures of three new diarylheptanoid glycosides from the bark of Myrica rubra. Chem. Pharm. Bull. (Tokyo) 2002, 50, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kwon, S.H.; Kim, S.B.; Lim, S.S. Inhibitory Activities of Stauntonia hexaphylla Leaf Constituents on Rat Lens Aldose Reductase and Formation of Advanced Glycation End Products and Antioxidant. BioMed Res. Int. 2017, 2017, 4273257. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Mishra, S. Isoquinoline alkaloids from Tinospora cordifolia inhibit rat lens aldose reductase. Phyther. Res. 2012, 26, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Fujimoto, K.; Matsumoto, T.; Nakashima, S.; Ohta, T.; Ogawa, K.; Tamura, H.; Matsuda, H.; Yoshikawa, M. Structures of Acylated Sucroses and Inhibitory Effects of Constituents on Aldose Reducatase from the Flower Buds of Prunus mume. Chem. Pharm. Bull. 2013, 61, 445–451. [Google Scholar]
- Nakamura, S.; Fujimoto, K.; Matsumoto, T.; Ohta, T.; Ogawa, K.; Tamura, H.; Matsuda, H.; Yoshikawa, M. Structures of acylated sucroses and an acylated flavonol glycoside and inhibitory effects of constituents on aldose reductase from the flower buds of Prunus mume. J. Nat. Med. 2013, 67, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, Y.S.; Lee, Y.M.; Jang, D.S.; Kim, J.S. Inhibition of aldose reductase and xylose-induced lens opacity by puerariafuran from the roots of Pueraria lobata. Biol. Pharm. Bull. 2010, 33, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Stobiecki, M.; Kachlicki, P. Isolation and identification of flavonoids. Sci. Flavonoids 2006, 27, 47–69. [Google Scholar]
- Lee, H.E.; Kim, J.A.; Whang, W.K. Chemical constituents of smilax China l. stems and their inhibitory activities against glycation, aldose reductase, α-glucosidase, and lipase. Molecules 2017, 22, 451. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Yasuko, H.; Goto, H.; Hollinshead, J.; Nash, R.J.; Adachi, I. Inhibitory effect of rhetsinine isolated from Evodia rutaecarpa on aldose reductase activity. Phytomedicine 2009, 16, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tang, Y.; Liu, Q.; Guo, N.; Zhang, J.; Xiao, Z.; Chen, R.; Shen, Z. Isolation, modification, and aldose reductase inhibitory activity of rosmarinic acid derivatives from the roots of Salvia grandifolia. Fitoterapia 2016, 112, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Hwang, S.H.; Kang, B.G.; Hong, J.S.; Lim, S.S. Inhibitory effects of Colocasia esculenta (L.) Schott constituents on aldose reductase. Molecules 2014, 19, 13212–13224. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, N.H.; Nam, J.W.; Lee, Y.M.; Jang, D.S.; Kim, Y.S.; Nam, S.H.; Seo, E.K.; Yang, M.S.; Kim, J.S. Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis Ex Vivo. Arch. Pharm. Res. 2010, 33, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kwon, S.B.; Heo, N.K.; Chun, W.J.; Kim, M.J.; Kwon, Y.S. Constituents of the stem of Angelica gigas with rat lens aldose reductase inhibitory activity. J. Appl. Biol. Chem. 2011, 54, 194–199. [Google Scholar] [CrossRef]
- Abou Assi, R.; Darwis, Y.; Abdulbaqi, I.; Khan, A.A.; Lim, V.; Laghari, M.H. Morinda citrifolia (Noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arab. J. Chem. 2017, 10, 691–707. [Google Scholar] [CrossRef]
- Wang, Q.; Qian, Y.; Wang, Q.; Yang, Y.F.; Ji, S.; Song, W.; Qiao, X.; Guo, D.; Liang, H.; Ye, M. Metabolites identification of bioactive licorice compounds in rats. J. Pharm. Biomed. Anal. 2015, 115, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, S.H.; Jung, S.H.; Kim, J.K.; Pan, C.H.; Lim, S.S. Aldose Reductase Inhibitory Compounds from Glycyrrhiza uralensis. Biol. Pharm. Bull. 2010, 33, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Hwang, J.K.; Koh, H.W.; Jang, K.Y.; Lee, J.H.; Park, J.W.; Park, B.H. Sulfuretin, a major flavonoid isolated from Rhus verniciflua, ameliorates experimental arthritis in mice. Life Sci. 2012, 90, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Song, D.G.; Lee, J.Y.; Pan, C.H.; Um, B.H.; Jung, S.H. Inhibitory Effect of the Compounds Isolated from Rhus verniciflua on Aldose Reductase and Advanced Glycation Endproducts. Biol. Pharm. Bull. 2008, 31, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Jung, S.H.; Ji, J.; Shin, K.H.; Keum, S.R. Inhibitory effects of 2′-hydroxychalcones on rat lens aldose reductase and rat platelet aggregation. Chem. Pharm. Bull. 2000, 48, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Yuan, H.; Yamazaki, Y.; Sasaki, T.; Oka, S. Alkaloids and Flavonoids from Peanut Skins. Planta Med. 2001, 67, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, D.; Yi, Y.; Qi, H.; Gao, X.; Fang, H.; Gu, Q.; Wang, L.; Gu, L. Syringic acid extracted from Herba dendrobii prevents diabetic cataract pathogenesis by inhibiting aldose reductase activity. Evid.-Based Complement Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.Y.; Liu, H.L.; Zhang, J.Y.; Yang, B. Phenolic glycosides and other constituents from the bark of Magnolia officinalis. J. Asian Nat. Prod. Res. 2014, 16, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Mishra, S.M. Aldose Reductase Inhibitory Activity of a Cglycosidic Flavonoid Derived from Enicostemma hyssopifolium. Inf. J. Complement. Integr. Med. 2009, 6, 1553–3840. [Google Scholar]
| Active Ingredient | Structure | IC50 Values | ||||||
|---|---|---|---|---|---|---|---|---|
| AGE | ARI | GLWW | RHAR | BLAR | HLAR | RLAR | ||
| 1-O-galloyl-β-d-glucose (β-Glucogallin) |  | NA | NA | NA | 17.00 µM [30] | NA | NA | NA |
| 1,3-di-O-caffeoylquinic acid |  | 24.85 µM [32] | NA | NA | 0.810 µM [33] | NA | NA | 0.22 µM [32] |
| 1,5-Di-hydroxy-1,5-di-[(E)-3-(4-hydroxyphenyl)-2-propenoic]-3-pentanonyl ester (DHDP) |  | NA | NA | NA | 194.67µM [34] | NA | NA | NA |
| 1,5-di-O-caffeoylquinic acid |  | NA | NA | NA | NA | NA | NA | 2.98 µM [35] |
| 1,3,6-trihydroxy-2-methoxymethylanthraquinone |  | 52.72 µM [36] | NA | NA | NA | NA | NA | 3.04 µM [36] |
| 1,2,3,6-tetra-O-galloyl-β-d-glucose |  | 1.99 µM [38] | NA | NA | NA | NA | NA | 0.70 µM [38] |
| 1,3,5,8-Tetrahydroxyxanthone |  | NA | NA | NA | NA | NA | NA | 0.0886 μM [39] |
| 2″,4″-O-Diacetylquercitrin |  | 11.46 µM [40] | NA | NA | NA | NA | NA | 0.077 µM [40] |
| 3-Isomangostin |  | NA | NA | NA | NA | NA | NA | 3.48 μM [41] |
| 3′,5′-dimethoxy-(1,1′-biphenyl)-3,4-diol 3-O-β-d-glucopyranoside |  | NA | NA | NA | NA | NA | NA | 3.80 µM [47] |
| 3,5-di-O-caffeoylquinic Acid |  | 6.06 µM [48] | NA | 153 g [33] | 1.34 µM [33] | NA | NA | 0.19 µM [33] |
| 4-O-butylpaeoniflorin |  | 10.80 µM [53] | NA | NA | NA | NA | NA | 36.20 µM [53] |
| 4,5-Di-O-trans-caffeoyl-d-quinic acid |  | NA | NA | NA | NA | NA | NA | 0.29 µM [55] |
| 5-O-Feruloly quinic acid |  | NA | NA | NA | NA | NA | NA | 14.19 µM [56] |
| 5,7,4′-trihydroxyisoflavone (Genistein) |  | NA | NA | NA | NA | NA | NA | 9.48 µM [60] |
| 20(S)-Ginsenoside Rh2 |  | NA | NA | NA | 147.40 µM [61] | NA | NA | NA |
| Acteoside |  | 5.11 µM [63] | NA | NA | NA | NA | NA | 0.83 µM [63] |
| Basilicumin [7-(3-hydroxypropyl)-3-methyl-8-β-O-d-glucoside-2H-chromen-2-one] |  | NA | NA | NA | NA | 2.09 µM | NA | NA |
| Caffeic acid |  | 7.56 µM [68] | NA | NA | 210.28µM [66] | NA | NA | 16.71 µM [65] |
| Canangafruiticoside E |  Glc=β-d-glucopyranoside | NA | NA | NA | NA | NA | NA | 0.80 µM [71] |
| Capsofulvesin A [((2S)-l-O-(6Z,9Z,12Z,15Zoctadecatetraenoyl)-2-O-(4Z,10Z,13Zhexadecatetraenoyl)-3-O-β-d-galactopyranosyl glycerol)] |  | NA | NA | NA | NA | NA | NA | 52.53 µM [72] |
| Davallialactone |  | NA | NA | NA | 0.56 µM [67] | NA | NA | 0.33 µM [67] |
| Delphinidin 3-O-β-galactopyranoside-3′-O-β-glucopyranoside |  Glc= β-glucopyranoside, Gal= β-galactopyranoside | NA | NA | NA | NA | NA | NA | 0.37 µM [83] |
| Desmethylanhydroicaritin |  | 294.60 µM [84] | NA | NA | 0.45 µM [84] | NA | NA | 0.95 µM [84] |
| Ellagic acid |  | 26.0 µM [86] | NA | 42.47% [85] | NA | NA | 1.37 µM [67] | 0.12 µM [87] |
| Epiberberine |  | NA | NA | NA | 168.10 µM [88] | NA | NA | 100.07 µM [88] |
| Geraniin |  | 21.00 µM 96% * [89] | 0.15 µM [89] | 39.87% [85] | NA | NA | NA | NA |
| Hipolon |  | NA | NA | NA | NA | NA | NA | 9.47 µM [90] |
| Hirsutrin |  | NA | NA | 33.78% [91] | NA | NA | NA | 4.78 µM [91] |
| Hopeafuran |  | NA | NA | NA | NA | NA | NA | 14.80 µM [92] |
| Hypolaetin 7-O-[6′′′-O-acetyl-β-d-allopyranosyl-(1→2)]-6′′-O-acetyl-β-d-glucopyranoside |  | NA | 0.66 µM [93] | NA | NA | NA | NA | NA |
| Isocampneoside II |  | NA | NA | NA | 9.72 µM [66] | NA | NA | NA |
| Kaempferol |  | 36.01 µM [100] | NA | NA | 45.58 µM [66] | NA | NA | 1.10 µM [98,100] |
| Kakkalide |  | NA | NA | NA | NA | NA | NA | 0.56 µM [101] |
| Lucidumol A [(24S)-24,25-Dihydroxylanost-8-ene-3,7-dione] |  | NA | NA | NA | NA | 19.10 µM [102] | NA | NA |
| Lupeol |  | NA | NA | NA | 3.60 µM [107] | NA | NA | NA |
| Luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromomenone) |  | 16.60 µM [111] | NA | NA | 6.34 µM [52] | NA | NA | 0.087 µM [111] |
| Luteolin-7-O-β-d-glucopyranoside |  | 117.80 µM [117] | NA | NA | NA | NA | NA | 7.34 µM [117] |
| Magnoflorine |  | NA | NA | NA | NA | NA | NA | 3.60 µM [118] |
| Methyl-3,5-di-O-caffeoylquinate |  | NA | NA | 117 g [33] | 0.67 µM [33] | NA | NA | 0.30 µM [33] |
| Mumeic acid-A |  | NA | NA | NA | NA | NA | NA | 0.40 µM [119] |
| Palbinone |  | >500 µM [53] | NA | NA | NA | NA | NA | 11.40 µM [53] |
| Puerariafuran |  | NA | NA | NA | NA | NA | NA | 22.20 µM [57,121] |
| Quercetin-3-O-β-d-glucoside |  | >1000 µM [117] | NA | NA | NA | NA | NA | 2.21 µM [122] |
| Quercitrin (Quercetin 3-O-α-l-rhamnoside) |  | 4.20 µM [100] | NA | NA | NA | NA | NA | 0.17 µM [40] |
| Rhetsinine |  | NA | NA | NA | NA | NA | NA | 24.10 µM [124] |
| Rosmarinic acid |  | NA | NA | 532.38g [70] | 2.77 µM [69] | NA | NA | 0.30 µM [125] |
| Scopoletin |  | 2.93 µM [127] | NA | NA | NA | NA | NA | 2.59 µM [128] |
| Semilicoisoflavone B |  | NA | NA | NA | 10.60 µM [131] | NA | NA | 1.80 µM [131] |
| Sulfuretin |  | 124.00 µM [133] | NA | NA | 1.30 µM [133] | NA | NA | NA |
| Syringic Acid |  | NA | NA | NA | NA | NA | NA | 1081.1 µm [136] |
| Swertisin (C-glycosidic flavonoid) |  Glu=glucose | NA | NA | NA | NA | NA | NA | 1.60 µm [138] |
| Valoneic acid dilactone |  | NA | NA | NA | NA | NA | NA | 0.075 µM [87] |
| Constituent Name (Class of Constituent) | Structure | Doses (IC50/EC50) | |||||
|---|---|---|---|---|---|---|---|
| AR Transgenic Mice | Selenite-Induced | AR Rat Lens | Galactose-Induced Lens Opacity | Xylose-Induced Lens Opacity | Ref | ||
| 1-O-galloyl-β-d-glucose (β-Glucogallin) |  | Ex vivo: 30.00 µM | NA | NA | NA | NA | [29] |
| 1,2,3,6-Tetra-O-galloyl-β-d-glucose |  | NA | NA | NA | NA | Ex vivo: 80.00 µM | [38] |
| 3,5-di-O-caffeoyl-epi-quinic Acid |  | NA | NA | NA | NA | Ex vivo: 10.00 μM | [48] |
| 5,7,4′-trihydroxyisoflavone (Genistein) |  | NA | NA | NA | NA | Ex vivo: 18.50 µM | [60] |
| Isorhamnetin-3-glucoside |  | NA | Ex vivo: 52.25 µM | NA | NA | NA | [97] |
| Lupeol |  | NA | In vivo: 126.15 μM | NA | NA | NA | [108] |
| Luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromomenone) |  | NA | Ex vivo: 6.98 µM | NA | NA | NA | [112] |
| Puerariafuran |  | NA | NA | NA | NA | Ex vivo: 15.00 µM | [121] |
| Scopoletin |  | NA | NA | NA | NA | Ex vivo: 25.00 µM | [127] |
| Syringic acid |  | NA | NA | NA | Ex vivo: 1075.70 μM In vivo: 2% syringic acid eye drop (131,197.80 μM) | NA | [136] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, V.; Schneider, E.; Wu, H.; Pang, I.-H. Cataract Preventive Role of Isolated Phytoconstituents: Findings from a Decade of Research. Nutrients 2018, 10, 1580. https://doi.org/10.3390/nu10111580
Lim V, Schneider E, Wu H, Pang I-H. Cataract Preventive Role of Isolated Phytoconstituents: Findings from a Decade of Research. Nutrients. 2018; 10(11):1580. https://doi.org/10.3390/nu10111580
Chicago/Turabian StyleLim, Vuanghao, Edward Schneider, Hongli Wu, and Iok-Hou Pang. 2018. "Cataract Preventive Role of Isolated Phytoconstituents: Findings from a Decade of Research" Nutrients 10, no. 11: 1580. https://doi.org/10.3390/nu10111580
APA StyleLim, V., Schneider, E., Wu, H., & Pang, I.-H. (2018). Cataract Preventive Role of Isolated Phytoconstituents: Findings from a Decade of Research. Nutrients, 10(11), 1580. https://doi.org/10.3390/nu10111580