Hesperidin Prevents Nitric Oxide Deficiency-Induced Cardiovascular Remodeling in Rats via Suppressing TGF-β1 and MMPs Protein Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals and Experimental Protocols
2.3. Blood Pressure Measurements
2.4. Collection of Blood and Organs
2.5. Assays of Vascular O2•− Production, Plasma Malondialdehyde (MDA), Plasma Nitric Oxide Metabolite (Nitrate/Nitrite, NOx), Plasma TNF-α and Plasma TGF- β1 Levels
2.6. Morphometric Analysis of Thoracic Aorta and Heart Tissue
2.7. Western Blot Analysis of Tumor Necrosis Factor Receptor 1 (TNF-R1), TGF- β1, MMP-2 and MMP-9 Protein Expressions in Cardiac and Aortic Tissues
2.8. Statistical Analysis
3. Results
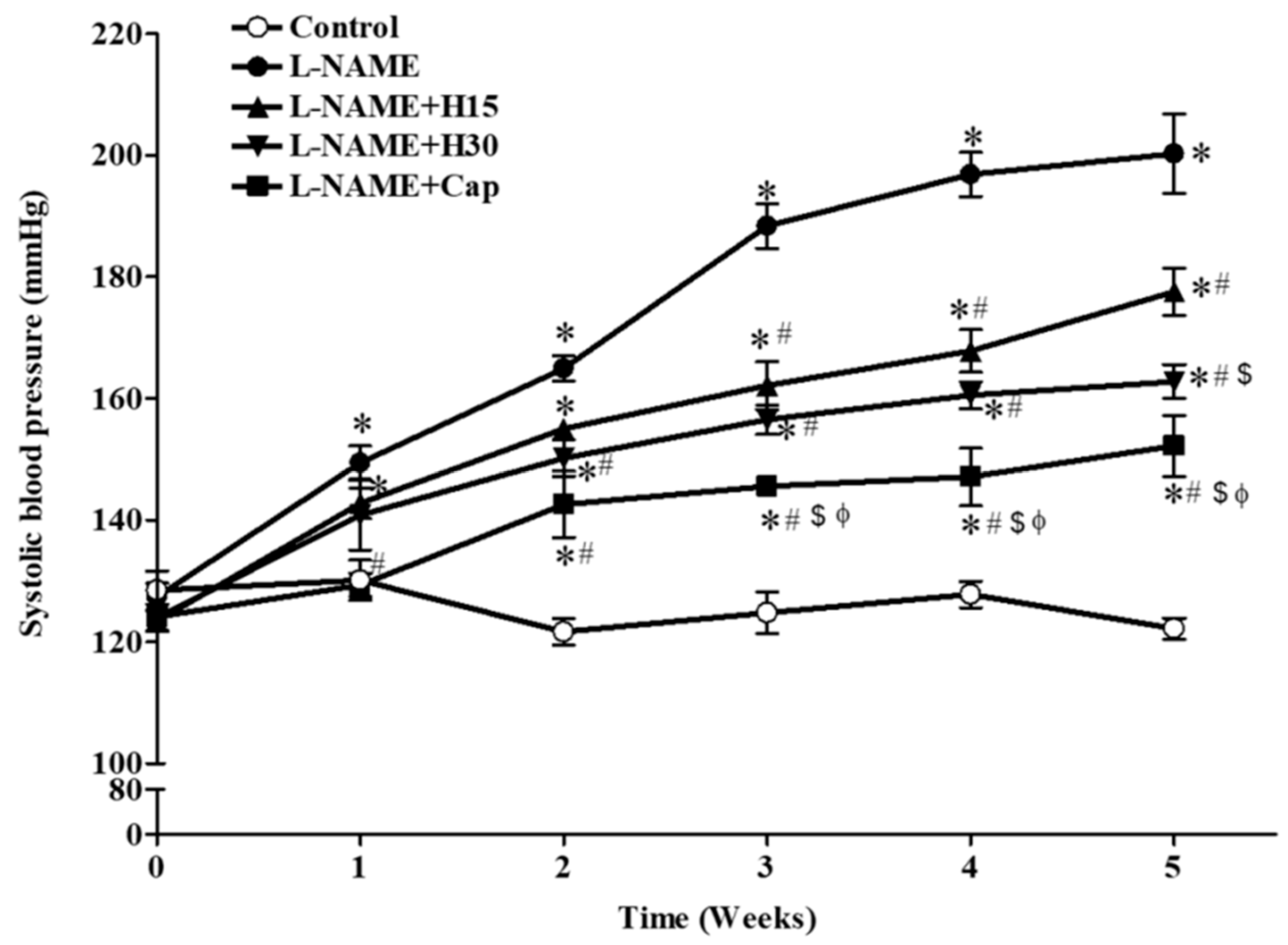
3.1. Effects of Hesperidin and Captopril on Blood Pressure in Conscious Rats
3.2. Effects of Hesperidin and Captopril on SP, DP, MAP, and HR in Anesthetized Rats
3.3. Effects of Hesperidin and Captopril on Left Ventricular (LV) Morphometry and Fibrosis
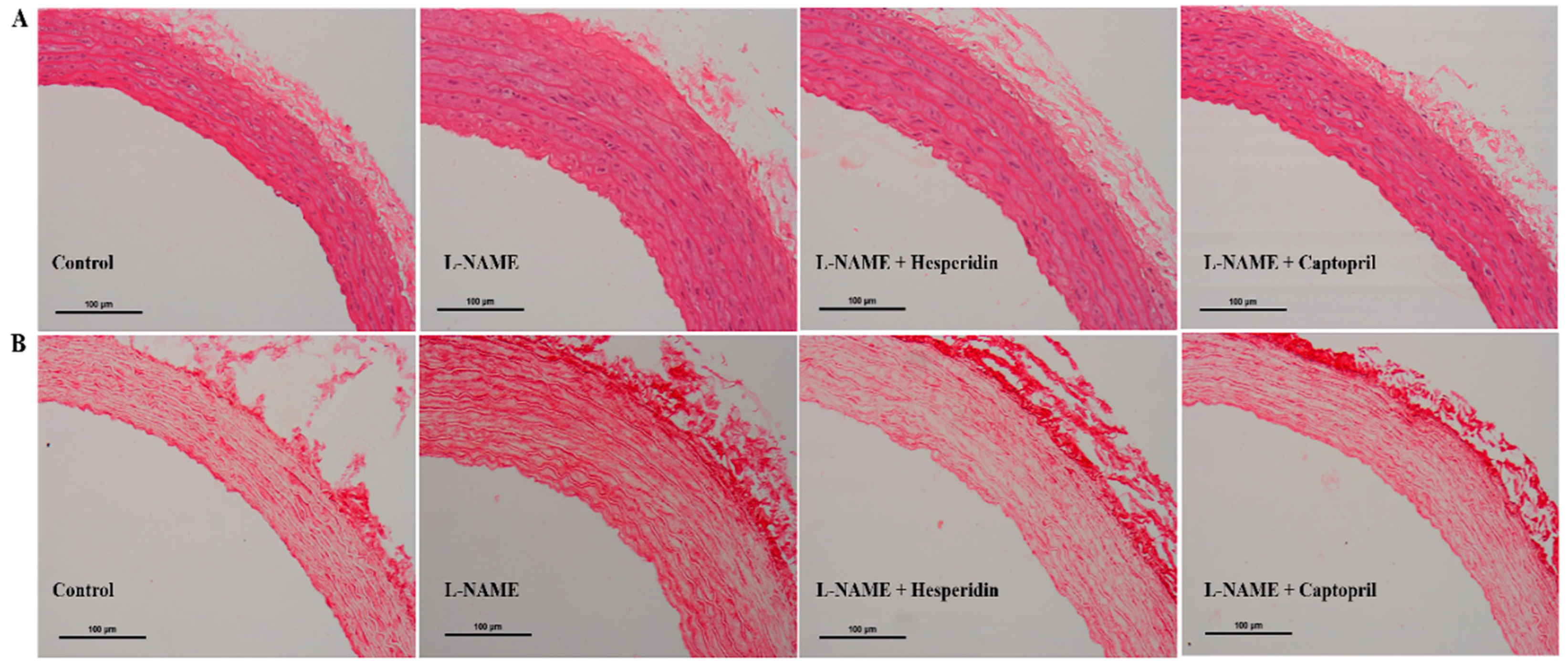
3.4. Effect of Hesperidin and Captopril on Vascular Morphology
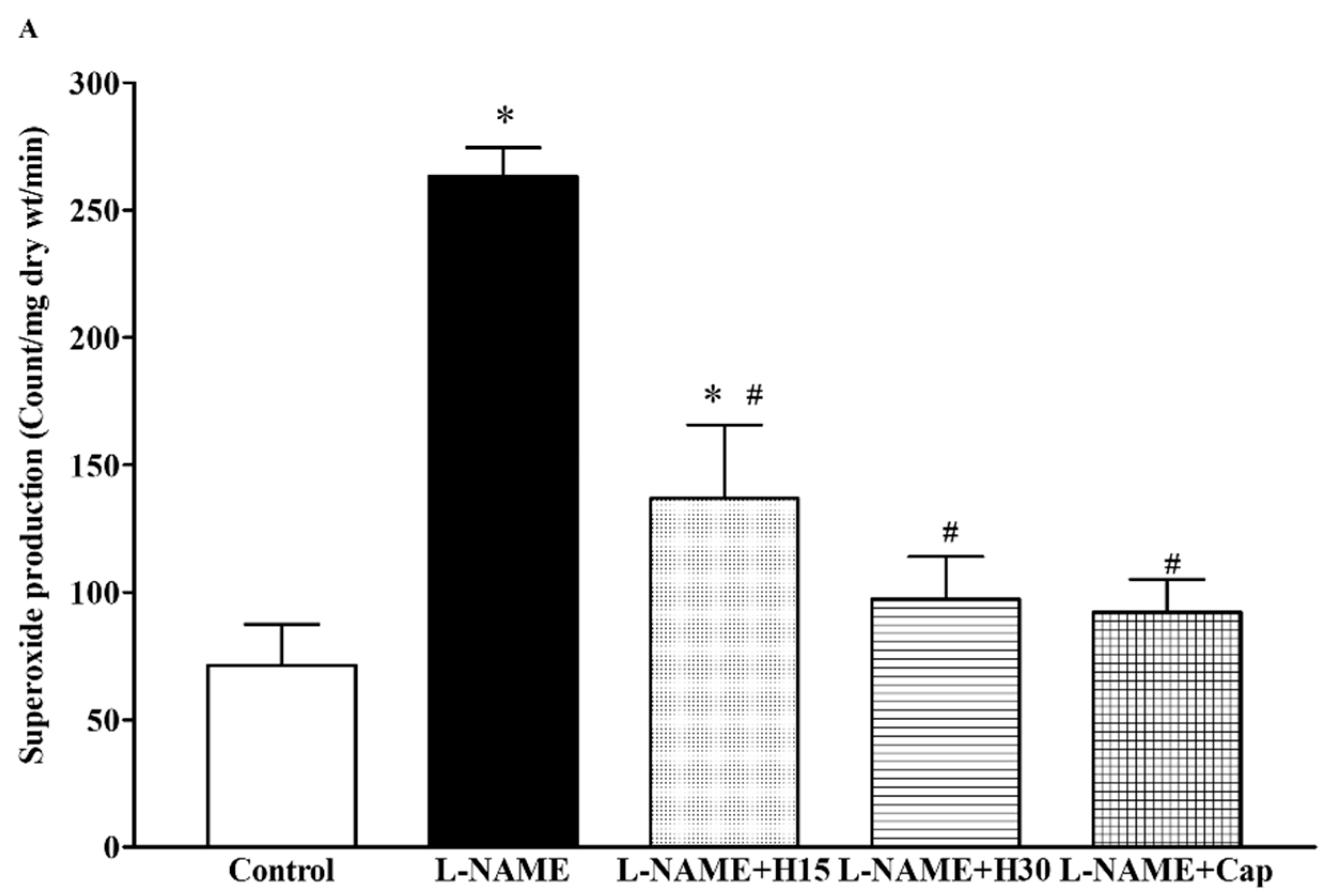
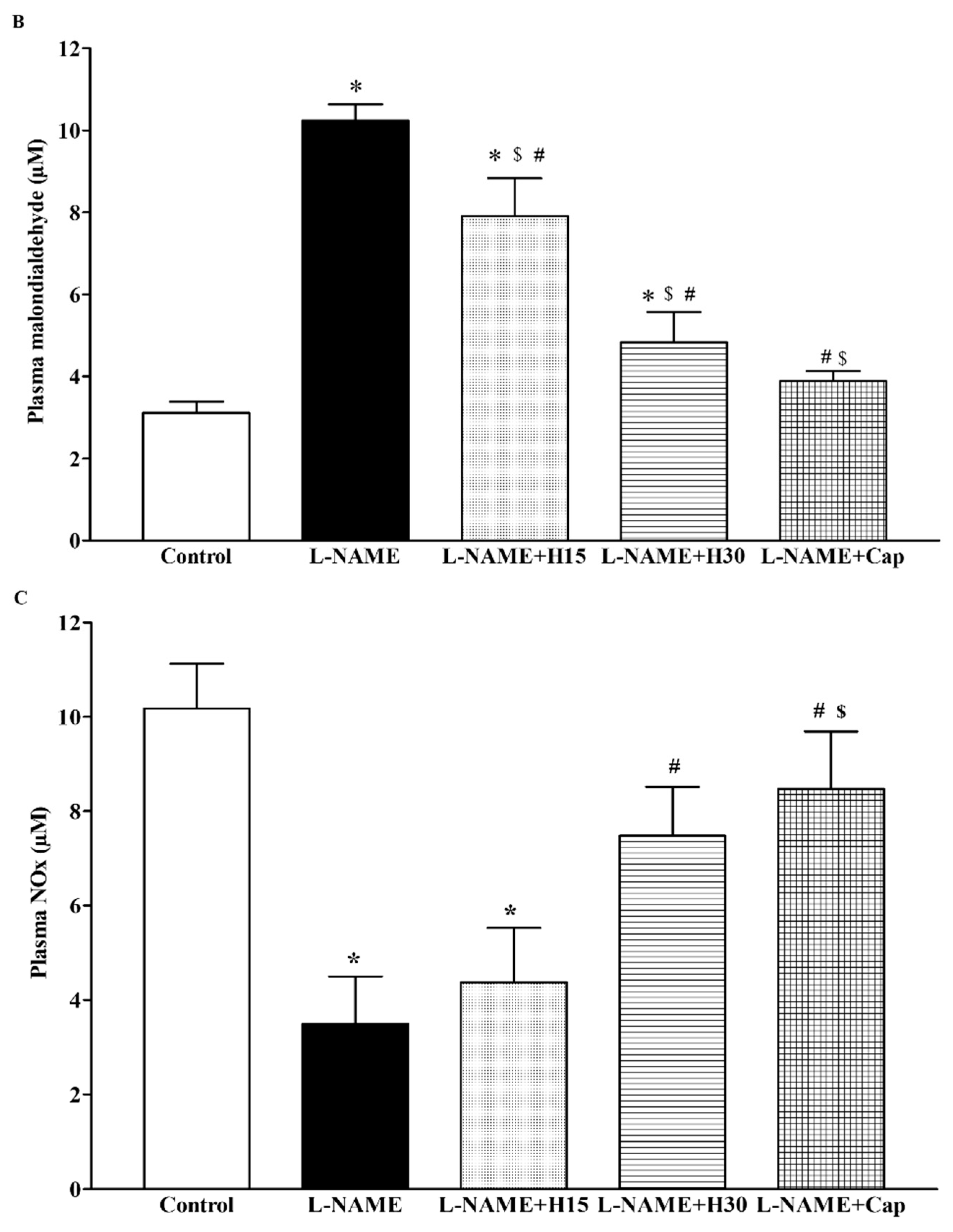
3.5. Effects of Hesperidin and Captopril Supplementation on Oxidative Stress Markers, Plasma Nitric Oxide Metabolites (NOx) Levels in l-NAME Treated Rats
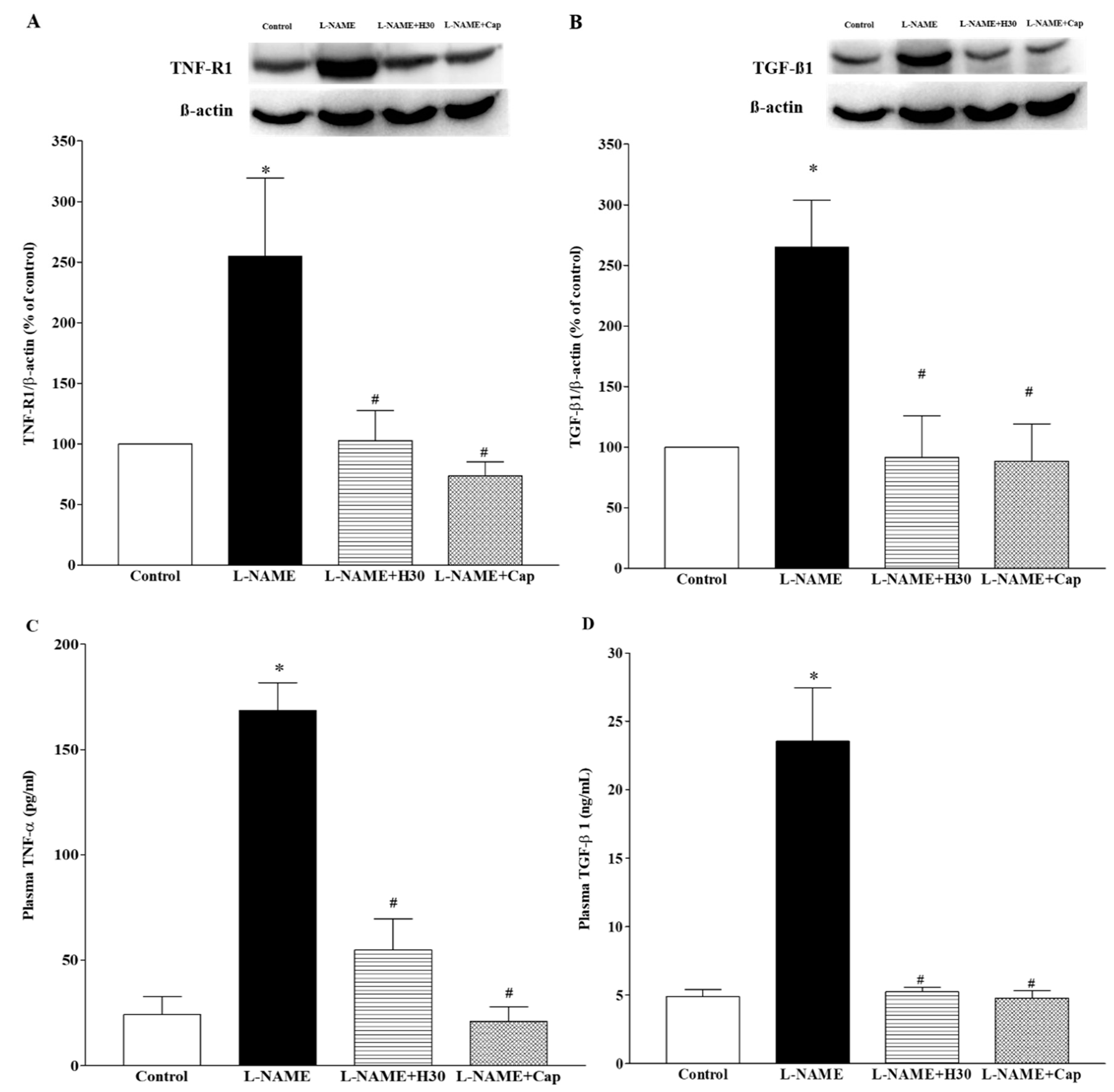
3.6. Effects of Hesperidin and Captopril on Protein Expression of TNF-R1 and TGF-β1 in Heart Tissues and Concentrations of TNF-α and TGF-β1 in Plasma
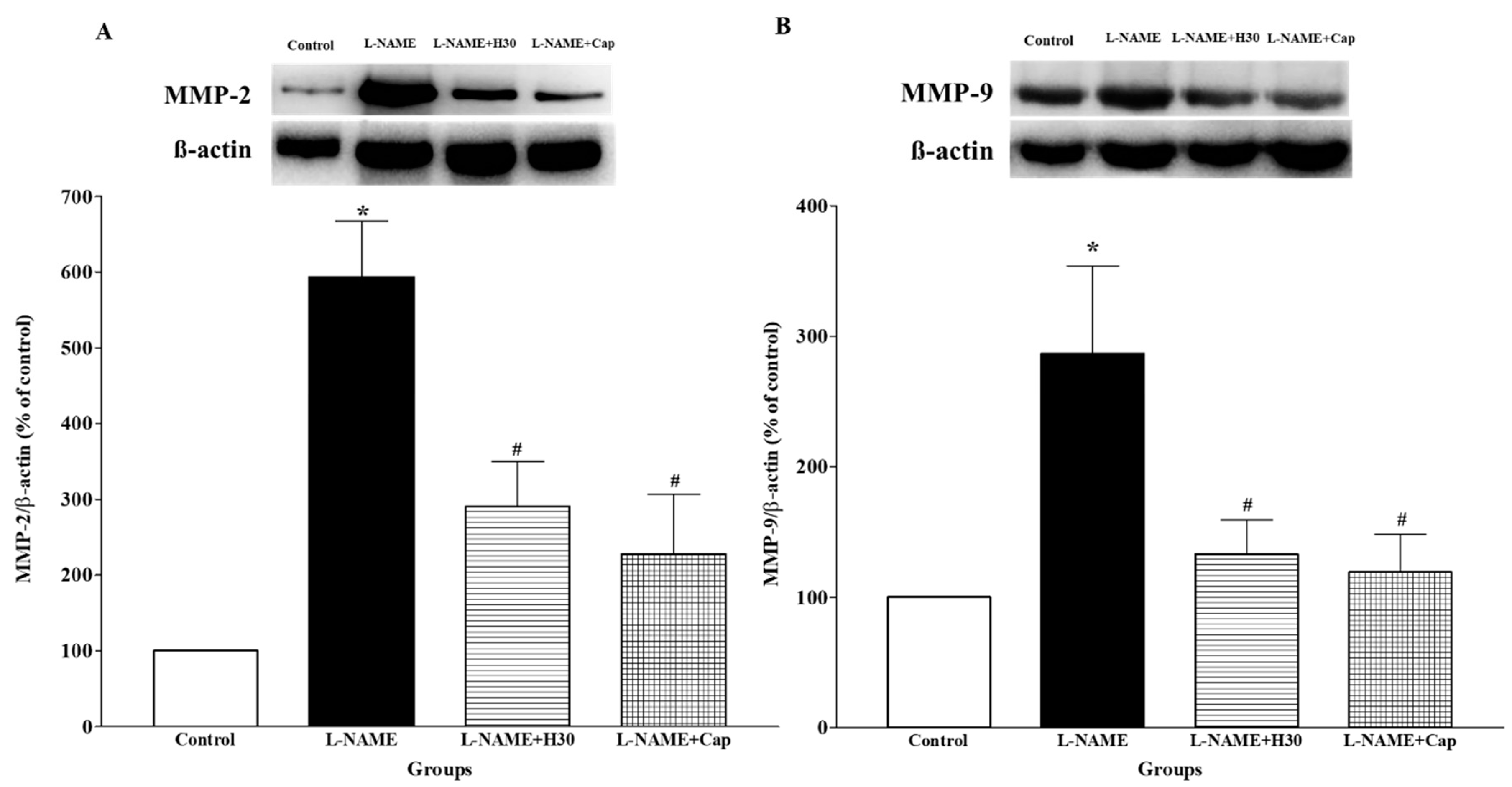
3.7. Effects of Hesperidin and Captopril on Protein Expression of MMP-2 and MMP-9 in Aortic Tissue
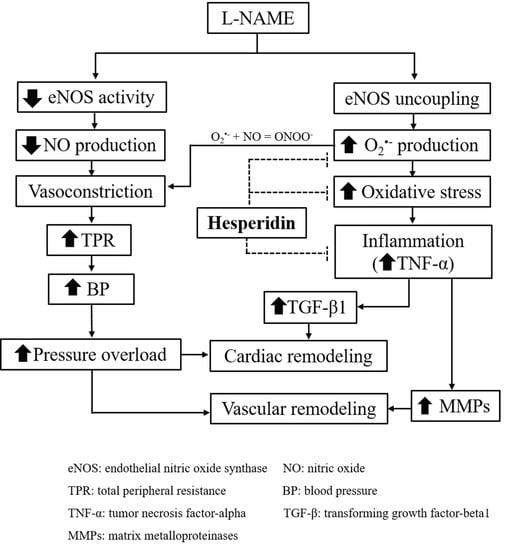
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Bunbupha, S.; Pakdeechote, P.; Kukongviriyapan, U.; Prachaney, P.; Kukongviriyapan, V. Asiatic acid reduces blood pressure by enhancing nitric oxide bioavailability with modulation of eNOS and p47phox expression in l-NAME-induced hypertensive rats. Phytother. Res. 2014, 28, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Bernatova, I.; Babal, P.; Martinez, M.C.; Kysela, S.; Stvrtina, S.; Andriantsitohaina, R. Red wine polyphenols prevent cardiovascular alterations in l-NAME-induced hypertension. J. Hypertens. 2004, 22, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Bunbupha, S.; Prachaney, P.; Kukongviriyapan, U.; Kukongviriyapan, V.; Welbat, J.U.; Pakdeechote, P. Asiatic acid alleviates cardiovascular remodelling in rats with l-NAME-induced hypertension. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I.; Pechanova, O.; Babal, P.; Kysela, S.; Stvrtina, S.; Andriantsitohaina, R. Wine polyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H942–H948. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Eisen, H.J. Epidemiology of heart failure and scope of the problem. Cardiol. Clin. 2014, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Babal, P.; Pechanova, O.; Bernatova, I.; Stvrtina, S. Chronic inhibition of NO synthesis produces myocardial fibrosis and arterial media hyperplasia. Histol. Histopathol. 1997, 12, 623–629. [Google Scholar] [PubMed]
- Mir, S.A.; Chatterjee, A.; Mitra, A.; Pathak, K.; Mahata, S.K.; Sarkar, S. Inhibition of signal transducer and activator of transcription 3 (STAT3) attenuates interleukin-6 (IL-6)-induced collagen synthesis and resultant hypertrophy in rat heart. J. Biol. Chem. 2012, 287, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Siwik, D.A.; Pagano, P.J.; Colucci, W.S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 2001, 280, C53–C60. [Google Scholar] [CrossRef] [PubMed]
- Boonprom, P.; Boonla, O.; Chayaburakul, K.; Welbat, J.U.; Pannangpetch, P.; Kukongviriyapan, U.; Kukongviriyapan, V.; Pakdeechote, P.; Prachaney, P. Garcinia mangostana pericarp extract protects against oxidative stress and cardiovascular remodeling via suppression of p47(phox) and iNOS in nitric oxide deficient rats. Ann. Anat. 2017, 212, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Chibbar, R.; Lane, N. Heme oxygenase suppresses markers of heart failure and ameliorates cardiomyopathy in l-NAME-induced hypertension. Eur. J. Pharmacol. 2014, 734, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zheng, B.; Chen, T.; Chang, X.; Yin, B.; Huang, Z.; Shuai, P.; Han, L. Semen brassicae ameliorates hepatic fibrosis by regulating transforming growth factor-beta1/Smad, nuclear factor-kappaB, and AKT signaling pathways in rats. Drug Des. Dev. Ther. 2018, 12, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.; Jenkins, R.H.; Fraser, D.J. MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. J. Pathol. 2013, 229, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Arribas, S.M.; Hinek, A.; Gonzalez, M.C. Elastic fibres and vascular structure in hypertension. Pharmacol. Ther. 2006, 111, 771–791. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.K.; Cha, B.Y.; Kim, C.H. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J. Cell. Physiol. 2004, 198, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Arenas, I.A.; Xu, Y.; Lopez-Jaramillo, P.; Davidge, S.T. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-alpha. Am. J. Physiol. Cell Physiol. 2004, 286, C779–C784. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, J.; Castrillon, D.H.; DePinho, R.A.; Olson, E.N.; Liu, Z.P. FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol. Cell. Biol. 2007, 27, 2676–2686. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.W.; Dubois, M.P. New drugs for the treatment of hypertension: Where do they fit? Can. Fam. Phys. 1987, 33, 2583–2589. [Google Scholar]
- Rodriguez-Garcia, J.L.; Villa, E.; Serrano, M.; Gallardo, J.; Garcia-Robles, R. Prostacyclin: Its pathogenic role in essential hypertension and the class effect of ACE inhibitors on prostaglandin metabolism. Blood Press 1999, 8, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Maneesai, P.; Prasarttong, P.; Bunbupha, S.; Kukongviriyapan, U.; Kukongviriyapan, V.; Tangsucharit, P.; Prachaney, P.; Pakdeechote, P. Synergistic Antihypertensive effect of Carthamus tinctorius L. extract and captopril in l-NAME-induced hypertensive rats via restoration of eNOS and AT(1)R expression. Nutrients 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Sato, H.; Shimada, H.; Takashima, N.; Arakawa, M. Comparative free radical scavenging action of angiotensin-converting enzyme inhibitors with and without the sulfhydryl radical. Pharmacology 1993, 47, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, K.; Ohtake, K.; Uchida, H.; Ito, J.; Uchida, M.; Natsume, H.; Tamada, H.; Kobayashi, J. Dietary nitrite supplementation attenuates cardiac remodeling in l-NAME-induced hypertensive rats. Nitric Oxide 2017, 67, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Emim, J.A.; Oliveira, A.B.; Lapa, A.J. Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. J. Pharm. Pharmacol. 1994, 46, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, P.; Pugalendi, K.V. Hesperidin, a flavanone glycoside, on lipid peroxidation and antioxidant status in experimental myocardial ischemic rats. Redox Rep. 2010, 15, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes; a randomized double-blind controlled clinical trial. Phytother. Res. 2018, 32, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Hao, J.; Liu, T.; Zhang, D.; Lv, H.; Song, E.; Zhu, C. Hesperetin suppresses inflammatory responses in lipopolysaccharide-induced raw 264.7 cells via the inhibition of NF-kappaB and activation of Nrf2/HO-1 pathways. Inflammation 2016, 39, 964–973. [Google Scholar] [PubMed]
- Giannini, I.; Amato, A.; Basso, L.; Tricomi, N.; Marranci, M.; Pecorella, G.; Tafuri, S.; Pennisi, D.; Altomare, D.F. Flavonoids mixture (diosmin, troxerutin, hesperidin) in the treatment of acute hemorrhoidal disease: A prospective, randomized, triple-blind, controlled trial. Tech. Coloproctol. 2015, 19, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Wunpathe, C.; Potue, P.; Maneesai, P.; Bunbupha, S.; Prachaney, P.; Kukongviriyapan, U.; Kukongviriyapan, V.; Pakdeechote, P. Hesperidin suppresses renin-angiotensin system mediated NOX2 over-expression and sympathoexcitation in 2K-1C hypertensive rats. Am. J. Chin. Med. 2018, 46, 751–767. [Google Scholar] [CrossRef]
- Lu, F.J.; Lin, J.T.; Wang, H.P.; Huang, W.C. A simple, sensitive, non-stimulated photon counting system for detection of superoxide anion in whole blood. Experientia 1996, 52, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Luangaram, S.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem. Toxicol. 2007, 45, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Verdon, C.P.; Burton, B.A.; Prior, R.L. Sample pretreatment with nitrate reductase and glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal. Biochem. 1995, 224, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.O.; Antunes, E.; de Nucci, G.; Lovisolo, S.M.; Zatz, R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 1992, 20, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Paulis, L.; Zicha, J.; Kunes, J.; Hojna, S.; Behuliak, M.; Celec, P.; Kojsova, S.; Pechanova, O.; Simko, F. Regression of l-NAME-induced hypertension: The role of nitric oxide and endothelium-derived constricting factor. Hypertens. Res. 2008, 31, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Simko, J. The potential role of nitric oxide in the hypertrophic growth of the left ventricle. Physiol. Res. 2000, 49, 37–46. [Google Scholar] [PubMed]
- Simko, F. Physiologic and pathologic myocardial hypertrophy--physiologic and pathologic regression of hypertrophy? Med. Hypotheses 2002, 58, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.I.; Kass, D.A. Phosphodiesterases and cyclic GMP regulation in heart muscle. Physiology 2012, 27, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, H. Response of the rat aortic media to hypertension: Morphological and chemical studies. Circ. Res. 1970, 26, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, G.H.; Dzau, V.J. The emerging concept of vascular remodeling. N. Engl. J. Med. 1994, 330, 1431–1438. [Google Scholar] [PubMed]
- Tronc, F.; Wassef, M.; Esposito, B.; Henrion, D.; Glagov, S.; Tedgui, A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.D.; Kandasamy, K.; Subramani, J.; Tandan, S.K.; Kumar, D. Involvement of inducible nitric oxide synthase and dimethyl arginine dimethylaminohydrolase in Nomega-nitro-l-arginine methyl ester (l-NAME)-induced hypertension. Cardiovasc. Pathol. 2015, 24, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zweier, J.L. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. USA 1997, 94, 6954–6958. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995, 268, L699–L722. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Garg, S.; Zaneveld, L.J.; Singla, A.K. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, K.J.; Choi, J.S.; Chung, H.Y. Hesperetin: A potent antioxidant against peroxynitrite. Free Radic. Res. 2004, 38, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, W.; Fontaine, V.; Pueyo, M.E.; Laquay, N.; Messika-Zeitoun, D.; Philippe, M.; Arnal, J.F.; Jacob, M.P.; Michel, J.B. Molecular plasticity of vascular wall during n(G)-nitro-l-arginine methyl ester-induced hypertension: Modulation of proinflammatory signals. Hypertension 2000, 36, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Luvara, G.; Pueyo, M.E.; Philippe, M.; Mandet, C.; Savoie, F.; Henrion, D.; Michel, J.B. Chronic blockade of NO synthase activity induces a proinflammatory phenotype in the arterial wall: Prevention by angiotensin II antagonism. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Carrasco, J.L.; Mate, A.; Monserrat, M.T.; Arias, J.L.; Aramburu, O.; Vazquez, C.M. The role of inflammatory markers in the cardioprotective effect of l-carnitine in l-NAME-induced hypertension. Am. J. Hypertens. 2008, 21, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Nyby, M.D.; Abedi, K.; Smutko, V.; Eslami, P.; Tuck, M.L. Vascular Angiotensin type 1 receptor expression is associated with vascular dysfunction, oxidative stress and inflammation in fructose-fed rats. Hypertens. Res. 2007, 30, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Jeong, J.J.; Xu, G.H.; Lee, S.H.; Kang, S.S.; Kim, Y.S.; Chang, K.C.; Kim, H.J. Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells. Int. Immunopharmacol. 2008, 8, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Cai, L.; Hu, C.M.; Zhang, L. Suppression of adjuvant arthritis by hesperidin in rats and its mechanisms. J. Pharm. Pharmacol. 2008, 60, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Belo, V.A.; Guimaraes, D.A.; Castro, M.M. Matrix Metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J. Vasc. Res. 2015, 52, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jin, M.; Yang, F.; Zhu, J.; Xiao, Q.; Zhang, L. Matrix metalloproteinases: Inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediat. Inflamm. 2013, 2013, 928315. [Google Scholar] [CrossRef] [PubMed]
- Del Mauro, J.S.; Prince, P.D.; Donato, M.; Fernandez Machulsky, N.; Moretton, M.A.; Gonzalez, G.E.; Bertera, F.M.; Carranza, A.; Gorzalczany, S.B.; Chiappetta, D.A.; et al. Effects of carvedilol or amlodipine on target organ damage in l-NAME hypertensive rats: Their relationship with blood pressure variability. J. Am. Soc. Hypertens. 2017, 11, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I.; Pechanova, O.; Simko, F. Captopril prevents NO-deficient hypertension and left ventricular hypertrophy without affecting nitric oxide synthase activity in rats. Physiol. Res. 1996, 45, 311–316. [Google Scholar] [PubMed]
- Bernatova, I.; Pechanova, O.; Simko, F. Effect of captopril in l-NAME-induced hypertension on the rat myocardium, aorta, brain and kidney. Exp. Physiol. 1999, 84, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, M.; Kedziora, J.; Bartosz, G. Antioxidant and prooxidant properties of captopril and enalapril. Free Radic. Biol. Med. 1997, 23, 729–735. [Google Scholar] [CrossRef]
- Okada, M.; Kikuzuki, R.; Harada, T.; Hori, Y.; Yamawaki, H.; Hara, Y. Captopril attenuates matrix metalloproteinase-2 and -9 in monocrotaline-induced right ventricular hypertrophy in rats. J. Pharmacol. Sci. 2008, 108, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Maneesai, P.; Bunbupha, S.; Kukongviriyapan, U.; Senggunprai, L.; Kukongviriyapan, V.; Prachaney, P.; Pakdeechote, P. Effect of asiatic acid on the Ang II-AT1R-NADPH oxidase-NF-kappaB pathway in renovascular hypertensive rats. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 1073–1083. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Control | l-NAME | l-NAME + H15 | l-NAME + H30 | l-NAME + Cap |
|---|---|---|---|---|---|
| SP (mmHg) | 120.92 ± 2.27 | 205.88 ± 3.19 * | 179.38 ± 16.51 *,# | 154.07 ± 4.88 *,#,$ | 140.14 ± 7.06 #,$ |
| DP (mmHg) | 72.68 ± 3.31 | 141.65 ± 5.73 * | 114.13 ± 16.57 *,# | 86.89 ± 5.74 *,#,$ | 91.48 ± 7.36 #,$ |
| MAP (mmHg) | 88.76 ± 2.47 | 161.41 ± 4.01 * | 135.88 ± 16.00 *,# | 109.28 ± 5.39 *,#,$ | 107.70 ± 6.27 #,$ |
| HR (beat/min) | 367.86 ± 11.90 | 419.30 ± 11.96 * | 391.93 ± 14.35 | 351.44 ± 13.47 #,$ | 384.28 ± 17.31 |
| Cardiac Mass Indices | ||||||
| Groups | Body Weight (g) | Heart Weight/BW (mg/g) | LVW/BW (mg/g) | |||
| Control | 434 ± 6.8 | 3.14 ± 0.17 | 2.06 ± 0.10 | |||
| l-NAME | 413 ± 16.9 | 4.21 ± 0.26 * | 3.04 ± 0.18 * | |||
| l-NAME + H30 | 406 ± 9.7 | 3.11 ± 0.23 # | 2.23 ± 0.17 # | |||
| l-NAME + Cap | 401 ± 9.7 | 3.12 ± 0.18 # | 2.07 ± 0.12 # | |||
| Left Ventricle | ||||||
| Groups | LV Wall Thickness (mm) | LV CSA (mm2) | LV Fibrosis (%) | |||
| Control | 2.72 ± 0.05 | 57.58 ± 1.05 | 0.69 ± 0.04 | |||
| l-NAME | 3.28 ± 0.04 * | 72.42 ± 0.51 * | 2.72 ± 0.15 * | |||
| l-NAME + H30 | 2.90 ± 0.06 # | 61.12 ± 1.75 # | 0.92 ± 0.09 # | |||
| l-NAME + Cap | 2.79 ± 0.09 # | 59.87 ± 1.63 # | 1.00 ± 0.06 # | |||
| Thoracic Aorta Structural Modifications | ||||||
| Groups | Wall Thickness (µm) | CSA (×103 µm2) | VSMCs (cells/CSA) | Collagen Deposition (% Area Fraction) | ||
| Control | 106.39 ± 1.02 | 579.00 ± 15.16 | 1298.00 ± 73.64 | 15.78 ± 0.70 | ||
| l-NAME | 150.58 ± 2.09 * | 810.50 ± 18.64 * | 2013.71 ± 51.62 * | 31.32 ± 1.00 * | ||
| l-NAME + H30 | 127.11 ± 2.90 *,# | 617.95 ± 18.65 # | 1540.16 ± 46.88 *,# | 24.84 ± 0.69 *,# | ||
| l-NAME + Cap | 129.91 ± 6.50 *,# | 658.38 ± 40.22 # | 1671.78 ± 24.90 *,# | 23.68 ± 0.63 *,# | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maneesai, P.; Bunbupha, S.; Potue, P.; Berkban, T.; Kukongviriyapan, U.; Kukongviriyapan, V.; Prachaney, P.; Pakdeechote, P. Hesperidin Prevents Nitric Oxide Deficiency-Induced Cardiovascular Remodeling in Rats via Suppressing TGF-β1 and MMPs Protein Expression. Nutrients 2018, 10, 1549. https://doi.org/10.3390/nu10101549
Maneesai P, Bunbupha S, Potue P, Berkban T, Kukongviriyapan U, Kukongviriyapan V, Prachaney P, Pakdeechote P. Hesperidin Prevents Nitric Oxide Deficiency-Induced Cardiovascular Remodeling in Rats via Suppressing TGF-β1 and MMPs Protein Expression. Nutrients. 2018; 10(10):1549. https://doi.org/10.3390/nu10101549
Chicago/Turabian StyleManeesai, Putcharawipa, Sarawoot Bunbupha, Prapassorn Potue, Thewarid Berkban, Upa Kukongviriyapan, Veerapol Kukongviriyapan, Parichat Prachaney, and Poungrat Pakdeechote. 2018. "Hesperidin Prevents Nitric Oxide Deficiency-Induced Cardiovascular Remodeling in Rats via Suppressing TGF-β1 and MMPs Protein Expression" Nutrients 10, no. 10: 1549. https://doi.org/10.3390/nu10101549
APA StyleManeesai, P., Bunbupha, S., Potue, P., Berkban, T., Kukongviriyapan, U., Kukongviriyapan, V., Prachaney, P., & Pakdeechote, P. (2018). Hesperidin Prevents Nitric Oxide Deficiency-Induced Cardiovascular Remodeling in Rats via Suppressing TGF-β1 and MMPs Protein Expression. Nutrients, 10(10), 1549. https://doi.org/10.3390/nu10101549