Gluten and Functional Abdominal Pain Disorders in Children
Abstract
:1. Introduction
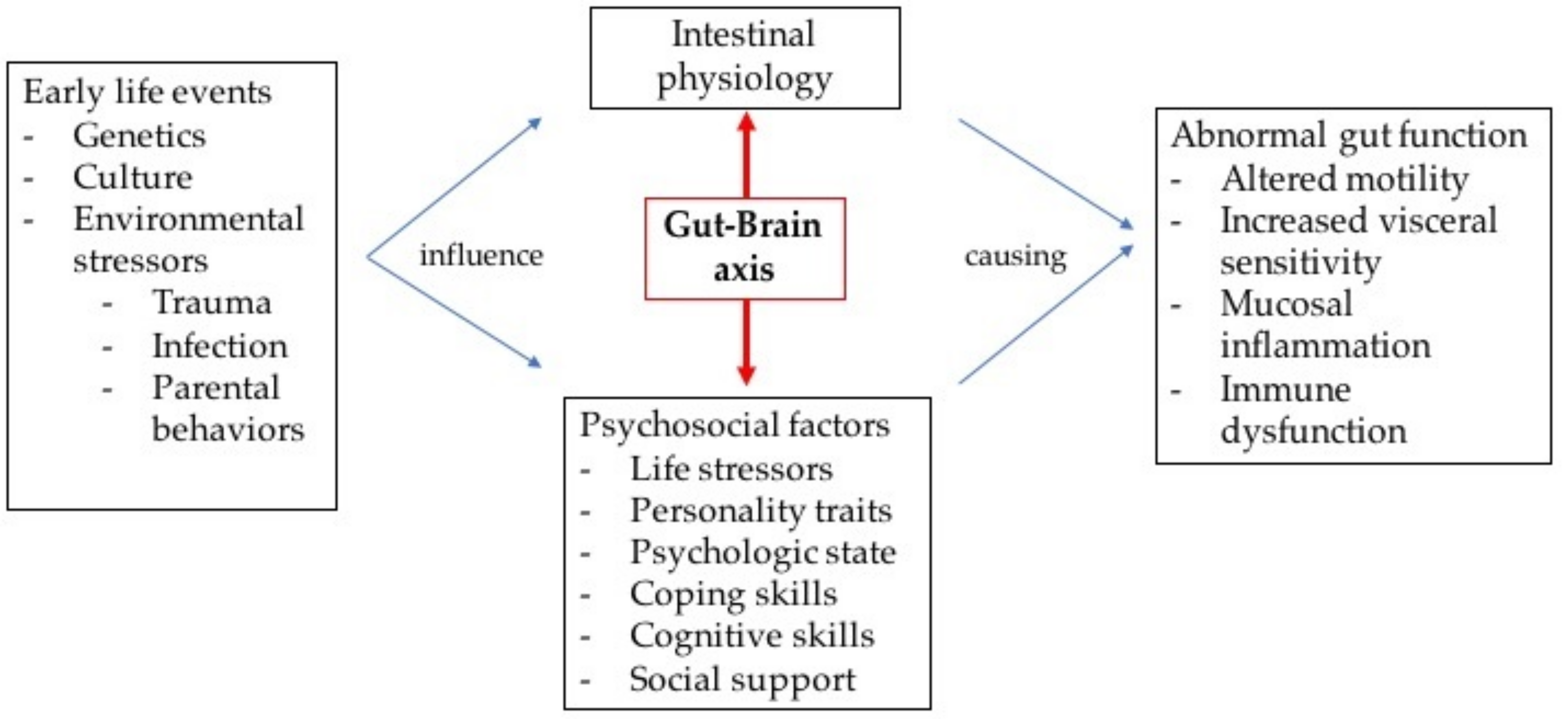
2. Pathogenesis of FGID and FAPD
3. Gluten as a Factor in FAPDs
4. Non-Celiac Gluten Sensitivity and FAPD
5. Celiac Disease and FAPD
6. Evidence to Recommend Gluten Elimination Diet as a Treatment in Patients with FAPD
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Volta, U.; Pinto-Sanchez, M.I.; Boschetti, E.; Caio, G.; De Giorgio, R.; Verdu, E.F. Dietary triggers in irritable bowel syndrome: Is there a role for gluten? J. Neurogastroenterol. Motil. 2016, 22, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.T.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC Med. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac gluten sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Van Tilburg, M.A.; Hyman, P.E.; Walker, L.; Rouster, A.; Palsson, O.S.; Kim, S.M.; Whitehead, W.E. Prevalence of functional gastrointestinal disorders in infants and toddlers. J. Pediatr. 2015, 166, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.L.; Palsson, O.S.; Whitehead, W.E.; van Tilburg, M.A.L. Prevalence of functional gastrointestinal disorders in children and adolescents. J. Pediatr. 2016, 177, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.M.; Benninga, M.A.; Di Lorenzo, C. Epidemiology of childhood constipation: A systematic review. Am. J. Gastroenterol. 2006, 101, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Abkari, A.; Bellaiche, M.; Benninga, M.; Chouraqui, J.P.; Cokura, F.; Hegar, B.; Lifschitz, C.; Ludwig, T.; Miqdady, M.; et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Chogle, A.; Velasco-Benitez, C.A.; Koppen, I.J.; Moreno, J.E.; Ramirez Hernandez, C.R.; Saps, M. A population-based study on the epidemiology of functional gastrointestinal disorders in young children. J. Pediatr. 2016, 179, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Functional disorders: Children and adolescents. Gastroenterology 2016, 150, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Faure, C.; Giguere, L. Functional gastrointestinal disorders and visceral hypersensitivity in children and adolescents suffering from Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, L.A.; Srinath, A.I.; Goyal, A.; Bousvaros, A.; Ducharme, P.; Szigethy, E.; Nurko, S. The overlap of functional abdominal pain in pediatric Crohn’s disease. Inflamm. Bowel Dis. 2013, 19, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Saps, M.; Nichols-Vinueza, D.X.; Rosen, J.M.; Velasco-Benitez, C.A. Prevalence of functional gastrointestinal disorders in Colombian school children. J. Pediatr. 2014, 164, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Van Tilburg, M.A.; Walker, L.S.; Palsson, O.S.; Kim, S.M.; Spiegel, B.M.; Spiller, R.C.; Tack, J.F.; Yang, Y. Prevalence of child/adolescent functional gastrointestinal disorders in a national U.S. community sample. Gastroenterology 2014, 146 (Suppl. 1), S143–S144. [Google Scholar] [CrossRef]
- Hyams, J.S.; Burke, G.; Davis, P.M.; Rzepski, B.; Andrulonis, P.A. Abdominal pain and irritable bowel syndrome in adolescents: A community-based study. J. Pediatr. 1996, 129, 220–226. [Google Scholar] [CrossRef]
- Devanarayana, N.M.; Mettananda, S.; Liyanarachchi, C.; Nanayakkara, N.; Mendis, N.; Perera, N.; Rajindrajith, S. Abdominal pain-predominant functional gastrointestinal diseases in children and adolescents: Prevalence, symptomatology, and association with emotional stress. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Saps, M.; Adams, P.; Bonilla, S.; Chogle, A.; Nichols-Vinueza, D. Parental report of abdominal pain and abdominal pain-related functional gastrointestinal disorders from a community survey. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Devanarayana, N.M.; Adhikari, C.; Pannala, W.; Rajindrajith, S. Prevalence of functional gastrointestinal diseases in a cohort of Sri Lankan adolescents: Comparison between Rome II and Rome III criteria. J. Trop. Pediatr. 2011, 57, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Robin, S.G.; Keller, C.; Zwiener, R.; Hyman, P.E.; Nurko, S.; Saps, M.; Lorenzo, C.D.; Shulman, R.J.; Hyams, J.S.; Palsson, O.; et al. Prevalence of pediatric functional gastrointestinal disorders utilizing the Rome IV criteria. J. Pediatr. 2018, 195, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Saps, M.; Velasco-Benitez, C.A.; Langshaw, A.H.; Ramirez-Hernandez, C.R. Prevalence of functional gastrointestinal disorders in children and adolescents: Comparison between Rome III and Rome IV criteria. J. Pediatr. 2018, 199, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Saps, M.; Moreno-Gomez, J.E.; Ramirez-Hernandez, C.R.; Rosen, J.M.; Velasco-Benitez, C.A. A nationwide study on the prevalence of functional gastrointestinal disorders in school-children. Bol. Med. Hosp. Infant. Mex. 2017, 74, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional Gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Van Oudenhove, L.; Crowell, M.D.; Drossman, D.A.; Halpert, A.D.; Keefer, L.; Lackner, J.M.; Murphy, T.B.; Naliboff, B.D. Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology 2016, 150, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Chogle, A.; Sztainberg, M.; Bass, L.; Youssef, N.N.; Miranda, A.; Nurko, S.; Hyman, P.; Cocjin, J.; Di Lorenzo, C.; Saps, M. Accuracy of pain recall in children. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Gaman, A.; Kuo, B. Neuromodulatory processes of the brain-gut axis. Neuromodulation 2008, 11, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.P.; Dilley, J.B.; Drossman, D.; Crowell, M.D. Brain-gut connections in functional GI disorders: Anatomic and physiologic relationships. Neurogastroenterol. Motil. 2006, 18, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Peripheral mechanisms in irritable bowel syndrome. N. Engl. J. Med. 2012, 367, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Gebhart, G.F. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 1994, 107, 271–293. [Google Scholar] [CrossRef]
- Ohman, L.; Simren, M. Pathogenesis of IBS: Role of inflammation, immunity and neuroimmune interactions. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Daulatzai, M.A. Non-celiac gluten sensitivity triggers gut dysbiosis, neuroinflammation, gut-brain axis dysfunction, and vulnerability for dementia. CNS Neurol. Disord. Drug Targets 2015, 14, 110–131. [Google Scholar] [CrossRef] [PubMed]
- Simren, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Lasch, K.; Zhou, W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am. J. Physiol. Gastrointest Liver Physiol. 2012, 303, G775–G785. [Google Scholar] [CrossRef] [PubMed]
- Matricon, J.; Meleine, M.; Gelot, A.; Piche, T.; Dapoigny, M.; Muller, E.; Ardid, D. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 36, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Ringel, Y.; Maharshak, N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 305, G529–G541. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.A.; Zola, H.; Penttila, I.A.; Blackshaw, L.A.; Andrews, J.M.; Krumbiegel, D. Immune activation in irritable bowel syndrome: Can neuroimmune interactions explain symptoms? Am. J. Gastroenterol. 2013, 108, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Morales, E.E.; Overington, J.; Guerrero-Alba, R.; Ochoa-Cortes, F.; Ibeakanma, C.O.; Spreadbury, I.; Bunnett, N.W.; Beyak, M.; Vanner, S.J. Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: A role for PAR2. Am. J. Gastroenterol. 2013, 108, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Everard, A.; Duparc, T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharmacol. 2013, 13, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Pigrau, M.; Rodino-Janeiro, B.K.; Casado-Bedmar, M.; Lobo, B.; Vicario, M.; Santos, J.; Alonso-Cotoner, C. The joint power of sex and stress to modulate brain-gut-microbiota axis and intestinal barrier homeostasis: Implications for irritable bowel syndrome. Neurogastroenterol. Motil. 2016, 28, 463–486. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. The neurobiology of stress and gastrointestinal disease. Gut 2000, 47, 861–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Simren, M.; Mansson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; James, R.; Smith, H.; Dudley, C.R.; Jewell, D.P. Food intolerance and the irritable bowel syndrome. Gut 1989, 30, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D. The role of food in the functional gastrointestinal disorders: Introduction to a manuscript series. Am. J. Gastroenterol. 2013, 108, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Rajilic-Stojanovic, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; de Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P.; et al. Intestinal microbiota and diet in IBS: Causes, consequences, or epiphenomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halpert, A.; Dalton, C.B.; Palsson, O.; Morris, C.; Hu, Y.; Bangdiwala, S.; Hankins, J.; Norton, N.; Drossman, D. What patients know about irritable bowel syndrome (IBS) and what they would like to know. National Survey on Patient Educational Needs in IBS and development and validation of the Patient Educational Needs Questionnaire (PEQ). Am. J. Gastroenterol. 2007, 102, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Walker, M.M. Celiac Disease and nonceliac gluten or wheat sensitivity: The risks and benefits of diagnosis. JAMA Intern. Med. 2017, 177, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.D.E.; Brienesse, S.C.; Walker, M.M.; Boyle, A.; Talley, N.J. Effect of the gluten-free diet on cardiovascular risk factors in patients with coeliac disease: A systematic review. J. Gastroenterol. Hepatol. 2018, 33, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Mansueto, P.; D’Alcamo, A.; Iacono, G. Non-celiac wheat sensitivity as an allergic condition: Personal experience and narrative review. Am. J. Gastroenterol. 2013, 108, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten causes gastrointestinal symptoms in subjects without celiac disease: A double-blind randomized placebo-controlled trial. Am. J. Gastroenterol. 2011, 106, 508–514. [Google Scholar] [CrossRef] [PubMed]
- van Tilburg, M.A.; Felix, C.T. Diet and functional abdominal pain in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, R.; Andreozzi, P.; Zito, F.P.; Passananti, V.; De Carlo, G.; Sarnelli, G. Irritable bowel syndrome and food interaction. World J. Gastroenterol. 2014, 20, 8837–8845. [Google Scholar] [PubMed]
- De Giorgio, R.; Volta, U.; Gibson, P.R. Sensitivity to wheat, gluten and FODMAPs in IBS: Facts or fiction? Gut 2016, 65, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Verdu, E.F.; Armstrong, D.; Murray, J.A. Between celiac disease and irritable bowel syndrome: The “no man’s land” of gluten sensitivity. Am. J. Gastroenterol. 2009, 104, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- de Punder, K.; Pruimboom, L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Verdu, E.F.; Huang, X.; Natividad, J.; Lu, J.; Blennerhassett, P.A.; David, C.S.; McKay, D.M.; Murry, J.A. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G217–G225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinova, J.; Palova-Jelinkova, L.; Smythies, L.E.; Cerna, M.; Pecharova, B.; Dvorak, M.; Fruhauf, P.; Tlaskalová-hogenová, H.; Smith, P.D.; Tučková, L. Gliadin peptides activate blood monocytes from patients with celiac disease. J. Clin. Immunol. 2007, 27, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Choung, R.S.; Unalp-Arida, A.; Ruhl, C.E.; Brantner, T.L.; Everhart, J.E.; Murray, J.A. Less hidden celiac disease but increased gluten avoidance without a diagnosis in the united states: Findings from the national health and nutrition examination surveys from 2009 to 2014. Mayo Clin. Proc. 2016. [Google Scholar] [CrossRef]
- Cabrera-Chavez, F.; Dezar, G.V.; Islas-Zamorano, A.P.; Espinoza-Alderete, J.G.; Vergara-Jimenez, M.J.; Magana-Ordorica, D.; Ontiveros, N. Prevalence of self-reported gluten sensitivity and adherence to a gluten-free diet in Argentinian adult population. Nutrients 2017, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Chavez, F.; Granda-Restrepo, D.M.; Aramburo-Galvez, J.G.; Franco-Aguilar, A.; Magana-Ordorica, D.; Vergara-Jimenez Mde, J.; Ontiveros, N. Self-reported prevalence of gluten-related disorders and adherence to gluten-free diet in Colombian adult population. Gastroenterol. Res. Pract. 2016, 2016, 4704309. [Google Scholar] [CrossRef] [PubMed]
- van Gils, T.; Nijeboer, P.; IJssennagger, C.E.; Sanders, D.S.; Mulder, C.J.; Bouma, G. Prevalence and characterization of self-reported gluten sensitivity in the Netherlands. Nutrients 2016, 8, 714. [Google Scholar] [CrossRef] [PubMed]
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public Health Nutr. 2015, 18, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; Lopez-Gallardo, J.A.; Vergara-Jimenez, M.J.; Cabrera-Chavez, F. Self-reported prevalence of symptomatic adverse reactions to gluten and adherence to gluten-free diet in an adult Mexican population. Nutrients 2015, 7, 6000–6015. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfield, S.N.; Rugg, N.; Kelsall, A.; Newrick, A.; Sanders, D.S. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur. J. Gastroenterol. Hepatol. 2014, 26, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanpowpong, P.; Ingham, T.R.; Lampshire, P.K.; Kirchberg, F.F.; Epton, M.J.; Crane, J.; Camarhgo, C.A. Coeliac disease and gluten avoidance in New Zealand children. Arch. Dis. Child. 2012, 97, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Giambalvo, O.; Blasca, F.; Iacobucci, R.; D’Alcamo, A.; Mansueto, P. Self-reported non-celiac wheat sensitivity in high school students: Demographic and clinical characteristics. Nutrients 2017, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Nikulina, M.; Habich, C.; Flohe, S.B.; Scott, F.W.; Kolb, H. Wheat gluten causes dendritic cell maturation and chemokine secretion. J. Immunol. 2004, 173, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Picascia, S.; Gianfrani, C. The cross-talk between enterocytes and intraepithelial lymphocytes. Mol. Cell. Pediatr. 2016, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Summary of the NIAID-Sponsored expert panel report. J. Allergy Clin. Immunol. 2010, 126, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Rocken, C.; Brasch, J.; Bethge, J.; Böttner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Rodes, L.; Khan, A.; Paul, A.; Coussa-Charley, M.; Marinescu, D.; Tomaro-Duchesneau, C.; Shao, W.; Kahouli, I.; Prakash, S. Effect of probiotics Lactobacillus and Bifidobacterium on gut-derived lipopolysaccharides and inflammatory cytokines: An in vitro study using a human colonic microbiota model. J. Microbiol. Biotechnol. 2013, 23, 18–26. [Google Scholar] [CrossRef]
- Sapone, A.; Lammers, K.M.; Mazzarella, G.; Mikhailenko, I.; Carteni, M.; Casolaro, V.; Fasano, A. Differential mucosal IL-17 expression in two gliadin-induced disorders: Gluten sensitivity and the autoimmune enteropathy celiac disease. Int. Arch. Allergy Immunol. 2010, 152, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Brottveit, M.; Beitnes, A.C.; Tollefsen, S.; Bratlie, J.E.; Jahnsen, F.L.; Johansen, F.E.; Sollid, L.M.; Lundin, K.E. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am. J. Gastroenterol. 2013, 108, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Zecchi, L.; Barbaro, R.; Cremon, C.; Bellacosa, L.; Marcellini, M.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J. Clin Gastroenterol. 2012, 46, S52–S55. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Cyrany, J.; Kohoutova, D.; Forstl, M.; Rejchrt, S.; Kvetina, J.; Vorisek, V.; Kopacova, M. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 2010, 16, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Giorgetti, G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am. J. Gastroenterol. 2003, 98, 839–843. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, D.V.; Tennyson, C.A.; Green, P.H.; Demmer, R.T. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: Results from the continuous national health and nutrition examination survey 2009–2010. Scand. J. Gastroenterol. 2013, 48, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S. Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabro, A.; Troncone, R.; Corazza, G.R.; Study Group for Non-Celiac Gluten, S. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; Cristofori, F.; Castellaneta, S.; Polloni, C.; Albano, V.; Dellatte, S.; Indrio, F.; Cavallo, L.; Catassi, C. Clinical, serologic, and histologic features of gluten sensitivity in children. J. Pediatr. 2014, 164, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Mastrototaro, L.; Castellaneta, S.; Gentile, A.; Fontana, C.; Tandoi, E.; Dellate, S.; Romagnoli, V.; Catassi, C.; Francavilla, R. Gluten sensitivity in children: Clinical, serological, genetic and histological description of the first paediatric series. Dig. Liver Dis. 2012, 44 (Suppl. 4), S254–S255. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; Dieterich, W.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The salerno experts’ criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bai, J.C.; Bonaz, B.; Bouma, G.; Calabro, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-Celiac gluten sensitivity: The new frontier of gluten related disorders. Nutrients 2013, 5, 3839–3853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, M.M.; Sapone, A.; Catassi, C.; Fasano, A. Celiac Disease and nonceliac gluten sensitivity: A Review. JAMA 2017, 318, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Santolaria Piedrafita, S.; Fernandez Banares, F. Gluten-sensitive enteropathy and functional dyspepsia. Gastroenterol. Hepatol. 2012, 35, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C. Gluten sensitivity. Ann. Nutr. Metab. 2015, 67 (Suppl. 2), 16–26. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Tovoli, F.; De Giorgio, R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol. 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Langshaw, A.H.; Rosen, J.M.; Pensabene, L.; Borrelli, O.; Salvatore, S.; Thapar, N.; Thpar, N.; Concolino, D.; Saps, M. Overlap between functional abdominal pain disorders and organic diseases in children. Rev. Gastroenterol. Mex. 2018, 83, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Chey, W.D.; Talley, N.J.; Malhotra, A.; Spiegel, B.M.; Moayyedi, P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: Systematic review and meta-analysis. Arch. Intern. Med. 2009, 169, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.S.; Carter, M.J.; Hurlstone, D.P.; Pearce, A.; Ward, A.M.; McAlindon, M.E.; Lobo, A.J. Association of adult coeliac disease with irritable bowel syndrome: A case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet 2001, 358, 1504–1508. [Google Scholar] [CrossRef]
- Zuo, X.L.; Li, Y.Q.; Li, W.J.; Guo, Y.T.; Lu, X.F.; Li, J.M.; Desmond, P.V. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin. Exp. Allergy 2007, 37, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, A.; Sanders, D.S.; Ford, A.C. Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: A meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 359–365. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Lomholt-Beck, B.; Gundersen, D. The prevalence of celiac disease in patients with irritable bowel syndrome. Mol. Med. Rep. 2011, 4, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Korkut, E.; Bektas, M.; Oztas, E.; Kurt, M.; Cetinkaya, H.; Ozden, A. The prevalence of celiac disease in patients fulfilling Rome III criteria for irritable bowel syndrome. Eur. J. Intern. Med. 2010, 21, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Jadallah, K.A.; Khader, Y.S. Celiac disease in patients with presumed irritable bowel syndrome: A case-finding study. World J. Gastroenterol. 2009, 15, 5321–5325. [Google Scholar] [CrossRef] [PubMed]
- Pulido, O.; Zarkadas, M.; Dubois, S.; Macisaac, K.; Cantin, I.; La Vieille, S.; Goderfroy, S.; Rashid, M. Clinical features and symptom recovery on a gluten-free diet in Canadian adults with celiac disease. Can. J. Gastroenterol. 2013, 27, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.; Watson, T.; Clearman, B.; Mitros, F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am. J. Clin. Nutr. 2004, 79, 669–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irvine, A.J.; Chey, W.D.; Ford, A.C. Screening for celiac disease in irritable bowel syndrome: An updated systematic review and meta-analysis. Am. J. Gastroenterol. 2017, 112, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Fontana, C.; Magista, A.; Capriati, T.; Indrio, F.; Castellaneta, S.; Cavallo, L.; Francavilla, R. Increased prevalence of celiac disease among pediatric patients with irritable bowel syndrome: A 6-year prospective cohort study. JAMA Pediatr. 2014, 168, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, K.P.; Sherman, P.M.; Ipp, M.; Saunders, N.; Macarthur, C. Screening for celiac disease in children with recurrent abdominal pain. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Treem, W.R.; Justinich, C.J.; Davis, P.; Shoup, M.; Burke, G. Characterization of symptoms in children with recurrent abdominal pain: Resemblance to irritable bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Turco, R.; Boccia, G.; Miele, E.; Giannetti, E.; Buonavolonta, R.; Quitadamo, P.; Auricchio, R.; Staiano, A. The association of coeliac disease in childhood with functional gastrointestinal disorders: A prospective study in patients fulfilling Rome III criteria. Aliment. Pharmacol. Ther. 2011, 34, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Saps, M.; Pensabene, L.; Di Martino, L.; Staiano, A.; Wechsler, J.; Zheng, X.; Di Lorenzo, C. Post-infectious functional gastrointestinal disorders in children. J. Pediatr. 2008, 152, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Pensabene, L.; Talarico, V.; Concolino, D.; Ciliberto, D.; Campanozzi, A.; Gentile, T.; Rutigliano, V.; Salvatore, S.; Staiano, A.; Di Lorenzo, C. Postinfectious functional gastrointestinal disorders in children: A multicenter prospective study. J. Pediatr. 2015, 166, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Saps, M.; Sansotta, N.; Bingham, S.; Magazzu, G.; Grosso, C.; Romano, S.; Pusatcioglu, C.; Guandalini, S. Abdominal pain-associated functional gastrointestinal disorder prevalence in children and adolescents with celiac disease on gluten-free diet: A multinational study. J. Pediatr. 2017, 182, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Saps, M.; Adams, P.; Bonilla, S.; Nichols-Vinueza, D. Abdominal pain and functional gastrointestinal disorders in children with celiac disease. J. Pediatr. 2013, 162, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Cranney, A.; Zarkadas, M.; Graham, I.D.; Butzner, J.D.; Rashid, M.; Warren, R.; Molly, M.; Case, S.; Burrows, V.; Switzer, C. Canadian Celiac Health Survey. Dig. Dis. Sci. 2007, 52, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.M.; Papanicolas, I.N. Impact of symptoms on quality of life before and after diagnosis of coeliac disease: Results from a UK population survey. BMC Health Serv. Res. 2010, 10, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, P.H.R.; Stavropoulos, S.N.; Panagi, S.G.; Goldstein, S.L.; McMahon, D.J.; Absan, H.; Neugut, A.I. Characteristics of adult celiac disease in the USA: Results of a national survey. Am. J. Gastroenterol. 2001, 96, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Wahnschaffe, U.; Schulzke, J.D.; Zeitz, M.; Ullrich, R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2007, 5, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; De Giorgio, R.; Di Stefano, M.; Corazza, G.R. Small amounts of gluten in subjects with suspected nonceliac gluten sensitivity: A randomized, double-blind, placebo-controlled, cross-over trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Zanini, B.; Basche, R.; Ferraresi, A.; Ricci, C.; Lanzarotto, F.; Marullo, M.; Villanacci, V.; Hidalgo, A.; Lanzini, A. Randomised clinical study: Gluten challenge induces symptom recurrence in only a minority of patients who meet clinical criteria for non-coeliac gluten sensitivity. Aliment. Pharmacol. Ther. 2015, 42, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: Results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, L.; Burden, S.T.; Bannerman, E. A dietary survey to determine if patients with coeliac disease are meeting current healthy eating guidelines and how their diet compares to that of the British general population. Eur. J. Clin Nutr. 2008, 62, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Bardella, M.T.; Fredella, C.; Prampolini, L.; Molteni, N.; Giunta, A.M.; Bianchi, P.A. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am. J. Clin. Nutr. 2000, 72, 937–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Asta, C.; Scarlato, A.P.; Galaverna, G.; Brighenti, F.; Pellegrini, N. Dietary exposure to fumonisins and evaluation of nutrient intake in a group of adult celiac patients on a gluten-free diet. Mol. Nutr. Food Res. 2012, 56, 632–640. [Google Scholar] [CrossRef] [PubMed]
|
| Type of FGID | United States n (%) | Colombia n (%) |
|---|---|---|
| Any FGID | 240 (25.00%) | 755 (21.20%) |
| Functional Dyspepsia—Postprandial Distress Syndrome | 69 (7.20%) | 97 (2.70%) |
| Functional Dyspepsia—Epigastric Pain Syndrome | 4 (0.40%) | 11 (0.30%) |
| Irritable Bowel Syndrome | 49 (5.10%) | 83 (2.30%) |
| Abdominal Migraine | 11 (1.10%) | 18 (0.50%) |
| Functional Abdominal Pain—Not Otherwise Specified | 30 (3.10%) | 85 (2.40%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llanos-Chea, A.; Fasano, A. Gluten and Functional Abdominal Pain Disorders in Children. Nutrients 2018, 10, 1491. https://doi.org/10.3390/nu10101491
Llanos-Chea A, Fasano A. Gluten and Functional Abdominal Pain Disorders in Children. Nutrients. 2018; 10(10):1491. https://doi.org/10.3390/nu10101491
Chicago/Turabian StyleLlanos-Chea, Alejandro, and Alessio Fasano. 2018. "Gluten and Functional Abdominal Pain Disorders in Children" Nutrients 10, no. 10: 1491. https://doi.org/10.3390/nu10101491