Regular Intake of a Usual Serving Size of Flavanol-Rich Cocoa Powder Does Not Affect Cardiometabolic Parameters in Stably Treated Patients with Type 2 Diabetes and Hypertension—A Double-Blinded, Randomized, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
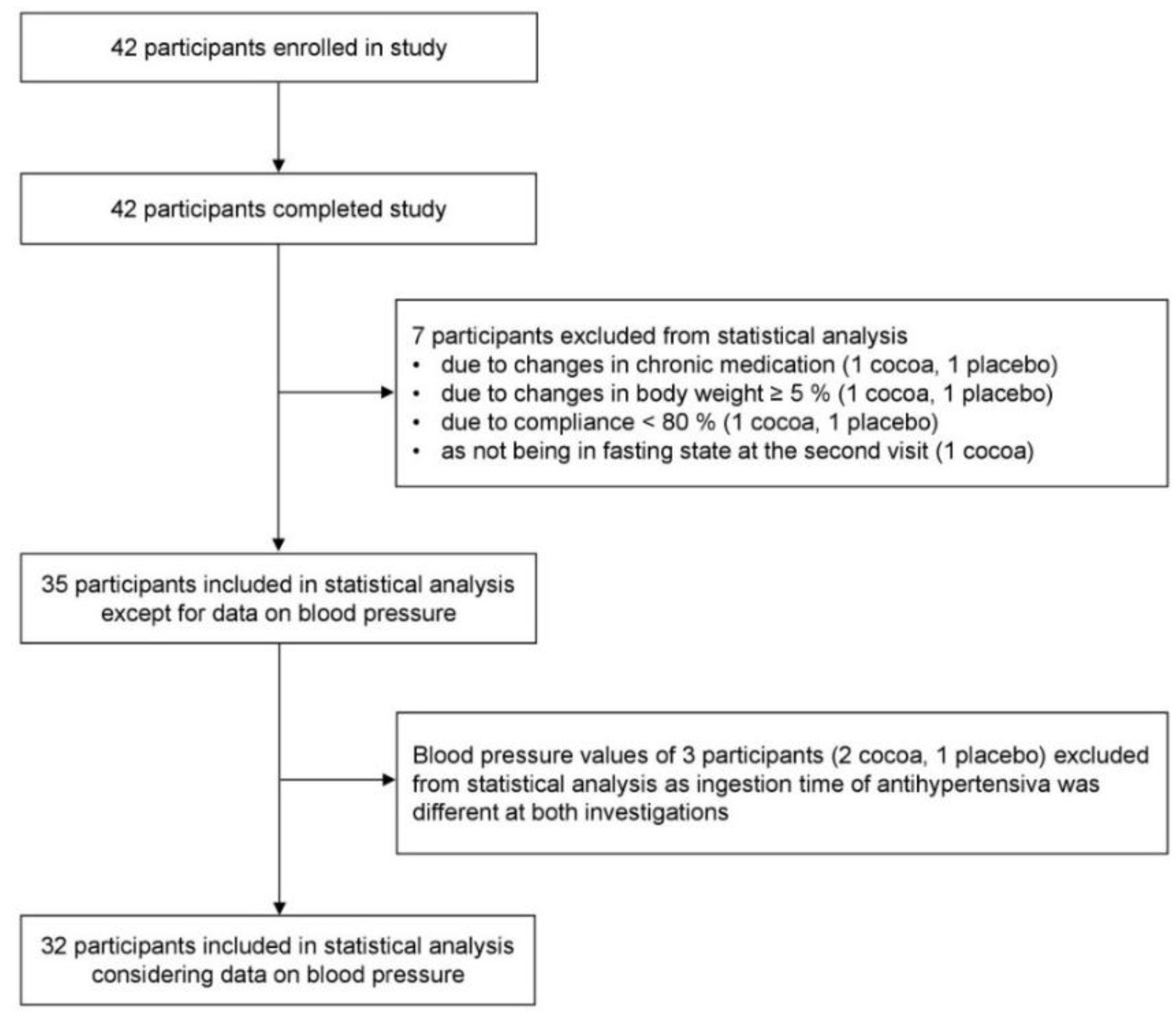
2.2. Participants
2.3. Cocoa Powder
2.4. Blood Pressure Investigation
2.5. Laboratory Investigations
2.6. Anthropometric Investigations
2.7. Food Intake
2.8. Compliance
2.9. Sample Size Calculation
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association (ADA). Standards of medical care in diabetes-2017. Diabetes Care 2017, 40, 1–135. [Google Scholar]
- Turner, R.C.; Millns, H.; Neil, H.A.W.; Stratton, I.M.; Manley, S.E.; Matthews, D.R.; Holman, R.R. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS: 23). BMJ 1998, 316, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Balzer, J.; Rassaf, T.; Heiss, C.; Kleinbongard, P.; Lauer, T.; Merx, M.; Heussen, N.; Gross, H.B.; Keen, C.L.; Schroeter, H.; et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008, 51, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.D.; Sathyapalan, T.; Kilpatrick, E.S.; Beckett, S.; Atkin, S.L. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet. Med. 2010, 27, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Khan, N.; Andres-Lacueva, C.; Casas, R.; Urpí-Sardà, M.; Llorach, R.; Lamuela-Raventós, R.M.; Estruch, R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2009, 90, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Parsaeyan, N.; Mozaffari-Khosravi, H.; Absalan, A.; Mozayan, M.R. Beneficial effects of cocoa on lipid peroxidation and inflammatory markers in type 2 diabetic patients and investigation of probable interactions of cocoa active ingredients with prostaglandin synthase-2 (PTGS-2/COX-2) using virtual analysis. J. Diabetes Metab. Disord. 2014, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Khalili, M.; Haghighat, N.; Eghtesadi, S.; Shidfar, F.; Heidari, I.; Ebrahimpour-Koujan, S.; Eghtesadi, M. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015, 11, 21–29. [Google Scholar] [PubMed]
- Ramos, S.; Martín, M.A.; Goya, L. Effects of cocoa antioxidants in type 2 diabetes mellitus. Antioxidants 2017, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on the modification of the authorisation of a health claim related to cocoa flavanols and maintenance of normal endotheliumdependent vasodilation pursu-ant to Article 13(5) of Regulation (EC) No 1924/2006 following request in accordance with Article 19 of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 1–13. [Google Scholar]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction-a major mediator of diabetic vascular disease. Biochim. Biophys. Acta. 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [PubMed]
- Al-Tabakha, M.M. HPMC capsules: Current status and future prospects. J. Pharm. Pharm. Sci. 2010, 13, 428–442. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO); International Diabetes Federation (IDF). Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO/IDF consultation. In Proceedings of the WHO Document Production Services, Geneva, Switzerland, 2006. [Google Scholar]
- European Society of Hypertension (ESH); European Society of Cardiology (ESC). 2013 ESH/ESC guidelines for the management of arterial hypertension: The task force for the management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef] [PubMed]
- Damm, I.; Enger, E.; Chrubasik-Hausmann, S.; Schieber, A.; Zimmermann, B.F. Fast and comprehensive analysis of secondary metabolites in cocoa products using ultra high-performance liquid chromatography directly after pressurized liquid extraction. J. Sep. Sci. 2016, 39, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Waist Ccrcumference and waist-hip ratio: Report of a WHO expert consultation. 2011. Available online: http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf (accessed on 7 May 2017).
- Kyle, U.G.; Bosaeus, I.; de Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gomez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Kirch, N.; Berk, L.; Liegl, Y.; Adelsbach, M.; Zimmermann, B.F.; Stehle, P.; Stoffel-Wagner, B.; Ludwig, N.; Schieber, A.; Helfrich, H.-P.; et al. A nutritive dose of pure (-)-epicatechin does not beneficially affect increased cardiometabolic risk factors in overweight-to-obese adults-a randomized, placebo-controlled, double-blind crossover study. Am. J. Clin. Nutr. 2018, 107, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Karsegard, L.; Slosman, D.O.; Pichard, C. Single prediction equation for bioelectrical impedance analysis in adults aged 20–94 years. Nutrition 2001, 17, 248–253. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA). Database for the Flavonoid Content of Selected Foods, Release 3.1 (December 2013). 2014. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md/belt-ville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-database-for-the-flavonoid-content-of-selected-foods-release-31-december-2013/ (accessed on 5 September 2016).
- Ott, L. An Introduction to Statistical Methods and Data Analysis; Duxbury Press: North Scituate, MA, USA, 1977. [Google Scholar]
- Ellinger, S.; Reusch, A.; Stehle, P.; Helfrich, H.-P. Epicatechin ingested via cocoa products reduces blood pressure in humans: A nonlinear regression model with a Bayesian approach. Am. J. Clin. Nutr. 2012, 95, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, F.; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet 2003, 362, 1527–1535. [Google Scholar] [PubMed]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Cooper, K.A.; Campos-Gimenez, E.; Jimenez Alvarez, D.; Nagy, K.; Donovan, J.L.; Williamson, G. Rapid reversed phase ultra-performance liquid chromatography analysis of the major cocoa polyphenols and inter-relationships of their concentrations in chocolate. J. Agric. Food Chem. 2007, 55, 2841–2847. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Di Giosia, P.; Barnabei, R.; Allegaert, L.; Bernaert, H.; Ferri, C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J. Hypertens. 2015, 33, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Meissner, K. Placebo responses on cardiovascular, gastrointestinal, and respiratory organ functions. Handb. Exp. Pharmacol. 2014, 225, 183–203. [Google Scholar] [PubMed]
- Escolà-Gil, J.C.; Julve, J.; Griffin, B.A.; Freeman, D.; Blanco-Vaca, F. HDL and lifestyle interventions. Handb. Exp. Pharmacol. 2015, 224, 569–592. [Google Scholar] [PubMed]
- Martin, M.Á.; Goya, L.; Ramos, S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016, 60, 1727–1896. [Google Scholar] [CrossRef] [PubMed]
- Strat, K.M.; Rowley, T.J.; Smithson, A.T.; Tessem, J.S.; Hulver, M.W.; Liu, D.; Davy, B.M.; Davy, K.P.; Neilson, A.P. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J. Nutr. Biochem. 2016, 35, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Miron, A.; Trifan, A.; Luca, V.S.; Costache, I.-I. The cardiovascular effects of cocoa polyphenols -an overview. Diseases 2016, 4, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Kwik-Uribe, C.; Keen, C.L.; Schroeter, H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am. J. Clin. Nutr. 2012, 95, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Borges, G.; Momma, T.Y.; Spencer, J.P.E.; Keen, C.L.; Crozier, A.; Schroeter, H. The metabolome of (2-14C) (-)-epicatechin in humans: Implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci. Rep. 2016, 6, 29034. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Content Per Daily Portion (2.5 g) |
|---|---|
| Manufacturer Analysis | |
| Energy (kJ/kcal) | 38/9 |
| Macronutrients | |
| Protein (g) | 0.6 |
| Fat (g) | 0.4 |
| Carbohydrates (g) | 0.6 |
| Micronutrients | |
| Sodium (mg) | 0.5 |
| Potassium (mg) | 37.5 |
| Calcium (mg) | 11.4 |
| Iron (mg) | 1.1 |
| Phosphorus (mg) | 18.1 |
| Magnesium (mg) | 11.4 |
| Methylxanthines | |
| Caffeine (mg) | 5.0 |
| Theobromine (mg) | 52.5 |
| Flavanols, degree of polymerization 1–10 (mg) | 207.5 |
| Laboratory Analysisa | |
| Flavanols, degree of polymerization (per NP-HPLC) | |
| Monomers (mg) | 49.7 |
| Dimers (mg) | 13.9 |
| Trimers (mg) | 5.5 |
| Tetramers (mg) | 4.7 |
| Pentamers (mg) | 3.1 |
| Individual flavanols (per RP-HPLC) | |
| Epicatechin (mg) | 40.4 |
| Catechin (mg) | 13.6 |
| A-Dimers (mg) | 4.3 |
| Procyanidin B2 (mg) | 12.3 |
| Procyanidin B5 (mg) | 1.3 |
| Procyanidin C1 (mg) | 3.1 |
| Trimers b (mg) | 4.1 |
| Trimers b (mg) | 4.4 |
| Procyanidin D (mg) | 4.1 |
| Cocoa group (n = 17) | Placebo group (n = 18) | p Baseline | |
|---|---|---|---|
| Sex (n; %) | |||
| Female | 10 (58.8) | 7 (38.9) | ns a |
| Male | 7 (41.2) | 11 (61.1) | ns a |
| Age (years) | 65.6 ± 2.6 | 62.8 ± 1.6 | ns c |
| Diabetes duration (years) | 6.7 ± 1.4 | 7.2 ± 1.0 | ns c |
| Antihyperglycemic drugs (n) | |||
| Metformin | 11 | 14 | ns b |
| DPP4 inhibitors | 5 | 3 | ns b |
| SGLT2 inhibitors | 2 | 2 | ns b |
| Antihypertensive drugs (n) | |||
| Beta-receptor blockers | 7 | 7 | ns a |
| AT1 receptor blockers | 3 | 10 | 0.020 a |
| ACE inhibitors | 8 | 9 | ns a |
| Calcium-channel blockers | 6 | 8 | ns a |
| Diuretics | 9 | 10 | ns a |
| Lipid-lowering drugs (n) | |||
| HMG-CoA reductase inhibitors | 6 | 11 | ns a |
| Fibrates | 1 | 0 | ns b |
| Cocoa group (n = 17) | Placebo group (n = 18) | p Baseline | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | p | Baseline | Week 12 | p | ||
| Nutrition status | |||||||
| Body weight (kg) | 89.9 ± 7.0 | 89.4 ± 7.0 | ns c | 91.3 ± 4.6 | 91.3 ± 4.6 | ns c | ns a |
| BMI (kg/m²) # | 30.2 (26.5; 34.7) | 29.8 (26.3; 34.8) | ns c | 29.3 (26.0; 33.8) | 29.5 (26.0; 33.4) | ns c | ns a |
| Waist circumference (cm) | 103.6 ± 4.8 | 102.3 ± 4.6 | 0.047 c | 103.4 ± 2.9 | 103.5 ± 2.9 | ns c | ns a |
| Waist-to-hip ratio | 0.97 ± 0.02 | 0.96 ± 0.02 | 0.011 c | 0.99 ± 0.02 | 0.99 ± 0.02 | ns c | ns a |
| Fat mass (kg) | 34.7 ± 3.8 | 34.2 ± 3.7 | ns c | 33.5 ± 3.0 | 33.5 ± 3.1 | ns c | ns a |
| Fat mass (% BW) | 37.7 ± 1.8 | 37.4 ± 1.8 | ns c | 36.0 ± 1.9 | 36.0 ± 2.0 | ns c | ns a |
| Nutritional intake § | |||||||
| Energy (kcal) | 2132 ± 227 | 2074 ± 153 | ns c | 1859 ± 128 | 2021 ± 149 | ns c | ns a |
| Protein (g) | 91 ± 11 | 85 ± 8 | ns c | 84 ± 6 | 89 ± 7 | ns c | ns a |
| Protein (g/kg BW) | 0.8 (0.7; 1.6) | 0.9 (0.7; 1.2) | ns d | 0.9 (0.8; 1.0) | 1.1 (0.7; 1.3) | ns d | ns b |
| Fat (g) | 98 ± 13 | 91 ± 8 | ns c | 81 ± 6 | 89 ± 9 | ns c | ns a |
| SFAs (g) # | 35 (22; 44) | 36 (20; 45) | ns c | 31 (22; 37) | 30 (25; 45) | ns c | ns a |
| MUFAs (g) | 33 ± 5 | 29 ± 3 | ns c | 28 ± 3 | 31 ± 4 | ns c | ns a |
| PUFAs (g) # | 14 (8; 24) | 15 (11; 32) | ns c | 14 (11; 21) | 13 (9; 24) | ns c | ns a |
| Cholesterol (mg) | 415 (300; 507) | 401 (254; 588) | ns d | 389 (197; 455) | 343 (254; 485) | ns d | ns b |
| Carbohydrates (g) # | 180 (122; 271) | 198 (161; 239) | ns c | 169 (123; 206) | 179 (143; 218) | ns c | ns a |
| Dietary fiber (g) | 22 ± 2 | 24 ± 2 | ns c | 20 ± 2 | 22 ± 2 | ns c | ns a |
| Saccharose (g) | 31 (22; 56) | 34 (29; 52) | ns d | 27 (19; 43) | 33 (24; 28) | ns d | ns b |
| Alcohol (g) | 0.5 (0.0; 8.2) | 0.6 (0.0; 4.3) | ns d | 0.2 (0.0; 7.7) | 0.5 (0.1; 11.6) | ns d | ns b |
| Sodium (mg) $ | 3140 ± 489 | 2944 ± 342 | ns c | 3203 ± 328 | 3270 ± 363 | ns c | ns a |
| Sodium chloride (g) $ | 7 ± 1 | 7 ± 1 | ns c | 7 ± 1 | 7 ± 1 | ns c | ns a |
| Epicatechin (mg) | 1.1 (0.3; 9.3) | 4.8 (0.7; 9.4) | ns d | 4.9 (0.8; 6.5) | 4.5 (0.8; 6.4) | ns d | ns b |
| Cocoa group (n = 17) | Placebo group (n = 18) | P Baseline | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | p | Baseline | Week 12 | p | ||
| Blood pressure | |||||||
| Systolic (mmHg) $ | 139.1 ± 3.2 | 138.5 ± 3.7 | ns c | 141.6 ± 4.2 | 140.4 ± 4.1 | ns c | ns a |
| Diastolic (mmHg) $ | 78.1 ± 2.9 | 78.2 ± 2.4 | ns c | 79.1 ± 1.8 | 78.2 ± 2.6 | ns c | ns a |
| Glucose metabolism | |||||||
| Fasting blood glucose (mmol/l) | 7.6 ± 0.3 | 7.5 ± 0.2 | ns c | 7.6 ± 0.3 | 7.8 ± 0.2 | ns c | ns a |
| HbA1c (mmol/mol) | 46.5 (43.2; 49.7) | 46.5(41.0; 50.8) | ns d | 47.5(44.3; 55.2) | 48.6(43.2; 53.0) | ns d | ns b |
| Insulin (pmol/l) | 99.6 ± 11.0 | 83.1 ± 9.0 | ns c | 89.6 ± 10.1 | 91.8 ± 7.7 | ns c | ns a |
| HOMA-IR | 4.7 ± 0.5 | 3.8 ± 0.4 | ns c | 4.4 ± 0.6 | 4.5 ± 0.4 | ns c | ns a |
| Lipid status | |||||||
| Total cholesterol (mmol/l) | 5.0 ± 0.2 | 4.9 ± 0.2 | ns c | 4.7 ± 0.2 | 4.6 ± 0.2 | ns c | ns a |
| LDL-cholesterol (mmol/l) | 3.0 ± 0.2 | 2.9 ± 0.2 | ns c | 2.8 ± 0.2 | 2.9 ± 0.2 | ns c | ns a |
| HDL-cholesterol (mmol/l) # | 1.3(1.2; 1.5) | 1.4(1.2; 1.8) | ns c | 1.3(1.1; 1.4) | 1.2(1.2; 1.4) | ns c | ns a |
| LDL/HDL cholesterol ratio | 2.3 ± 0.2 | 2.1 ± 0.2 | ns c | 2.3 ± 0.2 | 2.3 ± 0.2 | ns c | ns a |
| Triglycerides (mmol/l) # | 1.3(0.9; 1.9) | 1.4(0.9; 1.8) | ns c | 1.8(1.3; 2.3) | 1.5(1.1; 2.0) | ns c | ns a |
| Creatinine (µmol/l) | 61.0 ± 3.8 | 61.0 ± 3.8 | ns c | 61.0 ± 3.1 | 61.0 ± 3.1 | ns c | ns a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicks, L.; Kirch, N.; Gronwald, D.; Wernken, K.; Zimmermann, B.F.; Helfrich, H.-P.; Ellinger, S. Regular Intake of a Usual Serving Size of Flavanol-Rich Cocoa Powder Does Not Affect Cardiometabolic Parameters in Stably Treated Patients with Type 2 Diabetes and Hypertension—A Double-Blinded, Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 1435. https://doi.org/10.3390/nu10101435
Dicks L, Kirch N, Gronwald D, Wernken K, Zimmermann BF, Helfrich H-P, Ellinger S. Regular Intake of a Usual Serving Size of Flavanol-Rich Cocoa Powder Does Not Affect Cardiometabolic Parameters in Stably Treated Patients with Type 2 Diabetes and Hypertension—A Double-Blinded, Randomized, Placebo-Controlled Trial. Nutrients. 2018; 10(10):1435. https://doi.org/10.3390/nu10101435
Chicago/Turabian StyleDicks, Lisa, Natalie Kirch, Dorothea Gronwald, Kerstin Wernken, Benno F. Zimmermann, Hans-Peter Helfrich, and Sabine Ellinger. 2018. "Regular Intake of a Usual Serving Size of Flavanol-Rich Cocoa Powder Does Not Affect Cardiometabolic Parameters in Stably Treated Patients with Type 2 Diabetes and Hypertension—A Double-Blinded, Randomized, Placebo-Controlled Trial" Nutrients 10, no. 10: 1435. https://doi.org/10.3390/nu10101435
APA StyleDicks, L., Kirch, N., Gronwald, D., Wernken, K., Zimmermann, B. F., Helfrich, H.-P., & Ellinger, S. (2018). Regular Intake of a Usual Serving Size of Flavanol-Rich Cocoa Powder Does Not Affect Cardiometabolic Parameters in Stably Treated Patients with Type 2 Diabetes and Hypertension—A Double-Blinded, Randomized, Placebo-Controlled Trial. Nutrients, 10(10), 1435. https://doi.org/10.3390/nu10101435






