Potential Anticancer Properties of Osthol: A Comprehensive Mechanistic Review
Abstract
:1. Introduction
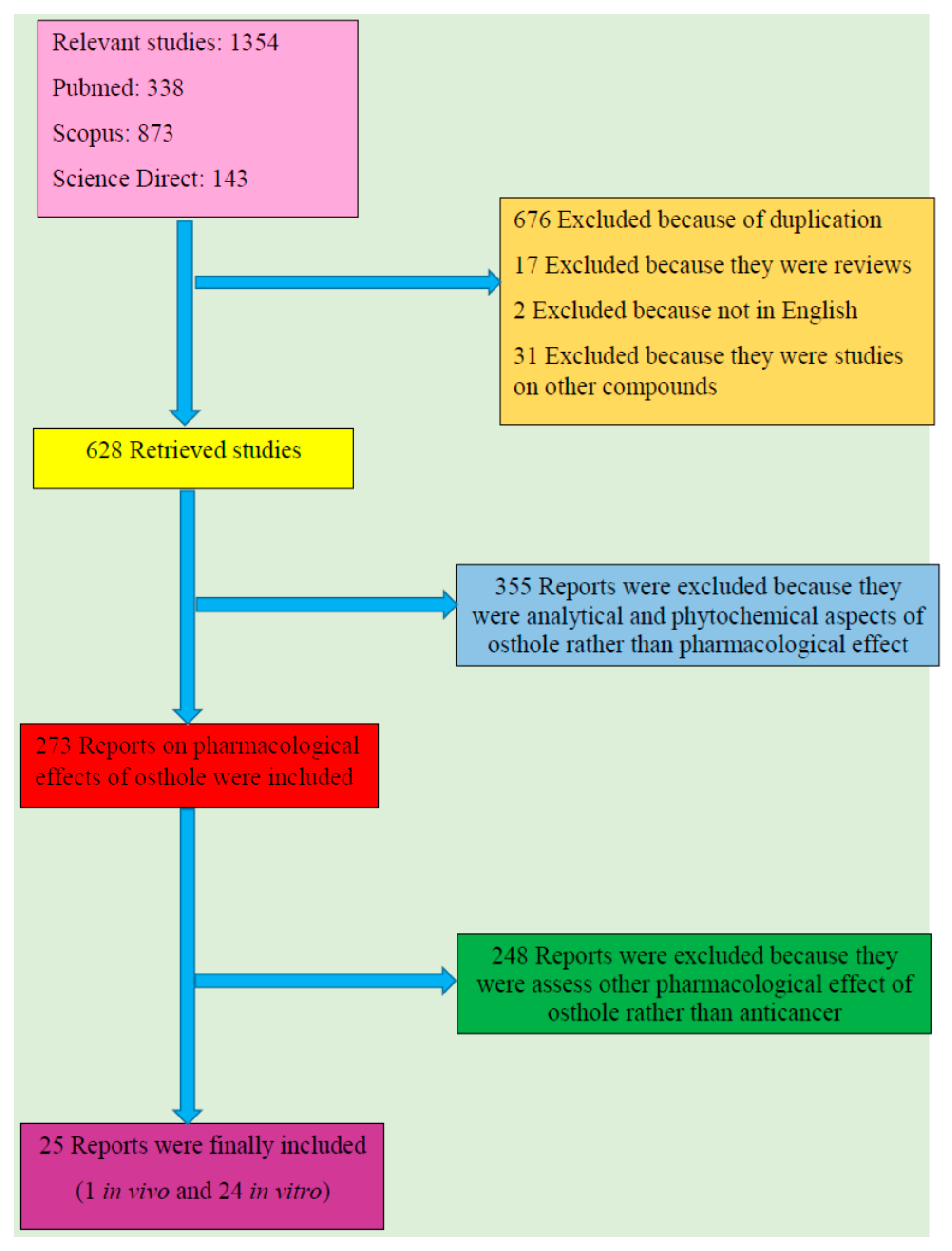
2. Literature Search Methodology
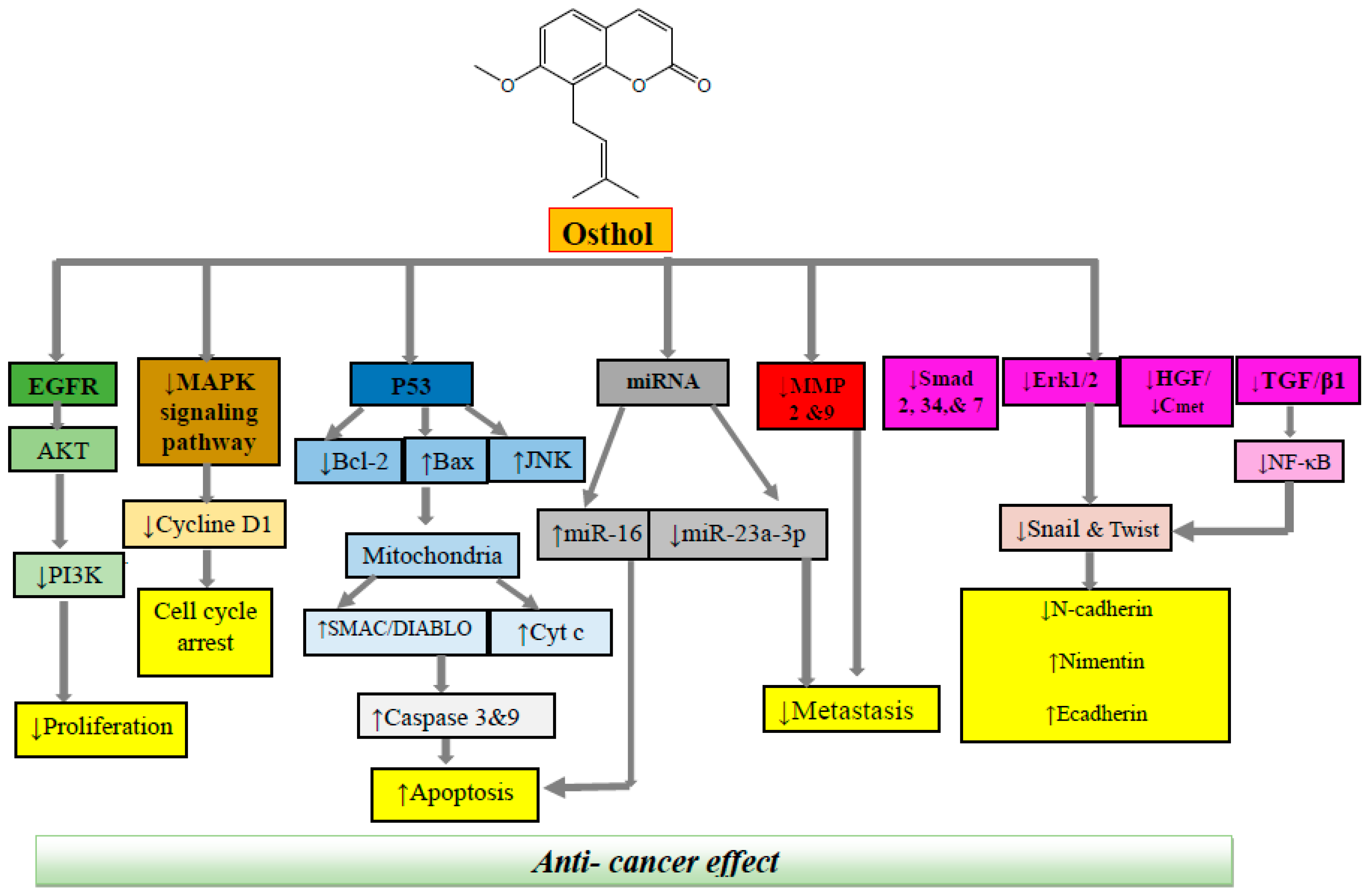
3. Cellular and Molecular Mechanisms of Anticancer Effects of Osthol
3.1. Colon Cancer
3.2. Prostate Cancer
3.3. Breast Cancer
3.4. Brain Cancer
3.5. Lung Cancer
3.6. Leukemia
3.7. Cervical Cancer
3.8. Ovarian Cancer
3.9. Renal Cancer
3.10. Liver Cancer
3.11. Protectice Effect against Toxicity of Chemotherpy
4. Toxicity of Osthol
5. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World-Health-Organization. Cancer: Fact Sheet No. 297. 2015. Available online: http://www.who.int (accessed on 2 October 2016).
- Jena, J.; Ranjan, R.; Ranjan, P.; Sarangi, M.K. A Study on Natural Anticancer Plants. Int. J. Pharm. Chem. Sci. 2012, 1, 365–368. [Google Scholar]
- Padmaja, G.; Vanlalhruaii, C.; Rana, S.; Nandinee, D.; Hariharan, M. Care givers’ depression, anxiety, distress, and somatization as predictors of identical symptoms in cancer patients. J. Cancer Res. Ther. 2016, 12, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Sandoghchian, S.S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar] [PubMed]
- HemaIswarya, S.; Doble, M. Potential synergism of natural products in the treatment of cancer. Phytother. Res. 2006, 20, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Sun, Z.; Chen, X.; Wu, H.; Zhang, X. Inhibitory effects of Zengshengping fractions on DMBA-induced buccal pouch carcinogenesis in hamsters. Chin. Med. J. 2012, 125, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Aliabadi, H.; Aliasgharluo, M.; Fattahi, A.; Mirian, M.; Ghanadian, M. In vitro cytotoxic evaluation of some synthesized COX-2 inhibitor derivatives against a panel of human cancer cell lines. Res. Pharm. Sci. 2013, 8, 298–303. [Google Scholar] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting microtubules by natural agents for cancer therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Basmadjian, C.; Zhao, Q.; Bentouhami, E.; Djehal, A.; Nebigil, C.G.; Johnson, R.A.; Serova, M.; De Gramont, A.; Faivre, S.; Raymond, E.; et al. Cancer wars: Natural products strike back. Front. Chem. 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; De Domenico, S.; Tinelli, A.; Stanca, E.; Del Mercato, L.L.; Giudetti, A.M.; Simeone, P.; Guazzelli, N.; Lessi, M.; Manzini, C.; et al. Anticancer effects of novel resveratrol analogues on human ovarian cancer cells. Mol. BioSyst. 2017, 13, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Derakhshandeh, K.; Jalalizadeh, A.; Mostafaie, A.; Hosseinzadeh, L. Encapsulation in PLGA-PEG enhances 9-nitro-camptothecin cytotoxicity to human ovarian carcinoma cell line through apoptosis pathway. Res. Pharm. Sci. 2015, 10, 161–168. [Google Scholar] [PubMed]
- Newman, D.J.; Giddings, L.-A. Natural products as leads to antitumor drugs. Phytochem. Rev. 2014, 13, 123–137. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Rahimi, R. Phytochemicals as adjunctive with conventional anticancer therapies. Curr. Pharm. Des. 2016, 22, 4201–4218. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Ashley, R.E.; Osheroff, N. Natural products as topoisomerase II poisons: Effects of thymoquinone on DNA cleavage mediated by human topoisomerase IIα. Chem. Res. Toxicol. 2014, 27, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Schafer, G.; Kaschula, C.H. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anti-Cancer Agents Med. Chem. 2014, 14, 233–240. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Hosseinzadeh, L.; Moieni-Arya, M.; Mostafaie, A.; Mohammadi-Motlagh, H.-R. Osthole attenuates doxorubicin-induced apoptosis in PC12 cells through inhibition of mitochondrial dysfunction and ROS production. BioMed Res. Int. 2014, 2014, 156848. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Block, K.I.; Gyllenhaal, C.; Lowe, L.; Amedei, A.; Amin, A.R.; Amin, A.; Aquilano, K.; Arbiser, J.; Arreola, A.; Arzumanyan, A. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin. Cancer Biol. 2015, 35, S276–S304. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.P.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Waksmundzka-Hajnos, M.; Sherma, J.; Kowalska, T. Thin Layer Chromatography in Phytochemistry; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Sajjadi, S.; Zeinvand, H.; Shokoohinia, Y. Isolation and identification of osthol from the fruits and essential oil composition of the leaves of Prangos asperula Boiss. Res. Pharm. Sci. 2009, 4, 19–23. [Google Scholar]
- Sajjadi, S.E.; Shokoohinia, Y.; Hemmati, S. Isolation and identification of furanocoumarins and a phenylpropanoid from the acetone extract and identification of volatile constituents from the essential oil of Peucedanum pastinacifolium. Chem. Nat. Compd. 2012, 48, 668–671. [Google Scholar] [CrossRef]
- Ahmadi, F.; Valadbeigi, S.; Sajjadi, S.; Shokoohinia, Y.; Azizian, H.; Taheripak, G. Grandivittin as a natural minor groove binder extracted from Ferulago macrocarpa to ct-DNA, experimental and in silico analysis. Chem. Biol. Interact. 2016, 258, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.-N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Gheibi, S.; Kiani, A.; Sadrjavadi, K.; Nowroozi, A.; Shahlaei, M. Multi-spectroscopic and molecular modeling investigation of the interactions between prantschimgin and matrix metalloproteinase 9 (MMP9). Luminescence 2015, 31, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Ghannadi, A.; Fattahian, K.; Shokoohinia, Y.; Behbahani, M.; Shahnoush, A. Anti-viral evaluation of sesquiterpene coumarins from Ferula assa-foetida against HSV-1. Iran. J. Pharm. Res. 2014, 13, 523–530. [Google Scholar] [PubMed]
- Sajjadi, S.E.; Eskandarian, A.-A.; Shokoohinia, Y.; Yousefi, H.-A.; Mansourian, M.; Asgarian-Nasab, H.; Mohseni, N. Antileishmanial activity of prenylated coumarins isolated from Ferulago angulata and Prangos asperula. Res. Pharm. Sci. 2016, 11, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.; Almasi, K.; Shokoohinia, Y.; Sadrjavadi, K.; Nowroozi, A.; Shahlaei, M. Combined spectroscopy and molecular modeling studies on the binding of galbanic acid and MMP9. Int. J. Biol. Macromol. 2015, 81, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Sadraei, H.; Shokoohinia, Y.; Sajjadi, S.E.; Mozafari, M. Antispasmodic effects of Prangos ferulacea acetone extract and its main component osthole on ileum contraction. Res. Pharm. Sci. 2013, 8, 137–144. [Google Scholar] [PubMed]
- Ceska, O.; Chaudhary, S.; Warrington, P.; Ashwood-Smith, M. Photoactive furocoumarins in fruits of some umbellifers. Phytochemistry 1986, 26, 165–169. [Google Scholar] [CrossRef]
- Ranjbar, S.; Shokoohinia, Y.; Ghobadi, S.; Bijari, N.; Gholamzadeh, S.; Moradi, N.; Ashrafi-Kooshk, M.R.; Aghaei, A.; Khodarahmi, R. Studies of the interaction between isoimperatorin and human serum albumin by multispectroscopic method: Identification of possible binding site of the compound using esterase activity of the protein. Sci. World J. 2013, 2013, 305081. [Google Scholar] [CrossRef] [PubMed]
- Bijari, N.; Shokoohinia, Y.; Ashrafi-Kooshk, M.R.; Ranjbar, S.; Parvaneh, S.; Moieni-Arya, M.; Khodarahmi, R. Spectroscopic study of interaction between osthole and human serum albumin: Identification of possible binding site of the compound. J. Lumin. 2013, 143, 328–336. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.; Sasano, H.; Chen, S.; Purohit, A. Steroid sulfatase inhibitors: Promising new tools for breast cancer therapy? J. Steroid Biochem. Mol. Biol. 2011, 125, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Jelodarian, Z.; Shokoohinia, Y.; Rashidi, M.; Ghiasvand, N.; Hosseinzadeh, L.; Iranshahi, M. New polyacetylenes from Echinophora cinerea (Boiss.) Hedge et Lamond. Nat. Prod. Res. 2017, 31, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Feng, S.; An, R.; Wang, X. Osthole: A promising lead compound for drug discovery from a traditional Chinese medicine (TCM). Nat. Prod. Commun. 2009, 4, 297–302. [Google Scholar] [PubMed]
- Zhang, Q.; Qin, L.; He, W.; Van Puyvelde, L.; Maes, D.; Adams, A.; Zheng, H.; De Kimpe, N. Coumarins from Cnidium monnieri and their antiosteoporotic activity. Planta Med. 2007, 73, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Lin, T.-Y.; Lu, C.-W.; Huang, W.-J. Osthole and imperatorin, the active constituents of Cnidium monnieri (L.) Cusson, facilitate glutamate release from rat hippocampal nerve terminals. Neurochem. Int. 2008, 53, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Sadraei, H.; Shokoohinia, Y.; Sajjadi, S.; Ghadirian, B. Antispasmodic effect of osthole and Prangos ferulacea extract on rat uterus smooth muscle motility. Res. Pharm. Sci. 2012, 7, 141–149. [Google Scholar] [PubMed]
- Resch, M.; Steigel, A.; Chen, Z.-L.; Bauer, R. 5-Lipoxygenase and cyclooxygenase-1 inhibitory active compounds from Atractylodes lancea. J. Nat. Prod. 1998, 61, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, D.; Zhao, Q.; Ishida, T. The effect of the major components of Fructus Cnidii on osteoblasts in vitro. J. Acupunct. Meridian Stud. 2010, 3, 32–37. [Google Scholar] [CrossRef]
- Huang, R.; Chen, C.; Huang, Y.; Hsieh, D.; Hu, C.; Chang, C. Osthole increases glycosylation of hepatitis B surface antigen and suppresses the secretion of hepatitis B virus in vitro. Hepatology 1996, 24, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Guh, J.-H.; Yu, S.-M.; Ko, F.-N.; Wu, T.-S.; Teng, C.-M. Antiproliferative effect in rat vascular smooth muscle cells by osthole, isolated from Angelica pubescens. Eur. J. Pharmacol. 1996, 298, 191–197. [Google Scholar] [CrossRef]
- Gholamzadeh, S.; Behbahani, M.; Fattahi, A.; Sajjadi, S.; Shokoohinia, Y. Antiviral evaluation of coumarins from Prangos ferulacea L. (Lindl). Res. Pharm. Sci. 2012, 7, S783. [Google Scholar]
- Kermani, E.-K.; Sajjadi, S.-E.; Hejazi, S.-H.; Arjmand, R.; Saberi, S.; Eskandarian, A.-A. Anti-Leishmania activity of osthole. Pharmacog. Res. 2016, 8 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.; Tsai, C.-F.; Chen, D.-R.; Wang, M.-Y.; Yeh, W.-L. p53 is a key regulator for osthole-triggered cancer pathogenesis. BioMed Res. Int. 2014, 2014, 175247. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Hosseinzadeh, L.; Alipour, M.; Mostafaie, A.; Mohammadi-Motlagh, H.-R. Comparative evaluation of cytotoxic and apoptogenic effects of several coumarins on human cancer cell lines: Osthole induces apoptosis in p53-deficient H1299 cells. Adv. Pharmacol. Sci. 2014, 2014, 847574. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-C.; Lee, W.-J.; Tan, P.; Yang, S.-F.; Hsiao, M.; Lee, L.-M.; Chien, M.-H. By inhibiting snail signaling and miR-23a-3p, osthole suppresses the EMT-mediated metastatic ability in prostate cancer. Oncotarget 2015, 6, 21120–21136. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Han, X.; Guo, B.; Sun, Z.; Liu, S. Combination treatment with platycodin D and osthole inhibits cell proliferation and invasion in mammary carcinoma cell lines. Environ. Toxicol. Pharmacol. 2013, 36, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Kuo, D.-H.; Chou, C.-H.; Su, Y.-C.; Ho, C.-T.; Way, T.-D. Osthole suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition via repression of the c-Met/Akt/mTOR pathway in human breast cancer cells. J. Agric. Food Chem. 2011, 59, 9683–9690. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.C.-H.; Chou, C.-H.; Lin, Y.-C.; Lin, J.-N.; Yu, C.-C.; Tang, C.-H.; Lin, H.-Y.; Way, T.-D. Osthole suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt/mTOR pathway. J. Agric. Food Chem. 2010, 58, 4786–4793. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, Z.; Guo, B.; Ye, Y.; Han, X.; Qin, Y.; Liu, S. Osthole inhibits bone metastasis of breast cancer. Oncotarget 2017, 8, 58480–58493. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Gao, Z.; Shang, B.; Sui, S.; Fu, Q. Osthole suppresses the proliferation and accelerates the apoptosis of human glioma cells via the upregulation of microRNA-16 and downregulation of MMP-9. Mol. Med. Rep. 2015, 12, 4592–4597. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Wei, S.; Song, Y.; Li, L.; Du, G.; Zhan, H.; Cao, Y. Osthole exhibits anti-cancer property in rat glioma cells through inhibiting PI3K/Akt and MAPK signaling pathways. Cell. Physiol. Biochem. 2013, 32, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Lin, J.-C.; Hung, C.-M.; Chen, Y.; Liu, L.-C.; Chang, T.-C.; Kao, J.-Y.; Ho, C.-T.; Way, T.-D. Osthole inhibits insulin-like growth factor-1-induced epithelial to mesenchymal transition via the inhibition of PI3K/Akt signaling pathway in human brain cancer cells. J. Agric. Food Chem. 2014, 62, 5061–5071. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Zhang, Y.; Qu, D.; Feng, X.-W.; Chen, Y.; Zhao, L. Osthole suppresses migration and invasion of A549 human lung cancer cells through inhibition of matrix metalloproteinase-2 and matrix metallopeptidase-9 in vitro. Mol. Med. Rep. 2012, 6, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Lu, J.-J.; Wang, Y.; Pei, L.; Chen, X. Osthole inhibited TGF β-induced epithelial–mesenchymal transition (EMT) by suppressing NF-κB mediated Snail activation in lung cancer A549 cells. Cell Adhes. Migr. 2017, 11, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jia, X.-H.; Chen, J.-R.; Wang, J.-Y.; Li, Y.-J. Osthole shows the potential to overcome P-glycoprotein-mediated multidrug resistance in human myelogenous leukemia K562/ADM cells by inhibiting the PI3K/Akt signaling pathway. Oncol. Rep. 2016, 35, 3659–3668. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.Y.; Hsu, C.S.; Wang, K.T.; Wang, M.C.; Wang, C.C. Antitumor effects of Osthol from Cnidium monnieri: An in vitro and in vivo study. Phytother. Res. 2007, 21, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Liu, J.; Ren, B.; Tang, Y.; Owusu, L.; Li, M.; Zhang, J.; Liu, L.; Li, W. Anti-tumor effects of osthole on ovarian cancer cells in vitro. J. Ethnopharmacol. 2016, 193, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Min, K.-J.; Han, M.; Kim, S.; Park, J.-W.; Kwon, T.K. Osthole enhances TRAIL-mediated apoptosis through downregulation of c-FLIP expression in renal carcinoma Caki cells. Oncol. Rep. 2017, 37, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-K.; Liu, J.; Jiang, G.-Q.; Tan, G.; Gong, P.; Luo, H.-F.; Li, H.-M.; Du, J.; Ning, Z.; Xin, Y. Osthole inhibits the tumorigenesis of hepatocellular carcinoma cells. Oncol. Rep. 2017, 37, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Friend, S. p53: A glimpse at the puppet behind the shadow play. Science 1994, 265, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, L.R.; White, A.; Sprouse, J.; Livanos, E.; Jacks, T.; Tlsty, T.D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell 1992, 70, 923–935. [Google Scholar] [CrossRef]
- Hosseinzadeh, L.; Behravan, J.; Mosaffa, F.; Bahrami, G.; Bahrami, A.R.; Karimi, G. Effect of curcumin on doxorubicin-induced cytotoxicity in H9c2 cardiomyoblast cells. Iran. J. Basic Med. Sci. 2011, 14, 49–56. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.D.; Xavier, J.M.; Steer, C.J.; Rodrigues, C.M. The role of p53 in apoptosis. Discov. Med. 2010, 9, 145–152. [Google Scholar] [PubMed]
- Mao, H.L.; Liu, P.S.; Zheng, J.F.; hai Zhang, P.; Zhou, L.G.; Xin, G.; Liu, C. Transfection of Smac/DIABLO sensitizes drug-resistant tumor cells to TRAIL or paclitaxel-induced apoptosis in vitro. Pharmacol. Res. 2007, 56, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Panza, E.; Armogida, C.; Ercolano, G.; Taglialatela-Scafati, O.; Shokoohinia, Y.; Camerlingo, R.; Pirozzi, G.; Calderone, V.; Cirino, G.; et al. The hydrogen sulfide releasing molecule acetyl deacylasadisulfide inhibits metastatic melanoma. Front. Pharmacol. 2017, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-C.; Wang, J.; Guo, C.-C.; Sai, K.; Wang, J.; Chen, F.-R.; Yang, Q.-Y.; Chen, Y.-S.; Wang, J.; To, T.S.-S.; et al. MiR-181b sensitizes glioma cells to teniposide by targeting MDM2. BMC Cancer 2014, 14, 611. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Shokohinia, Y.; Kiani, A.; Sadrjavadi, K.; Nowroozi, A.; Shahlaei, M. Molecular insight into the Grandivitin-matrix metalloproteinase 9 interactions. J. Photochem. Photobiol. B 2016, 162, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Tatevossian, R.G.; Lawson, A.R.; Forshew, T.; Hindley, G.F.; Ellison, D.W.; Sheer, D. MAPK pathway activation and the origins of pediatric low-grade astrocytomas. J. Cell. Physiol. 2010, 222, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Balmanno, K.; Cook, S. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009, 16, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Manning, B.D.; Cantley, L.C. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell 2003, 4, 257–262. [Google Scholar] [CrossRef]
- Buckner, J.C. Factors influencing survival in high-grade gliomas. In Seminars in Oncology; WB Saunders: Philadelphia, PA, USA, 2003; Volume 30, pp. 10–14. [Google Scholar]
- Chen, Y.-H.; Hung, M.-C.; Shyu, W.-C. Role of cancer stem cells in brain tumors. Biomedicine 2012, 2, 84–91. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, T.; Zhang, J.; Mao, Q.; Li, S.; Xiong, W.; Qiu, Y.; Xie, Q.; Ge, J. Notch1 promotes glioma cell migration and invasion by stimulating β-catenin and NF-κB signaling via AKT activation. Cancer Sci. 2012, 103, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Qu, D.; Jiang, T.; Li, S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2011, 30, 33. [Google Scholar] [CrossRef] [PubMed]
- Mathisen, M.S.; Kantarjian, H.M.; Cortes, J.; Jabbour, E. Mutant BCR-ABL clones in chronic myeloid leukemia. Haematologica 2011, 96, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.S.; Vasconcelos, F.C.; De Souza Reis, F.R.; De Moraes, G.N.; Maia, R.C. P-glycoprotein and survivin simultaneously regulate vincristine-induced apoptosis in chronic myeloid leukemia cells. Int. J. Oncol. 2011, 39, 925–933. [Google Scholar] [PubMed]
- Echelman, D.; Feldman, S. Management of cervical precancers: A global perspective. Hematol. Oncol. Clin. N. Am. 2012, 26, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Chen, H.; Wei, D.M.; Fang, F.; Liu, G.J.; Xie, H.Y.; Wang, X.; Zou, J.; Han, X.; Feng, D. Maintenance chemotherapy for ovarian cancer. Curr. Oncol. Rep. 2013, 5, 454–458. [Google Scholar]
- Park, J.T.; Chen, X.; Trope, C.G.; Davidson, B.; Shih, I.-M.; Wang, T.-L. Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am. J. Pathol. 2010, 177, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Grahovac, J.; Wheeler, S.; Ma, B.; Lauffenburger, D. Targeting tumor cell motility as a strategy against invasion and metastasis. Trends Pharmacol. Sci. 2013, 34, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Shokoohinia, Y.; Javaheri, S.; Azizian, H. Proposed binding mechanism of galbanic acid extracted from Ferula assa–foetida to DNA. J. Photochem. Photobiol. B 2017, 166, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Khajouei, S.; Ahmadi, F.; Ghiasvand, N.; Hosseinzadeh, L. Protective effect of bioactive compounds from Echinophora cinerea against cisplatin-induced oxidative stress and apoptosis in the PC12 cell line. Iran. J. Basic Med. Sci. 2017, 20, 438–445. [Google Scholar] [PubMed]
- Shokoohinia, Y.; Bazargan, S.; Miraghaee, S.; Javadirad, E.; Hosseinzadeh, L. Safety assessment of osthole isolated from Prangos ferulacea: Acute and subchronic toxicities and modulation of cytochrome P450. Jundishapur J. Nat. Pharm. Prod. 2017. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Peiris-Pagès, M.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Metastasis and oxidative stress: Are antioxidants a metabolic driver of progression? Cell Metabol. 2015, 22, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liaison in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.K.; Melendez, J.A. Mitochondrial redox control of matrix metallopro-teinases. Free Radic. Biol. Med. 2004, 37, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef] [PubMed]
| Type of Cancer | Conc. or Dose | Cancer Model Used | Anticancer Effects and Mechanisms | Reference |
|---|---|---|---|---|
| Colon | 1, 3 & 10 mM | In vitro (HCT116 & SW480 cells) | ↓Cell motility; ↑apoptosis; ↑phosphorylation of p53 on Ser15 (p-p53); ↑acetylation of p53; ↑ROS; ↑JNK | [48] |
| Prostate | 100 mM | In vitro (PC3 cells) | ↑Apoptosis; ↓Bcl-; ↑Bax; ↑Smac/DIABLO | [49] |
| Prostate | 20~80 μM | In vitro (AIPC, DU145 & PC3 cells) | ↓TGF-β, ↓Akt, JNK& ERK ↓miR-23a-3p | [50] |
| Breast | 15 mM | In vitro (MDA-MB-231 & 4T1) | ↓TbetaRII; ↓Smad2; ↓Smad3; ↓Smad4 | [51] |
| Breast | 20 mM | In vitro (MCF-7, MDA-MB-453, MDA-MB-231 & BT-20 cells) | ↓c-Met signaling; ↓FASN; ↓HGF- induced EMT; ↓c-Met protein levels; ↓cell migration; ↓invasion; ↓c-Met/Akt/mTOR | [52] |
| Breast | 5, 10, 24, 40 & 80 mM | In vitro (MDA-MB-231, MCF-7, HBL-100 & HER2-overexpressing human cancer cell lines) | ↓proliferation; ↑apoptosis; ↓FASN; ↓Akt; ↓mTOR; ↑paclitaxel-induced cytotoxicity | [53] |
| Breast | 5.25 mg/kg | In vivo (Mice treated orally twice weekly) | ↑IL-8; ↑M-CSF; ↑PTHrP; ↓OPG/RANKL | [54] |
| Breast | 20–90 mM | In vitro (MDA-231BO cells) | ↓Cell viability; ↓proliferation; ↑apoptosis; ↓TGF-β/Smads | [54] |
| Brain | 50, 100 & 200 mM | In vitro (U87 cells) | ↓proliferation; ↑apoptosis; ↑miR16; ↓MMP9 | [55] |
| Brain | 25, 50 & 100 mM | In vitro (Rat glioma cells) | ↓Proliferation; ↓PI3K/Akt/MAPK | [56] |
| Brain | 10–100 mM | In vitro (GBM8401 cells) | ↓EMT; ↓Akt and GSK3β; ↓Snail; ↓Twist; ↓I3K/Akt | [57] |
| Brain | 100 mM | In vitro (SKNMC cells) | ↑Apoptosis by ↑Bcl; ↑Bax; ↑Smac/DIABLO | [49] |
| Lung | 50, 100 & 150 mM | In vitro (A549 cells) | ↑G2/M arrest; ↑apoptosis; ↓Cyclin B1; ↓p-Cdc2; ↓Bcl-2; ↑Bax, ↓PI3K/Akt signaling pathway | [58] |
| Lung | 20, 40, 60 mM 80 mM | In vitro (A549 cells) | ↓MMP-2; ↓MMP-9 | [58] |
| Lung | 5–20 mM | In vitro (A549 cells) | ↓NF-κB mediated snail activation; ↓invasion; ↓migration; ↓adhesion | [59] |
| Lung | 100 mM | In vitro (H1299 cells) | ↑Apoptosis; ↓Bcl; ↑Bax; ↑Smac/DIABLO | [49] |
| Leukemia | 5 mM 15 mM | In vitro (K562/ADM cells) | ↓MDR in myelogenous leukemia | [60] |
| Leukemia | 30 mg/kg for 8 days | In vivo (CDF1 female mice transplanted with P-388 D1 cells) | ↑Apoptosis; ↓P-388 D1 cells | [61] |
| Cervix | 77.96 mM 64.94 mM | In vitro (HeLa cells) | ↑Apoptosis | [61] |
| Ovary | 20, 40, 80, 120, 160 and 200 | In vitro (A2780 & OV2008 cells) | ↓Cells proliferation; ↑apoptosis | [62] |
| Ovary | 5, 10, 24, 40 mM 80 mM | In vitro (SKOV3 human cancer cells) | ↓FASN; ↓proliferation; ↑apoptosis; ↓Akt; ↓mTOR; ↑paclitaxel-induced cytotoxicity | [53] |
| Renal | 20–30 mM | In vitro (Caki & U251MG cells) | ↑Apoptosis; ↓MMP level; ↑cytochrome c; ↓c-FLIP | [63] |
| Liver | 20, 40, 80, 120, 160 or 200 mM | In vitro (SMCC-7721, MHCC-97H, HCC-LM3 & BEL-7402 cells) | ↓Proliferation; ↑DNA damage; ↓migration; ↓Cdc2; ↓cyclin B1; ↑ERCC1 | [64] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokoohinia, Y.; Jafari, F.; Mohammadi, Z.; Bazvandi, L.; Hosseinzadeh, L.; Chow, N.; Bhattacharyya, P.; Farzaei, M.H.; Farooqi, A.A.; Nabavi, S.M.; et al. Potential Anticancer Properties of Osthol: A Comprehensive Mechanistic Review. Nutrients 2018, 10, 36. https://doi.org/10.3390/nu10010036
Shokoohinia Y, Jafari F, Mohammadi Z, Bazvandi L, Hosseinzadeh L, Chow N, Bhattacharyya P, Farzaei MH, Farooqi AA, Nabavi SM, et al. Potential Anticancer Properties of Osthol: A Comprehensive Mechanistic Review. Nutrients. 2018; 10(1):36. https://doi.org/10.3390/nu10010036
Chicago/Turabian StyleShokoohinia, Yalda, Fataneh Jafari, Zeynab Mohammadi, Leili Bazvandi, Leila Hosseinzadeh, Nicholas Chow, Piyali Bhattacharyya, Mohammad Hosein Farzaei, Ammad Ahmad Farooqi, Seyed Mohammad Nabavi, and et al. 2018. "Potential Anticancer Properties of Osthol: A Comprehensive Mechanistic Review" Nutrients 10, no. 1: 36. https://doi.org/10.3390/nu10010036
APA StyleShokoohinia, Y., Jafari, F., Mohammadi, Z., Bazvandi, L., Hosseinzadeh, L., Chow, N., Bhattacharyya, P., Farzaei, M. H., Farooqi, A. A., Nabavi, S. M., Yerer, M. B., & Bishayee, A. (2018). Potential Anticancer Properties of Osthol: A Comprehensive Mechanistic Review. Nutrients, 10(1), 36. https://doi.org/10.3390/nu10010036







