Predictive Benthic Habitat Mapping Reveals Significant Loss of Zostera marina in the Puck Lagoon, Baltic Sea, over Six Decades
Highlights
- Binary machine learning classification models (Random Forest, Support Vector Machine, and K-Nearest Neighbors) achieved 93.3% accuracy for Zostera marina presence/absence detection, while substrate-level classification (EUNIS Level 3) reached 86.7% accuracy; however, fine-scale habitat classifications (EUNIS Level 4/5) achieved only 43–62% accuracy due to severe class imbalance in training data, demonstrating that classification performance is fundamentally constrained by data representation rather than algorithmic complexity.
- Object-based image analysis (OBIA) combined with Boruta feature selection identified geomorphometric variables (slope, aspect, and terrain ruggedness index) and optical features (airborne LiDAR intensity, and spectral bands) as the most significant discriminators for benthic habitat classification; ALB intensity, MBES backscatter, and DEM proved critical for substrate characterization, while geometric descriptors (roundness and compactness) enhanced finer-scale habitat discrimination.
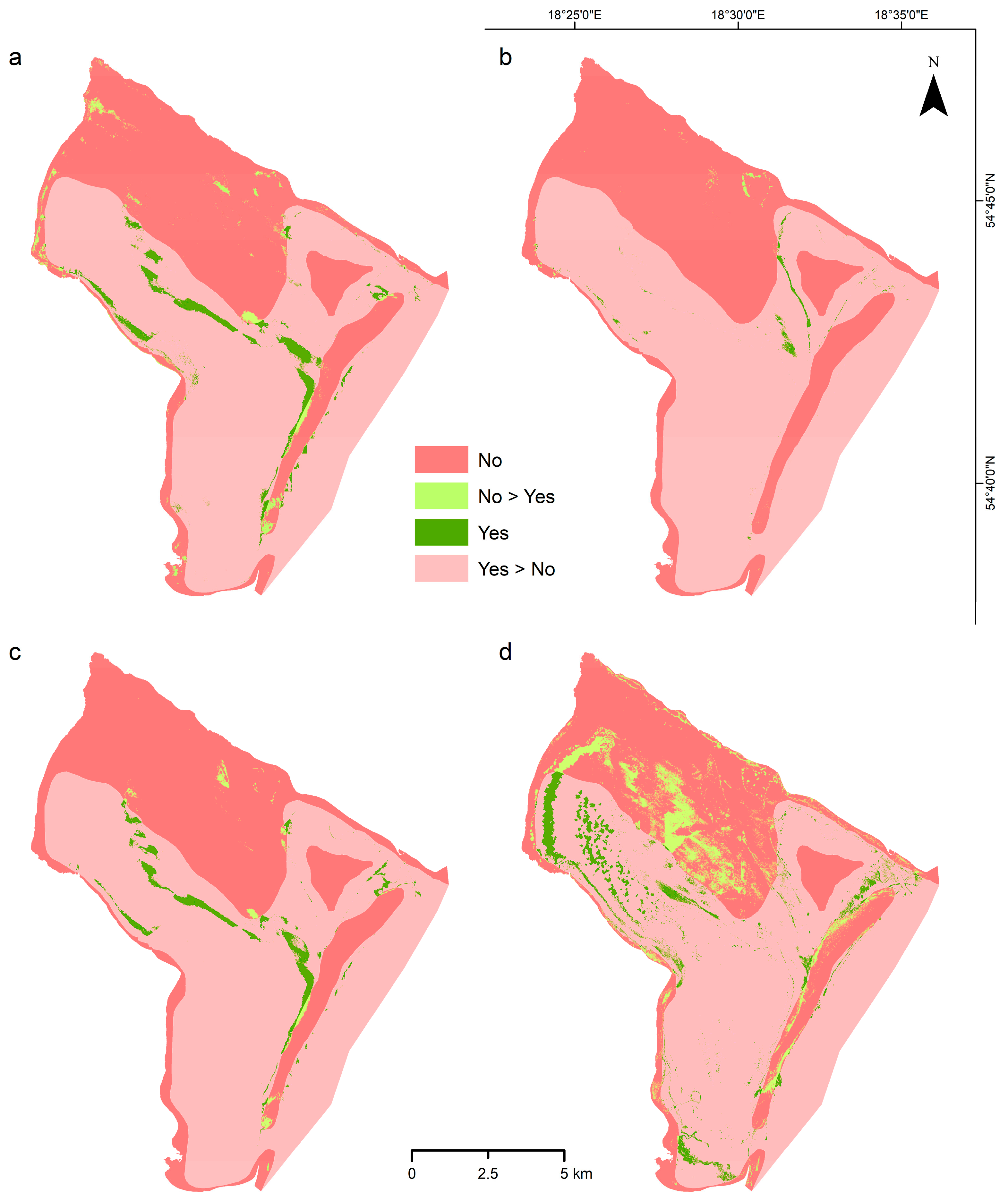
- Change detection analysis revealed catastrophic Zostera marina habitat loss in the Puck Lagoon of 84–99% over the 66-year period (1957–2023), with seagrass coverage declining from 61.15% of the study area to just 9.70% or 0.63% depending on the classification model; even accounting for seasonal phenological mismatch, corrected estimates indicate minimum 69% net loss, confirming severe ecosystem degradation and emphasizing the imperative for immediate conservation and restoration action at the landscape scale.
- Future operational benthic habitat mapping programs must prioritize balanced sampling design with a minimum of 50–80 samples per rare habitat type, multi-temporal ground-truth campaigns rather than single-season surveys, and dynamic oceanographic predictors (temperature, light, and nutrients) to advance beyond the current capability limitations; this study establishes critical baselines and provides a reproducible methodology framework for analogous habitat monitoring in shallow nearshore environments globally.
Abstract
1. Introduction
- Which are the most significant environmental predictors and derived features for benthic habitat classification, as determined by the Boruta [20] feature selection algorithm, and how does their significance vary across classification scenarios?
- What are the capabilities and limitations of object-based image analysis (OBIA) for benthic habitat mapping in the Puck Lagoon when applied to heterogeneous remote sensing datasets, and how do object-based geometric features contribute to habitat classification accuracy?
- How have the area and distribution of Zostera marina meadows in the Puck Lagoon changed since 1957, and what are the patterns of habitat loss, gain, and spatial redistribution exhibited by change detection analysis?
- How do class imbalance and sampling strategy influence benthic habitat classification accuracy, and how do temporal sampling strategies rank compared to spatial sampling density for monitoring certain types of habitats?
- What are the ecosystem implications of the observed distributional shifts in Zostera marina on Baltic Sea ecosystem function, and what are the management actions necessary to reduce habitat loss?
Related Work
2. Materials and Methods
2.1. Study Area
2.1.1. Geographic Setting and Geological Context
2.1.2. Hydrodynamic Environment
2.2. Data Acquisition and Processing
2.2.1. Remote Sensing Data Collection
2.2.2. Data Processing and Integration
2.3. Ground-Truth Data Collection
2.3.1. Field Sampling Strategy
2.3.2. Habitat Classification Scheme
2.4. Object-Based Image Analysis (OBIA) and Machine Learning Classification
2.4.1. Segmentation of Remote Sensing Datasets and Extraction of Object-Based Features
2.4.2. Feature Selection
2.4.3. Machine Learning Classification
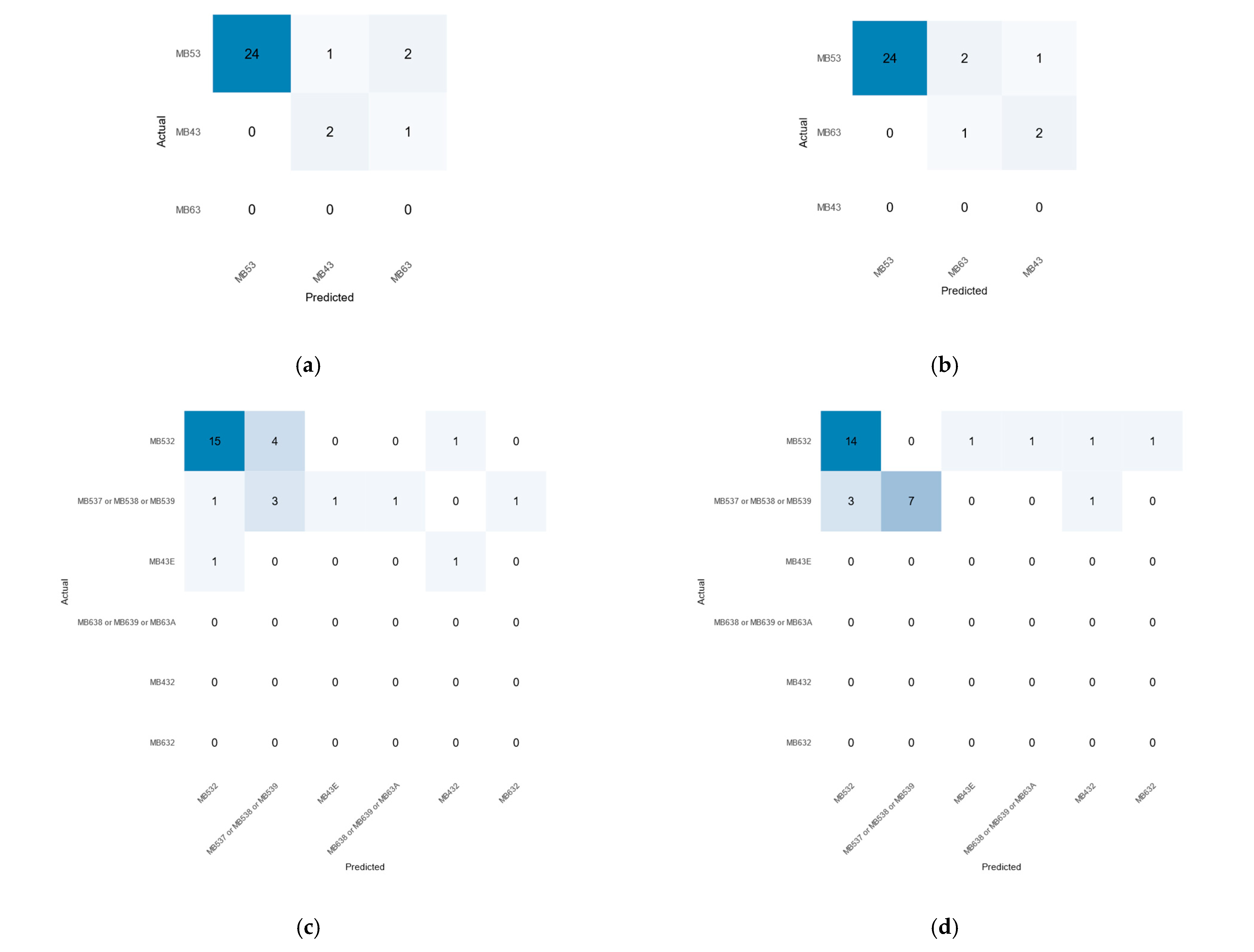
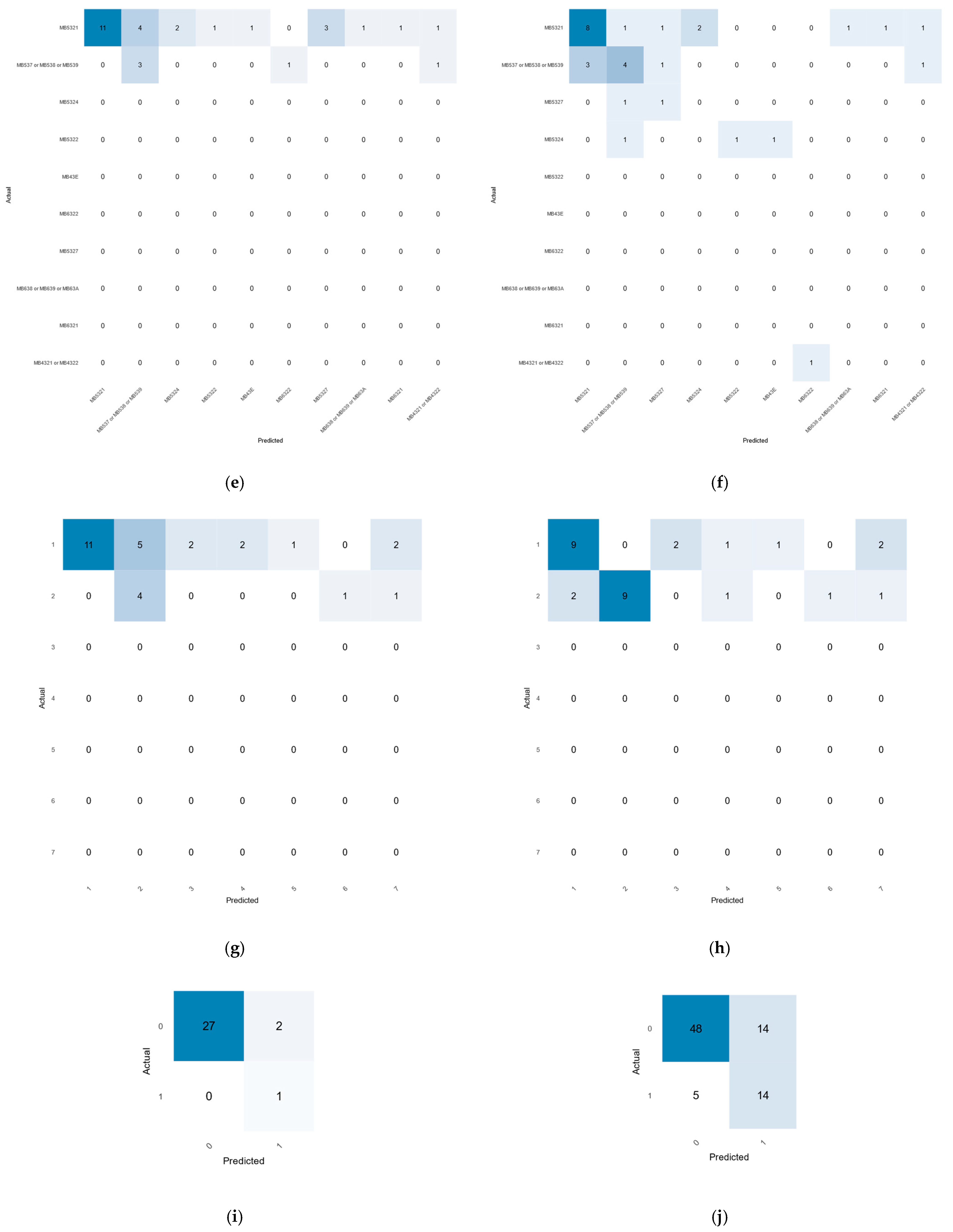
2.5. Accuracy Assessment
2.6. Map Generation and Spatial Analysis
Change Detection Analysis
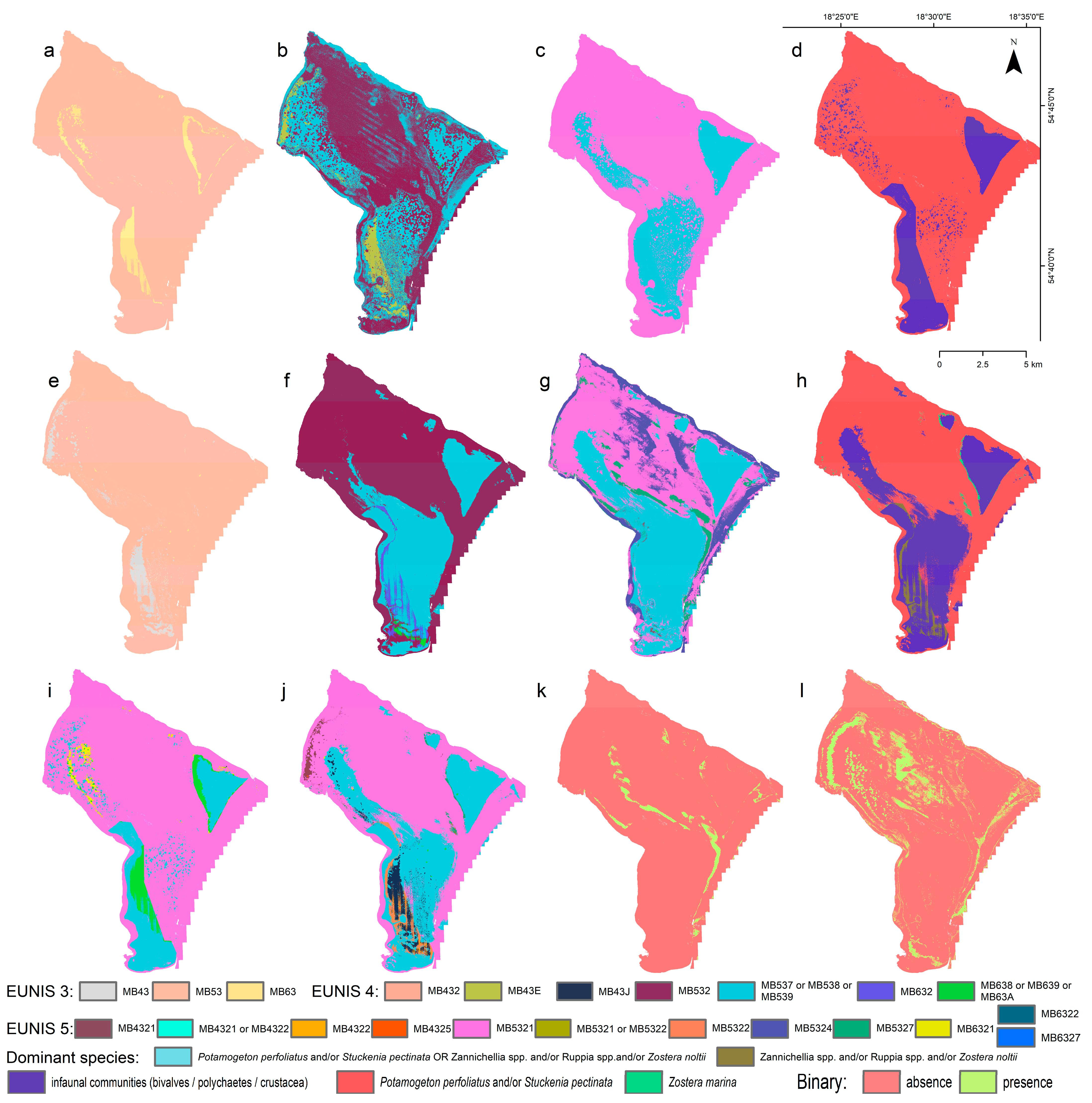
3. Results
3.1. Feature Selection
3.2. Predictive Modeled Maps of Benthic Habitats
3.3. Accuracy Assessment of the Machine Learning Results
3.4. Change Detection Analysis
4. Discussion
4.1. Classification Performance Hierarchy: From Operationally Defensible to Exploratory Findings
4.2. Feature Selection and Environmental Predictors
Temporal Dynamics and Environmental Predictors
4.3. Effectiveness of Object-Based Image Analysis
4.4. Class Imbalance as Fundamental Constraint on Fine-Scale Classifications: Data Requirements for Advancement
4.5. Change Detection Analysis and Temporal Habitat Dynamics
4.6. Methodological Limitations and Uncertainty Assessment
4.7. Ecological Implications and Conservation Significance
Anthropogenic Drivers and Spatiotemporal Correlation Analysis
4.8. Technological Integration and Future Directions
4.9. Management Applications and Operational Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALB | Airborne LiDAR Bathymetry |
| MBES | Multibeam Echosounder |
| KNN | K-Nearest Neighbors |
| RF | Random Forest |
| SVM | Support Vector Machine |
| GT | Ground-Truth |
| TRI | Terrain Ruggedness Index |
| EUNIS | European Nature Information System |
| OBIA | Object-Based Image Analysis |
Appendix A
| No | Depth | X | Y | Sediment Analyses | Description of the Video Recordings |
| 1 | 1.6 | 18.3955 | 54.7299 | fine SAND with medium sand | vascular plants rooted in the seabed: Stuckenia pectinata, Cerastoderma glaucum shells, Stuckenia pectinata covered with mats of Zostera marina brown algae |
| 2 | 2.4 | 18.3988 | 54.7350 | fine SAND | vascular plants in the seabed: Stuckenia pectinata; Charales green algae; all covered with dense algal mat |
| 3 | 2.5 | 18.4102 | 54.7235 | medium SAND and fine SAND | Mya arenaria shells; single stems of Stuckenia pectinata |
| 4 | 3.3 | 18.4126 | 54.7406 | fine SAND with medium sand | vascular plants in the bottom: Stuckenia pectinata; all covered with dense algal mat; shells of Cerastoderma glaucum |
| 5 | 2.9 | 18.4126 | 54.7549 | fine SAND with medium sand | vascular plants: Stuckenia pectinata; prominent Charales green algae; all covered with dense algal mat |
| 6 | 3.0 | 18.4138 | 54.7298 | sandy SILT | vascular plants: Stuckenia pectinata; all covered with dense algal mat; |
| 7 | 4.0 | 18.4254 | 54.7330 | sandy SILT | vascular plants: Stuckenia pectinata; all covered with dense algal mat; |
| 8 | 3.4 | 18.4166 | 54.7500 | silty SAND | vascular plants: Stuckenia pectinata; all covered with dense algal mat; |
| 9 | 4.2 | 18.4244 | 54.7404 | fine SAND | plants in the bottom: Stuckenia pectinata; prominent Charales green algae and probably Zostera marina; all covered with dense algal mat |
| 10 | 4.2 | 18.4277 | 54.7263 | sandy SILT | shells mainly of Mya arenaria; single stems of Stuckenia pectinata covered with algal mats |
| 11 | 3.4 | 18.4243 | 54.7557 | silty SAND | vascular plants: Stuckenia pectinata; all covered with dense algal mat |
| 12 | 3.5 | 18.4256 | 54.7518 | silty SAND | vascular plants: Stuckenia pectinata; visible Charales green algae; all covered with dense algal mat |
| 13 | 3.4 | 18.4367 | 54.7446 | fine SAND | vascular plants: Stuckenia pectinata; all covered with dense algal mat |
| 14 | 4.5 | 18.4393 | 54.7337 | fine SAND | vascular plants in the seabed: Stuckenia pectinata, Zannichellia palustris; all covered with dense algal mat |
| 15 | 4.4 | 18.4395 | 54.7198 | medium SAND with fine sand | single stems of vascular plants in the seabed: Stuckenia pectinata; all covered with dense algal mat; shells of Cerastoderma glaucum |
| 16 | 4.6 | 18.4424 | 54.7250 | sandy clayey SILT | shells of Cerastoderma glaucum and Mya arenaria |
| 17 | 2.1 | 18.4406 | 54.7593 | fine SAND with medium sand | vascular plants in bottom: Stuckenia pectinata; Charales green algae; all covered with algal mat |
| 18 | 2.9 | 18.4422 | 54.7516 | fine SAND with medium sand | vascular plants in bottom: Stuckenia pectinata, Zannichellia palustris; Charales green algae; all covered with dense algal mat |
| 19 | 2.7 | 18.4428 | 54.7521 | fine SAND with medium sand | vascular plants: Stuckenia pectinata; Charales green algae; all covered with algal mat |
| 20 | 3.1 | 18.4495 | 54.7286 | medium SAND with fine sand | vascular plants: Stuckenia pectinata; Charales green algae; all covered with algal mat |
| 21 | 4.1 | 18.4500 | 54.7047 | medium SAND with fine sand | single stems of vascular plants in the seabed: Stuckenia pectinata; all covered with algal mat; shells of Cerastoderma glaucum; remains of Zostera marina |
| 22 | 1.6 | 18.4508 | 54.7679 | fine SAND with medium sand | vascular plants in bottom: Stuckenia pectinata; Charales green algae; all covered with algal mat |
| 23 | 3.1 | 18.4502 | 54.7342 | fine SAND and medium SAND | vascular plants in bottom: Stuckenia pectinata; Charales green algae; all covered with dense algal mat |
| 24 | 1.6 | 18.4571 | 54.7697 | fine SAND with medium sand | Charales green algae; covered with algal mat |
| 25 | 2.2 | 18.4539 | 54.7519 | fine SAND with medium sand | Charales green algae; single stems of Stuckenia pectinata; covered with algal mat |
| 26 | 3.3 | 18.4562 | 54.7285 | fine SAND and medium SAND | vascular plants: Stuckenia pectinata; all covered with algal mat |
| 27 | 3.6 | 18.4581 | 54.7074 | medium SAND with coarse and fine sand | vascular plants: Zostera marina; covered with algal mat and overgrown with Hydrozoa |
| 28 | 2.7 | 18.4584 | 54.7211 | medium SAND with fine sand | vascular plants: Zostera marina, Stuckenia pectinata; covered with algal mat |
| 29 | 2.1 | 18.4513 | 54.7568 | fine SAND | vascular plants in bottom: Stuckenia pectinata; Charales green algae; covered with algal mat |
| 30 | 2.3 | 18.4605 | 54.7463 | fine SAND | vestigial stems of vascular plants in bottom: Stuckenia pectinata; Charales green algae; all covered with algal mat |
| 31 | 2.2 | 18.4648 | 54.7429 | fine SAND | Charales green algae; covered with algal mat |
| 32 | 4.2 | 18.4660 | 54.7154 | medium SAND with fine sand | vascular plants in the seabed, probably Stuckenia pectinata, Zannichellia palustris; all covered with dense algal mat; remains of Zostera marina |
| 33 | 1.6 | 18.4673 | 54.7629 | fine SAND with medium sand | vascular plants in the seabed: Stuckenia pectinata; Charales green algae |
| 34 | 2.3 | 18.4704 | 54.6614 | SAND | single stems of vascular plants in the seabed, probably Stuckenia pectinata, Zannichellia palustris; all covered with algal mat |
| 35 | 3.4 | 18.4707 | 54.6843 | silty SAND | vascular plants in the seabed, probably Stuckenia pectinata, Zannichellia palustris; all covered with dense algal mat |
| 36 | 2.0 | 18.4726 | 54.7364 | fine SAND with medium sand | vascular plants in the seabed: Stuckenia pectinata; Charales green algae; small amounts of algal mat; visible Zostera marina |
| 37 | 2.9 | 18.4742 | 54.7011 | medium SAND with fine sand | vascular plants: Zostera marina and Stuckenia pectinata; covered with algal mat |
| 38 | 3.4 | 18.4755 | 54.6645 | medium SAND with fine sand | vascular plants: Zostera marina and Stuckenia pectinata; covered with algal mat |
| 39 | 1.9 | 18.4759 | 54.6368 | fine SAND and medium SAND | vascular plants: single stems of Stuckenia pectinata; covered with algal mat |
| 40 | 2.4 | 18.4756 | 54.7243 | fine SAND with medium sand | vascular plants: Stuckenia pectinata and Myriophyllum spicatum; Charales green algae; covered with algal mat |
| 41 | 4.1 | 18.4778 | 54.7071 | medium SAND | vascular plants: Zostera marina (overgrown with Hydrozoa), Zannichellia palustris and Stuckenia pectinata; covered with algal mat; Cerastoderma glaucum shells |
| 42 | 3.8 | 18.4778 | 54.6590 | sandy SILT | vascular plants: Zannichellia palustris and probably Stuckenia pectinata; all covered with dense algal mat |
| 43 | 2.2 | 18.4793 | 54.6477 | medium SAND with coarse sand | single stems of the bottom vascular plants, probably Stuckenia pectinata; covered with algal mat |
| 44 | 1.8 | 18.4808 | 54.7475 | medium SAND and fine SAND | vascular plants: Stuckenia pectinata; Charales green algae; covered with algal mat |
| 45 | 1.7 | 18.4832 | 54.7382 | fine SAND with medium sand | vascular plants in the bottom: Stuckenia pectinata; Charales green algae; covered with algal mat in small amount |
| 46 | 5.4 | 18.4837 | 54.6875 | silty SAND | loose mat; Cerastoderma glaucum shells |
| 47 | 4.9 | 18.4868 | 54.6732 | sandy SILT | vascular plants in the bottom: Zannichellia palustris; all covered with dense algal mat |
| 48 | 1.7 | 18.4869 | 54.6586 | medium SAND with coarse sand | vestigial stems of vascular plants covered with algal mat |
| 49 | 5.0 | 18.4873 | 54.7078 | medium SAND | single stems of vascular plants: Stuckenia pectinata; covered with algal mat; Cerastoderma glaucum shells |
| 50 | 4.0 | 18.4842 | 54.6561 | sandy SILT | algal mats, Cerastoderma glaucum shells |
| 51 | 1.5 | 18.4881 | 54.6368 | medium SAND with fine sand | vestigial stems of vascular plants: probably Stuckenia pectinata; covered with algal mat |
| 52 | 2.7 | 18.4879 | 54.6409 | fine SAND with medium sand | vascular plants in the bottom: Stuckenia pectinata; all covered with dense algal mat |
| 53 | 3.7 | 18.4888 | 54.6473 | medium SAND with fine sand | single stems of vascular plants: Stuckenia pectinata; small amount of algal mat; shells mainly of Cerastoderma glaucum |
| 54 | 5.2 | 18.4889 | 54.6969 | fine SAND and medium SAND | shells of Cerastoderma glaucum |
| 55 | 3.3 | 18.4915 | 54.7450 | fine SAND with medium sand | vascular plants in the bottom: Stuckenia pectinata; overgrown with Hydrozoa; covered with algal mat |
| 56 | 4.7 | 18.4931 | 54.6585 | sandy SILT | single stems of Zannichellia palustris, covered with algal mat, remains of Zostera marina |
| 57 | 2.6 | 18.4971 | 54.7139 | fine SAND with medium sand | vascular plants in the bottom: Stuckenia pectinata; overgrown with Hydrozoa; covered with algal mat |
| 58 | 2.0 | 18.4945 | 54.6358 | medium SAND with fine sand | algal mat, shells of Cerastoderma glaucum, vascular plant stalk remains, probably Stuckenia pectinata |
| 59 | 1.8 | 18.4959 | 54.7501 | fine SAND and medium SAND | vascular plants in the bottom: Stuckenia pectinata; Charales green algae; covered with algal mat in small amount; peat outcrop |
| 60 | 4.5 | 18.4955 | 54.6545 | silty SAND | vascular plant stalk remains: probably Stuckenia pectinata and/or Zannichellia palustris; covered with algal mat |
| 61 | 2.8 | 18.4978 | 54.7440 | fine SAND with medium sand | Charales green algae; covered with algal mat, single stems of Stuckenia pectinata |
| 62 | 5.2 | 18.5001 | 54.6764 | silty SAND | loose mat; shells of Cerastoderma glaucum |
| 63 | 2.9 | 18.5006 | 54.7331 | fine SAND | vascular plants: Stuckenia pectinata; covered with algal mat in small quantity |
| 64 | 2.9 | 18.5017 | 54.7308 | fine SAND with medium sand | vascular plants: Stuckenia pectinata (overgrown with Hydrozoa); Charales green algae visible; covered with algal mat |
| 65 | 1.8 | 18.5022 | 54.7184 | medium SAND and fine SAND | vascular plants: Stuckenia pectinata; covered with algal mat |
| 66 | 2.1 | 18.5040 | 54.7225 | fine SAND and medium SAND | vascular plants: Stuckenia pectinata (overgrown Hydrozoa); covered with algal mat |
| 67 | 2.7 | 18.5046 | 54.7112 | fine SAND with medium sand | vascular plants in the bottom: Stuckenia pectinata (overgrown Hydrozoa); covered with algal mat |
| 68 | 5.5 | 18.5038 | 54.6927 | fine SAND with medium sand | loose mat; Cerastoderma glaucum shells |
| 69 | 2.6 | 18.5057 | 54.7350 | fine SAND with medium sand | vascular plants in the bottom: Stuckenia pectinata (single stems); Charales green algae visible; covered with algal mat |
| 70 | 5.2 | 18.5048 | 54.6644 | fine SAND | loose mat in small quantity; Cerastoderma glaucum shells |
| 71 | 3.7 | 18.5070 | 54.7055 | fine SAND with medium sand | single stems of vascular plants: Stuckenia pectinata and Zannichellia palustris, covered with algal mat |
| 72 | 4.7 | 18.5062 | 54.6522 | silty SAND | shells of Cerastoderma glaucum |
| 73 | 3.9 | 18.5076 | 54.6459 | SAND | vascular plants: Zannichellia palustris and Stuckenia pectinata, covered with algal mat |
| 74 | 2.4 | 18.5110 | 54.7224 | fine SAND with medium sand | single stems of vascular plants: Stuckenia pectinata, probably remains of green algae visible; covered with algal mat |
| 75 | 8.0 | 18.5234 | 54.7427 | medium SAND with fine sand | vascular plants: Zannichellia palustris and Stuckenia pectinata, covered with algal mat |
| 76 | 2.9 | 18.5126 | 54.6370 | fine SAND | shells mainly of Cerastoderma glaucum |
| 77 | 5.8 | 18.5000 | 54.6833 | medium SAND and fine SAND | shells mainly of Cerastoderma glaucum |
| 78 | 5.2 | 18.5160 | 54.6689 | fine SAND | vascular plants: Zannichellia palustris, covered with algal mat |
| 79 | 5.0 | 18.5160 | 54.6642 | fine SAND | loose algal mats in small amount, shells |
| 80 | 2.7 | 18.5184 | 54.7129 | fine SAND with medium sand | vascular plants in the bottom: Stuckenia pectinata; Charales green algae visible; covered with algal mat |
| 81 | 2.8 | 18.5194 | 54.6603 | coarse SAND with medium sand and medium gravel | Sandy bottom |
| 82 | 2.8 | 18.5201 | 54.7166 | fine SAND with medium sand | vascular plants in the bottom: Stuckenia pectinata; covered with algal mat |
| 83 | 3.0 | 18.5223 | 54.7385 | fine SAND with medium sand | loose algal mats, Cerastoderma glaucum shells |
| 84 | 4.5 | 18.5232 | 54.6759 | fine SAND with medium sand | loose algal mats, Cerastoderma glaucum shells, remains of Zostera marina |
| 85 | 5.3 | 18.5216 | 54.6939 | fine SAND with medium sand | vascular plants: Zannichellia palustris and Stuckenia pectinata, covered with dense algal mat |
| 86 | 3.4 | 18.5290 | 54.7100 | fine SAND | vascular plants: Stuckenia pectinata, covered with algal mats |
| 87 | 5.0 | 18.5286 | 54.6867 | fine SAND | sandy bottom, shells |
| 88 | 5.9 | 18.5354 | 54.7394 | sandy SILT | Sandy bottom |
| 89 | 3.4 | 18.5331 | 54.6995 | fine SAND with medium sand | vascular plants: Zostera marina, covered with algal mats |
| 90 | 2.6 | 18.5338 | 54.7075 | medium SAND and fine SAND | vascular plants: Zostera marina and Stuckenia pectinata, covered with algal mat |
| 91 | 3.3 | 18.5360 | 54.6871 | fine SAND | vascular plants: Zostera marina and Stuckenia pectinata, covered with algal mat |
| 92 | 5.3 | 18.5401 | 54.7178 | sandy SILT | sandy bottom, shells |
| 93 | 2.0 | 18.5400 | 54.7017 | medium SAND with fine sand | vascular plants: Zostera marina, covered with algal mat |
| 94 | 5.9 | 18.5427 | 54.7381 | fine SAND and medium SAND | sandy bottom |
| 95 | 6.0 | 18.5453 | 54.7088 | fine SAND and medium SAND | sandy bottom, shells |
| 96 | 7.0 | 18.5453 | 54.7278 | SAND | sandy bottom, shells |
| 97 | 8.0 | 18.5636 | 54.7349 | medium SAND | vascular plants: Stuckenia pectinata, covered with dense algal mats |
| 98 | 7.0 | 18.5631 | 54.7278 | fine SAND with medium sand | sandy bottom, shells mainly of Cerastoderma glaucum |
| 99 | 2.7 | 18.5709 | 54.7212 | medium SAND with fine sand | vascular plants: Zostera marina, covered with algal mats |
| 100 | 1.3 | 18.5783 | 54.7290 | medium SAND | vascular plants: Zostera marina and Stuckenia pectinata, covered with dense algal mats |
References
- Uścinowicz, S.; Witak, M.; Miotk-Szpiganowicz, G.; Burska, D.; Cieślikiewicz, W.; Jegliński, W.; Jurys, L.; Sydor, P.; Pawlyta, J.; Piotrowska, N. Climate and sea level variability on a centennial time scale over the last 1500 years as inferred from the Coastal Peatland of Puck Lagoon (southern Baltic Sea). Holocene 2020, 30, 1801–1816. [Google Scholar] [CrossRef]
- Janowski, Ł.; Skarlatos, D.; Agrafiotis, P.; Tysiąc, P.; Pydyn, A.; Popek, M.; Kotarba-Morley, A.M.; Mandlburger, G.; Gajewski, Ł.; Kołakowski, M.; et al. High resolution optical and acoustic remote sensing datasets of the Puck Lagoon. Sci. Data 2024, 11, 360. [Google Scholar] [CrossRef]
- Wicaksono, P.; Aryaguna, P.A.; Lazuardi, W. Benthic Habitat Mapping Model and Cross Validation Using Machine-Learning Classification Algorithms. Remote Sens. 2019, 11, 1279. [Google Scholar] [CrossRef]
- Strong, J.A.; Clements, A.; Lillis, H.; Galparsoro, I.; Bildstein, T.; Pesch, R. A review of the influence of marine habitat classification schemes on mapping studies: Inherent assumptions, influence on end products, and suggestions for future developments. ICES J. Mar. Sci. 2019, 76, 10–22. [Google Scholar] [CrossRef]
- Coveney, S.; Monteys, X. Integration Potential of INFOMAR Airborne LIDAR Bathymetry with External Onshore LIDAR Data Sets. J. Coast. Res. 2011, 62, 19–29. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Z.; Zhang, Z.; Chu, Y.; Xu, C.; Yao, S.; García-Fernández, Á.F.; Zhu, X.; Yue, Y.; Levers, A.; et al. Exploring modern bathymetry: A comprehensive review of data acquisition devices, model accuracy, and interpolation techniques for enhanced underwater mapping. Front. Mar. Sci. 2023, 10, 1178845. [Google Scholar] [CrossRef]
- Ierodiaconou, D.; Schimel, A.C.G.; Kennedy, D.; Monk, J.; Gaylard, G.; Young, M.; Diesing, M.; Rattray, A. Combining pixel and object based image analysis of ultra-high resolution multibeam bathymetry and backscatter for habitat mapping in shallow marine waters. Mar. Geophys. Res. 2018, 39, 271–288. [Google Scholar] [CrossRef]
- Benz, U.C.; Hofmann, P.; Willhauck, G.; Lingenfelder, I.; Heynen, M. Multi-resolution, object-oriented fuzzy analysis of remote sensing data for GIS-ready information. ISPRS J. Photogramm. Remote Sens. 2004, 58, 239–258. [Google Scholar] [CrossRef]
- Blaschke, T.; Hay, G.J.; Kelly, M.; Lang, S.; Hofmann, P.; Addink, E.; Queiroz Feitosa, R.; van der Meer, F.; van der Werff, H.; van Coillie, F.; et al. Geographic Object-Based Image Analysis—Towards a new paradigm. ISPRS J. Photogramm. Remote Sens. 2014, 87, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, A.; Jankowska, E.; Balazy, P.; Jędruch, A. Distribution and extent of benthic habitats in Puck Bay (Gulf of Gdańsk, southern Baltic Sea). Oceanologia 2021, 63, 301–320. [Google Scholar] [CrossRef]
- Ciszewski, P.; Kruk-Dowgiałło, L.; Andrulewicz, E. A study on pollution of the Puck Lagoon and possibility of restoring the lagoon′s original ecological state. Acta Ichthyol. Piscat. 1991, 21, 29–37. [Google Scholar] [CrossRef]
- Bockelmann, A.-C.; Tams, V.; Ploog, J.; Schubert, P.R.; Reusch, T.B. Quantitative PCR reveals strong spatial and temporal variation of the wasting disease pathogen, Labyrinthula zosterae in northern European eelgrass (Zostera marina) beds. PLoS ONE 2013, 8, e62169. [Google Scholar] [CrossRef]
- Dybowski, D.; Dzierzbicka-Głowacka, L. Analysis of the impact of nutrients deposited from the land side on the waters of Puck Lagoon (Gdańsk Basin, Southern Baltic): A model study. Oceanologia 2023, 65, 386–397. [Google Scholar] [CrossRef]
- Galparsoro, I.; Connor, D.W.; Borja, Á.; Aish, A.; Amorim, P.; Bajjouk, T.; Chambers, C.; Coggan, R.; Dirberg, G.; Ellwood, H. Using EUNIS habitat classification for benthic mapping in European seas: Present concerns and future needs. Mar. Pollut. Bull. 2012, 64, 2630–2638. [Google Scholar] [CrossRef]
- Vasquez, M.; Manca, E.; Inghilesi, R.; Martin, S.; Agnesi, S.; Al Hamdani, Z.; Annunziatellis, A.; Bekkby, T.; Pesch, R.; Askew, N.; et al. EUSeaMap 2019, A European Broad-Scale Seabed Habitat Map; Technical Report; EMODnet: Oostende, Belgium, 2020. [Google Scholar]
- Populus, J.; Vasquez, M.; Albrecht, J.; Manca, E.; Agnesi, S.; Al Hamdani, Z.; Andersen, J.; Annunziatellis, A.; Bekkby, T.; Bruschi, A.; et al. EUSeaMap. A European Broad-Scale Seabed Habitat Map; Ifremer: Plouzané, France, 2017. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Bremner, D.; Demaine, E.; Erickson, J.; Iacono, J.; Langerman, S.; Morin, P.; Toussaint, G. Output-Sensitive Algorithms for Computing Nearest-Neighbour Decision Boundaries. Discret. Comput. Geom. 2005, 33, 593–604. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Pontius, R.G.; Millones, M. Death to Kappa: Birth of quantity disagreement and allocation disagreement for accuracy assessment. Int. J. Remote Sens. 2011, 32, 4407–4429. [Google Scholar] [CrossRef]
- Agrafiotis, P.; Janowski, Ł.; Skarlatos, D.; Demir, B. MAGICBATHYNET: A Multimodal Remote Sensing Dataset for Bathymetry Prediction and Pixel-Based Classification in Shallow Waters. In Proceedings of the IGARSS 2024 IEEE International Geoscience and Remote Sensing Symposium, Athens, Greece, 7–12 July 2024; pp. 249–253. [Google Scholar]
- Janowski, Ł. Advancing Seabed Bedform Mapping in the Kuźnica Deep: Leveraging Multibeam Echosounders and Machine Learning for Enhanced Underwater Landscape Analysis. Remote Sens. 2025, 17, 373. [Google Scholar] [CrossRef]
- Janowski, L.; Wroblewski, R.; Dworniczak, J.; Kolakowski, M.; Rogowska, K.; Wojcik, M.; Gajewski, J. Offshore benthic habitat mapping based on object-based image analysis and geomorphometric approach. A case study from the Slupsk Bank, Southern Baltic Sea. Sci. Total Environ. 2021, 801, 149712. [Google Scholar] [CrossRef] [PubMed]
- Trzcinska, K.; Janowski, L.; Nowak, J.; Rucinska-Zjadacz, M.; Kruss, A.; Schneider von Deimling, J.; Pocwiardowski, P.; Tegowski, J. Spectral features of dual-frequency multibeam echosounder data for benthic habitat mapping. Mar. Geol. 2020, 427, 106239. [Google Scholar] [CrossRef]
- Si-Moussi, S.; Hennekens, S.; Mücher, S.; De Keersmaecker, W.; Chytrý, M.; Agrillo, E.; Attorre, F.; Biurrun, I.; Bonari, G.; Čarni, A. EUNIS Habitat Maps: Enhancing Thematic and Spatial Resolution for Europe through Machine Learning. arXiv 2025, arXiv:2506.13649. [Google Scholar] [CrossRef]
- Leblanc, C.; Bonnet, P.; Servajean, M.; Chytrý, M.; Aćić, S.; Argagnon, O.; Bergamini, A.; Biurrun, I.; Bonari, G.; Campos, J.A. A deep-learning framework for enhancing habitat identification based on species composition. Appl. Veg. Sci. 2024, 27, e12802. [Google Scholar] [CrossRef]
- Diesing, M.; Mitchell, P.; Stephens, D. Image-based seabed classification: What can we learn from terrestrial remote sensing? ICES J. Mar. Sci. 2016, 73, 2425–2441. [Google Scholar] [CrossRef]
- Montereale Gavazzi, G.; Madricardo, F.; Janowski, L.; Kruss, A.; Blondel, P.; Sigovini, M.; Foglini, F. Evaluation of seabed mapping methods for fine-scale classification of extremely shallow benthic habitats—Application to the Venice Lagoon, Italy. Estuar. Coast. Shelf Sci. 2016, 170, 45–60. [Google Scholar] [CrossRef]
- Anderson, R.O.D. High Resolution Remote Sensing of Eelgrass (Zostera marina) in South Slough, Oregon. Master’s Thesis, University of Oregon, Eugene, OR, USA, 2020. [Google Scholar]
- Moniruzzaman, M.; Islam, S.; Lavery, P.; Bennamoun, M.; Lam, C.P. Imaging and classification techniques for seagrass mapping and monitoring: A comprehensive survey. arXiv 2019, arXiv:1902.11114. [Google Scholar] [CrossRef]
- Dunic, J.C.; Brown, C.J.; Connolly, R.M.; Turschwell, M.P.; Côté, I.M. Long-term declines and recovery of meadow area across the world’s seagrass bioregions. Glob. Change Biol. 2021, 27, 4096–4109. [Google Scholar] [CrossRef]
- Lyons, M.B.; Phinn, S.R.; Roelfsema, C.M. Long term land cover and seagrass mapping using Landsat and object-based image analysis from 1972 to 2010 in the coastal environment of South East Queensland, Australia. ISPRS J. Photogramm. Remote Sens. 2012, 71, 34–46. [Google Scholar] [CrossRef]
- Lango, M.; Stefanowski, J. What makes multi-class imbalanced problems difficult? An experimental study. Expert Syst. Appl. 2022, 199, 116962. [Google Scholar] [CrossRef]
- Lecours, V.; Devillers, R.; Schneider, D.C.; Lucieer, V.L.; Brown, C.J.; Edinger, E.N. Spatial scale and geographic context in benthic habitat mapping: Review and future directions. Mar. Ecol. Prog. Ser. 2015, 535, 259–284. [Google Scholar] [CrossRef]
- Lamarche, G.; Orpin, A.; Mitchell, J.; Pallentin, A. Chapter 5: Benthic habitat mapping. In Biological Sampling in the Deep Sea; Clark, M.R., Consalvey, M., Rowden, A.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 80–102. [Google Scholar]
- Mohamed, H.; Nadaoka, K.; Nakamura, T. Semiautomated mapping of benthic habitats and seagrass species using a convolutional neural network framework in shallow water environments. Remote Sens. 2020, 12, 4002. [Google Scholar] [CrossRef]
- Menandro, P.S.; Misiuk, B.; Brown, C.J.; Bastos, A.C. Multispectral multibeam backscatter response of heterogeneous rhodolith beds. Sci. Rep. 2023, 13, 20220. [Google Scholar] [CrossRef]
- Zhang, C. Applying data fusion techniques for benthic habitat mapping and monitoring in a coral reef ecosystem. ISPRS J. Photogramm. Remote Sens. 2015, 104, 213–223. [Google Scholar] [CrossRef]
- Hasan, R.; Ierodiaconou, D.; Laurenson, L.; Schimel, A. Integrating multibeam backscatter angular response, mosaic and bathymetry data for benthic habitat mapping. PLoS ONE 2014, 9, e97339. [Google Scholar] [CrossRef]
- Rucińska, M.; Wróblewski, R.; Pałgan, D.; Trzcińska, K.; Belicki, J. Exploring sand ridge formation and preservation in a semi-enclosed tideless basin, Puck Bay, Baltic Sea. Earth Surf. Process. Landf. 2025, 50, e70098. [Google Scholar] [CrossRef]
- Szymczak, E.; Rucińska, M. Characteristics of morphodynamic conditions in the shallows of Puck Bay (southern Baltic Sea). Oceanol. Hydrobiol. Stud. 2021, 50, 220–231. [Google Scholar] [CrossRef]
- Moskalewicz, D. Hel Peninsula-development and future of sandy barrier under sea level rise. In Quaternary Geology of North-Central Poland: From the Baltic Coast to the LGM Limit; University of Gdańsk: Gdańsk, Poland, 2016; Volume 5. [Google Scholar]
- Miotk-Szpiganowicz, G. Holocene shoreline migrations in the Puck Lagoon (Southern Baltic Sea) based on the Rzucewo Headland case study. Landf. Anal. 2003, 4, 3–97. [Google Scholar]
- Janowski, Ł.; Wróblewski, R. Application and Evaluation of the AI-Powered Segment Anything Model (SAM) in Seafloor Mapping: A Case Study from Puck Lagoon, Poland. Remote Sens. 2024, 16, 2638. [Google Scholar] [CrossRef]
- Urbański, J.A.; Grusza, G.; Chlebus, N.; Kryla, L. A GIS-based WFD oriented typology of shallow micro-tidal soft bottom using wave exposure and turbidity mapping. Estuar. Coast. Shelf Sci. 2008, 78, 27–37. [Google Scholar] [CrossRef]
- Sokołowski, A.; Lasota, R.; Alias, I.S.; Piłczyńska, J.; Wołowicz, M. Prospects and opportunities for mussel Mytilus trossulus farming in the southern Baltic Sea (the Gulf of Gdańsk). Oceanol. Hydrobiol. Stud. 2022, 51, 53–73. [Google Scholar] [CrossRef]
- Tysiac, P. Bringing Bathymetry LiDAR to Coastal Zone Assessment: A Case Study in the Southern Baltic. Remote Sens. 2020, 12, 3740. [Google Scholar] [CrossRef]
- Montereale-Gavazzi, G.; Roche, M.; Degrendele, K.; Lurton, X.; Terseleer, N.; Baeye, M.; Francken, F.; Van Lancker, V. Insights into the Short-Term Tidal Variability of Multibeam Backscatter from Field Experiments on Different Seafloor Types. Geosciences 2019, 9, 34. [Google Scholar] [CrossRef]
- Sagawa, T.; Yamashita, Y.; Okumura, T.; Yamanokuchi, T. Satellite derived bathymetry using machine learning and multi-temporal satellite images. Remote Sens. 2019, 11, 1155. [Google Scholar] [CrossRef]
- Zevenbergen, L.W.; Thorne, C.R. Quantitative analysis of land surface topography. Earth Surf. Process. Landf. 1987, 12, 47–56. [Google Scholar] [CrossRef]
- Riley, S.J.; De Gloria, S.D.; Elliot, R. A Terrain Ruggedness that Quantifies Topographic Heterogeneity. Intermt. J. Sci. 1999, 5, 23–27. [Google Scholar]
- Lecours, V.; Dolan, M.F.J.; Micallef, A.; Lucieer, V.L. A review of marine geomorphometry, the quantitative study of the seafloor. Hydrol. Earth Syst. Sci. 2016, 20, 3207–3244. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Package ‘Boruta’. Wrapper Algorithm for All Relevant Feature Selection. 2016. Available online: https://cran.r-project.org/web/packages/Boruta/Boruta.pdf (accessed on 1 September 2025).
- Menandro, P.S.; Lavagnino, A.C.; Vieira, F.V.; Boni, G.C.; Franco, T.; Bastos, A.C. The role of benthic habitat mapping for science and managers: A multi-design approach in the Southeast Brazilian Shelf after a major man-induced disaster. Front. Mar. Sci. 2022, 9, 1004083. [Google Scholar] [CrossRef]
- Ilich, A.R.; Brizzolara, J.L.; Grasty, S.E.; Gray, J.W.; Hommeyer, M.; Lembke, C.; Locker, S.D.; Silverman, A.; Switzer, T.S.; Vivlamore, A.; et al. Integrating Towed Underwater Video and Multibeam Acoustics for Marine Benthic Habitat Mapping and Fish Population Estimation. Geosciences 2021, 11, 176. [Google Scholar] [CrossRef]
- Nemani, S.; Cote, D.; Misiuk, B.; Edinger, E.; Mackin-McLaughlin, J.; Templeton, A.; Shaw, J.; Robert, K. A multi-scale feature selection approach for predicting benthic assemblages. Estuar. Coast. Shelf Sci. 2022, 277, 108053. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth: Belmont, CA, USA, 1984. [Google Scholar]
- Foody, G.M. Status of land cover classification accuracy assessment. Remote Sens. Environ. 2002, 80, 185–201. [Google Scholar] [CrossRef]
- Stehman, S.V.; Czaplewski, R.L. Design and Analysis for Thematic Map Accuracy Assessment—An application of Satellite Imagery. Remote Sens. Environ. 1998, 64, 331–344. [Google Scholar] [CrossRef]
- Story, M.; Congalton, R.G. Accuracy assessment: A user’s perspective. Photogramm. Eng. Remote Sens. 1986, 52, 397–399. [Google Scholar]
- Ciszewski, P.; Demel, K.; Ringher, Z.; Szatybełko, M. Zasoby widlika w Zatoce Puckiej oszacowane metodą nurkowania. Pr. MIR 1962, 11/A, 9–36. [Google Scholar]
- Rattray, A.; Ierodiaconou, D.; Monk, J.; Versace, V.L.; Laurenson, L.J.B. Detecting patterns of change in benthic habitats by acoustic remote sensing. Mar. Ecol. Prog. Ser. 2013, 477, 1–13. [Google Scholar] [CrossRef]
- Janowski, L.; Madricardo, F.; Fogarin, S.; Kruss, A.; Molinaroli, E.; Kubowicz-Grajewska, A.; Tegowski, J. Spatial and Temporal Changes of Tidal Inlet Using Object-Based Image Analysis of Multibeam Echosounder Measurements: A Case from the Lagoon of Venice, Italy. Remote Sens. 2020, 12, 2117. [Google Scholar] [CrossRef]
- Brown, C.J.; Smith, S.J.; Lawton, P.; Anderson, J.T. Benthic habitat mapping: A review of progress towards improved understanding of the spatial ecology of the seafloor using acoustic techniques. Estuar. Coast. Shelf Sci. 2011, 92, 502–520. [Google Scholar] [CrossRef]
- Vassallo, P.; Bianchi, C.N.; Paoli, C.; Holon, F.; Navone, A.; Bavestrello, G.; Cattaneo Vietti, R.; Morri, C. A predictive approach to benthic marine habitat mapping: Efficacy and management implications. Mar. Pollut. Bull. 2018, 131, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Köppen, M. The curse of dimensionality. In Proceedings of the 5th Online World Conference on Soft Computing in Industrial Applications (WSC5), Online, 4–18 September 2000; pp. 4–8. [Google Scholar]
- Li, J.; Tran, M.; Siwabessy, J. Selecting Optimal Random Forest Predictive Models: A Case Study on Predicting the Spatial Distribution of Seabed Hardness. PLoS ONE 2016, 11, e0149089. [Google Scholar] [CrossRef]
- Phinn, S.R.; Roelfsema, C.M.; Mumby, P.J. Multi-scale, object-based image analysis for mapping geomorphic and ecological zones on coral reefs. Int. J. Remote Sens. 2012, 33, 3768–3797. [Google Scholar] [CrossRef]
- Rhoads, D.C.; Germano, J.D. Interpreting long-term changes in benthic community structure: A new protocol. In Proceedings of the Long-Term Changes in Coastal Benthic Communities: Proceedings of a Symposium, Brussels, Belgium, 9–12 December 1985; pp. 291–308. [Google Scholar]
- Torn, K.; Herkül, K.; Martin, G.; Oganjan, K. Assessment of quality of three marine benthic habitat types in northern Baltic Sea. Ecol. Indic. 2017, 73, 772–783. [Google Scholar] [CrossRef]
- Bekkby, T.; Gerovasileiou, V.; Papadopoulou, N.; Sevastou, K.; Dailianis, T.; Fiorentino, D.; McOwen, C.; Smith, C.; Amaro, T.; Bakran-Petricioli, T. State of the Knowledge on European Marine Habitat Mapping and Degraded Habitats. MERCES Project. 2017. Available online: https://research.abo.fi/en/publications/state-of-the-knowledge-on-european-marine-habitat-mapping-and-deg/ (accessed on 1 September 2025).
- Alexander, T.J.; Vonlanthen, P.; Seehausen, O. Does eutrophication-driven evolution change aquatic ecosystems? Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160041. [Google Scholar] [CrossRef]
- Boström, C.; Bonsdorff, E.; Kangas, P.; Norkko, A. Long-term changes of a brackish-water eelgrass (Zostera marina L.) community indicate effects of coastal eutrophication. Estuar. Coast. Shelf Sci. 2002, 55, 795–804. [Google Scholar] [CrossRef]
- Foody, G.M. Explaining the unsuitability of the kappa coefficient in the assessment and comparison of the accuracy of thematic maps obtained by image classification. Remote Sens. Environ. 2020, 239, 111630. [Google Scholar] [CrossRef]
- Jankowska, E.; Jankowska, K.; Włodarska-Kowalczuk, M. Seagrass vegetation and meiofauna enhance the bacterial abundance in the Baltic Sea sediments (Puck Bay). Environ. Sci. Pollut. Res. 2015, 22, 14372–14378. [Google Scholar] [CrossRef] [PubMed]
- Lacharité, M.; Brown, C.J. Utilizing benthic habitat maps to inform biodiversity monitoring in marine protected areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 938–951. [Google Scholar] [CrossRef]
- Bolałek, J.; Falkowska, L.; Korzeniewski, K. The hydrochemistry of gulfs. In Gulf of Gdańsk and Puck Bay; Springer: Berlin/Heidelberg, Germany, 1993; pp. 222–303. [Google Scholar]
- Kruk-Dowgiałło, L.; Szaniawska, A. Gulf of Gdańsk and Puck Bay. In Ecology of Baltic Coastal Waters; Springer: Berlin/Heidelberg, Germany, 2008; pp. 139–165. [Google Scholar]
- Trokowicz, D.; Andrulewicz, E. Wydobycie i przetwórstwo wodorostów z Zatoki Puckiej. Wiad. Ryb. Pismo Mor. Inst. Ryb. Panstw. Inst. Badaw 2019, 32, 11–12. [Google Scholar]
- Kruk-Dowgiałło, L. Wieloletnie zmiany składu taksonomicznego oraz biomasy fitobentosu Zatoki Gdańskiej. Inst. Mor. W Gdańsku 2004. [Google Scholar]
- Schönke, M.; Wiesenberg, L.; Schulze, I.; Wilken, D.; Darr, A.; Papenmeier, S.; Feldens, P. Impact of Sparse Benthic Life on Seafloor Roughness and High-Frequency Acoustic Scatter. Geosciences 2019, 9, 454. [Google Scholar] [CrossRef]
- Tęgowski, J.; Trzcińska, K.; Janowski, Ł.; Kruss, A.; Kusek, K.; Nowak, J. Comparison of Backscatter and Seabed Topographic Characteristics Recorded by Multibeam Echosounder at Rewal Area—Southern Baltic Sea. In Proceedings of the 2018 Joint Conference-Acoustics, Ustka, Poland, 11–14 September 2018; pp. 1–4. [Google Scholar]
- Misiuk, B.; Brown, C.J. Benthic habitat mapping: A review of three decades of mapping biological patterns on the seafloor. Estuar. Coast. Shelf Sci. 2024, 296, 108599. [Google Scholar] [CrossRef]
- Ilich, A.R.; Misiuk, B.; Lecours, V.; Murawski, S.A. MultiscaleDTM: An open-source R package for multiscale geomorphometric analysis. Trans. GIS 2023, 27, 1164–1204. [Google Scholar] [CrossRef]
- Pearman, T.R.R.; Robert, K.; Callaway, A.; Hall, R.; Iacono, C.L.; Huvenne, V.A.I. Improving the predictive capability of benthic species distribution models by incorporating oceanographic data—Towards holistic ecological modelling of a submarine canyon. Prog. Oceanogr. 2020, 184, 102338. [Google Scholar] [CrossRef]
- Montereale-Gavazzi, G.; Roche, M.; Lurton, X.; Degrendele, K.; Terseleer, N.; Van Lancker, V. Seafloor change detection using multibeam echosounder backscatter: Case study on the Belgian part of the North Sea. Mar. Geophys. Res. 2018, 39, 229–247. [Google Scholar] [CrossRef]
- Janowski, Ł.; Skarlatos, D.; Agrafiotis, P.; Tysiąc, P.; Pydyn, A.; Popek, M.; Kotarba-Morley, A.; Mandlburger, G.; Gajewski, Ł.; Kolakowski, M.; et al. Bathymetry and remote sensing data of the Puck Lagoon, Southern Baltic Sea. 2023. Available online: https://www.marine-geo.org/doi/10.26022/IEDA/331456 (accessed on 1 September 2025).
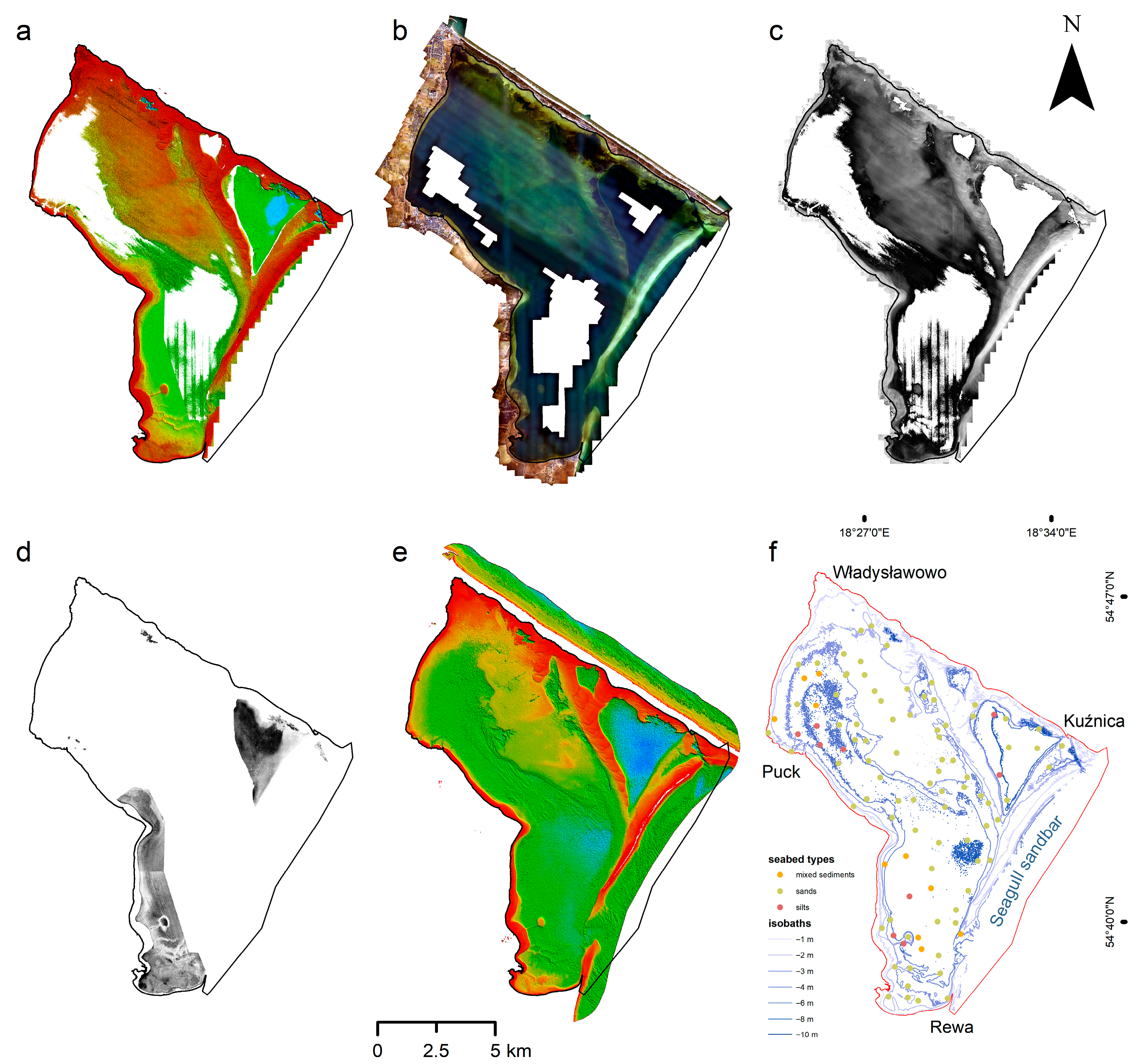
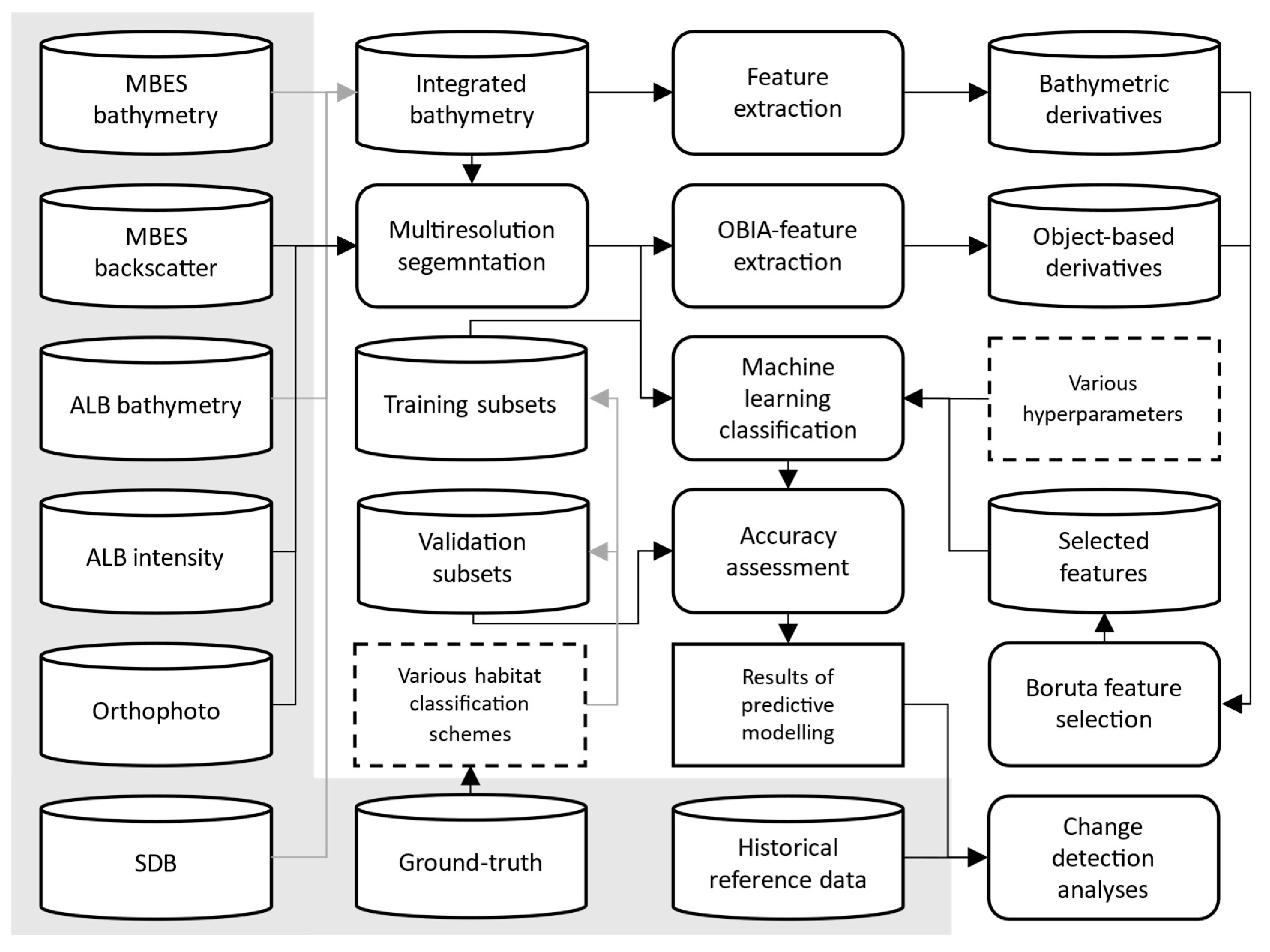
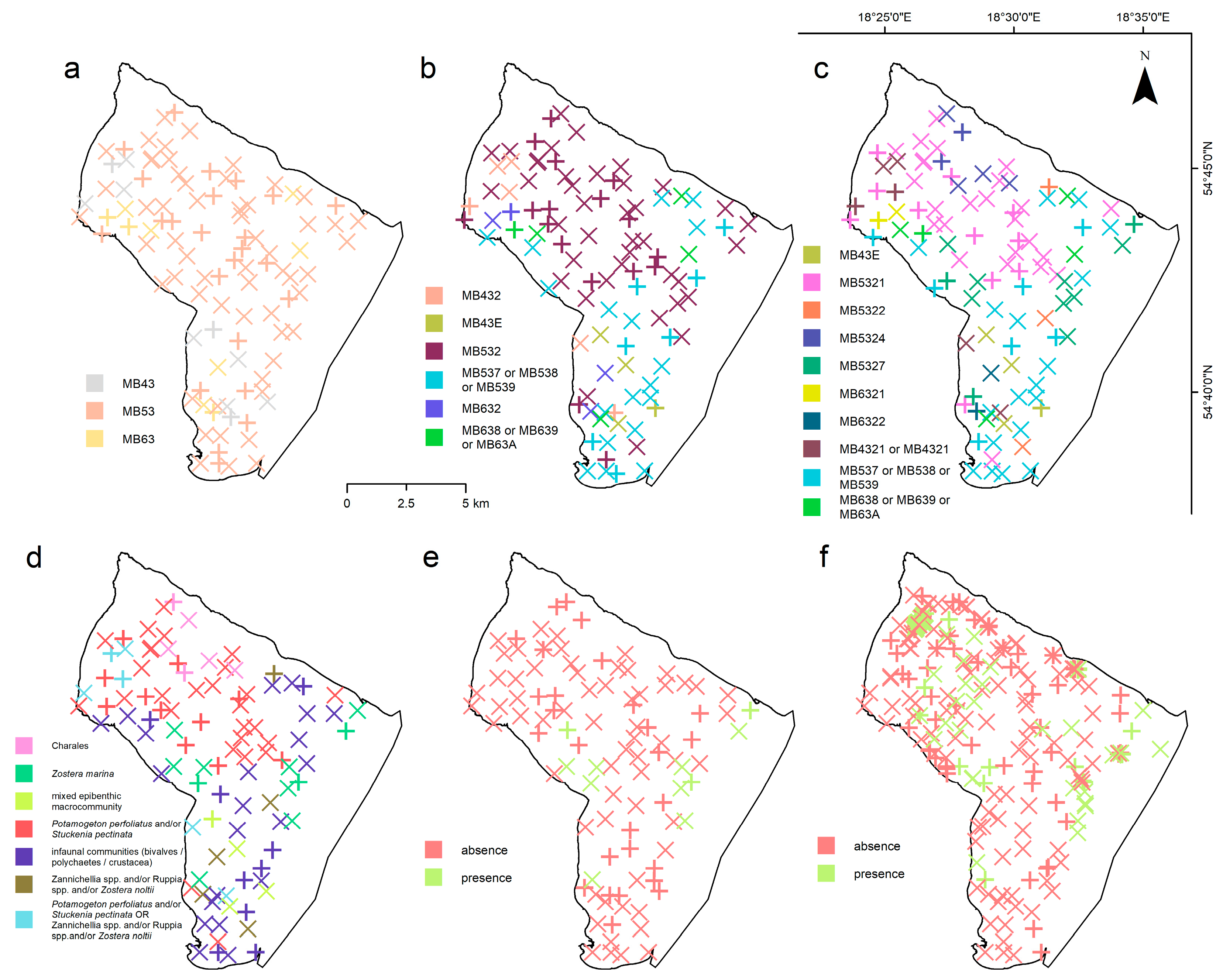
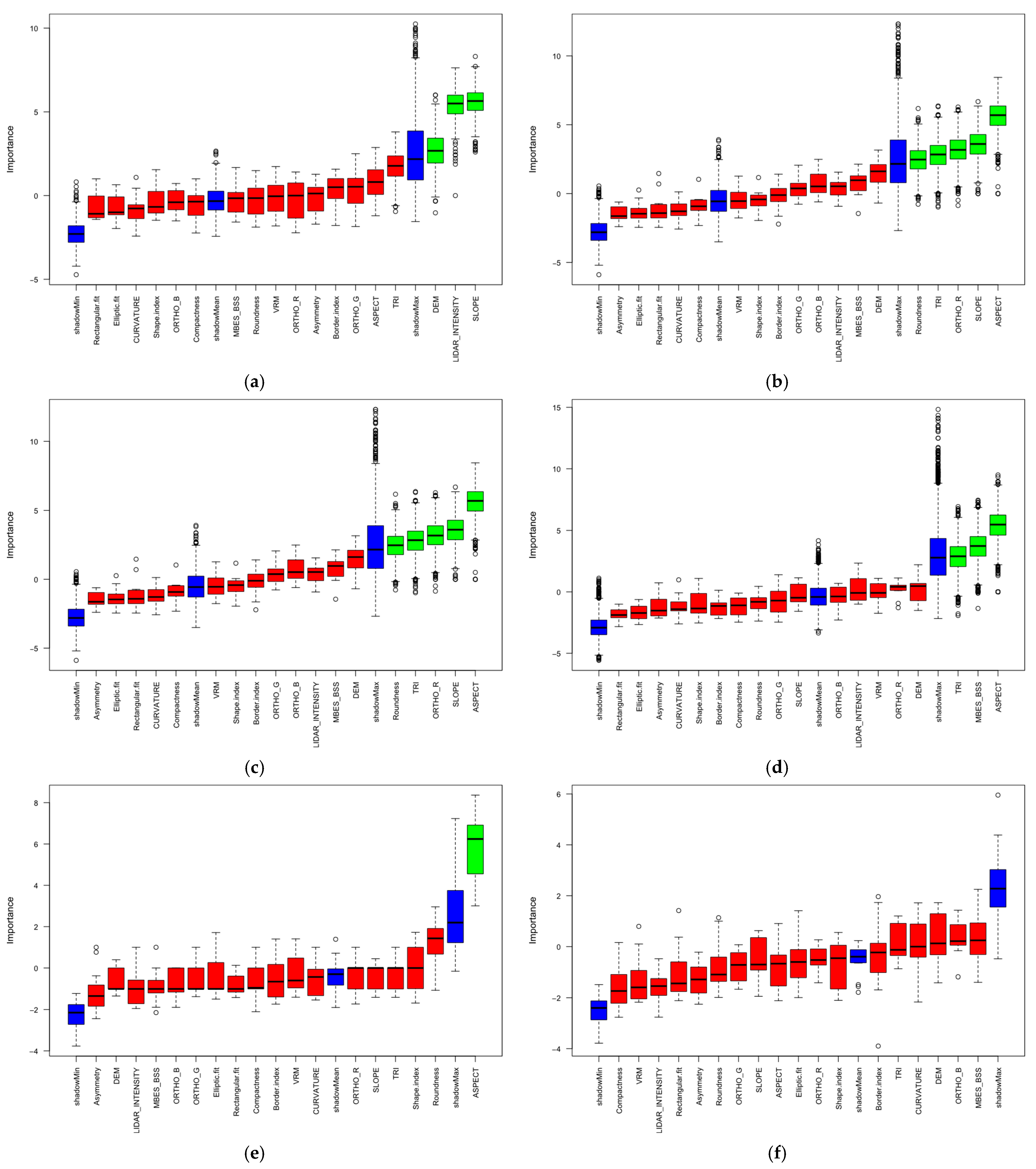

| Sensor | Platform | Type | Resolution | Wavelength/Frequency | Date of Acquisition | Conditions |
|---|---|---|---|---|---|---|
| Riegl VQ-880-GII | SP-PRO | Bathymetry | 0.5 m | 532/1064 nm | 27 February–2 March 2022; 7–10 March 2025 | Clear water, optimal light conditions |
| Riegl VQ-880-GII | SP-PRO | Intensity | 0.5 m | 532/1064 nm | 27 February–2 March 2022; 7–10 March 2025 | |
| Riegl VQ-880-GII | SP-PRO | Photogrammetry | 0.5 m | RGB | 27 February–2 March 2022; 7–10 March 2025 | |
| Reson T20/T50 | R/V IMOROS 2/3 | Bathymetry | 0.5 m | 420 kHz | 22 March–22 June 2022 | Sea state ≤ 2, calm weather |
| Reson T20/T50 | R/V IMOROS 2/3 | Backscatter | 0.5 m | 420 kHz | 22 March–22 June 2022 | |
| NAOMI | SPOT-6 | SDB | 5.2 × 8.3 m | RGB | 19 April 2021 | Cloud-free |
| EUNIS Level | EUNIS Code | Description |
|---|---|---|
| Level 3 | MB43 | Baltic infralittoral mixed sediment |
| Level 3 | MB53 | Baltic infralittoral sand |
| Level 3 | MB63 | Baltic infralittoral mud |
| Level 4 | MB432 | Baltic infralittoral mixed sediment characterized by submerged rooted plants |
| Level 4 | MB43E | Baltic infralittoral mixed sediment characterized by mixed epibenthic macrocommunity |
| Level 4 | MB532 | Baltic infralittoral sand characterized by submerged rooted plants |
| Level 4 | MB537 | Baltic infralittoral sand characterized by infaunal bivalves |
| Level 4 | MB538 | Baltic infralittoral sand characterized by infaunal polychaetes |
| Level 4 | MB539 | Baltic infralittoral sand characterized by infaunal crustacea |
| Level 4 | MB632 | Baltic infralittoral mud sediment characterized by submerged rooted plants |
| Level 4 | MB638 | Baltic infralittoral mud characterized by infaunal bivalves |
| Level 4 | MB639 | Baltic infralittoral mud characterized by infaunal polychaetes |
| Level 4 | MB63A | Baltic infralittoral mud characterized by infaunal crustacea |
| Level 5 | MB4321 | Baltic infralittoral mixed sediment dominated by Potamogeton perfoliatus and/or Stuckenia pectinata |
| Level 5 | MB4322 | Baltic infralittoral mixed sediment dominated by Zannichellia spp. and/or Ruppia spp. and/or Zostera noltii |
| Level 5 | MB4325 | Baltic infralittoral mixed sediment dominated by Zostera marina |
| Level 5 | MB5321 | Baltic infralittoral sand dominated by Potamogeton perfoliatus and/or Stuckenia pectinata |
| Level 5 | MB5322 | Baltic infralittoral sand dominated by Zannichellia spp. and/or Ruppia spp. and/or Zostera noltii |
| Level 5 | MB5324 | Baltic infralittoral sand dominated by Charales |
| Level 5 | MB5327 | Baltic infralittoral sand dominated by Zostera marina |
| Level 5 | MB6321 | Baltic infralittoral mud sediment dominated by Potamogeton perfoliatus and/or Stuckenia pectinata |
| Level 5 | MB6322 | Baltic infralittoral mud sediment dominated by Zannichellia spp. and/or Ruppia spp. and/or Zostera noltii |
| Level 5 | MB6327 | Baltic infralittoral mud sediment dominated by Zostera marina |
| Model/Parameter | k | Number of Trees | Tree Depth | C | Gamma |
|---|---|---|---|---|---|
| K-Nearest Neighbors | 1–9 | - | - | - | - |
| Classification and Regression Trees | - | 0–20 | 0–25 | - | - |
| Random Forest | - | 0–20 | 0–25 | - | - |
| Support Vector Machine | - | - | - | 2–4 | 0–1.2 |
| Model/Parameter | k | Number of Trees | Tree Depth | C | Gamma |
|---|---|---|---|---|---|
| EUNIS 3, KNN | 3 | - | - | - | - |
| EUNIS 4, RF | - | 10 | 15 | - | - |
| EUNIS 4/5, SVM | - | - | - | 2 | 0 |
| Dominant species descriptor, SVM | - | - | - | 3 | 0 |
| EUNIS 3, RF | - | 8 | 8 | - | - |
| EUNIS 4, KNN | 8 | - | - | - | - |
| EUNIS 4/5, RF | - | 5 | 6 | - | - |
| Dominant species descriptor, KNN | 8 | - | - | - | - |
| Zostera modeling, SVM | - | - | - | 2 | 1 |
| Zostera modeling, RF | - | 11 | 5 | - | - |
| Model | Total 1957 | Total Recent | Gain | Loss | Total Change | Swap (Location) | Net (Quantity) |
|---|---|---|---|---|---|---|---|
| EUNIS 4/5, RF | 61.15 | 4.05 | 1.31 | 58.40 | 59.71 | 2.62 | 57.09 |
| Combined model | 61.15 | 0.63 | 0.15 | 60.66 | 60.81 | 0.30 | 60.51 |
| GT samples ′23 | 61.15 | 2.69 | 0.53 | 58.98 | 59.50 | 1.05 | 58.45 |
| GT samples ′10–23 | 61.15 | 9.70 | 5.52 | 56.97 | 62.50 | 11.05 | 51.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowski, Ł.; Barańska, A.; Załęski, K.; Kubacka, M.; Michałek, M.; Tarała, A.; Niemkiewicz, M.; Gajewski, J. Predictive Benthic Habitat Mapping Reveals Significant Loss of Zostera marina in the Puck Lagoon, Baltic Sea, over Six Decades. Remote Sens. 2025, 17, 3725. https://doi.org/10.3390/rs17223725
Janowski Ł, Barańska A, Załęski K, Kubacka M, Michałek M, Tarała A, Niemkiewicz M, Gajewski J. Predictive Benthic Habitat Mapping Reveals Significant Loss of Zostera marina in the Puck Lagoon, Baltic Sea, over Six Decades. Remote Sensing. 2025; 17(22):3725. https://doi.org/10.3390/rs17223725
Chicago/Turabian StyleJanowski, Łukasz, Anna Barańska, Krzysztof Załęski, Maria Kubacka, Monika Michałek, Anna Tarała, Michał Niemkiewicz, and Juliusz Gajewski. 2025. "Predictive Benthic Habitat Mapping Reveals Significant Loss of Zostera marina in the Puck Lagoon, Baltic Sea, over Six Decades" Remote Sensing 17, no. 22: 3725. https://doi.org/10.3390/rs17223725
APA StyleJanowski, Ł., Barańska, A., Załęski, K., Kubacka, M., Michałek, M., Tarała, A., Niemkiewicz, M., & Gajewski, J. (2025). Predictive Benthic Habitat Mapping Reveals Significant Loss of Zostera marina in the Puck Lagoon, Baltic Sea, over Six Decades. Remote Sensing, 17(22), 3725. https://doi.org/10.3390/rs17223725





