Assessing Spectral Reflectance in African Smallholder Cereal Farms Using Sentinel-2 Imagery
Abstract
1. Introduction
- Determine the effectiveness of Sentinel-2 in extracting spectral reflectance values from African smallholder cereal farms (1 ha or smaller).
- Assess the capability of Sentinel-2 spectral reflectance to detect differences in crop yield potential at the smallholder scale.
2. Materials and Methods
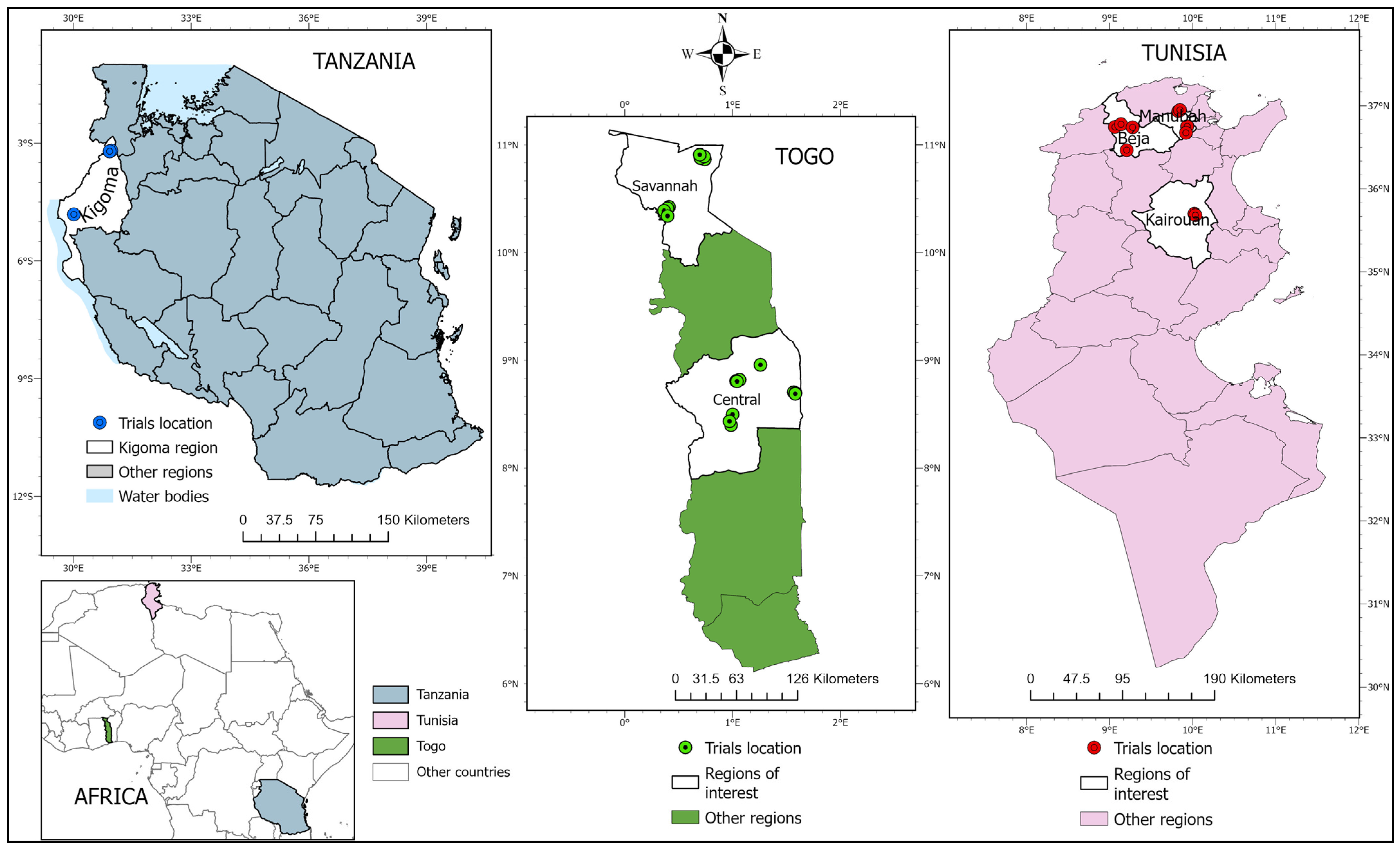
2.1. Site Selection
2.2. Study Design
2.3. Ground Data
2.4. Satellite Data
2.5. Spectral Reflectance Curves
2.6. Statistical Analysis
3. Results
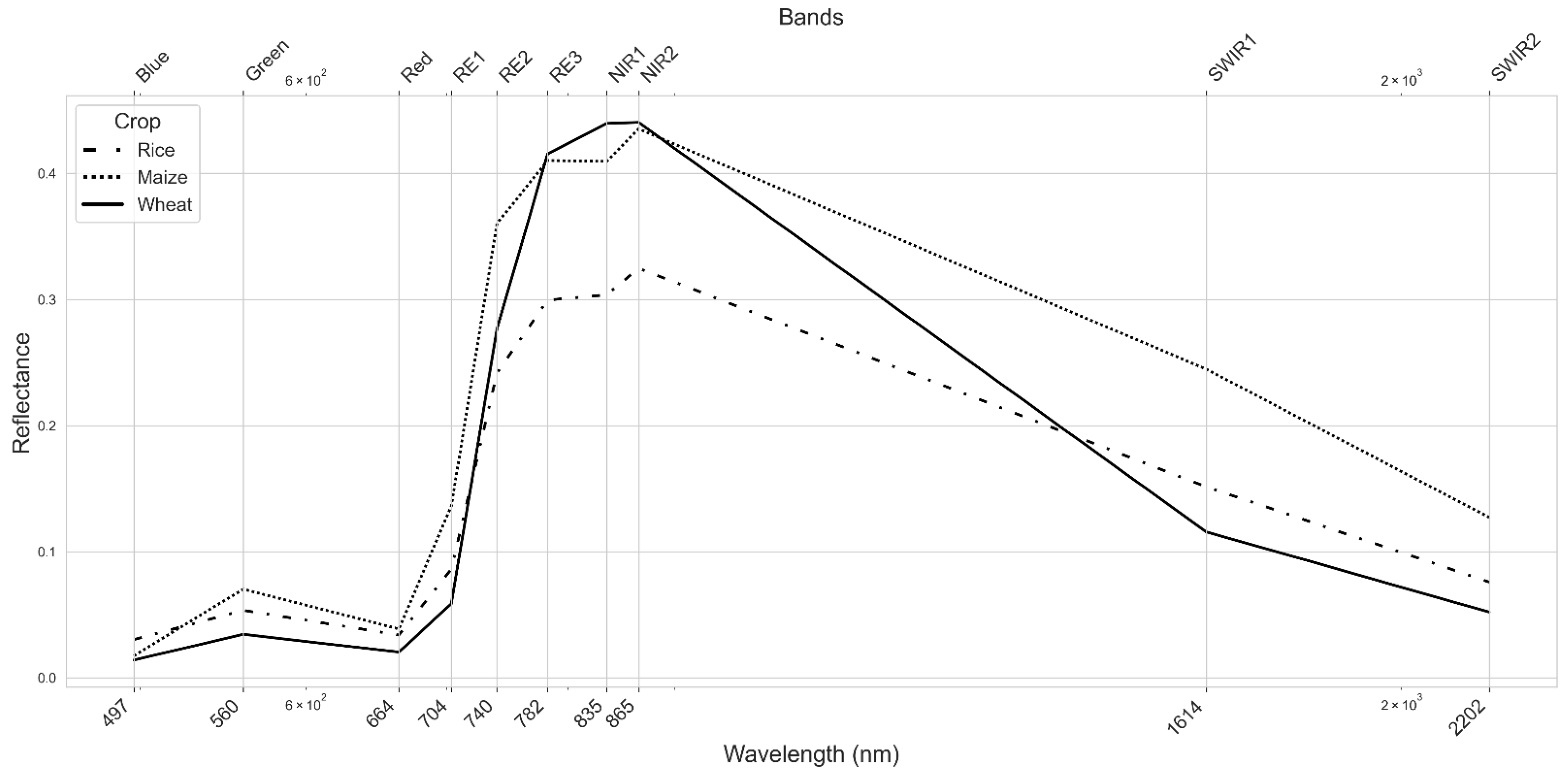
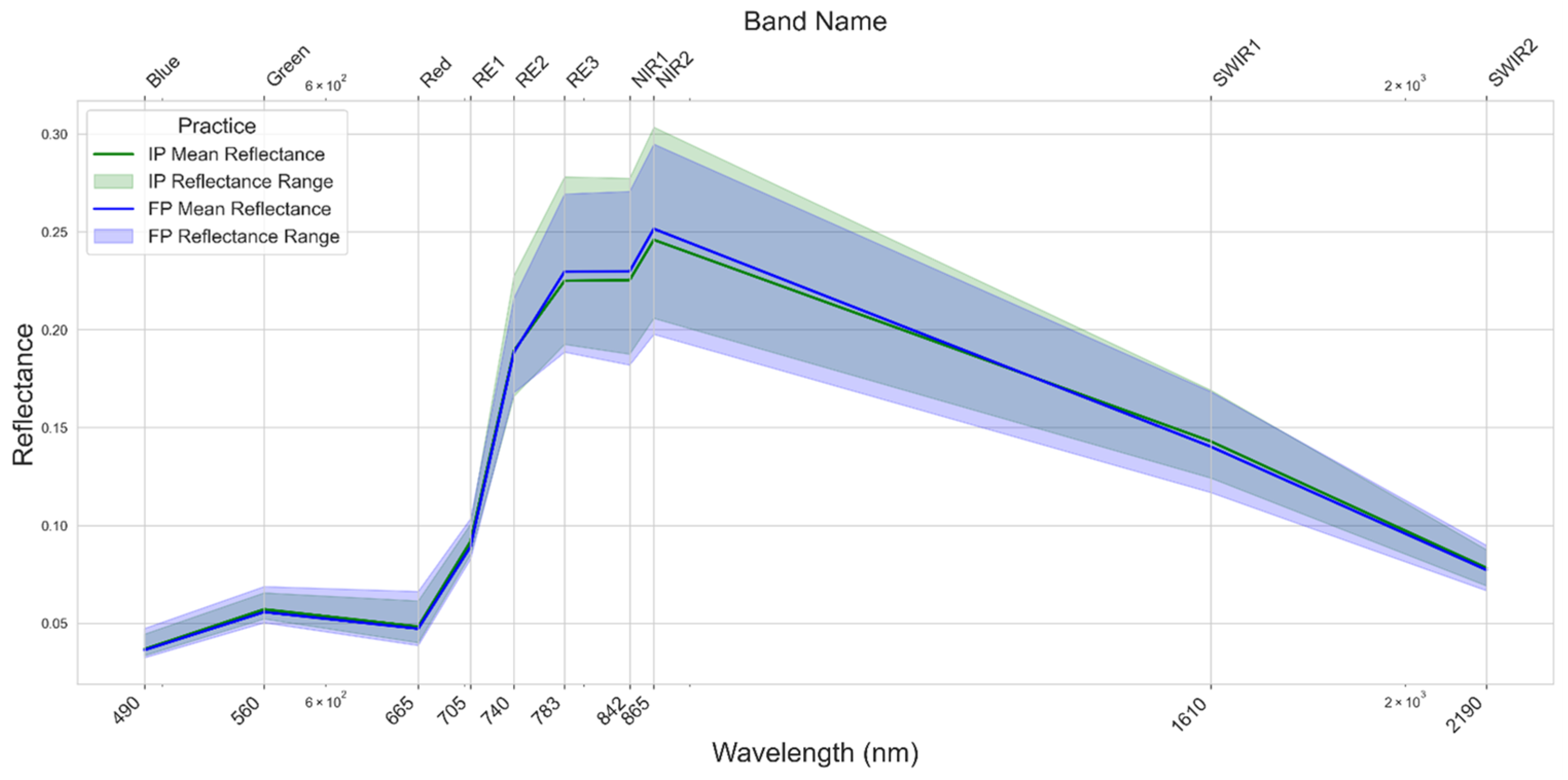
3.1. Spectral Reflectance of Maize, Wheat, and Rice
3.2. Spectral Reflectance of Maize
3.3. Spectral Reflectance of Wheat
3.4. Spectral Reflectance of Rice
4. Discussion
4.1. Implications of Spectral Reflectance Patterns for Crop Discrimination
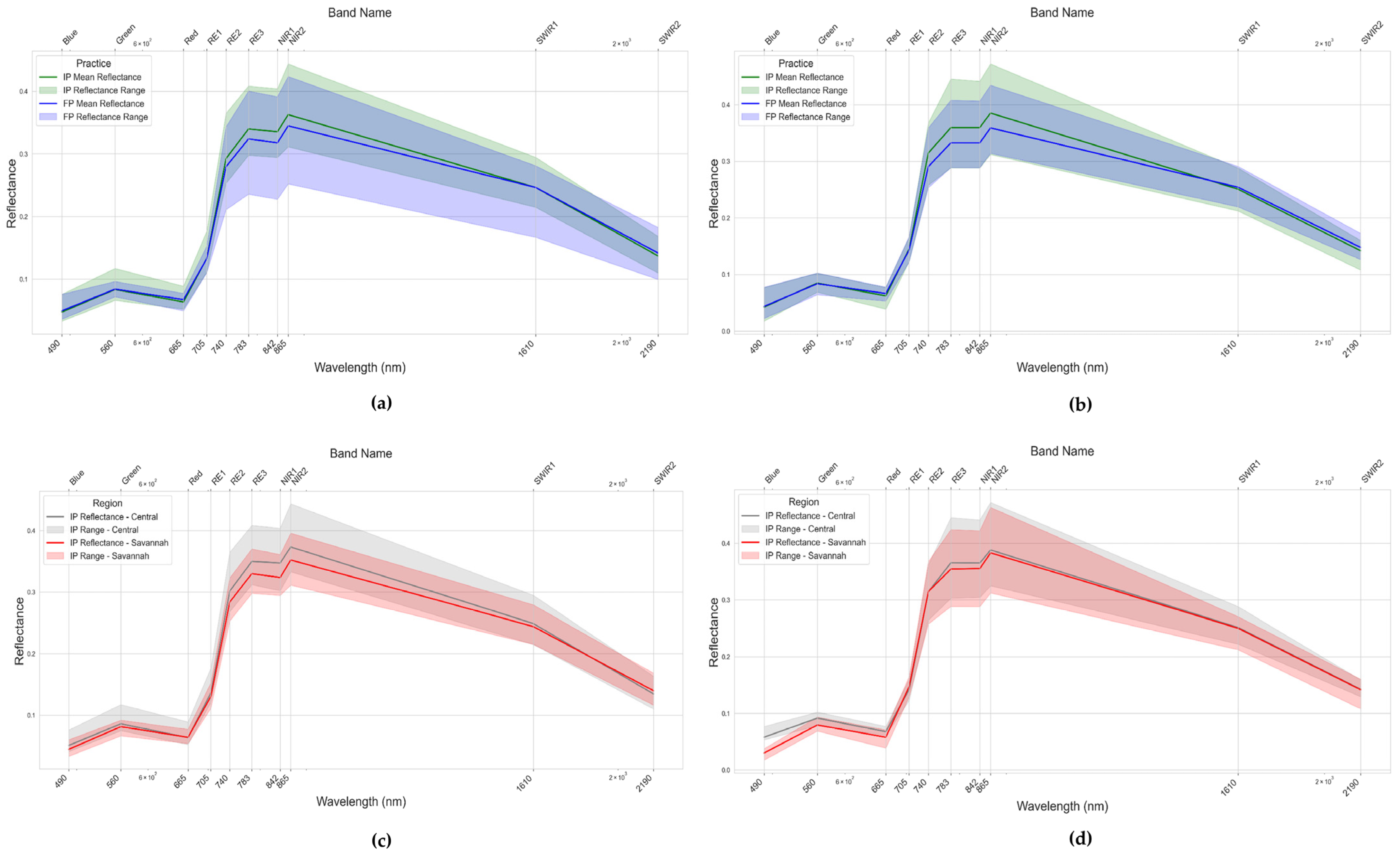
4.2. Management and Regional Effects on Maize Spectral Reflectance in Togo
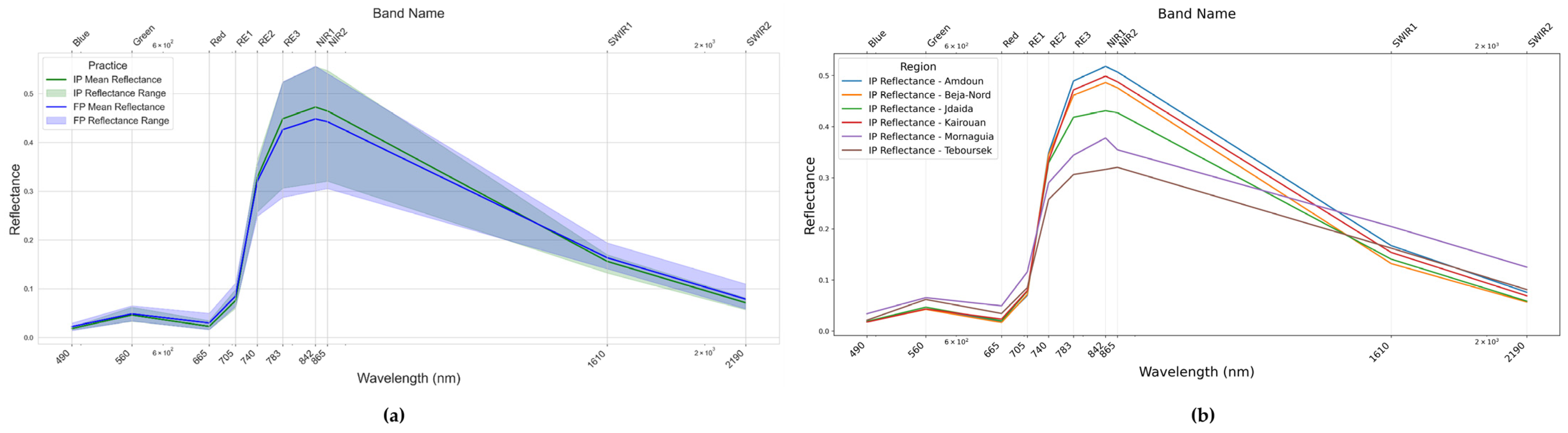
4.3. Management and Regional Effects on Wheat Spectral Reflectance in Tunisia
4.4. Management Effects on Rice Spectral Reflectance in Tanzania
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Alahira, J. Atlas of African Agriculture. Available online: https://www.agriculturenigeria.com/atlas-of-african-agriculture/ (accessed on 19 January 2025).
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.J. Global Trends in Wheat Production, Consumption and Trade; Springer Nature: London, UK, 2022; Chapter 4; ISBN 978-3-030-90672-6. [Google Scholar]
- Rapsomanikis, G. The Economic Lives of Smallholder Farmers; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Jayne, T.S.; Mather, D.; Mghenyi, E. Principal Challenges Confronting Smallholder Agriculture in Sub-Saharan Africa. World Dev. 2010, 38, 1384–1398. [Google Scholar] [CrossRef]
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting Maize Production to Climate Change in Sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]
- Nyambo, P.; Nyambo, P.; Mavunganidze, Z.; Nyambo, V. Sub-Saharan Africa Smallholder Farmers Agricultural Productivity: Risks and Challenges. In Food Security for African Smallholder Farmers; Mupambwa, H.A., Nciizah, A.D., Nyambo, P., Muchara, B., Gabriel, N.N., Eds.; Springer Nature: Singapore, 2022; pp. 47–58. ISBN 9789811667718. [Google Scholar]
- Mpandeli, S.; Maponya, P. Constraints and Challenges Facing the Small Scale Farmers in Limpopo Province, South Africa. J. Agric. Sci. 2014, 6, 135. [Google Scholar] [CrossRef]
- Dhillon, R.; Moncur, Q. Small-Scale Farming: A Review of Challenges and Potential Opportunities Offered by Technological Advancements. Sustainability 2023, 15, 15478. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y. Practices, Challenges, and Future of Digital Transformation in Smallholder Agriculture: Insights from a Literature Review. Agriculture 2024, 14, 2193. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Aker, J.C. Dial “A” for Agriculture: A Review of Information and Communication Technologies for Agricultural Extension in Developing Countries. Agric. Econ. 2011, 42, 631–647. [Google Scholar] [CrossRef]
- Feder, G.; Savastano, S. The Role of Opinion Leaders in the Diffusion of New Knowledge: The Case of Integrated Pest Management. World Dev. 2006, 34, 1287–1300. [Google Scholar] [CrossRef]
- Davis, K.; Nkonya, E.; Kato, E.; Mekonnen, D.A.; Odendo, M.; Miiro, R.; Nkuba, J. Impact of Farmer Field Schools on Agricultural Productivity and Poverty in East Africa. World Dev. 2012, 40, 402–413. [Google Scholar] [CrossRef]
- Anderson, J.R.; Feder, G. Agricultural Extension: Good Intentions and Hard Realities. World Bank Res. Obs. 2004, 19, 41–60. [Google Scholar] [CrossRef]
- Omia, E.; Bae, H.; Park, E.; Kim, M.S.; Baek, I.; Kabenge, I.; Cho, B.-K. Remote Sensing in Field Crop Monitoring: A Comprehensive Review of Sensor Systems, Data Analyses and Recent Advances. Remote Sens. 2023, 15, 354. [Google Scholar] [CrossRef]
- Li, M.; Shamshiri, R.R.; Weltzien, C.; Schirrmann, M. Crop Monitoring Using Sentinel-2 and UAV Multispectral Imagery: A Comparison Case Study in Northeastern Germany. Remote Sens. 2022, 14, 4426. [Google Scholar] [CrossRef]
- Segarra, J.; Buchaillot, M.L.; Araus, J.L.; Kefauver, S.C. Remote Sensing for Precision Agriculture: Sentinel-2 Improved Features and Applications. Agronomy 2020, 10, 641. [Google Scholar] [CrossRef]
- Guin, A.; Sahoo, S.; Samanta, S.; Maity, N.; Bera, B. Advancements in Agricultural Technology: A Historical Perspective. In Smart and Sustainable Agricultural Technology; Lovely Professional University (LPU): Phagwara, India, 2023; ISBN 978-81-19-33475-9. [Google Scholar]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Crespin-Boucaud, A.; Lebourgeois, V.; Lo Seen, D.; Castets, M.; Bégué, A. Agriculturally Consistent Mapping of smallholder Farming Systems Using Remote Sensing and Spatial Modelling. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2020, XLII-3/W11, 35–42. [Google Scholar] [CrossRef]
- Bowker, E.; Davis, E.; Myrick, L. Spectral Reflectances of Natural Targets for Use in Remote Sensing Studies; National Aeronautics and Space Administration: Washington, DC, USA, 1985.
- Lammy Nkuna, B.; George Chirima, J.; Newete, S.W.; Nyamugama, A.; van der Walt, A.J. Developing Models to Detect Maize Diseases Using Spectral Vegetation Indices Derived from Spectral Signatures. Egypt. J. Remote Sens. Space Sci. 2024, 27, 597–603. [Google Scholar] [CrossRef]
- Zhou, Z.; Plauborg, F.; Thomsen, A.G.; Andersen, M.N. A RVI/LAI-Reference Curve to Detect N Stress and Guide N Fertigation Using Combined Information from Spectral Reflectance and Leaf Area Measurements in Potato. Eur. J. Agron. 2017, 87, 1–7. [Google Scholar] [CrossRef]
- Raypah, M.E.; Nasru, M.I.M.; Nazim, M.H.H.; Omar, A.F.; Zahir, S.A.D.M.; Jamlos, M.F.; Muncan, J. Spectral Response to Early Detection of Stressed Oil Palm Seedlings Using Near-Infrared Reflectance Spectra at Region 900-1000 Nm. Infrared Phys. Technol. 2023, 135, 104984. [Google Scholar] [CrossRef]
- Peroni Venancio, L.; Chartuni Mantovani, E.; do Amaral, C.H.; Usher Neale, C.M.; Zution Gonçalves, I.; Filgueiras, R.; Coelho Eugenio, F. Potential of Using Spectral Vegetation Indices for Corn Green Biomass Estimation Based on Their Relationship with the Photosynthetic Vegetation Sub-Pixel Fraction. Agric. Water Manag. 2020, 236, 106155. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, X.; Ma, Y.; Liu, T.; He, L.; Yang, F.; Shu, C.; Li, L.; Fu, H.; Li, B.; et al. Combining Canopy Spectral Reflectance and RGB Images to Estimate Leaf Chlorophyll Content and Grain Yield in Rice. Comput. Electron. Agric. 2024, 221, 108975. [Google Scholar] [CrossRef]
- Lambert, M.-J.; Traoré, P.C.S.; Blaes, X.; Baret, P.; Defourny, P. Estimating Smallholder Crops Production at Village Level from Sentinel-2 Time Series in Mali’s Cotton Belt. Remote Sens. Environ. 2018, 216, 647–657. [Google Scholar] [CrossRef]
- World Bank Group Climate in Togo. Available online: https://climateknowledgeportal.worldbank.org/country/togo (accessed on 20 January 2025).
- Renaud, A. 1.5 Million People Cultivate Maize in Togo. Available online: https://www.togofirst.com/en/agriculture/0209-3774-1-5-million-people-cultivate-maize-in-togo (accessed on 20 June 2024).
- Food and Agriculture Organization of the United Nations. FAO Country Profiles: Togo. Available online: http://www.fao.org/countryprofiles/index/en/?iso3=TGO (accessed on 19 January 2025).
- World Bank. Climate Change Knowledge Portal. Tunisia Climate. Available online: https://climateknowledgeportal.worldbank.org/country/tunisia (accessed on 20 January 2025).
- Latiri, K.; Lhomme, J.-P.; Annabi, M.; Setter, T. Wheat Production in Tunisia: Progress, Inter-Annual Variability and Relation to Rainfall. Eur. J. Agron. 2010, 33, 33–42. [Google Scholar] [CrossRef]
- USDA. Rice Explorer—Tanzania. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/comm_chartview.aspx?ftypeid=47&fattributeid=1&fctypeid=60&fcattributeid=1®ionid=safrica&cntryid=TZA&cropid=0422110&nationalgraph=False&sel_year=2022&startrow=1 (accessed on 20 January 2025).
- USDA, Foreign Agricultural Service. Tanzania: Grain and Feed Annual. Available online: https://www.fas.usda.gov/data/tanzania-grain-and-feed-annual-7 (accessed on 19 January 2025).
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- DEAfrica Team. Analysis Sandbox—Digital Earth Africa 2021 Documentation. Available online: https://docs.digitalearthafrica.org/en/latest/sandbox/index.html (accessed on 19 January 2025).
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated Narrow-Band Vegetation Indices for Prediction of Crop Chlorophyll Content for Application to Precision Agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Baret, F.; Guyot, G. Potentials and Limits of Vegetation Indices for LAI and APAR Assessment. Remote Sens. Environ. 1991, 35, 161–173. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M. Remote Sensing of Chlorophyll Concentration in Higher Plant Leaves. Adv. Space Res. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Thenkabail, P. Biophysical and Yield Information for Precision Farming from Near-Real-Time and Historical Landsat TM Images. Int. J. Remote Sens. 2003, 24, 2879–2904. [Google Scholar] [CrossRef]
- Shibayama, M.; Sakamoto, T.; Takada, E.; Inoue, A.; Morita, K.; Takahashi, W.; Kimura, A. Continuous Monitoring of Visible and Near-Infrared Band Reflectance from a Rice Paddy for Determining Nitrogen Uptake Using Digital Cameras. Plant Prod. Sci. 2009, 12, 293–306. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Huete, A.R. A Soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.; Gonzalez-Dugo, V.; Berni, J.A.J. Fluorescence, Temperature and Narrow-Band Indices Acquired from a UAV Platform for Water Stress Detection Using a Micro-Hyperspectral Imager and a Thermal Camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Alonso, L.; Moreno, J. Evaluation of Sentinel-2 Red-Edge Bands for Empirical Estimation of Green LAI and Chlorophyll Content. Sensors 2011, 11, 7063–7081. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Hunt, E.R.; Jackson, T.J. Remote Sensing of Vegetation Water Content from Equivalent Water Thickness Using Satellite Imagery. Remote Sens. Environ. 2008, 112, 2514–2522. [Google Scholar] [CrossRef]
- Ghulam, A.; Li, Z.-L.; Qin, Q.; Yimit, H.; Wang, J. Estimating Crop Water Stress with ETM+ NIR and SWIR Data. Agric. For. Meteorol. 2008, 148, 1679–1695. [Google Scholar] [CrossRef]
- Xiao, X.; Boles, S.; Frolking, S.; Li, C.; Babu, J.Y.; Salas, W.; Moore, B. Mapping Paddy Rice Agriculture in South and Southeast Asia Using Multi-Temporal MODIS Images. Remote Sens. Environ. 2006, 100, 95–113. [Google Scholar] [CrossRef]
- Ceccato, P.; Flasse, S.; Tarantola, S.; Jacquemoud, S.; Grégoire, J.-M. Detecting Vegetation Leaf Water Content Using Reflectance in the Optical Domain. Remote Sens. Environ. 2001, 77, 22–33. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of Spectral Remote Sensing for Agronomic Decisions. Agron. J. 2008, 100, S-117–S-131. [Google Scholar] [CrossRef]
- Stournaras, S.; Arvanitis, K.; Loukatos, D.; Kalatzis, N. Crop Identification by Machine Learning Algorithm and Sentinel-2 Data. Chem. Proc. 2022, 10, 20. [Google Scholar] [CrossRef]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.-M.; Tucker, C.J.; Stenseth, N.C. Using the Satellite-Derived NDVI to Assess Ecological Responses to Environmental Change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Gao, B. NDWI—A Normalized Difference Water Index for Remote Sensing of Vegetation Liquid Water from Space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Small, C. The Landsat ETM+ Spectral Mixing Space. Remote Sens. Environ. 2004, 93, 1–17. [Google Scholar] [CrossRef]
| Countries † | Average Yield FP ‡ | Average Yield IP ¶ | Yield Gap |
|---|---|---|---|
| ---------------------------------------- kg ha−1 -------------------------------- | |||
| Tanzania | 3452 A | 4585 B | 1133 |
| Togo 2021 | 2144 A | 4402 B | 2258 |
| Togo 2022 | 2323 A | 4663 B | 2340 |
| Tunisia | 2016 A | 2200 A | 184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biaou, A.; Phillips, S.; Adolwa, I.; Sogbedji, J.; Mechri, M.; Kavishe, B. Assessing Spectral Reflectance in African Smallholder Cereal Farms Using Sentinel-2 Imagery. Remote Sens. 2025, 17, 3135. https://doi.org/10.3390/rs17183135
Biaou A, Phillips S, Adolwa I, Sogbedji J, Mechri M, Kavishe B. Assessing Spectral Reflectance in African Smallholder Cereal Farms Using Sentinel-2 Imagery. Remote Sensing. 2025; 17(18):3135. https://doi.org/10.3390/rs17183135
Chicago/Turabian StyleBiaou, Aicha, Steve Phillips, Ivan Adolwa, Jean Sogbedji, Mouna Mechri, and Basil Kavishe. 2025. "Assessing Spectral Reflectance in African Smallholder Cereal Farms Using Sentinel-2 Imagery" Remote Sensing 17, no. 18: 3135. https://doi.org/10.3390/rs17183135
APA StyleBiaou, A., Phillips, S., Adolwa, I., Sogbedji, J., Mechri, M., & Kavishe, B. (2025). Assessing Spectral Reflectance in African Smallholder Cereal Farms Using Sentinel-2 Imagery. Remote Sensing, 17(18), 3135. https://doi.org/10.3390/rs17183135





