Anthropogenic Forcing on the Coevolution of Tidal Creeks and Vegetation in the Dongtan Wetland, Changjiang Estuary
Abstract
1. Introduction
2. Materials and Methods
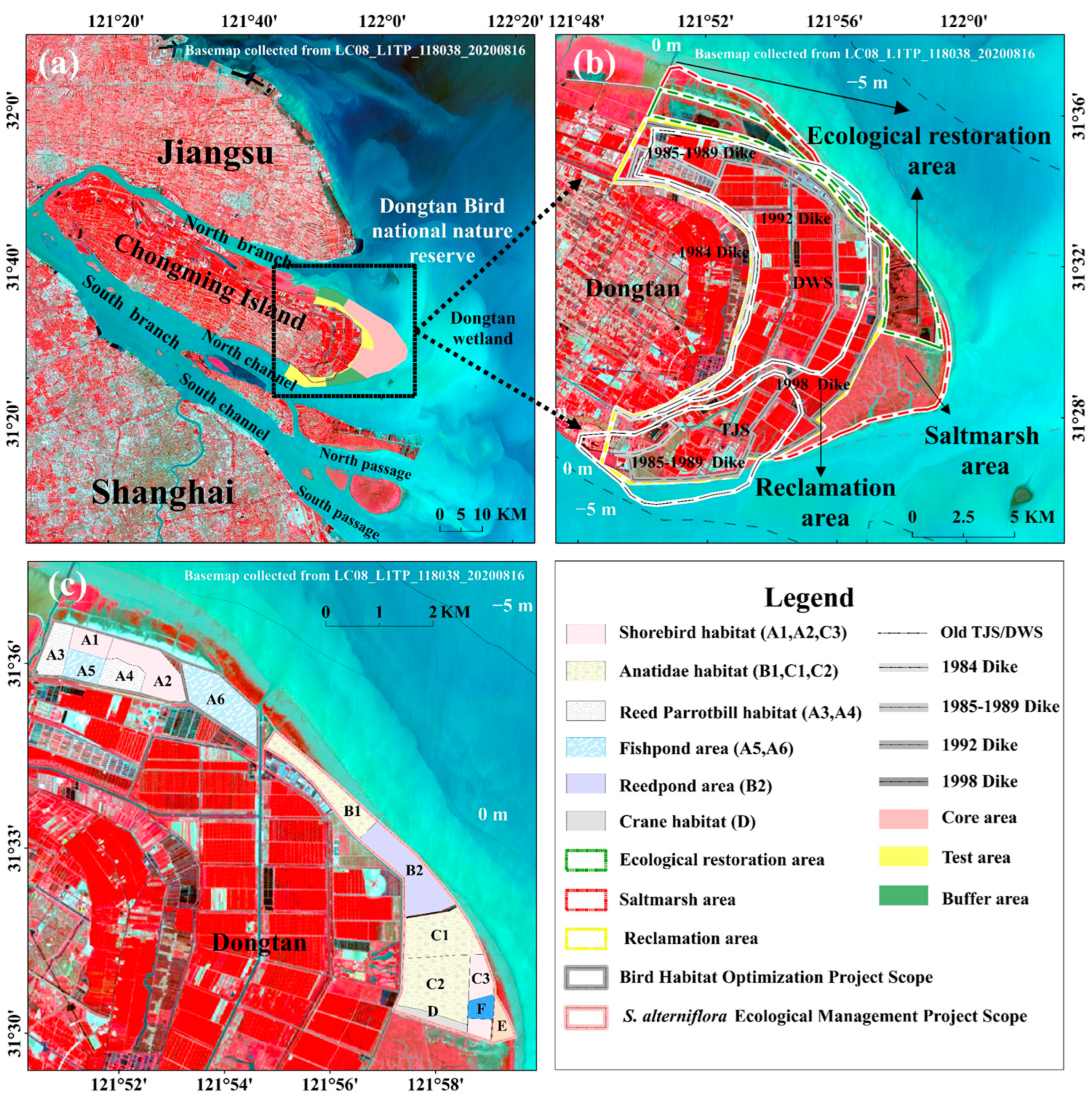
2.1. Study Area
2.2. Data Sources
2.3. Vegetation Information Extraction
2.3.1. Preprocessing Workflow
2.3.2. Feature Extraction and Hierarchical Classification
2.3.3. Accuracy Assessment
2.4. Tidal Creek Morphometric Analysis
2.4.1. Object-Based Segmentation
2.4.2. Horton–Strahler Classification
2.4.3. Morphometric Quantification
2.4.4. Fractal Dimension Quantification
2.5. Spatiotemporal Dynamics Analysis
2.5.1. Markov Transition Matrix
2.5.2. TCD-NDVI Coupling Analysis
3. Results
3.1. Vegetation Pattern Evolution
3.1.1. Spatial and Temporal Changes in the Landscape Pattern of the Dongtan Wetland
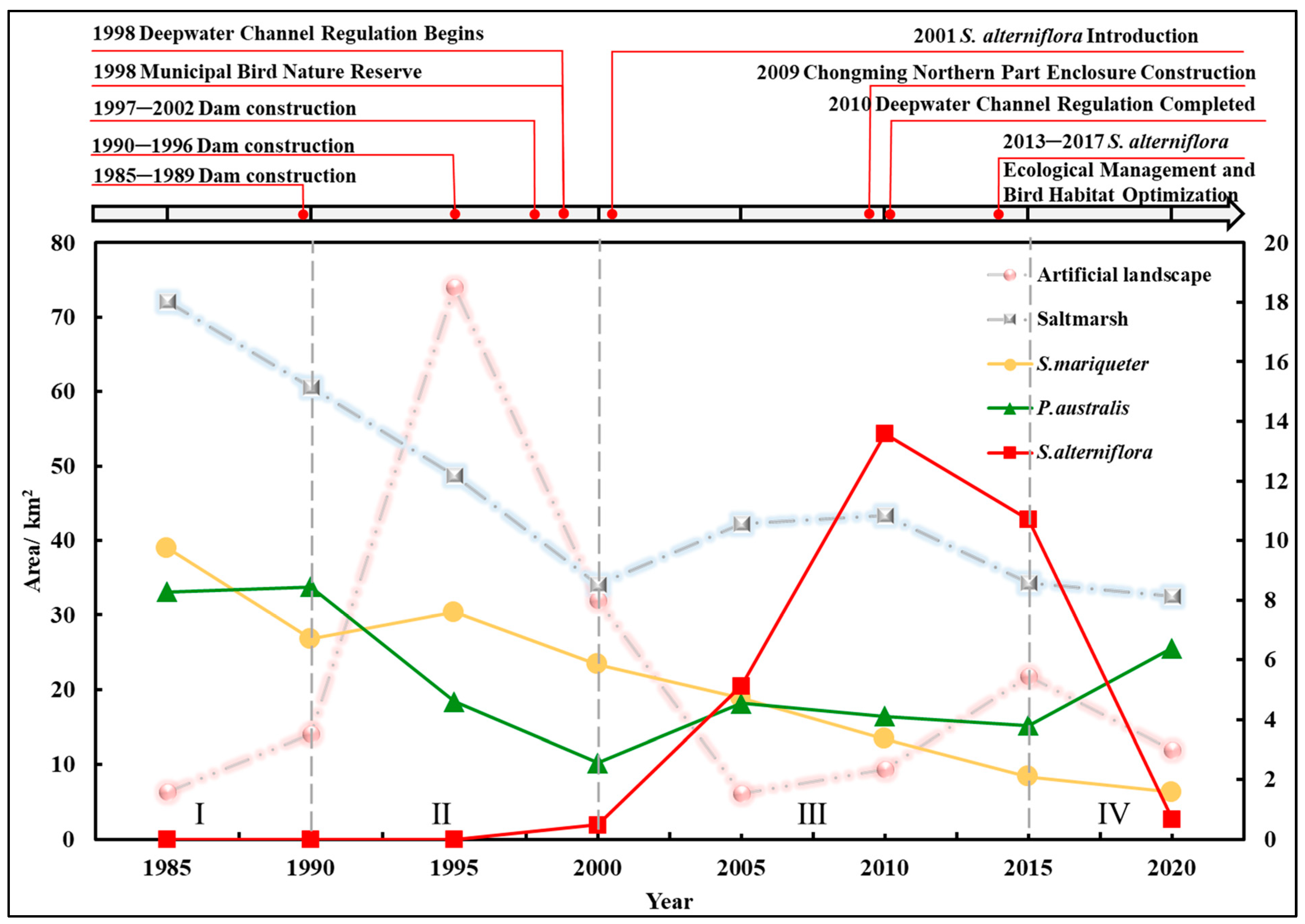
3.1.2. Changes in the Area of Dominant Vegetation
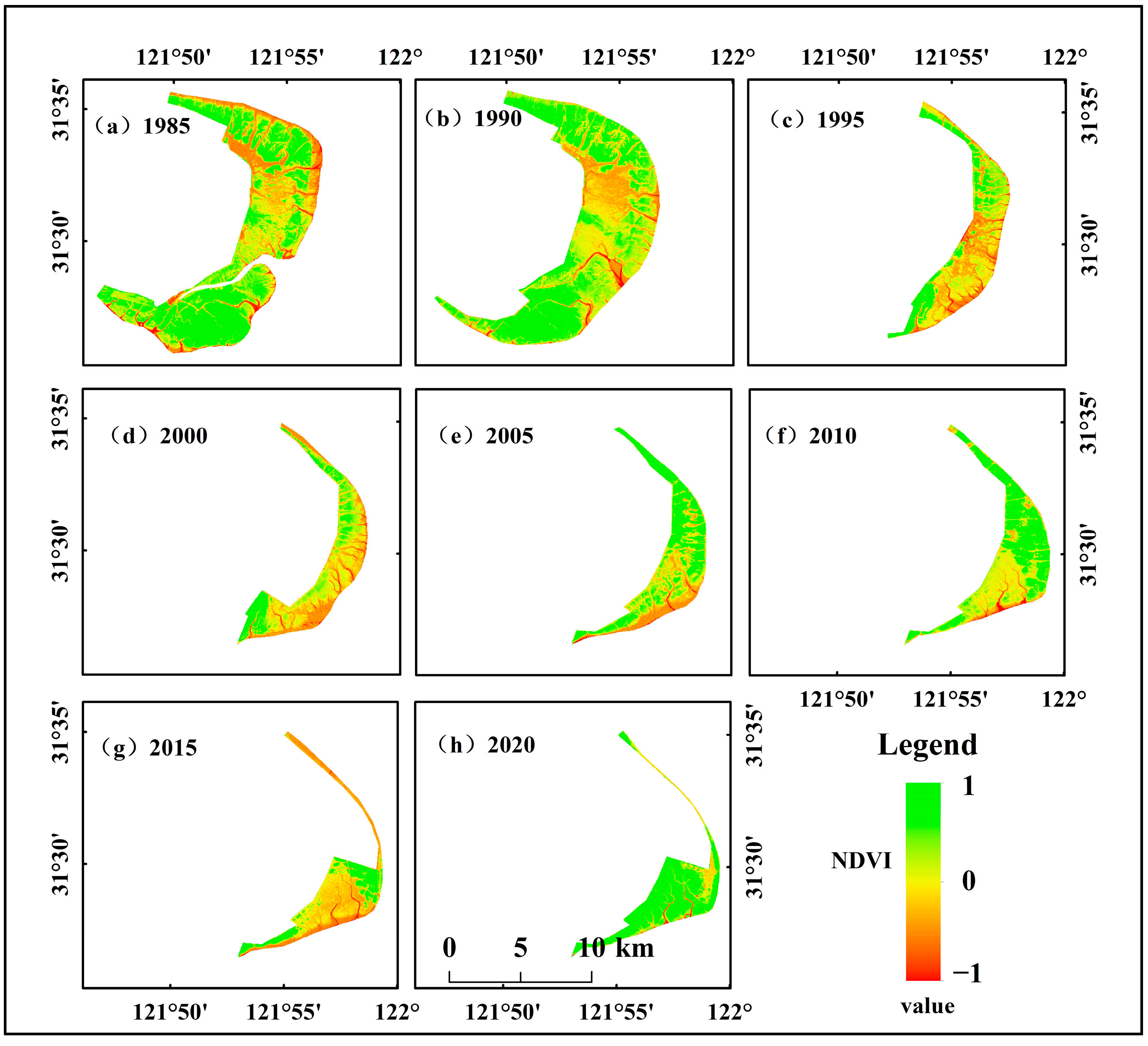
3.1.3. Changes in Vegetation Growth in the Dongtan Wetland
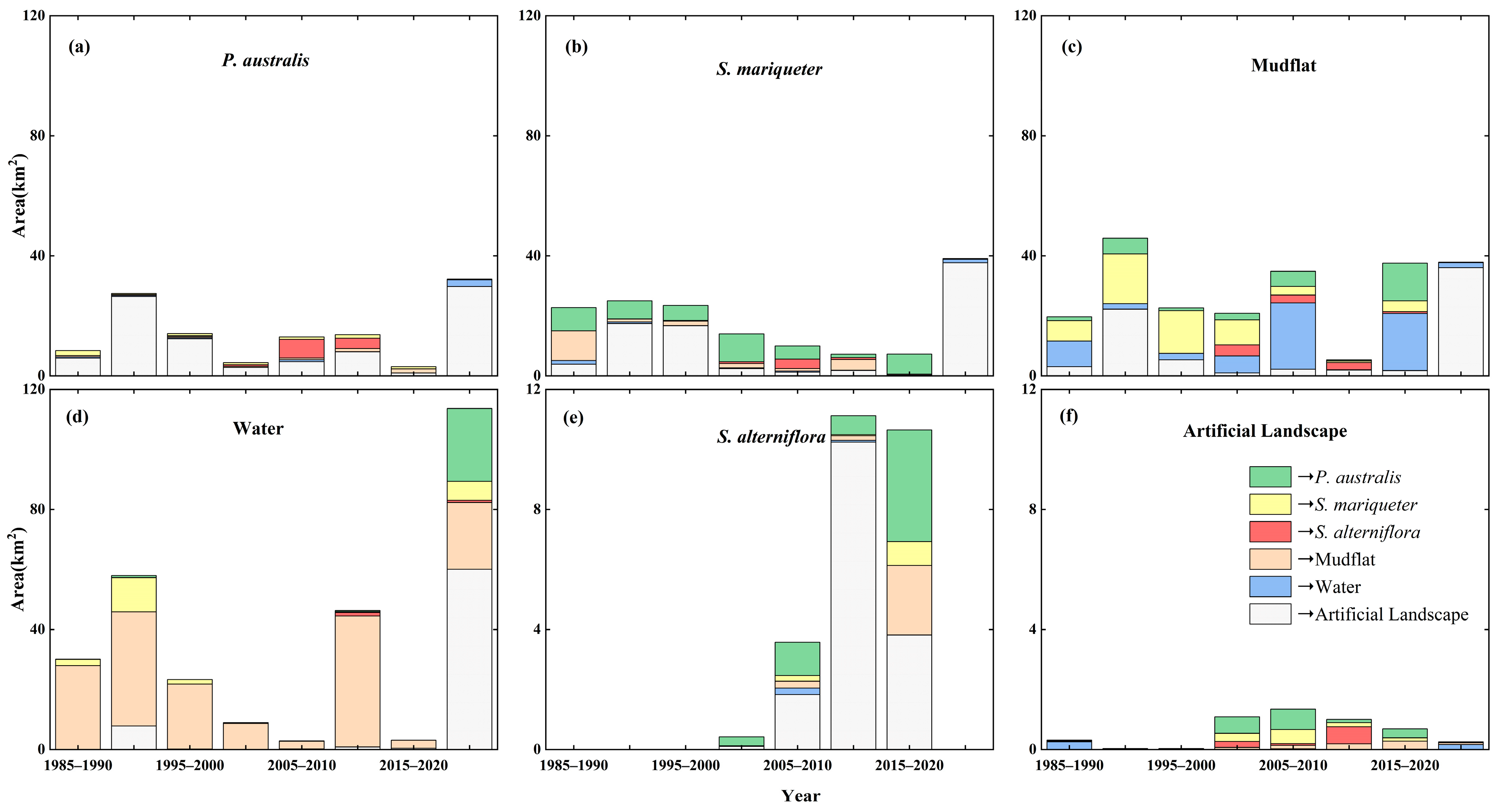
3.1.4. Landscape Transfer Patterns Under the Influence of Human Activities and Natural Factors
3.2. Development of the Tidal Creek System
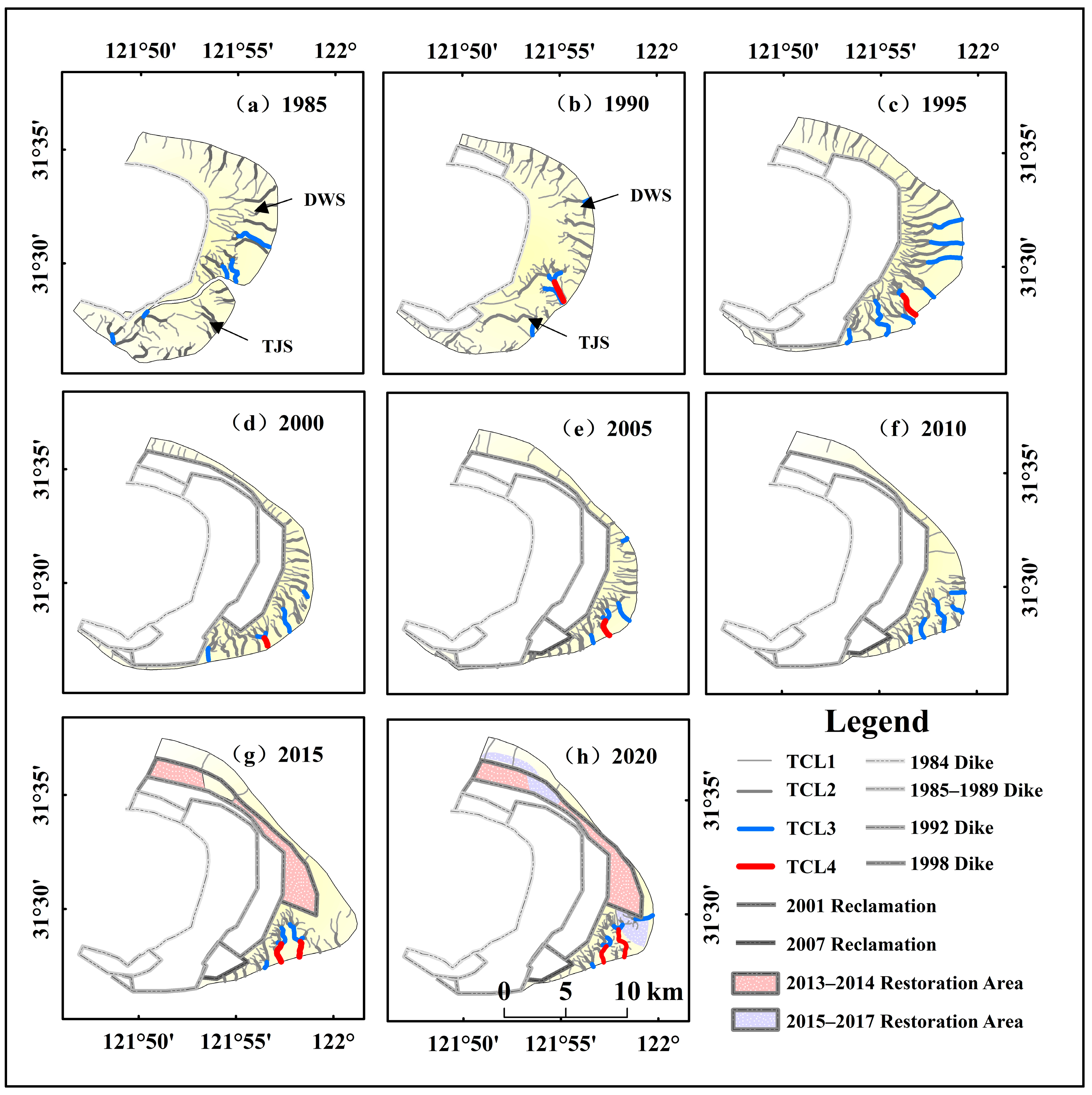
3.2.1. Spatial and Temporal Variations in Tidal Creek Distribution
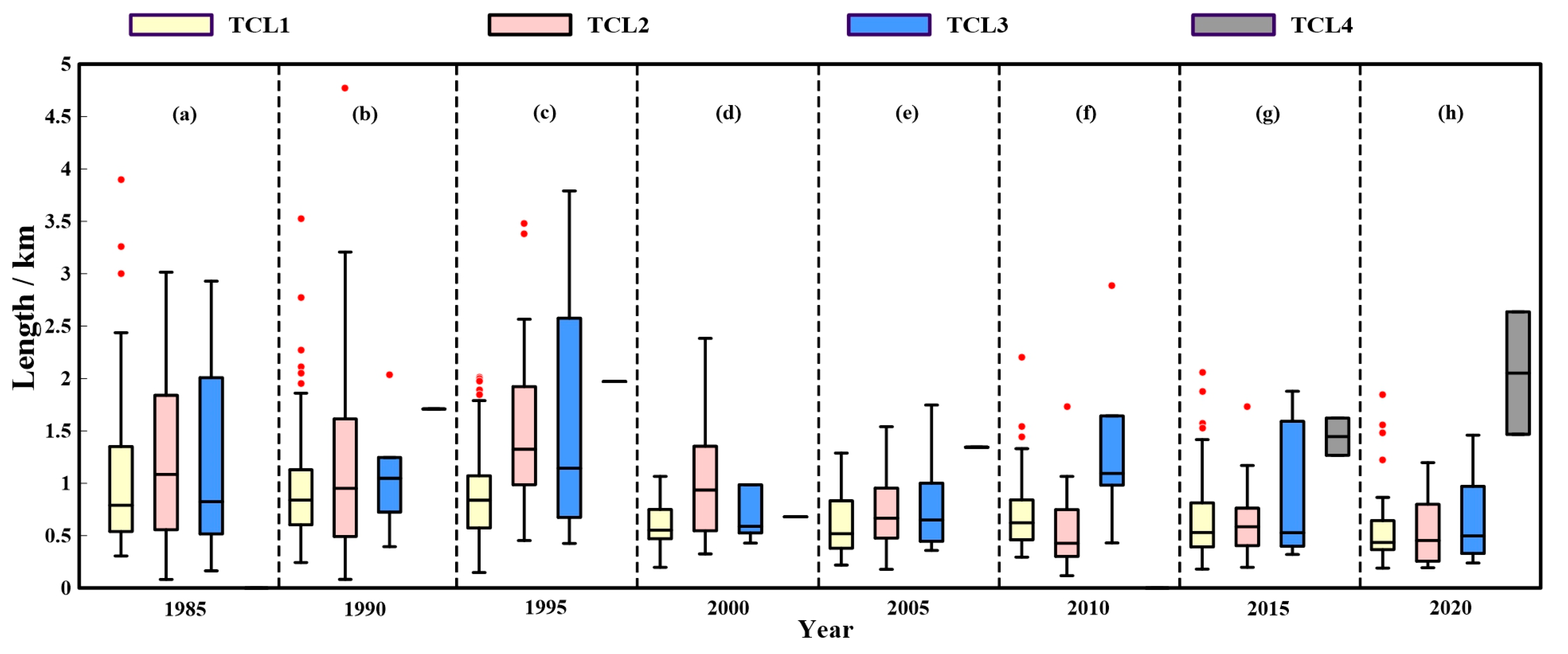
3.2.2. Changes in Length of Each Tidal Creek Level
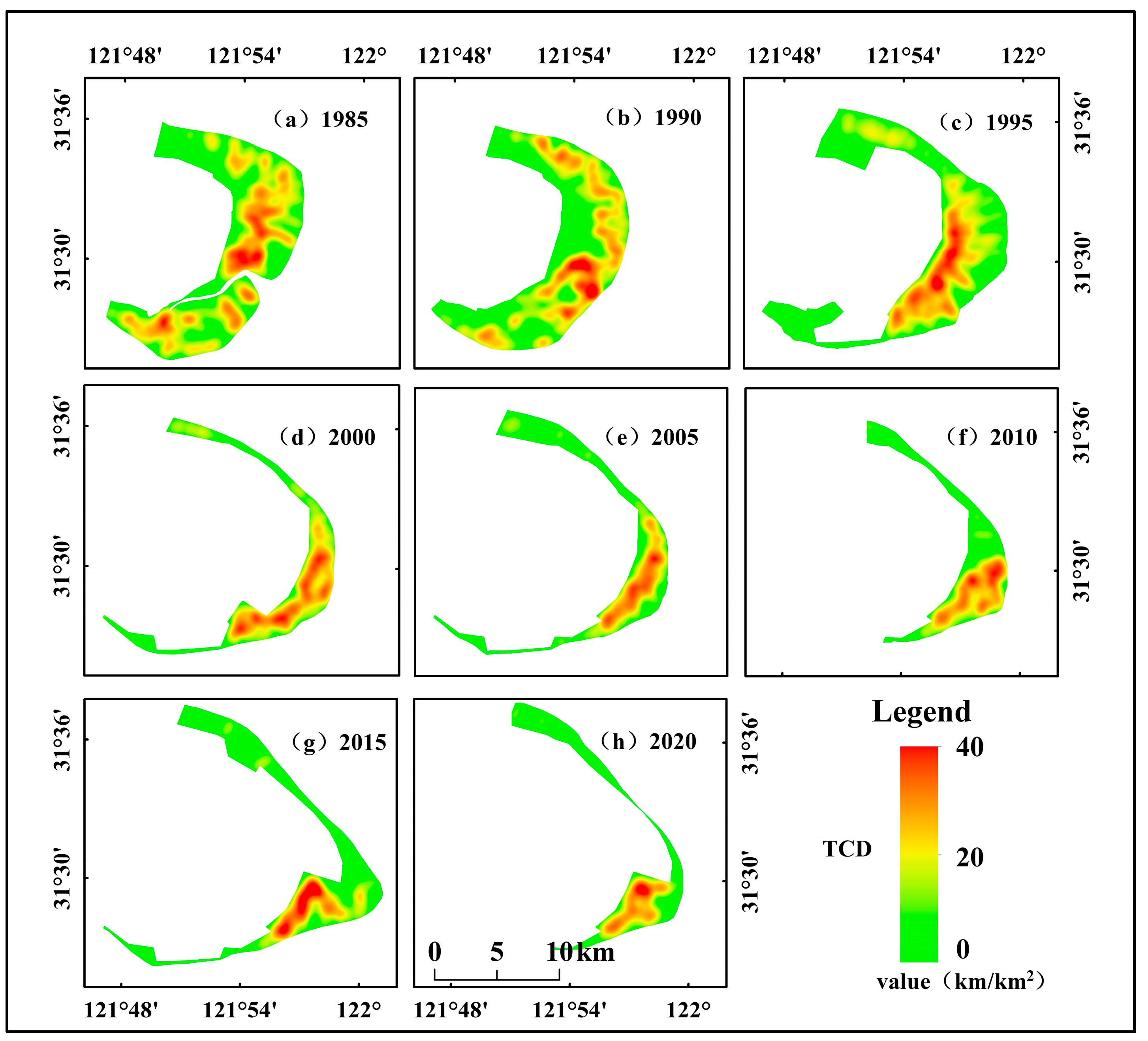
3.2.3. Changes in Tidal Creek Density Distribution
3.2.4. Characterization of Changes in Tidal Creek Properties Due to Human Activities and Natural Factors
4. Discussion
4.1. Interactions Between Vegetation and Tidal Creeks Under the Influence of Human Activities and Natural Factors
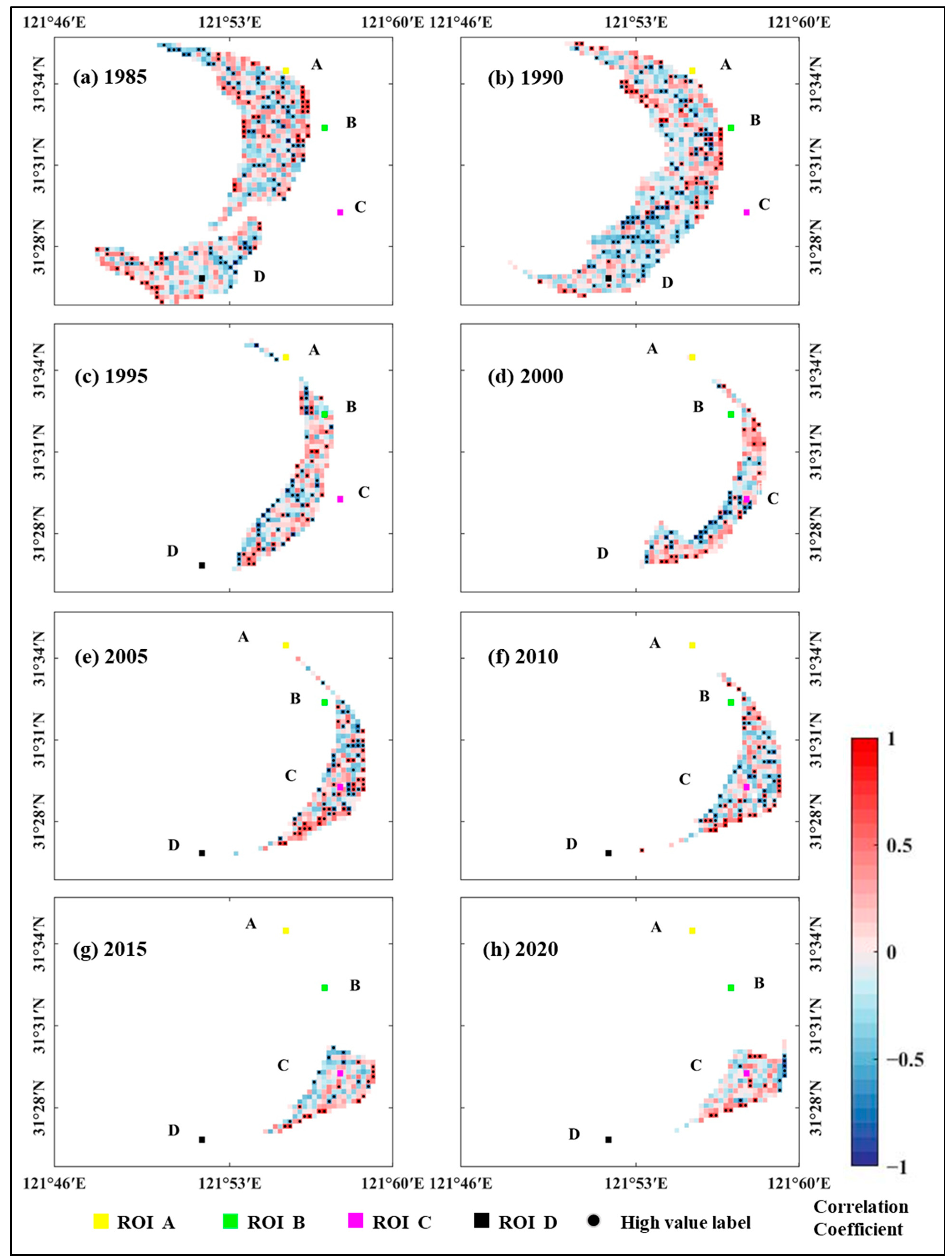
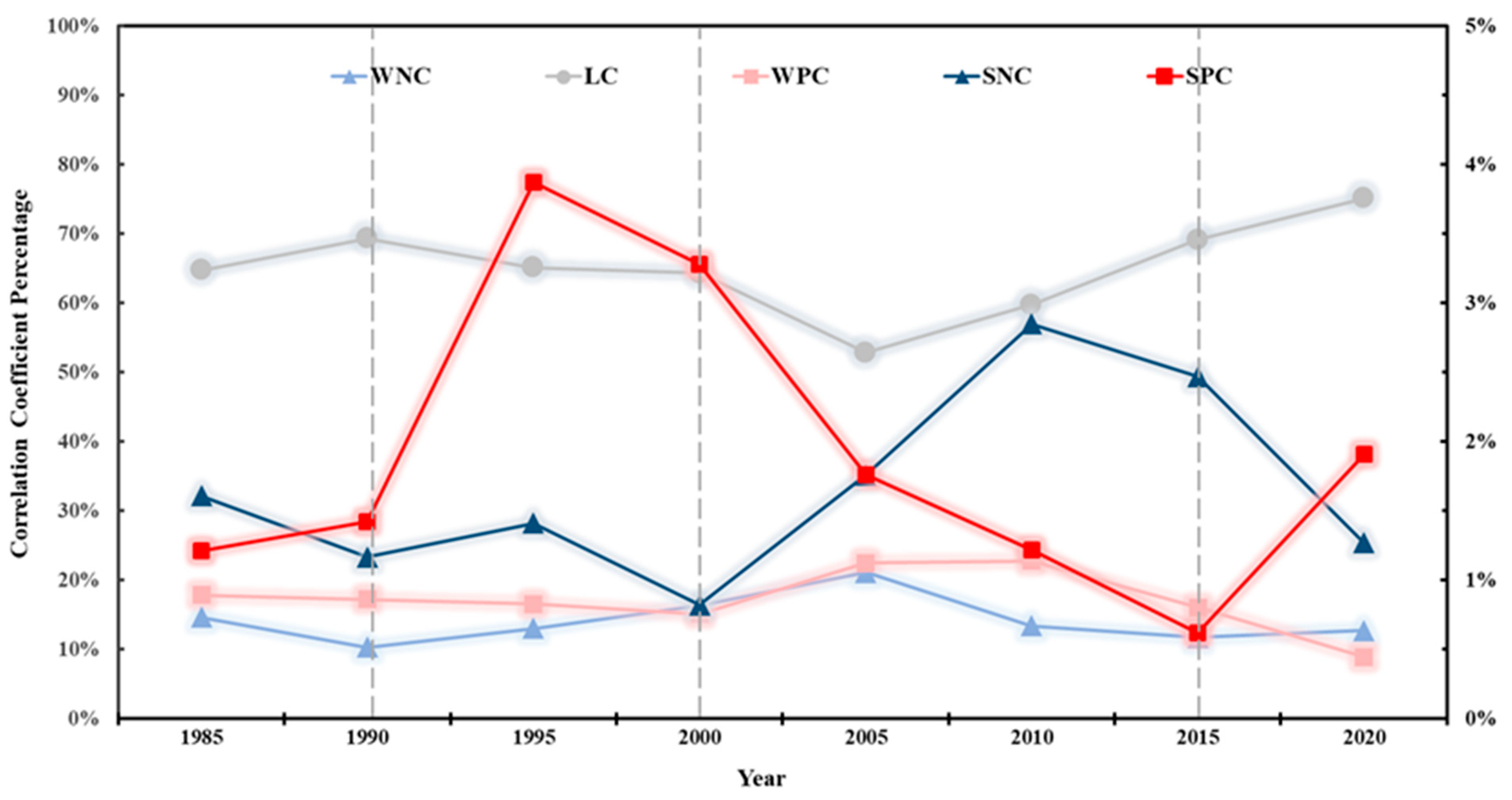
4.1.1. Spatial and Temporal Evolution of the Correlation Between Tidal Creek Attributes and Vegetation Indices
4.1.2. Temporal Variation of Correlation Coefficients in Areas of Interest
4.2. Mechanisms of Interaction Between Vegetation and Tidal Creeks
4.2.1. Mechanism of Vegetation Affecting the Development of Tidal Creeks
4.2.2. Mechanisms of Tidal Creeks Affecting the Evolution of Vegetation Patterns
4.3. Implications of Human Activities on the Dongtan Wetland
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odum, H.T.; Odum, E.P. The Energetic Basis for Valuation of Ecosystem Services. Ecosystems 2000, 3, 21–23. [Google Scholar] [CrossRef]
- Daidu, F.; Yuan, W.; Min, L. Classifications, sedimentary features and facies associations of tidal flats. J. Palaeogeogr. 2013, 2, 66–80. [Google Scholar] [CrossRef]
- Fan, D.; Li, C.; Wang, D.; Wang, P.; Greb, A.S.F. Morphology and Sedimentation on Open-Coast Intertidal Flats of the Changjiang Delta, China. J. Coast. Res. 2004, 20, 23–35. [Google Scholar]
- Wang, X.; Xiao, X.; Xu, X.; Zou, Z.; Chen, B.; Qin, Y.; Zhang, X.; Dong, J.; Liu, D.; Pan, L.; et al. Rebound in China’s coastal wetlands following conservation and restoration. Nat. Sustain. 2021, 4, 1076–1083. [Google Scholar] [CrossRef]
- Xu, Y.; Esposito, C.R.; Beltran-Burgos, M.; Nepf, H.M. Competing effects of vegetation density on sedimentation in deltaic marshes. Nat. Commun. 2022, 13, 4641. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Wong, P.P.; Burkett, V.; Codignotto, J.; Saito, Y. Coastal systems and low-lying areas. J. Bacteriol. 2007, 2, 313–356. [Google Scholar]
- Gedan, K.B.; Silliman, B.R.; Bertness, M.D. Centuries of human-driven change in salt marsh ecosystems. Ann. Rev. Mar. Sci. 2009, 1, 117–141. [Google Scholar] [CrossRef]
- Coco, G.; Zhou, Z.; van Maanen, B.; Olabarrieta, M.; Tinoco, R.; Townend, I. Morphodynamics of tidal networks: Advances and challenges. Mar. Geol. 2013, 346, 1–16. [Google Scholar] [CrossRef]
- Murray, N.J.; Phinn, S.R.; DeWitt, M.; Ferrari, R.; Johnston, R.; Lyons, M.B.; Clinton, N.; Thau, D.; Fuller, R.A. The global distribution and trajectory of tidal flats. Nature 2019, 565, 222–225. [Google Scholar] [CrossRef]
- Blum, M.D.; Roberts, H.H. Drowning of the Mississippi Delta due to insufficient sediment supply and global sea-level rise. Nat. Geosci. 2009, 2, 488–491. [Google Scholar] [CrossRef]
- Francalanci, S.; Bendoni, M.; Rinaldi, M.; Solari, L. Ecomorphodynamic evolution of salt marshes: Experimental observations of bank retreat processes. Geomorphology 2013, 195, 53–65. [Google Scholar] [CrossRef]
- Davy, A.J. Development and Structure of Salt Marshes: Community Patterns in Time and Space. In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 137–156. [Google Scholar]
- Weinstein, M.P.; Kreeger, D.A. Concepts and Controversies in Tidal Marsh Ecology || Twenty More Years of Marsh and Estuarine Flux Studies: Revisiting Nixon (1980); Springer: Cham, Switzerland, 2002; pp. 391–423. [Google Scholar]
- Shi, Z.; Lamb, H.F.; Collin, R.L. Geomorphic change of saltmarsh tidal creek networks in the Dyfi Estuary, Wales. Mar. Geol. 1995, 128, 73–83. [Google Scholar] [CrossRef]
- Huang, H.; Fan, H. Change Detection of Tidal Flats and Tidal Creeks in the Yellow River Delta Using Landsat TM/ETM+ Images. Acta Geogr. Sin. 2004, 59, 723–730. [Google Scholar] [CrossRef]
- Chen, Y.; He, Z.; Li, B. Spatial Distribution of Tidal Creeks and Quantitative Analysis of Its Driving Factors in Chongming Dongtan, Shanghai. J. Jilin Univ. 2013, 43, 212–219. [Google Scholar]
- Zhou, Z.; Wei, Y.; Geng, L.; Zhang, Y.; Gu, Y.; Finotello, A.; D’Alpaos, A.; Gong, Z.; Xu, F.; Zhang, C.; et al. Cross-shore parallel tidal channel systems formed by alongshore currents. Nat. Commun. 2024, 15, 4732. [Google Scholar] [CrossRef] [PubMed]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 4th ed.; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Marani, M.; Belluco, E. On the drainage density of tidal networks. Water Resour. Res. 2003, 39, 1040. [Google Scholar] [CrossRef]
- Glaser, C.; Frei, S.; Massmann, G.; Gilfedder, B.S. Tidal creeks as hot-spots for hydrological exchange in a coastal landscape. J. Hydrol. 2021, 597, 126158. [Google Scholar] [CrossRef]
- Wallace, K.J.; Zedler, C.J.B. Evolution of tidal creek networks in a high sedimentation environment: A 5-year experiment at Tijuana Estuary, California. Estuaries 2005, 28, 795–811. [Google Scholar] [CrossRef]
- Schwarz, C.; Cox, T.; Van Engeland, T.; Van Oevelen, D.; Van Belzen, J.; Van de Koppel, J.; Soetaert, K.; Bouma, T.J.; Meire, P.; Temmerman, S. Field estimates of floc dynamics and settling velocities in a tidal creek with significant along-channel gradients in velocity and SPM. Estuar. Coast. Shelf Sci. 2017, 197, 221–235. [Google Scholar] [CrossRef]
- Gong, Z.; Zhao, K.; Zhang, C.; Dai, W.; Coco, G.; Zhou, Z. The role of bank collapse on tidal creek ontogeny: A novel process-based model for bank retreat. Geomorphology 2018, 311, 13–26. [Google Scholar] [CrossRef]
- Sun, J.; van Prooijen, B.; Wang, X.; Zhao, Z.; He, Q.; Wang, Z. Sediment fluxes within salt marsh tidal creek systems in the Yangtze Estuary. Geomorphology 2024, 449, 109031. [Google Scholar] [CrossRef]
- Sanger, D.M.; Holland, A.F.; Scott, G.I. Tidal creek and salt marsh sediments in South Carolina coastal estuaries: II. Distribution of organic contaminants. Arch. Environ. Contam. Toxicol. 1999, 37, 458–471. [Google Scholar]
- Kim, D. Modeling spatial and temporal dynamics of plant species richness across tidal creeks in a temperate salt marsh. Ecol. Indic. 2018, 93, 188–195. [Google Scholar] [CrossRef]
- Voulgaris, G.; Meyers, S.T. Temporal variability of hydrodynamics, sediment concentration and sediment settling velocity in a tidal creek. Cont. Shelf Res. 2004, 15, 1659–1683. [Google Scholar] [CrossRef]
- Larcombe, P.; Ridd, P.V. Megaripple dynamics and sediment transport in a mesotidal mangrove creek: Implications for palaeoflow reconstructions. Sedimentology 2010, 42, 593–606. [Google Scholar] [CrossRef]
- Catharine, P.; Denise, S.; Edward, W. An Assessment of Southeast United States Headwater Tidal Creek Sediment Contamination Over a Twenty-Year Period in Relation to Coastal Development. Environ. Manag. 2023, 72, 883–901. [Google Scholar]
- Dybiec, J.M.; Sharbaugh, M.; Cherry, R.J.A. Seasonal Sediment Dynamics in a Constructed and Natural Tidal Marsh in the Northern Gulf of Mexico. Wetl. J. Soc. Wetl. Sci. 2023, 43, 70.71–70.79. [Google Scholar] [CrossRef]
- Watson, E.B.; Wigand, C.; Davey, E.W.; Andrews, H.M.; Bishop, J.; Raposa, K.B. Wetland Loss Patterns and Inundation-Productivity Relationships Prognosticate Widespread Salt Marsh Loss for Southern New England. Estuaries Coasts 2017, 40, 662–681. [Google Scholar] [CrossRef]
- Hinkle, R.L.; Mitsch, W.J. Salt marsh vegetation recovery at salt hay farm wetland restoration sites on Delaware Bay. Ecol. Eng. 2005, 25, 240–251. [Google Scholar] [CrossRef]
- Tognin, D.; Finotello, A.; D’Alpaos, A.; Viero, D.P.; Pivato, M.; Mel, R.A.; Defina, A.; Bertuzzo, E.; Marani, M.; Carniello, L. Loss of geomorphic diversity in shallow tidal embayments promoted by storm-surge barriers. Sci. Adv. 2022, 8, eabm8446. [Google Scholar] [CrossRef]
- Moeller, I. Quantifying saltmarsh vegetation and its effect on wave height dissipation: Results from a UK East coast saltmarsh. Estuar. Coast. Shelf Sci. 2006, 69, 337–351. [Google Scholar] [CrossRef]
- Belluco, E.; Camuffo, M.; Ferrari, S.; Modenese, L.; Silvestri, S.; Marani, A.; Marani, M. Mapping salt-marsh vegetation by multispectral and hyperspectral remote sensing. Remote Sens. Environ. 2006, 105, 54–67. [Google Scholar] [CrossRef]
- Eastwood, J.A.; Yates, M.G.; Thomson, A.G.; Fuller, R.M. The reliability of vegetation indices for monitoring saltmarsh vegetation cover. Int. J. Remote Sens. 1997, 18, 3901–3907. [Google Scholar] [CrossRef]
- Beijma, S.V.; Comber, A.; Lamb, A. Random forest classification of salt marsh vegetation habitats using quad-polarimetric airborne SAR, elevation and optical RS data. Remote Sens. Environ. 2014, 149, 118–129. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, Y.; Liu, M.; Chang, Y.; Yan, X.; Bu, R.; Zhao, D.; Li, Z. Introduction and Spread of an Exotic Plant, Spartina alterniflora, Along Coastal Marshes of China. Wetlands 2017, 37, 1181–1193. [Google Scholar] [CrossRef]
- Adamowicz, S.C.; Roman, C.T. New England salt marsh pools: A quantitative analysis of geomorphic and geographic features. Wetlands 2005, 25, 279–288. [Google Scholar] [CrossRef]
- Balke, T.; Webb, E.L.; Elzen, E.V.D.; Galli, D.; Herman, P.M.J.; Bouma, T.J. Seedling establishment in a dynamic sedimentary environment: A conceptual framework using mangroves. J. Appl. Ecol. 2013, 50, 740–747. [Google Scholar] [CrossRef]
- Li, S.H.; Ge, Z.M.; Xin, P.; Tan, L.S.; Li, Y.L.; Xie, L.N. Interactions between biotic and abiotic processes determine biogeomorphology in Yangtze Estuary coastal marshes: Observation with a modeling approach. Geomorphology 2021, 395, 107970. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Wang, J.; Zhang, Y. Does the effect of flowering time on biomass allocation across latitudes differ between invasive and native salt marsh grass Spartina alterniflora? Ecol. Evol. 2022, 12, e8681. [Google Scholar] [CrossRef]
- Dusseux, P.; Hubert-Moy, L.; Corpetti, T.; Vertès, F. Evaluation of SPOT imagery for the estimation of grassland biomass. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 72–77. [Google Scholar] [CrossRef]
- Chen, J.; Gu, S.; Shen, M.; Tang, Y.; Matsushita, B. Estimating aboveground biomass of grassland having a high canopy cover: An exploratory analysis of hyperspectral data. Int. J. Remote Sens. 2009, 30, 6497–6517. [Google Scholar] [CrossRef]
- Jackson, M.V.; Fuller, R.A.; Gan, X.; Li, J.; Choi, C.Y. Dual threat of tidal flat loss and invasive Spartina alterniflora endanger important shorebird habitat in coastal mainland China. J. Environ. Manag. 2021, 278, 111549. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.H.; Ma, Y.J. Evolution of tidal flats in China and ecological exploitation of tidal flat resources. Environ. Earth Sci. 2012, 67, 1639–1649. [Google Scholar] [CrossRef]
- Cui, B.; He, Q.; Gu, B.; Bai, J.; Liu, X. China’s Coastal Wetlands: Understanding Environmental Changes and Human Impacts for Management and Conservation. Wetlands 2016, 36, 1–9. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, Z.L.; Meng, W.Q.; Mo, X.Q.; Hu, B.B. Temporal-spatial variations and driving factors analysis of coastal reclamation in China. Estuar. Coast. Shelf Sci. 2017, 191, 39–49. [Google Scholar]
- Wang, W.; Liu, H.; Li, Y.; Su, J. Development and management of land reclamation in China. Ocean Coast. Manag. 2014, 102, 415–425. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Wen, Y.; Fan, W.; Liu, Q.; Tarolli, P. Monitoring the coastal wetlands dynamics in Northeast Italy from 1984 to 2016. Ecol. Indic. 2021, 129, 107906. [Google Scholar] [CrossRef]
- Allen, J.R.L. Retreat rates of soft-sediment cliffs: The contribution from dated fishweirs and traps on Holocene coastal outcrops. Proc. Geol. Assoc. 2002, 113, 1–8. [Google Scholar] [CrossRef]
- Yim, J.; Kwon, B.O.; Nam, J.; Hwang, J.H.; Choi, K.; Khim, J.S. Analysis of forty years long changes in coastal land use and land cover of the Yellow Sea: The gains or losses in ecosystem services. Environ. Pollut. 2018, 241, 74–84. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, T.; Zhou, Z.; Cao, H.; Qiu, J.; Huang, X. Increased river flow enhances the resilience of spatially patterned mudflats to erosion. Water Res. 2022, 220, 118660. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Li, J. Quantifying the spatial impact of landscape fragmentation on habitat quality: A multi-temporal dimensional comparison between the Yangtze River Economic Belt and Yellow River Basin of China. Land Use Policy 2023, 125, 106463. [Google Scholar] [CrossRef]
- Yuan, S.; Kuang, R.; Liao, Q. Remote sensing extraction and dynamic change of wetland vegetation—A case study of Eastern Chongming Island. J. Jiangxi Univ. Sci. Technol. 2018, 39, 44–51. [Google Scholar]
- Xie, D.F.; Fan, D.D.; Gao, S. Flat of Chongming island and its impacts on the sediment distribution. Mar. Geol. Quat. Geol. 2006, 26, 9–16. [Google Scholar]
- Wang, H.; Ge, Z.; Yuan, L.; Zhang, L. Evaluation of the combined threat from sea-level rise and sedimentation reduction to the coastal wetlands in the Yangtze Estuary, China. Ecol. Eng. 2014, 71, 346–354. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Li, Y.; Zhang, W.; Fu, X.; Le, Y. Effect of Spartina alterniflora invasion and its controlling technologies on soil microbial respiration of a tidal wetland in Chongming Dongtan, China. Ecol. Eng. 2012, 41, 52–59. [Google Scholar] [CrossRef]
- Gu, Y.F. Technologies and their effects on ecological control of Spartina alterniflora in Chongming Dongtan. J. Shanghai Jiaotong Univ. 2019, 37, 6. [Google Scholar]
- Xie, W.M. Study on Morphodynamic Processes and the Influence of Vegetation in a High-Turbidity Estuarine Tidal Flat; East China Normal University: Shanghai, China, 2018. [Google Scholar]
- Tang, C.D. Ecological Control of Spartina alterniflora and Improvement of Birds Habitats in Chongming Dongtan Wetland, Shanghai. Wetl. Sci. Manag. 2016, 12, 4–8. [Google Scholar]
- Zhao, G.Q.; Zhang, L.Q.; Liang, X. A comparison of photosynthetic characteristics between an invasive plant Spartina alterniflora and an indigenous plant Phragmites australis. Acta Ecol. Sin. 2005, 25, 1604–1611. [Google Scholar]
- Horton, R.E.; Horton, R.; Horton, H. Erosional development of streams and their drainage basins; hydrophysical approach to quantitative morphology. J. Jpn. For. Soc. 1945, 56, 275–370. [Google Scholar] [CrossRef]
- Han, X.; Fan, D.; Huang, J.; Tu, J.; Meng, L.; Chen, S. Cumulative impact of human activities on hydro-sediment dynamics and morphodynamics in the highly altered Yangtze Estuary. J. Hydrol. 2025, 651, 132625. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L. An experimental study on physical controls of an exotic plant Spartina alterniflora in Shanghai, China. Ecol. Eng. 2008, 32, 11–21. [Google Scholar] [CrossRef]
- Zhao, C.; Li, J.; Zhao, X. Mowing plus shading as an effective method to control the invasive plant Spartina alterniflora. Flora 2019, 257, 151408. [Google Scholar] [CrossRef]
- Strahler, A.N. Dynamic basis of geomorphology. Geol. Soc. Am. Bull. 1952, 63, 923–938. [Google Scholar] [CrossRef]
- Kearney, W.S.; Fagherazzi, S. Salt marsh vegetation promotes efficient tidal channel networks. Nat. Commun. 2016, 7, 12287. [Google Scholar] [CrossRef]
- Novakowski, K.I.; Torres, R.; Gardner, L.R.; Voulgaris, G. Geomorphic analysis of tidal creek networks. Water Resour. Res. 2004, 40, W05401. [Google Scholar] [CrossRef]
- Chen, L.; Han, Z. Study of the Influence of the Deep-Water Channel Project in the Yangtze River Estuary on Ecological Landscape and Fractal Dimensions of Jiuduan Shoal Tidal Channels. J. Coast. Res. 2015, 73, 146–154. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, Y.; Fan, D.; Wu, G.; Luo, F.; Yao, P.; Gong, Z.; Coco, G. Sediment sorting and bedding dynamics of tidal flat wetlands: Modeling the signature of storms. J. Hydrol. 2022, 610, 127913. [Google Scholar] [CrossRef]
- Ai, X.H.; Xin, K.; Zeng, S.J. A Review on Evolution and Ecological Effects of Tidal Creeks. Wetl. Sci. 2024, 22, 603–616. [Google Scholar] [CrossRef]
- Bush, N.; Bush, E.; Sokolova, Y.; Bush, N.; Blanchard, P. Utilizing the physiological adaptation mechanisms of coastal plants for vegetative restoration of barrier islands. Ocean Coast. Manag. 2018, 161, 222–227. [Google Scholar] [CrossRef]
- Qin, D.U.; Zhong, Q.C.; Kai-Yun, W.A.N.G. Root Effect of Three Vegetation Types on Shoreline Stabilization of Chongming Island, Shanghai. Pedosphere 2010, 20, 692–701. [Google Scholar]
- Andreoli, A.; Bischetti, G.B.; Chiaradia, E.; Cislaghi, A.; Comiti, F. The Roots of River Restoration: Role of Vegetation Recover in Bed Stabilization. In Proceedings of the 37th IAHR World Congress: Managing Water for Sustainable Development, Learning from the Past for the Future, Kuala Lumpur, Malaysia, 13–18 August 2017. [Google Scholar]
- Davidson-Arnott, R.G.D.; Proosdij, D.V.; Ollerhead, J.; Schostak, L. Hydrodynamics and sedimentation in salt marshes: Examples from a macrotidal marsh, Bay of Fundy. Geomorphology 2002, 48, 209–231. [Google Scholar] [CrossRef]
- Eiliott, A.H. Settling of fine sediment in a channel with emergent vegetation. J. Hydraul. Eng. 2000, 126, 570–577. [Google Scholar] [CrossRef]
- Gyssels, G.; Poesen, J.; Bochet, E.; Li, Y. Impact of plant roots on the resistance of soils to erosion by water: A review. Prog. Phys. Geogr. 2005, 29, 189–217. [Google Scholar] [CrossRef]
- Hughes, Z.J. Tidal Channels on Tidal Flats and Marshes; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Rominger, J.T.; Lightbody, A.F.; Nepf, H.M. Effects of Added Vegetation on Sand Bar Stability and Stream Hydrodynamics. J. Hydraul. Eng. 2010, 136, 994–1002. [Google Scholar] [CrossRef]
- James, C.S.; Jordanova, A.A.; Nicolson, C.R. Flume experiments and modelling of flow-sediment-vegetation interactions. Int. Assoc. Hydrol. Sci. 2002, 276, 3–9. [Google Scholar]
- Bouma, T.J.; Vries, M.B.D.; Low, E.; Kusters, L.; Herman, P.M.J.; Tánczos, I.C.; Temmerman, S.; Hesselink, A.; Meire, P.; Regenmortel, S.V. Flow hydrodynamics on a mudflat and in salt marsh vegetation: Identifying general relationships for habitat characterisations. Hydrobiologia 2005, 540, 259–274. [Google Scholar] [CrossRef]
- Yang, S.L.; Li, H.; Ysebaert, T.; Bouma, T.J.; Zhang, W.X.; Wang, Y.Y.; Li, P.; Li, M.; Ding, P.X. Spatial and temporal variations in sediment grain size in tidal wetlands, Yangtze Delta: On the role of physical and biotic controls. Estuar. Coast. Shelf Sci. 2008, 77, 657–671. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, S.-s.; Wang, P.-f.; Hou, J. Interactions between vegetation, water flow and sediment transport: A review. J. Hydrodyn. 2015, 27, 24–37. [Google Scholar] [CrossRef]
- Lvarez-Rogel, J.; Jiménez-Cárceles, F.J.; Roca, M.J.; Ortiz, R. Changes in soils and vegetation in a Mediterranean coastal salt marsh impacted by human activities. Estuar. Coast. Shelf Sci. 2007, 73, 510–526. [Google Scholar] [CrossRef]
- Xin, P.; Li, L.; Barry, D.A. Tidal influence on soil conditions in an intertidal creek-marsh system. Water Resour. Res. 2013, 49, 137–150. [Google Scholar] [CrossRef]
- Koretsky, C.M.; Cuellar, A.; Haveman, M.; Beuving, L.; Shattuck, T.; Wagner, M. Influence of Spartina and Juncus on saltmarsh sediments. II. Trace element geochemistry. Chem. Geol. 2008, 255, 100–113. [Google Scholar] [CrossRef]
- Rosso, P.H.; Ustin, S.L.; Hastings, A. Use of lidar to study changes associated with Spartina invasion in San Francisco Bay marshes. Remote Sens. Environ. 2006, 99, 295–306. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, D.; Ke, Y.; Wang, Y.; Wang, Q.; Zhang, L. Quantifying the Correlated Spatial Distributions between Tidal Creeks and Coastal Wetland Vegetation in the Yellow River Estuary. Wetlands 2020, 40, 2701–2711. [Google Scholar] [CrossRef]




| Satellite | Acquisition Date | Tidal Level | Resolution |
|---|---|---|---|
| LANDSAT5 | 23 August 1985 | LTL | 30 m |
| 5 July 1984 | LTL | 30 m | |
| SPOT1 | 27 August 1987 | LTL | 20 m |
| LANDSAT5 | 11 September 1990 | LTL | 30 m |
| 23 July 1989 | LTL | 30 m | |
| SPOT3 | 19 March 1995 | LTL | 20 m |
| LANDSAT5 | 12 August 1995 | LTL | 30 m |
| 03 June 1995 | LTL | 30 m | |
| LANDSAT7 | 18 September 2000 | LTL | 30 m |
| 25 July 2000 | LTL | 30 m | |
| SPOT4 | 22 July 2000 | LTL | 20 m |
| LANDSAT7 | 15 August 2005 | LTL | 30 m |
| 18 October 2004 | LTL | 30 m | |
| SPOT5 | 15 October 2005 | LTL | 10 m |
| LANDSAT5 | 08 August 2010 | LTL | 20 m |
| 12 July 2009 | LTL | 30 m | |
| SPOT4 | 16 August 2010 | LTL | 20 m |
| LANDSAT8 | 03 August 2015 | LTL | 30 m |
| 25 July 2015 | LTL | 30 m | |
| 23 October 2015 | LTL | 30 m | |
| LANDSAT8 | 16 August 2020 | LTL | 30 m |
| 24 June 2019 | LTL | 30 m | |
| 28 September 2021 | LTL | 30 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Fan, D.; Du, Y.; Li, B. Anthropogenic Forcing on the Coevolution of Tidal Creeks and Vegetation in the Dongtan Wetland, Changjiang Estuary. Remote Sens. 2025, 17, 1692. https://doi.org/10.3390/rs17101692
Sun Y, Fan D, Du Y, Li B. Anthropogenic Forcing on the Coevolution of Tidal Creeks and Vegetation in the Dongtan Wetland, Changjiang Estuary. Remote Sensing. 2025; 17(10):1692. https://doi.org/10.3390/rs17101692
Chicago/Turabian StyleSun, Yi, Daidu Fan, Yunfei Du, and Bing Li. 2025. "Anthropogenic Forcing on the Coevolution of Tidal Creeks and Vegetation in the Dongtan Wetland, Changjiang Estuary" Remote Sensing 17, no. 10: 1692. https://doi.org/10.3390/rs17101692
APA StyleSun, Y., Fan, D., Du, Y., & Li, B. (2025). Anthropogenic Forcing on the Coevolution of Tidal Creeks and Vegetation in the Dongtan Wetland, Changjiang Estuary. Remote Sensing, 17(10), 1692. https://doi.org/10.3390/rs17101692






