Cutting the Greenness Index into 12 Monthly Slices: How Intra-Annual NDVI Dynamics Help Decipher Drought Responses in Mixed Forest Tree Species
Abstract
1. Introduction
2. Materials and Methods
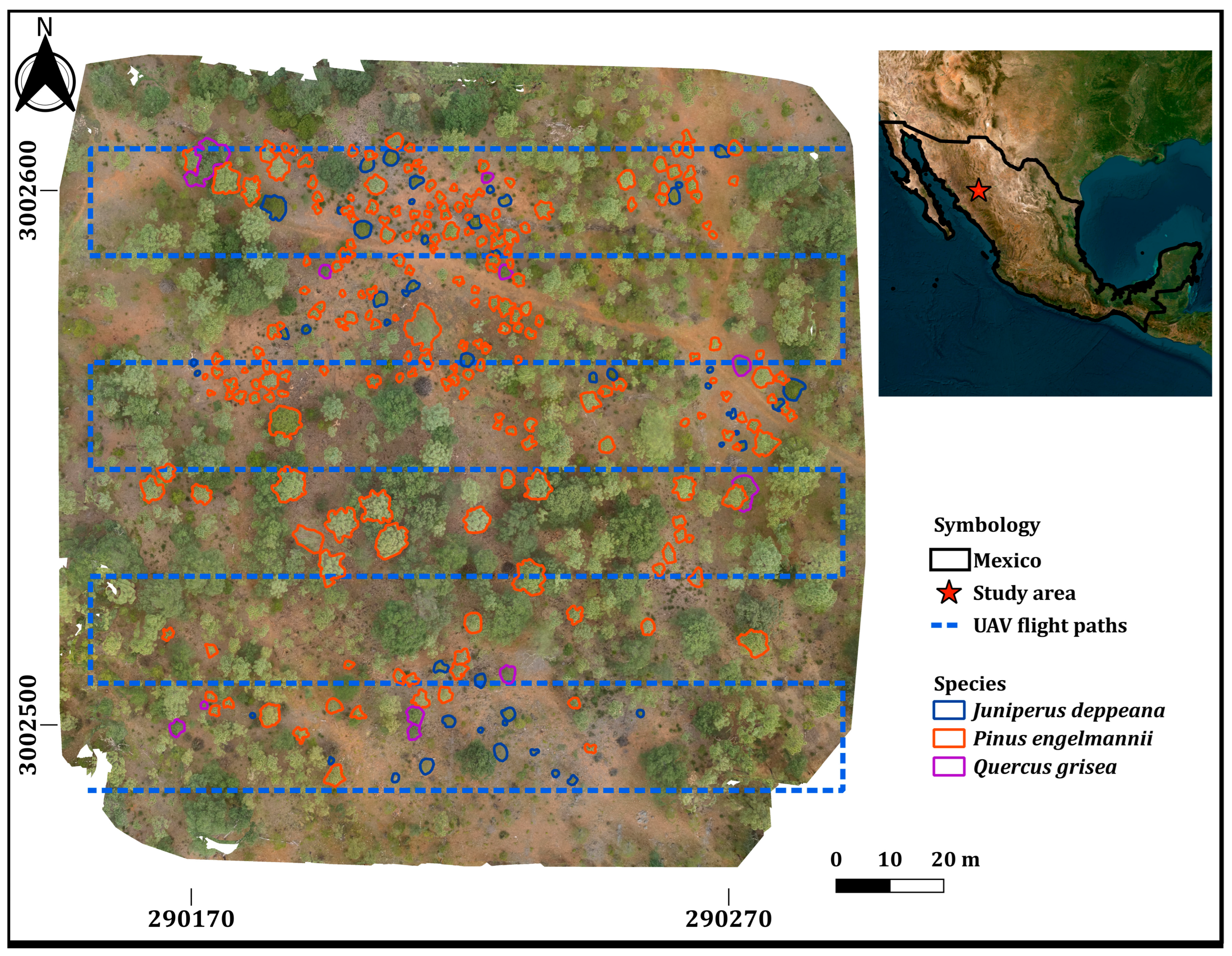
2.1. Description of the Study Area
2.2. Dendrochronological Data and Processing
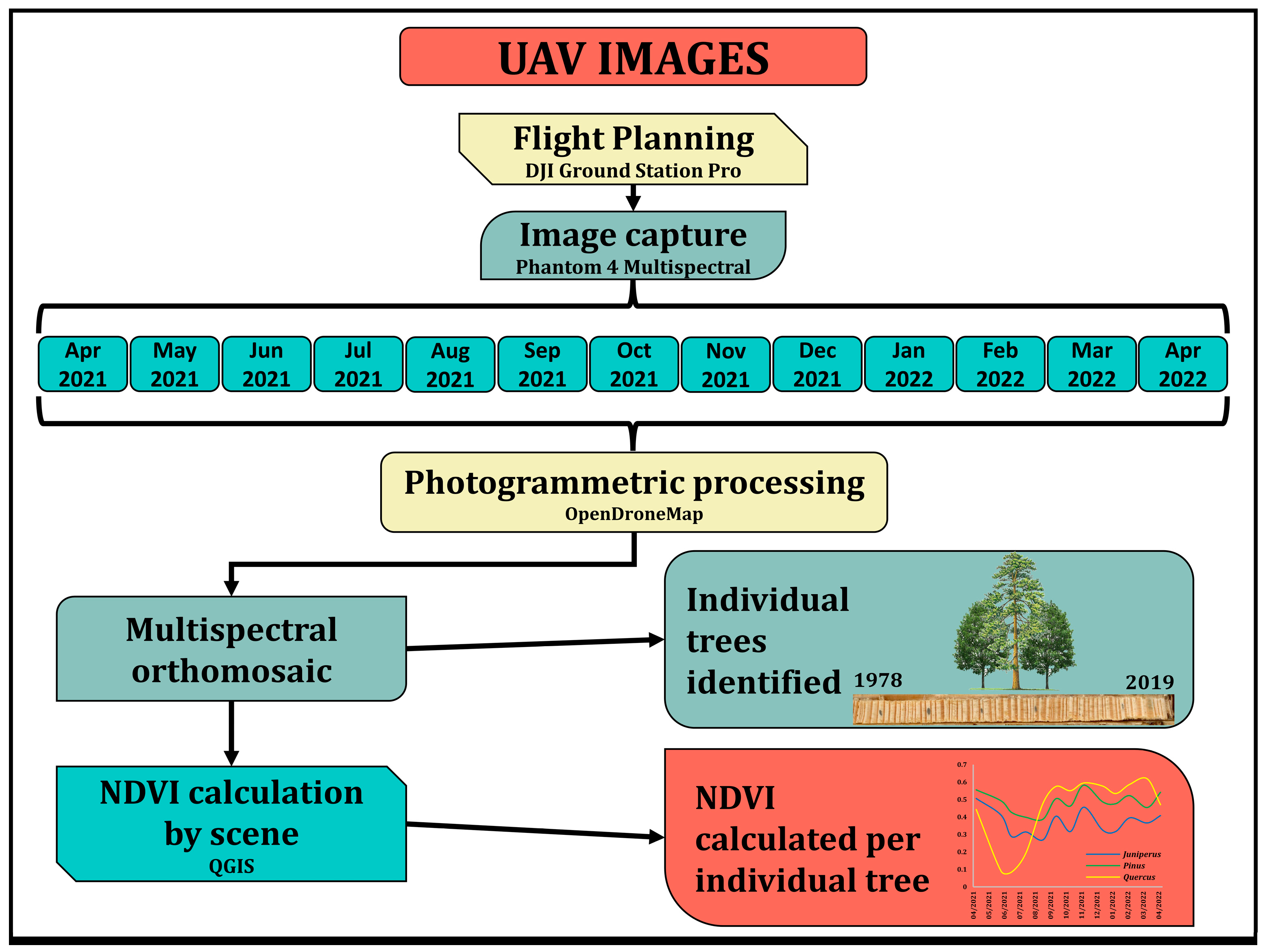
2.3. Remote Sensing Data and Procedures
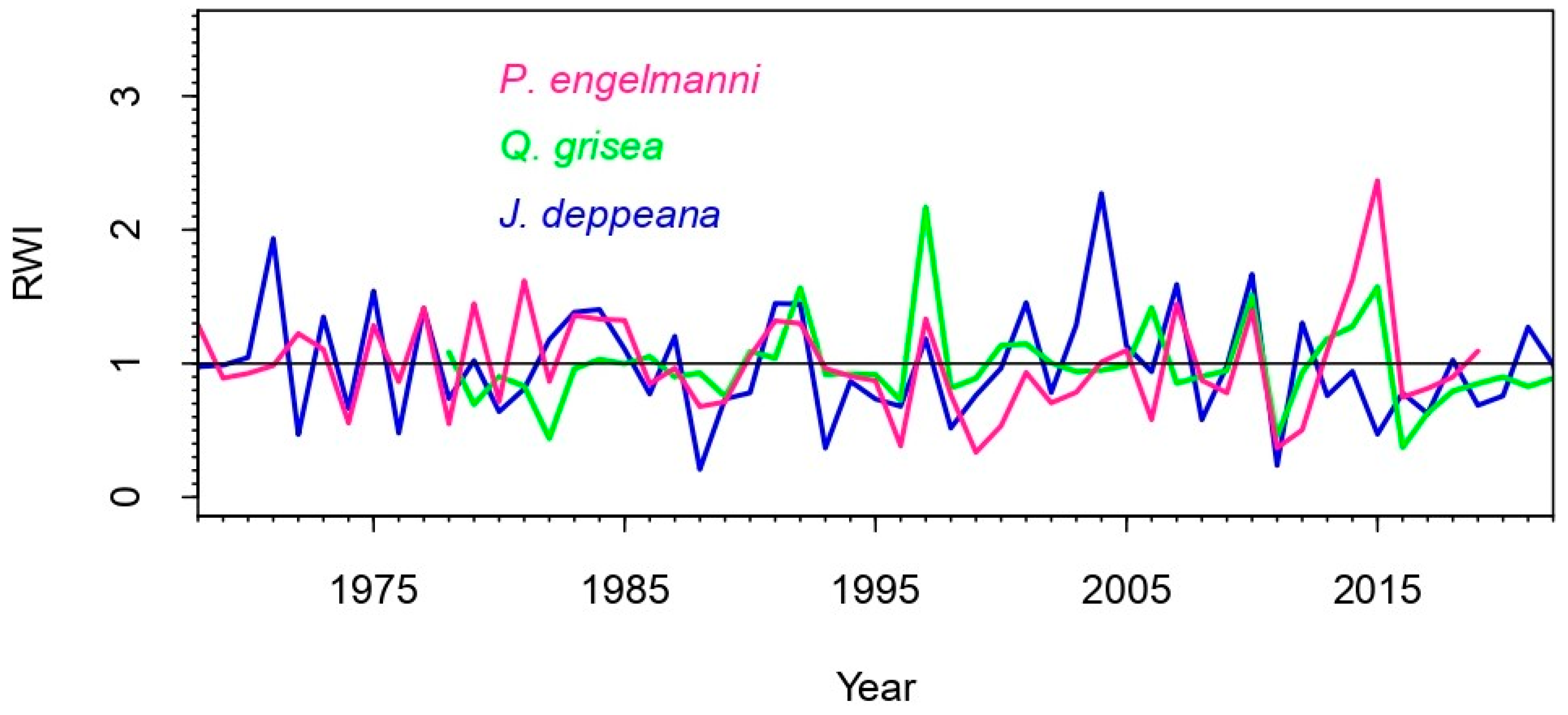
3. Results
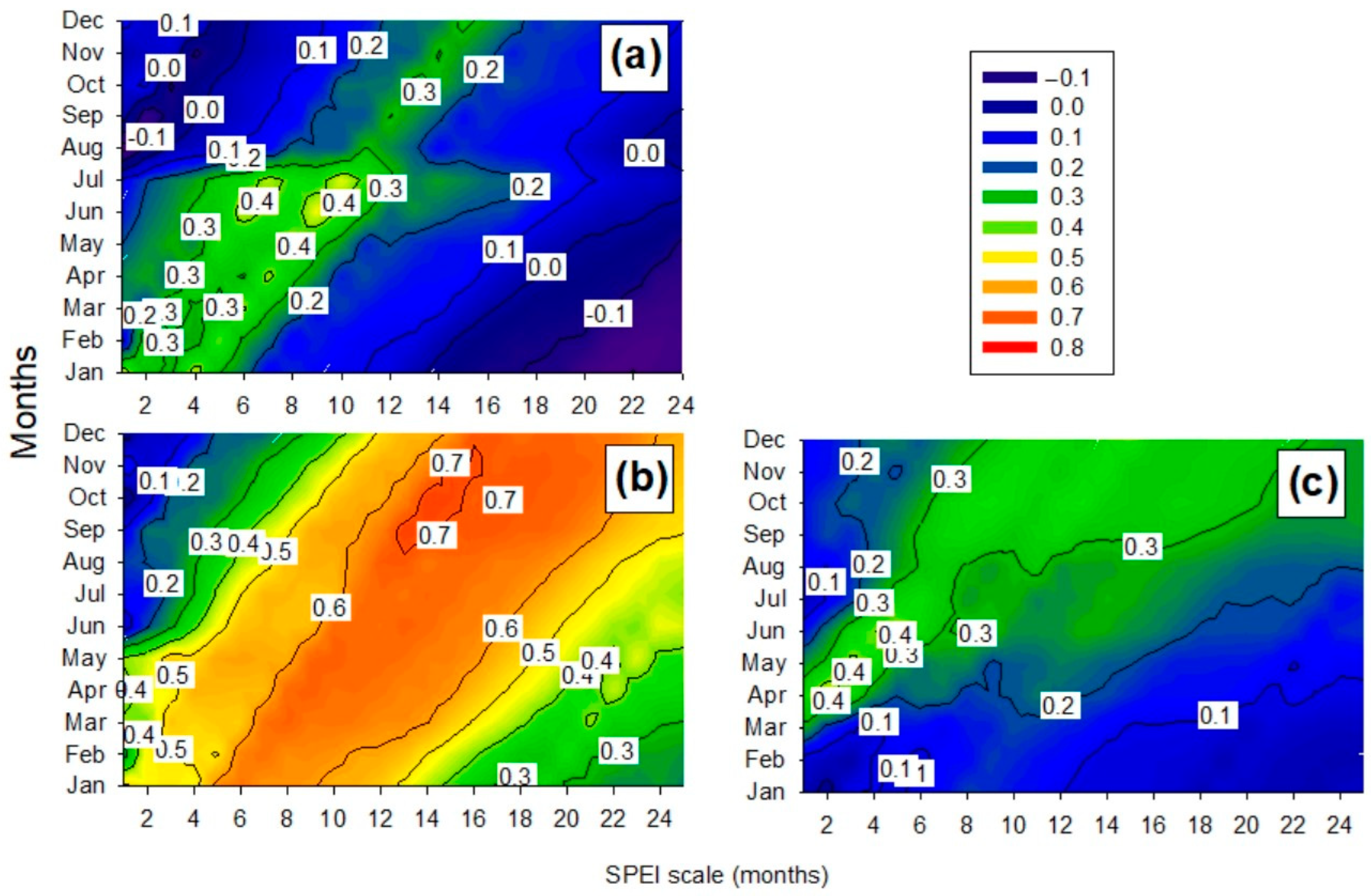
3.1. Variable Climate–Growth Relationships
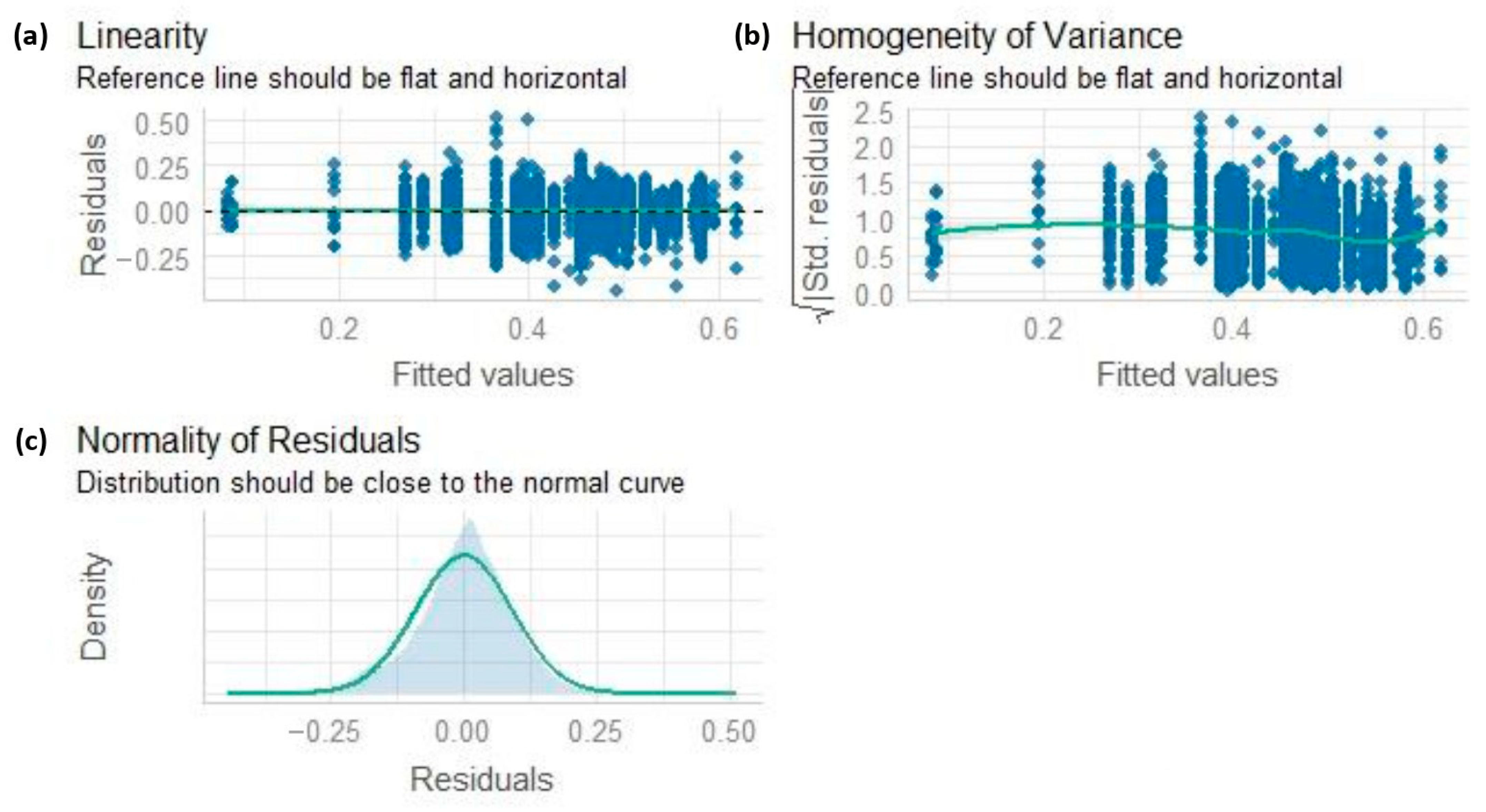
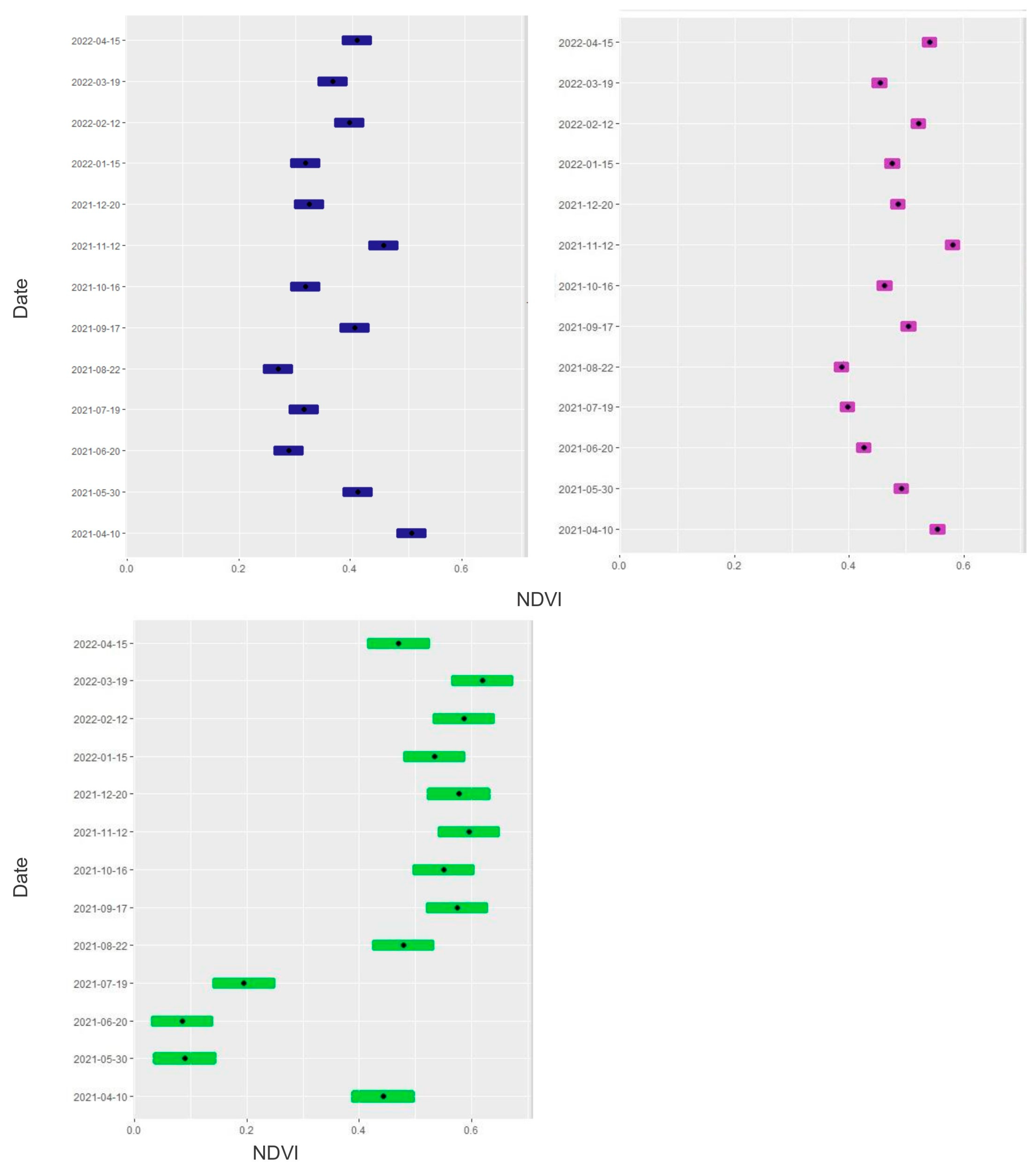
3.2. NDVI and Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Date | 10 April 2021: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.0492 | 0.0145 | 3471 | −3.402 | 0.002 |
| J. deppeana | - | Q. grisea | 0.0634 | 0.0304 | 3471 | 2.084 | 0.1116 |
| P. engelmannii | - | Q. grisea | 0.1127 | 0.0282 | 3471 | 3.993 | 0.0002 |
| Date | 30 May 2021: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.0817 | 0.0145 | 3471 | −5.647 | <0.0001 |
| J. deppeana | - | Q. grisea | 0.3214 | 0.0304 | 3471 | 10.561 | <0.0001 |
| P. engelmannii | - | Q. grisea | 0.4031 | 0.0282 | 3471 | 14.288 | <0.0001 |
| Date | 20 June 2021: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.1379 | 0.0145 | 3471 | −9.531 | <0.0001 |
| J. deppeana | - | Q. grisea | 0.2035 | 0.0304 | 3471 | 6.687 | <0.0001 |
| P. engelmannii | - | Q. grisea | 0.3415 | 0.0282 | 3471 | 12.102 | <0.0001 |
| Date | 19 July 2021: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.0833 | 0.0145 | 3471 | −5.759 | <0.0001 |
| J. deppeana | - | Q. grisea | 0.1195 | 0.0304 | 3471 | 3.926 | 0.0003 |
| P. engelmannii | - | Q. grisea | 0.2028 | 0.0282 | 3471 | 7.189 | <0.0001 |
| Date | 22 August 2021: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.1178 | 0.0145 | 3471 | −8.143 | <0.0001 |
| J. deppeana | - | Q. grisea | −0.209 | 0.0304 | 3471 | −6.867 | <0.0001 |
| P. engelmannii | - | Q. grisea | −0.0911 | 0.0282 | 3471 | −3.23 | 0.0037 |
| Date | 17 September 2021: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.1 | 0.0145 | 3471 | −6.909 | <0.0001 |
| J. deppeana | - | Q. grisea | −0.1702 | 0.0304 | 3471 | −5.593 | <0.0001 |
| P. engelmannii | - | Q. grisea | −0.0702 | 0.0282 | 3471 | −2.49 | 0.0385 |
| Date | 16 October 2021: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.1456 | 0.0145 | 3471 | −10.063 | <0.0001 |
| J. deppeana | - | Q. grisea | −0.2334 | 0.0304 | 3471 | −7.669 | <0.0001 |
| P. engelmannii | - | Q. grisea | −0.0878 | 0.0282 | 3471 | −3.111 | 0.0056 |
| Date | 12 November 2021: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.1258 | 0.0145 | 3471 | −8.691 | <0.0001 |
| J. deppeana | - | Q. grisea | −0.1385 | 0.0304 | 3471 | −4.55 | <0.0001 |
| P. engelmannii | - | Q. grisea | −0.0127 | 0.0282 | 3471 | −0.45 | 1 |
| Date | 20 December 2021: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.1618 | 0.0145 | 3471 | −11.178 | <0.0001 |
| J. deppeana | - | Q. grisea | −0.2522 | 0.0304 | 3471 | −8.288 | <0.0001 |
| P. engelmannii | - | Q. grisea | −0.0905 | 0.0282 | 3471 | −3.206 | 0.0041 |
| Date | 15 January 2022: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.1587 | 0.0145 | 3471 | −10.969 | <0.0001 |
| J. deppeana | - | Q. grisea | −0.2169 | 0.0304 | 3471 | −7.128 | <0.0001 |
| P. engelmannii | - | Q. grisea | −0.0582 | 0.0282 | 3471 | −2.062 | 0.1178 |
| Date | 12 February 2022: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.1268 | 0.0145 | 3471 | −8.764 | <0.0001 |
| J. deppeana | - | Q. grisea | −0.1914 | 0.0304 | 3471 | −6.288 | <0.0001 |
| P. engelmannii | - | Q. grisea | −0.0646 | 0.0282 | 3471 | −2.288 | 0.0666 |
| Date | 19 March 2022: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.0878 | 0.0145 | 3471 | −6.07 | <0.0001 |
| J. deppeana | - | Q. grisea | −0.2529 | 0.0304 | 3471 | −8.309 | <0.0001 |
| P. engelmannii | - | Q. grisea | −0.165 | 0.0282 | 3471 | −5.849 | <0.0001 |
| Date | 15 April 2022: | ||||||
| Interaction | Estimate | SE | df | t.ratio | p-Value | ||
| J. deppeana | - | P. engelmannii | −0.133 | 0.0145 | 3471 | −9.19 | <0.0001 |
| J. deppeana | - | Q. grisea | −0.0621 | 0.0304 | 3471 | −2.039 | 0.1244 |
| P. engelmannii | - | Q. grisea | 0.0709 | 0.0282 | 3471 | 2.514 | 0.036 |

References
- Bosela, M.; Tumajer, J.; Cienciala, E.; Dobor, L.; Kulla, L.; Marčiš, P.; Popa, I.; Sedmák, R.; Sedmáková, D.; Sitko, R.; et al. Climate warming induced synchronous growth decline in Norway spruce populations across biogeographical gradients since 2000. Sci. Total Environ. 2021, 752, 141794. [Google Scholar] [CrossRef] [PubMed]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.R.; Zellweger, F.; Aalto, J.; Ashcroft, M.B.; Christiansen, D.M.; Decocq, G.; De Pauw, K.; et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Chang. Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
- Batllori, E.; Lloret, F.; Aakala, T.; Anderegg, W.R.L.; Aynekulu, E.; Bendixsen, D.P.; Bentouati, A.; Bigler, C.; Burk, C.J.; Camarero, J.J.; et al. Forest and woodland replacement patterns followingdrought-related mortality. Proc. Nat. Acad. Sci. USA 2020, 117, 29720–29729. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Carbone, G.J.; Huang, X.; Lackstrom, K.; Gao, P. Mapping the sensitivity of agriculture to drought and estimating the effect of irrigation in the United States, 1950–2016. Agric. For. Meteorol. 2020, 108124, 292–293. [Google Scholar] [CrossRef]
- Bradford, J.B.; Schlaepfer, D.R.; Lauenroth, W.K.; Palmquist, K.A. Robust ecological drought projections for drylands in the 21st century. Glob. Chang. Biol. 2020, 26, 3906–3919. [Google Scholar] [CrossRef] [PubMed]
- Breshears, D.D.; Fontaine, J.B.; Ruthrof, K.X.; Field, J.P.; Feng, X.; Burger, J.R.; Law, D.J.; Kala, J.; Hardy, G.E.S.J. Underappreciated plant vulnerabilities to heat waves. New Phytol. 2021, 231, 32–39. [Google Scholar] [CrossRef] [PubMed]
- 2021 Disasters in Numbers. Brussels: CRED. 2022. Available online: https://cred.be/sites/default/files/2021_EMDAT_report.pdf (accessed on 1 January 2023).
- Mishra, A.K.; Singh, V.P. A review of drought concepts. J. Hydrol. 2010, 391, 202–216. [Google Scholar] [CrossRef]
- Italiano, S.S.P.; Camarero, J.J.; Colangelo, M.; Borghetti, M.; Castellaneta, M.; Pizarro, M.; Ripullone, F. Assessing Forest Vulnerability to Climate Change Combining Remote Sensing and Tree-Ring Data: Issues, Needs and Avenues. Forests 2023, 14, 1138. [Google Scholar] [CrossRef]
- Férriz, M.; Martin-Benito, D.; Cañellas, I.; Gea-Izquierdo, G. Sensitivity to water stress drives differential decline and mortality dynamics of three co-occurring conifers with different drought tolerance. For. Ecol. Manag. 2021, 486, 118964. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; González-Moreno, P.; Ruiz-Gómez, F.J.; Sánchez-Cuesta, R.; Gazol, A.; Camarero, J.J. Drought stress and pests increase defoliation and mortality rates in vulnerable Abies pinsapo forests. For. Ecol. Manag. 2022, 504, 119824. [Google Scholar] [CrossRef]
- Meyer, B.F.; Buras, A.; Rammig, A.; Zang, C.S. Higher susceptibility of beech to drought in comparison to oak. Dendrochronologia 2020, 64, 125780. [Google Scholar] [CrossRef]
- Brehaut, L.; Danby, R.K. Inconsistent relationships between annual tree ring-widths and satellite-measured NDVI in a mountainous subarctic environment. Ecol. Indic. 2018, 91, 698–711. [Google Scholar] [CrossRef]
- Mašek, J.; Tumajer, J.; Lange, J.; Kaczka, R.; Fišer, P.; Treml, V. Variability in Tree-ring Width and NDVI Responses to Climate at a Landscape Level. Ecosystems 2023, 26, 1144–1157. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Bolyn, C.; Lejeune, P.; Michez, A.; Latte, N. Mapping Tree Species Proportions from Satellite Imagery Using Spectral–Spatial Deep Learning. Remote Sens. Environ. 2022, 280, 113205. [Google Scholar] [CrossRef]
- Wang, H.; Müller, J.; Tatarinov, F.; Yakir, D.; Rotenberg, E. Disentangling Soil, Shade, and Tree Canopy Contributions to Mixed Satellite Vegetation Indices in a Sparse Dry Forest. Remote Sens. 2022, 14, 3681. [Google Scholar] [CrossRef]
- Gamon, J.A.; Field, C.B.; Goulden, M.L.; Griffin, K.L.; Hartley, A.E.; Joel, G.; Penuelas, J.; Valentini, R. Relationships between NDVI, Canopy Structure, and Photosynthesis in Three Californian Vegetation Types. Ecol. Appl. 1995, 5, 28–41. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Camarero, J.J.; Olano, J.M.; Martín-Hernández, N.; Peña-Gallardo, M.; Tomás-Burguera, M.; Gazol, A.; Azorin-Molina, C.; Bhuyan, U.; Kenawy, A.E. Diverse relationships between forest growth and the Normalized Difference Vegetation Index at a global scale. Remote Sens. Environ. 2016, 187, 14–29. [Google Scholar] [CrossRef]
- Gallardo-Salazar, J.L.; Lindig-Cisneros, R.A.; Lopez-Toledo, L.; Endara-Agramont, A.R.; Blanco-García, A.; Sáenz-Romero, C. Analysis of the Vigor of Pinus hartwegii Lindl. along an Altitudinal Gradient Using UAV Multispectral Images: Evidence of Forest Decline Possibly Associated with Climatic Change. Forests 2023, 14, 1176. [Google Scholar] [CrossRef]
- Xu, P.; Fang, W.; Zhou, T.; Zhao, X.; Luo, H.; Hendrey, G.; Yi, C. Spatial Upscaling of Tree-Ring-Based Forest Response to Drought with Satellite Data. Remote Sens. 2019, 11, 2344. [Google Scholar] [CrossRef]
- Acosta-Hernández, A.C.; Pompa-García, M.; Camarero, J.J. An Updated Review of Dendrochronological Investigations in Mexico, a Megadiverse Country with a High Potential for Tree-Ring Sciences. Forests 2017, 8, 160. [Google Scholar] [CrossRef]
- Farjon, A.; Styles, B.T. Pinus (Pinaceae). Flora Neotropica; New York Botanical Garden: New York, NY, USA, 1997. [Google Scholar]
- González, B.A. La Sierra Tarahumara, El Bosque Y Los Pueblos Originarios: Estudio de Caso de Chihuahua (México). 2012. Available online: http://www.fao.org/forestry/17194-0381f923a6bc236aa91ecf614d92e12e0.pdf (accessed on 7 November 2023).
- González-Elizondo, M.S.; González-Elizondo, M.; Tena-Flores, J.A.; Ruacho-González, L.; López-Enríquez, I.L. Vegetación de la Sierra Madre Occidental, México: Una síntesis. Acta Bot. Mex. 2012, 100, 351–403. [Google Scholar] [CrossRef]
- Fallon, B.; Cavender-Bares, J. Leaf-level trade-offs between drought avoidance and desiccation recovery drive elevation stratification in arid oaks. Ecosphere 2018, 9, e02149. [Google Scholar] [CrossRef]
- Stokes, M.A.; Smiley, T.L. Tree-Ring Dating; The University of Chicago Press: Chicago, IL, USA, 1968. [Google Scholar]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Bunn, A.; Korpela, M.; Bindi, F.; Campelo, F.; Mérian, P.; Queadan, F.; Zand, C.; Buras, A.; Cecile, A.; Mudelsee, M.; et al. Package ‘dplR’. Dendronhronology Program Library in R, Version 1.6.3. 2015. Available online: https://CRAN.R-project.org/package=dplR (accessed on 21 January 2015).
- Mérian, P.; Pierrat, J.-C.; Lebourgeois, F. Effect of sampling effort on the regional chronology statistics and climate–growth relationships estimation. Dendrochronologia 2013, 31, 58–67. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Stefanidis, S.; Rossiou, D.; Proutsos, N. Drought Severity and Trends in a Mediterranean Oak Forest. Hydrology 2023, 10, 167. [Google Scholar] [CrossRef]
- Daniels, L.; Eeckhout, E.; Wieme, J.; Dejaegher, Y.; Audenaert, K.; Maes, W.H. Identifying the Optimal Radiometric Calibration Method for UAV-Based Multispectral Imaging. Remote Sens. 2023, 15, 2909. [Google Scholar] [CrossRef]
- Kedzierski, M.; Wierzbicki, D.; Sekrecka, A.; Fryskowska, A.; Walczykowski, P.; Siewert, J. Influence of Lower Atmosphere on the Radiometric Quality of Unmanned Aerial Vehicle Imagery. Remote Sens. 2019, 11, 1214. [Google Scholar] [CrossRef]
- DJI P4 Multispectral Specs. Available online: https://www.dji.com/p4-multispectral/specs (accessed on 2 May 2022).
- Rouse, J.W., Jr.; Deering, D.W.; Schell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA: Greenbelt, MD, USA, 1974. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time Series, with Applications in Dendroclimatology and Hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Liu, F.; Qin, Q.; Zhan, Z. A novel dynamic stretching solution to eliminate saturation effect in NDVI and its application in drought monitoring. Chin. Geogr. Sci. 2012, 22, 683–694. [Google Scholar] [CrossRef]
- Zhen, Z.; Chen, S.; Yin, T.; Gastellu-Etchegorry, J.P. Globally quantitative analysis of the impact of atmosphere and spectral response function on 2-band enhanced vegetation index (EVI2) over Sentinel-2 and Landsat-8. ISPRS J. Photogramm. Remote Sens 2023, 205, 206–226. [Google Scholar] [CrossRef]
- Yousefpour, R.; Jacobsen, J.B.; Thorsen, B.J.; Meilby, H.; Hanewinkel, M.; Oehler, K. A review of decision-making approaches to handle uncertainty and risk in adaptive forest management under climate change. Ann. For. Sci. 2012, 69, 1–15. [Google Scholar] [CrossRef]
- Berner, L.T.; Beck, P.S.A.; Bunn, A.G.; Lloyd, A.H.; Goetz, S.J. High-latitude tree growth and satellite vegetation indices: Correlations and trends in Russia and Canada (1982–2008). J. Geophys. Res. Biogeosci. 2011, 116, 1–13. [Google Scholar] [CrossRef]
- González-Cásares, M.; Pompa-García, M.; Camarero, J.J. Differences in climate–growth relationship indicate diverse drought tolerances among five pine species coexisting in Northwestern Mexico. Trees 2017, 31, 531–544. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef]
- Sass-Klaassen, U.; Sabajo, C.R.; den Ouden, J. Vessel formation in relation to leaf phenology in pedunculate oak and European ash. Dendrochronologia 2011, 29, 171–175. [Google Scholar] [CrossRef]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 2016, 23, 1675–1690. [Google Scholar] [CrossRef]
- Hagedorn, F.; Joseph, J.; Peter, M.; Luster, J.; Pritsch, K.; Geppert, U.; Kerner, R.; Molinier, V.; Egli, S.; Schaub, M.; et al. Recovery of trees from drought depends on belowground sink control. Nat. Plants 2016, 2, 16111. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.C.; Härdtle, W.; Bruelheide, H.; Geißler, C.; Nadrowski, K.; Schuldt, A.; Yu, M.; von Oheimb, G. Tree morphology responds to neighbourhood competition and slope in species-rich forests of subtropical China. For. Ecol. Manag. 2010, 260, 1708–1715. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.; Montesharrei, S.; Mu, Q.; Kalnay, E.; Li, S. Local cooling and warming effects of forests based on satellite observations. Nat. Commun. 2015, 6, 6603. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, A.; Girardin, M.P.; Metsaranta, J.; Campbell, E.M.; Arsenault, A.; Reich, P.B.; Way, D. New tree-ring data from Canadian boreal and hemi-boreal forests provide insight for improving the climate sensitivity of terrestrial biosphere models. Sci. Total Environ. 2022, 851, 158062. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; Šimůnek, V.; Castellaneta, M.; Vacek, Z.; Vacek, S.; Pericolo, O.; Zito, R.G.; Ripullone, F. Mismatch between Annual Tree-Ring Width Growth and NDVI Index in Norway Spruce Stands of Central Europe. Forests 2022, 13, 1417. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef]
- Suzuki, M.; Yoda, K.; Suzuki, H. Phenological Comparison of the Onset of Vessel Formation Between Ring-Porous and Diffuse-Porous Deciduous Trees in a Japanese Temperate Forest. IAWA J. 1996, 17, 431–444. [Google Scholar] [CrossRef]
- Yeom, J.; Jung, J.; Chang, A.; Ashapure, A.; Maeda, M.; Maeda, A.; Landivar, J. Comparison of Vegetation Indices Derived from UAV Data for Differentiation of Tillage Effects in Agriculture. Remote Sens. 2019, 11, 1548. [Google Scholar] [CrossRef]
- Li, M.; Shamshiri, R.R.; Weltzien, C.; Schirrmann, M. Crop Monitoring Using Sentinel-2 and UAV Multispectral Imagery: A Comparison Case Study in Northeastern Germany. Remote Sens. 2022, 14, 4426. [Google Scholar] [CrossRef]
- Damiano, N.; Bonfante, A.; Cirillo, C.; Amitrano, C.; Erbaggio, A.; Brook, A.; De Micco, V. Retrospective Reconstruction of the Ecophysiological Grapevine Behaviour Through the Analysis of Tree-Ring Series to Validate an Approach to Extract Data From Space-Born and UAV Techniques. In Proceedings of the 2019 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Portici, Italy, 24–26 October 2019; pp. 191–195. [Google Scholar] [CrossRef]
- Vélez, S.; Barajas, E.; Rubio, J.A.; Vacas, R.; Poblete-Echeverría, C. Effect of Missing Vines on Total Leaf Area Determined by NDVI Calculated from Sentinel Satellite Data: Progressive Vine Removal Experiments. Appl. Sci. 2020, 10, 3612. [Google Scholar] [CrossRef]
- Zhen, Z.; Chen, S.; Yin, T.; Han, C.; Chavanon, E.; Lauret, N.; Guilleux, J.; Gastellu-Etchegorry, J.P. A Dynamic L-system based Architectural Maize Model for 3D Radiative Transfer Simulation. IEEE Trans. Geosci. Remote Sens. 2024. early access. [Google Scholar] [CrossRef]
- Charrier, G.; Martin-StPaul, N.; Damesin, C.; Delpierre, N.; Hänninen, H.; Torres-Ruiz, J.M.; Davi, H. Interaction of drought and frost in tree ecophysiology: Rethinking the timing of risks. Ann. For. Sci. 2021, 78, 40. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Battipaglia, G.; Borghetti, M.; De Micco, V.; Gentilesca, T.; Ripullone, F. A multi-proxy assessment of dieback causes in a Mediterranean oak species. Tree Physiol. 2017, 37, 617–631. [Google Scholar] [CrossRef]

| Species | DBH (cm) | Total Height (m) | Age at DBH (Years) | No. of Trees Sampled/No. of Cores Measured |
|---|---|---|---|---|
| J. deppeana | 17.04 ± 1.8 | 5.7 ± 0.4 | 45 ± 3 | 15/29 |
| P. engelmannii | 36.2 ± 1.4 | 11.8 ± 0.3 | 60 ± 2 | 22/42 |
| Q. grisea | 13.47 ± 0.7 | 6.01 ± 0.2 | 42 ± 1 | 14/28 |
| Tree Species | TRW Mean ± SD (mm) | AC | Rbar | EPS |
|---|---|---|---|---|
| J. deppeana | 1.40 ± 0.09 | 0.40 | 0.45 | 0.96 |
| P. engelmannii | 2.13 ± 0.08 | 0.38 | 0.66 | 0.99 |
| Q. grisea | 1.20 ± 0.06 | 0.23 | 0.38 | 0.95 |
| Species | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| J. deppeana | 49 | 0.37 | 0.12 | 0.14 | 0.61 |
| P. engelmannii | 210 | 0.48 | 0.08 | 0.18 | 0.68 |
| Q. grisea | 11 | 0.45 | 0.09 | 0.30 | 0.58 |
| Species | Var | N | Min | Max | Median | Q1 | Q3 | IQR | Mode | Mean | SD | SE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J. deppeana | DB | 49 | 2.80 | 28.80 | 10.50 | 8.20 | 15.90 | 7.70 | 5.78 | 11.71 | 5.44 | 0.22 |
| DBH | 49 | 0.00 | 22.60 | 7.70 | 5.00 | 11.40 | 6.40 | 5.19 | 7.94 | 4.89 | 0.19 | |
| CH | 49 | 0.71 | 3.36 | 1.78 | 1.45 | 2.12 | 0.67 | 0.49 | 1.77 | 0.49 | 0.02 | |
| TH | 49 | 1.51 | 6.34 | 3.70 | 2.67 | 4.49 | 1.82 | 1.26 | 3.63 | 1.17 | 0.05 | |
| CA | 49 | 0.01 | 14.90 | 1.86 | 0.86 | 4.01 | 3.15 | 1.87 | 2.87 | 2.86 | 0.11 | |
| NDVI | 49 | 0.02 | 0.88 | 0.36 | 0.27 | 0.47 | 0.20 | 0.14 | 0.37 | 0.14 | 0.01 | |
| P. engelmannii | DB | 210 | 6.00 | 66.40 | 12.80 | 10.90 | 16.40 | 5.50 | 3.34 | 15.23 | 7.94 | 0.15 |
| DBH | 210 | 3.70 | 56.40 | 9.10 | 7.60 | 12.30 | 4.70 | 2.97 | 11.37 | 6.91 | 0.13 | |
| CH | 210 | 1.42 | 12.55 | 2.51 | 2.20 | 2.84 | 0.64 | 0.45 | 3.03 | 1.96 | 0.04 | |
| TH | 210 | 2.21 | 20.76 | 5.47 | 4.38 | 7.04 | 2.66 | 1.89 | 6.43 | 3.24 | 0.06 | |
| CA | 210 | 0.58 | 35.41 | 2.63 | 1.67 | 4.63 | 2.96 | 1.67 | 4.69 | 5.72 | 0.11 | |
| NDVI | 210 | 0.00 | 0.89 | 0.49 | 0.42 | 0.56 | 0.13 | 0.10 | 0.48 | 0.10 | 0.00 | |
| Q. grisea | DB | 11 | 9.80 | 52.30 | 18.00 | 14.30 | 21.70 | 7.40 | 5.49 | 21.86 | 12.36 | 1.03 |
| DBH | 11 | 5.80 | 44.50 | 11.40 | 9.60 | 15.80 | 6.20 | 4.89 | 15.86 | 10.73 | 0.90 | |
| CH | 11 | 0.96 | 4.52 | 1.91 | 1.72 | 2.43 | 0.71 | 0.43 | 2.15 | 0.86 | 0.07 | |
| TH | 11 | 3.81 | 11.70 | 5.84 | 5.49 | 7.53 | 2.04 | 0.65 | 6.55 | 1.95 | 0.16 | |
| CA | 11 | 1.39 | 37.21 | 6.33 | 4.26 | 9.51 | 5.25 | 3.07 | 9.33 | 9.52 | 0.80 | |
| NDVI | 11 | 0.00 | 0.91 | 0.52 | 0.34 | 0.58 | 0.24 | 0.12 | 0.45 | 0.21 | 0.02 |
| SV | Df | Sum Sq | Mean Sq | F Value | Pr(>F) | Signif. |
|---|---|---|---|---|---|---|
| Species | 2 | 6.999 | 3.5 | 420.58 | <2 × 10−16 | *** |
| Date | 12 | 11.803 | 0.984 | 118.21 | <2 × 10−16 | *** |
| Species × Date | 24 | 4.685 | 0.195 | 23.46 | <2 × 10−16 | *** |
| Residuals | 3471 | 28.882 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Hernández, A.C.; Pompa-García, M.; Martínez-Rivas, J.A.; Vivar-Vivar, E.D. Cutting the Greenness Index into 12 Monthly Slices: How Intra-Annual NDVI Dynamics Help Decipher Drought Responses in Mixed Forest Tree Species. Remote Sens. 2024, 16, 389. https://doi.org/10.3390/rs16020389
Acosta-Hernández AC, Pompa-García M, Martínez-Rivas JA, Vivar-Vivar ED. Cutting the Greenness Index into 12 Monthly Slices: How Intra-Annual NDVI Dynamics Help Decipher Drought Responses in Mixed Forest Tree Species. Remote Sensing. 2024; 16(2):389. https://doi.org/10.3390/rs16020389
Chicago/Turabian StyleAcosta-Hernández, Andrea Cecilia, Marín Pompa-García, José Alexis Martínez-Rivas, and Eduardo Daniel Vivar-Vivar. 2024. "Cutting the Greenness Index into 12 Monthly Slices: How Intra-Annual NDVI Dynamics Help Decipher Drought Responses in Mixed Forest Tree Species" Remote Sensing 16, no. 2: 389. https://doi.org/10.3390/rs16020389
APA StyleAcosta-Hernández, A. C., Pompa-García, M., Martínez-Rivas, J. A., & Vivar-Vivar, E. D. (2024). Cutting the Greenness Index into 12 Monthly Slices: How Intra-Annual NDVI Dynamics Help Decipher Drought Responses in Mixed Forest Tree Species. Remote Sensing, 16(2), 389. https://doi.org/10.3390/rs16020389











