Abstract
The occurrence, frequency, and severity of drought are accelerating due to global warming. Understanding the vulnerability of plantation forests to climate change, particularly to drought events, is critical to revealing the underlying mechanisms of tree resilience, recovery, and acclimation, which are important for plantation management. How the stand age affects the climate sensitivity of tree growth, as well as the direction, magnitude, and duration of the drought legacy, in plantation forests in northeast China is still unclear. In this study, we used MODIS-derived NDVI time series with gridded climate data from 2000 to 2020 to fill this knowledge gap. The selected plantation forests were dominated by four coniferous species: Korean pine (Pinus koraiensis), Scots pine (Pinus sylvestris), Japanese larch (Larix kaempferi), and Dahurian larch (Larix gmelinii). The results show that the climate sensitivity of tree growth differed among species and age groups. The growth of Korean pine and Scots pine was mostly dependent upon precipitation, while the growth of Japanese larch and Dahurian larch was determined primarily by temperature. Old Japanese larch (21–40 years) and Dahurian larch trees (31–60 years) were more sensitive to temperature and precipitation than young conspecifics, whereas old Korean pine (41–60 years) and Scots pine (31–60 years) were less sensitive to precipitation and temperature than young conspecifics. Furthermore, the legacy of drought lasted one year for Korean pine, Japanese larch, and Dahurian larch and over three years for Scots pine. Old trees were more severely affected by drought, particularly Scots pine and Dahurian larch. The findings of the study can help improve plantation forest management for better adaptation to future climate change.
1. Introduction
Global warming is accelerating the occurrence, frequency, and severity of extreme climate events, including drought [1,2]. Drought generally has a strong effect on forest ecosystem processes and functioning by altering plant transpiration and increasing the risk of gas embolism in the xylem system, which may result in hydraulic failure [3,4]. Moreover, drought can increase the risk of carbon starvation by reducing carbohydrate transport and can be associated with heatwaves and wildfires to further enhance the risk of tree mortality [5,6]. Therefore, a prolonged drought event can reduce vegetation greenness, aboveground biomass, and the carbon sequestration capacity [7,8,9]. Understanding the vulnerability of forests to drought is critical to revealing the underlying mechanisms of tree resilience, recovery, and acclimation.
Recent studies have emphasized that forests generally require an extended period to recover from drought, which is referred to as the drought legacy [10]. Quantifying the drought legacy can uncover the response of forests to climatic change and improve the accuracy and robustness of process-based vegetation and climate models. Therefore, an increasing number of studies have been conducted to determine the duration and magnitude of drought legacies [11,12,13]. Furthermore, resilience to drought, defined as the duration of tree recovery to pre-drought levels, was found to generally differ among conifer species [14]. For example, Scots pine (Pinus sylvestris) and white spruce (Picea glauca) tended to recover more rapidly from drought than green ash (Fraxinus pennsylvanica) [15]. Korean pine (Pinus koraiensis) has a low drought resistance but a high recovery capacity [16]. In addition to the variable drought response of tree species, tree age may alter the magnitude and duration of the drought legacy. Yet, this has rarely been explored.
Peltier and Ogle [17] proposed that tree growth sensitivity to climate can be temporally variable, suggesting the age-specific sensitivity of growth to climate. However, large uncertainties remain in species- and age-specific tree-growth–climate sensitivity (i.e., the response of tree growth to variable climate). For example, in southern Italy, younger coniferous trees (Abies alba and Pinus leucodermis) had greater tree-growth–climate sensitivity than older conspecifics [18]. By contrast, on the Chinese Loess Plateau, older Pinus tabulaeformis tended to be more sensitive to climate than younger trees [19]. And in a temperate monsoon region, relatively old Pinus tabulaeformis trees had higher temperature but lower precipitation sensitivity [20]. Plantation forests play an important role in global carbon sequestration [21], but the role of stand age in determining the climate sensitivity and post-drought recovery of different tree species requires further research.
To close this knowledge gap, this study aimed to better understand how tree species and stand age modify tree-growth–climate sensitivity, as well as the magnitude and duration of the drought legacy of plantation forests in northeast China, which is the key region for national ecological security and home to native secondary and plantation forests [22]. We chose plantation forests for the present study on tree-growth–climate sensitivity and drought legacy because of the absence of inter-species competition and differences in stand density [23,24]. The study focused on four common tree species, namely, Korean pine, Scots pine, Japanese larch, and Dahurian larch, of varying ages because they are the major plantation species in northeast China. The growing-season Normalized Difference Vegetation Index (NDVI) was chosen as a proxy for tree growth. The research objectives were to quantify whether (a) tree-growth–climate sensitivity and drought legacy effects on tree growth vary among tree species and (b) stand age modifies tree-growth–climate sensitivity and the direction and the magnitude of drought legacy effects on tree growth. Our first hypothesis was that tree growth sensitivity to climate and the magnitude and duration of the drought legacy differ among tree species. This may be because tree-species-specific drought tolerance is highly variable [25,26]. The second hypothesis was that the drought legacy is modified by stand age and that the drought legacy increases with tree age. This is because, in contrast to young trees, older trees might have a lower photosynthetic capacity per unit of leaf area and lower water-use efficiency, therefore requiring more time to recover from drought [27].
2. Materials and Methods
2.1. Study Area
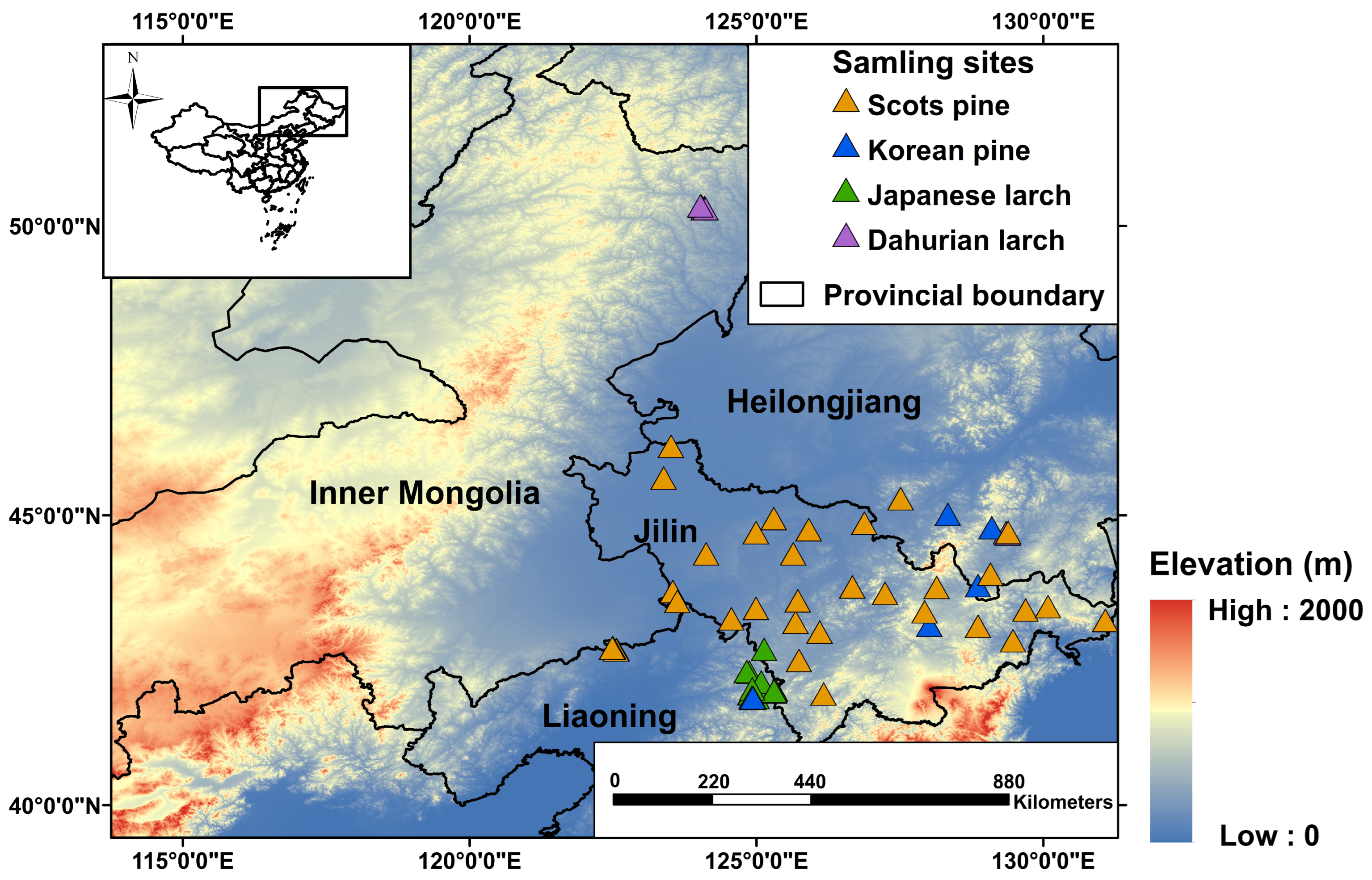
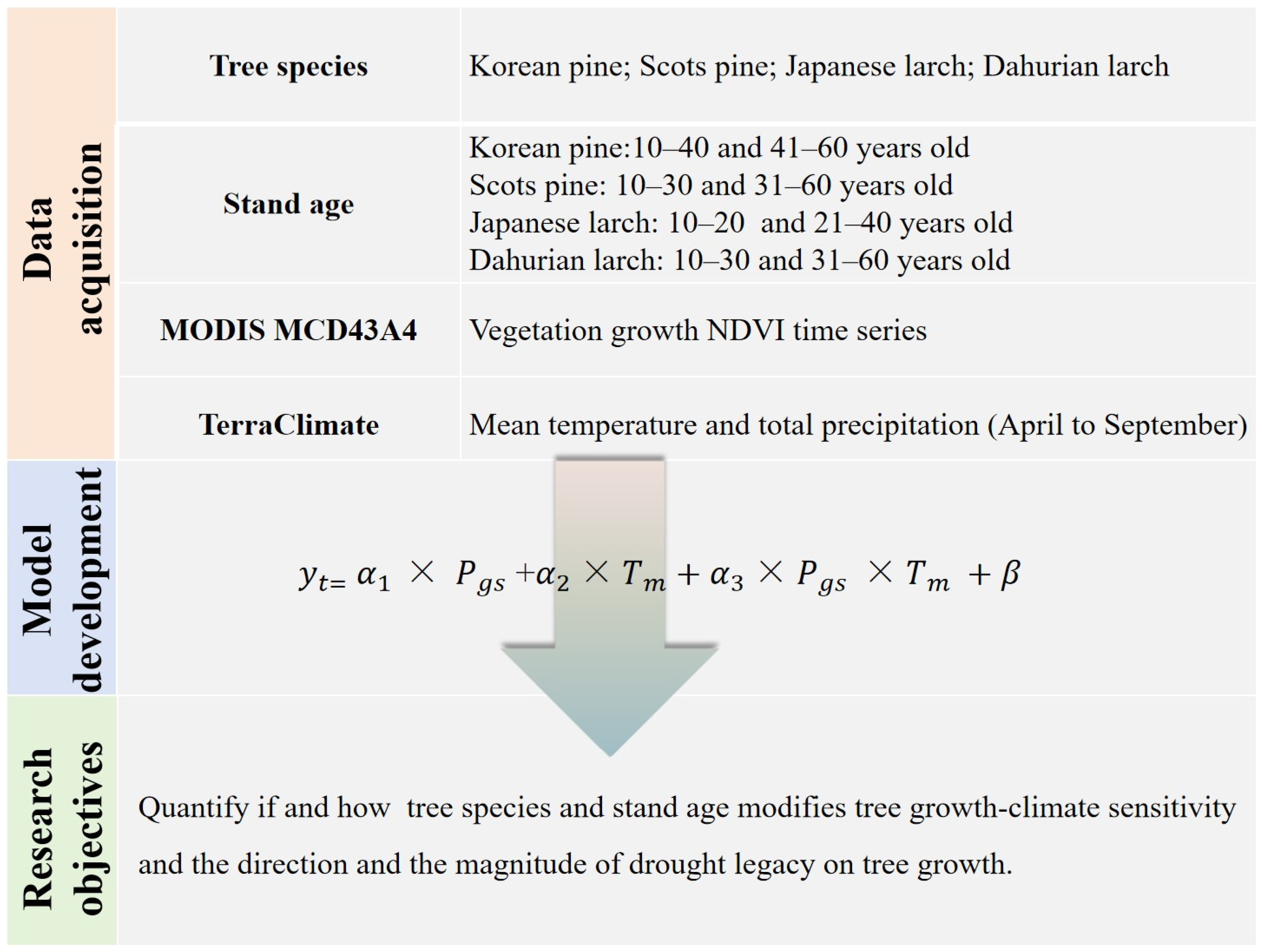
We collected detailed information about plantation forest stands in northeast China from peer-reviewed papers, theses, and dissertations (see Figure 1) from the ISI Web of Science, Scopus, and China National Knowledge Infrastructure (CNKI) databases. Specifically, dominant conifer species, geographical locations (i.e., latitude, longitude, elevation), stand age, topography, soil type, precipitation, and temperature in each forest stand were recorded. In total, we identified 82 plantation forest stands with tree ages that were directly reported or can be calculated (Figure 1, Table 1). Four common conifer species, namely, Korean pine (Pinus koraiensis), Scots pine (Pinus sylvestris), Japanese larch (Larix kaempferi), and Dahurian larch (Larix gmelinii), were the dominant species at these sites. The selected sites were distributed across three provinces (i.e., Heilongjiang, Jilin, and Liaoning) and one autonomous region (Inner Mongolia) of China, all of which are home to primary boreal and temperate forests and are priority areas for returning farmland to forests. The minimum area of a patchy plantation forest stand was 0.33 km2. For 90% of the plantation stands, the elevation was relatively comparable, ranging from 200 to 664 m above sea level. Annual mean precipitation over the past 20 years (2000–2020) was lower in pine stands (634 mm) than in larch stands (720 mm). The average annual mean temperature was higher in pine stands (5.31 °C) than in larch stands (4.03 °C). More detailed site-specific information is shown in the supplementary materials (Table S1). The overview of the methodology for this study is shown in Figure 2.
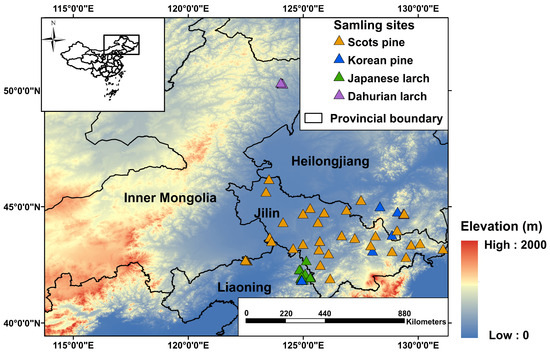
Figure 1.
Location of plantation forest stands used in this study. Elevation information was collected from NASA’s Shuttle Radar Topography Mission (SRTM) Version 3.0 Global 1 arc-second dataset (SRTMGL1) with a spatial resolution of about 30 m.

Table 1.
Summary of the 82 plantation forest stands with four common tree species in northeast China. Stand age was mean ± standard deviation. Annual mean precipitation and average of total annual precipitation were calculated by species across all sampling sites.
Stand age was determined by considering both the stand age reported and the field sampling date/time in the selected papers to calculate stand age in 2020, when the present study was carried out. For example, if one of the selected study cases stated that the forest stand was 35 years old and that the study was conducted in 2015, then the stand age was estimated to be 40 years old in the year 2020. For a small proportion of the peer-reviewed papers that only provided stand age intervals, such as 40–50 years old, the stand age of the plantation forest was assumed to be the average of the interval (i.e., 45 years old) for the subsequent analysis. We further constructed a general linear regression model in R [28] to test the relationship between annual tree growth and the year to ensure that plantation forests were not severely impacted by disturbances such as harvest or wildfire between 2000 and 2020.
We divided all trees into relatively young and old groups based upon the tree age distribution of each species and Chinese National Forest Inventory Standards (i.e., technical regulations for continuous forest inventory) [29]. It should be noted that plantation forests in China in general are relatively young (<60 years old). Here, relatively young and old trees are defined as 10–40 and 41–60 years old for Korean pine, 10–20 and 21–40 years old for Japanese larch, and 10–30 and 31–60 years old for Scots pine and Dahurian larch, respectively. And the ratios of young to old plantation stands were approximately 1.22, 1.00, 0.24, and 0.82 for these species, respectively.
2.2. MODIS-Derived NDVI Time-Series Data
NDVI can be used to estimate vegetation productivity and accurately detect vegetation phenology through changes in leaf area [30,31]. The NDVI has been extensively used to quantify the vegetation response to drought, e.g., [32,33]. We used NDVI time-series data derived from the daily Moderate Resolution Imaging Spectroradiometer (MODIS) Nadir Bidirectional Adjusted Reflectance (NBAR) product (MCD43A4) with a spatial resolution of 500 m from February 2000 to December 2020. Near-infrared reflectance (NIR: 841–876 nm) and red reflectance (Red: 620–670 nm) from the MCD43A4 (V6) product were downloaded from Google Earth Engine. Then, the daily NDVI was calculated as NDVI = (NIR − Red)/(NIR + Red) [34]. The growing-season NDVIs of plantation forest stands across all species varied from 0.56 to 0.80.
2.3. Climate Data
Monthly maximum and minimum temperatures and precipitation were obtained from the TerraClimate dataset for 2000–2020 with a spatial resolution of 4 km [35]. TerraClimate uses climatically aided interpolation to combine high-spatial resolution climatological normals from the WorldClim dataset with time-varying data from other sources. From this, we calculated the growing-season (i.e., April–September) and annual total precipitation input and mean temperature for each site and year. Growing-season precipitation was used because it has been shown to have a strong influence on the NDVI in northeast China [36], and our study sites received more than 85% of the annual precipitation input during these months. The mean growing-season temperature and precipitation were 17 °C and 501 mm, with standard deviations of 1.9 °C and 135.7 mm over the past 20 years. We identified periods of drought and extremely wet years when total growing-season precipitation was less than and greater than 1.5 standard deviations from the average of the 20-year growing-season precipitation input, respectively. To avoid the impacts of subsequent droughts and drying–wetting cycles in the following years after one episode of drought, we only used single drought events that lasted no more than one year and did not recur in the following 3 years.
2.4. Statistical Analysis
To quantify tree-growth–climate sensitivity, a general linear regression model was used for each species in R. To do so, we first detrended and normalized the growing-season NDVI time series for each site by using the “detrend” and “range” functions in MATLAB (MathWorks, R2019a, U.S.). This is because the growing-season NDVIs in the plantation forest stands increased with time. Then, the detrended and normalized NDVIs in “normal” years (i.e., after removing the extremely wet years, drought years, and 1–3 years post-drought) and associated climate variables (i.e., precipitation, temperature, and their interaction) were used in the linear models (see Equation (1)). This minimizes the potential impacts of extreme climate events on NDVI values. Dominance analysis was conducted to determine the relative contributions of the climatic variables using the “domir” package [37] in R (V 4.2.2; R Core Team 2021).
where represents the mean NDVI during the growing season; and are the total precipitation input and mean temperature over the growing season; , , and are the regression coefficients; and is the intercept.
To further compare species-specific climate sensitivity, we extracted the coefficient (slope) from each general linear regression model output, as shown by Li et al. [38]. To calculate the temperature sensitivity of tree growth, the precipitation variable in the generated models (Equation (1)) was removed first by providing the mean value of growing-season precipitation input. This allowed us to determine the model coefficient for the temperature variable (“slope”) as the temperature sensitivity of tree growth. Similarly, we obtained the precipitation sensitivity of tree growth using the same procedures described above, i.e., removing the temperature and using the average growing-season temperature. The larger the absolute value of the slope, the greater the tree-growth–climate sensitivity.
Next, to quantify the drought legacy effects on each tree species, we used Equation (1) again but replaced the growing-season mean temperature with the annual mean temperature. This is because a warmer autumn and spring can result in an extended growing season, which enhances tree growth and the NDVI, e.g., [39,40]. Thus, we included temperatures collected over the entire year as a predictor in the linear models. Then, we used these generated linear equations to predict the growing-season NDVI in the drought year and one to three years post-drought. The drought legacy for each species was quantified as the difference (∆NDVI) between the measured and the predicted growing-season NDVIs (∆NDVI = measured growing-season NDVI − predicted growing-season NDVI) [11,32,33]. A negative ∆NDVI indicates a reduction in post-drought growth, i.e., a legacy effect. One-way ANOVA was performed to compare differences in ∆NDVI one year prior to drought, the year of drought, and one to three years post-drought. Multiple comparisons among the five years were also conducted using Levene’s Test using the “leveneTest” package [41] in R.
The age-specific tree-growth–climate sensitivity and drought legacy were determined as described above. General linear regression models (as shown in Equation (1)) were conducted again using the age subsets defined above. Then, a linear mixed model was used to quantify whether and how stand age modified the drought legacy. The effects of stand age (for Korean pine: 10–40 and 41–60 years old; for Japanese larch: 10–20 and 21–40 years old; for Scots pine and Dahurian larch: 10–30 and 31–60 years old), time since drought (the year of drought, the first year post-drought, the second year post-drought, and the third year post-drought), and their interactions were treated as fixed effects, and the forest stand was treated as a random effect. The analysis was performed using the “lmer()” function, fitted into the “lme4” package [42], and p values were obtained by using the “lmerTest” package [43] in R. Significance was set at p < 0.05.
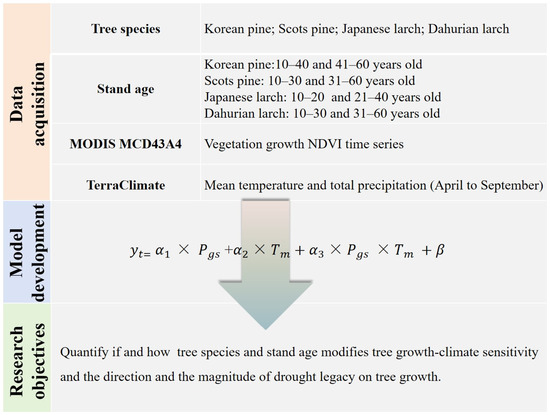
Figure 2.
Flowchart for analyzing climate sensitivity and drought legacy on tree growth used in this study.
Figure 2.
Flowchart for analyzing climate sensitivity and drought legacy on tree growth used in this study.
3. Results
3.1. Tree-Growth–Climate Sensitivity for Each Species and Age Group
Precipitation, temperature, and their interaction together affected the growing-season NDVI of all species (p < 0.05) (Table 2). According to dominance statistics, precipitation was the primary controller of Korean pine and Scots pine growth, whereas temperature was the main factor influencing Japanese larch and Dahurian larch growth (Table 3). At a given temperature (i.e., average July–August temperature), Scots pine tended to be more water-dependent than the other species, estimated by the steeper NDVI–precipitation slope (see Figure S1). The growth of Japanese larch and Dahurian larch was determined by the interaction between precipitation and temperature (p < 0.001).

Table 2.
ANOVA outputs of general linear regression model analyses of responses of MODIS-derived NDVI time series to precipitation, temperature, and their interaction for each of the species (Korean pine, Scots pine, Japanese larch, and Dahurian larch) and for their corresponding age groups (young and old).

Table 3.
Relative importance of three climatic variables in general linear regression model for each tree species. “General dominance statistics” are the contribution values of each predictor, and “Ranking” is the order of importance.
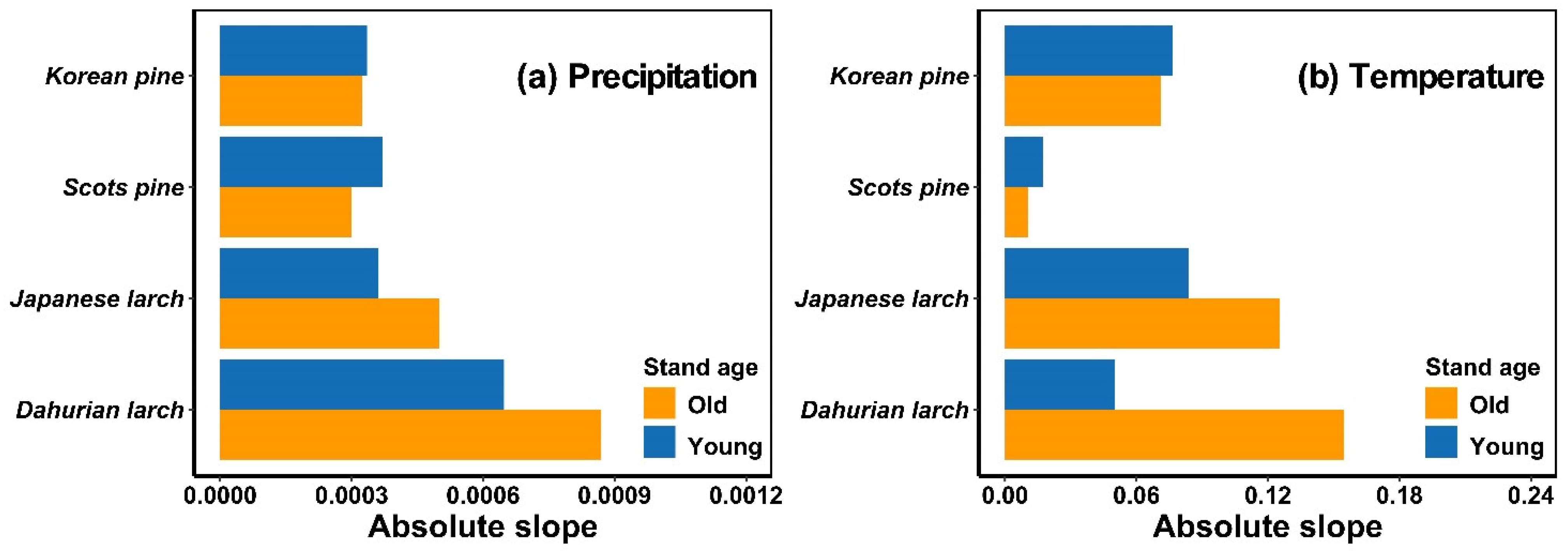
Similarly, precipitation, temperature, and their interaction affected the growing-season NDVI of both age groups of the four forest stands (p < 0.05) (Table 2). The tree-growth–climate sensitivity of Korean pine, Scots pine, Japanese larch, and Dahurian larch differed among age groups (Figure 3). Old Japanese larch and Dahurian larch trees were more sensitive to temperature and precipitation than young conspecifics, whereas old Korean pine and Scots pine were less sensitive to precipitation and temperature than young conspecifics.
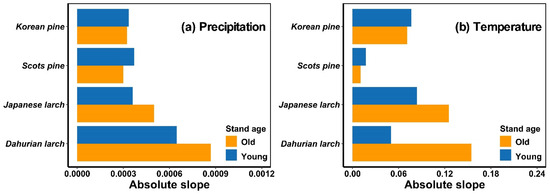
Figure 3.
The absolute coefficient value (slope) from general linear regression models for relationships between precipitation and growing-season NDVI at given air temperatures (i.e., averaged July–August temperature) (a) and between temperature and growing-season NDVI at given precipitation (i.e., cumulative precipitation from April to September) (b) of each tree species. Blue and orange bars represent trees in relatively young and old age groups, respectively.
3.2. Drought Legacy Effects on NDVI Varied among Species
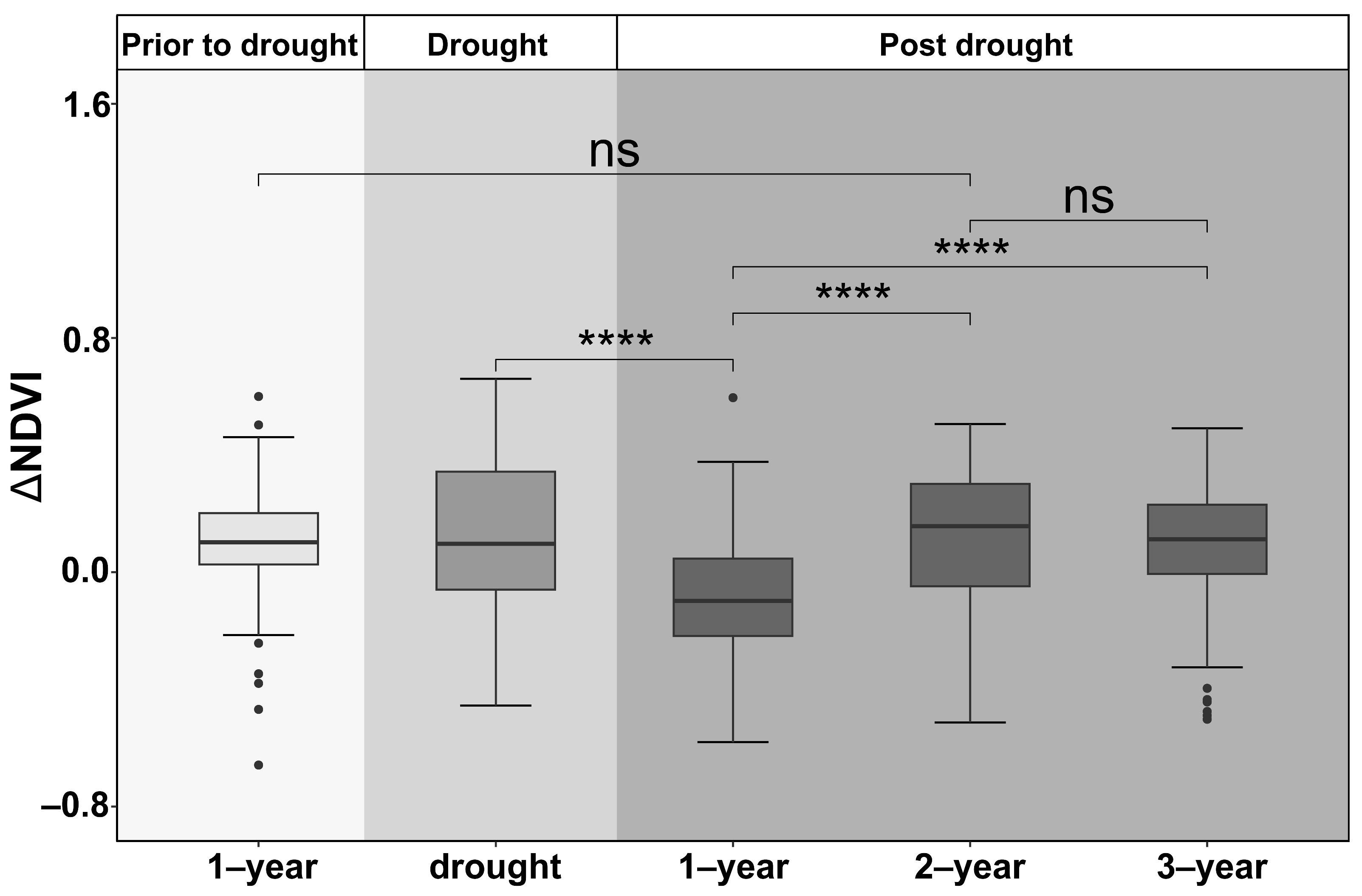
ΔNDVI, as a proxy for the drought legacy across all species and age groups, differed significantly among one year prior to drought, the year of drought, and one to three years post-drought across all plantation stands (p < 0.01) (Figure 4). On average, ΔNDVI was lowest in the first year post-drought and then recovered gradually and returned to the pre-drought level in about two years.
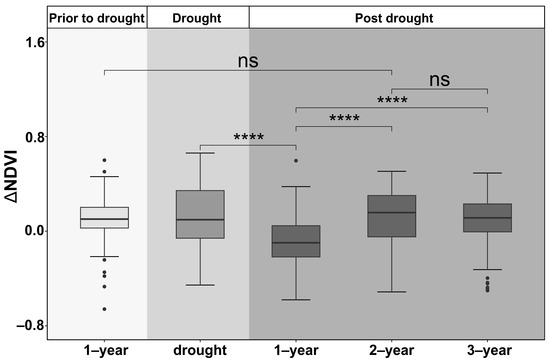
Figure 4.
Average residuals of growing-season NDVI across all species and age groups one year before the drought and drought legacies in the drought year and one to three years post-drought in plantation forests in northeast China. The stars (“****”) indicate significant difference at 0.001, and “ns” indicates no significant difference.
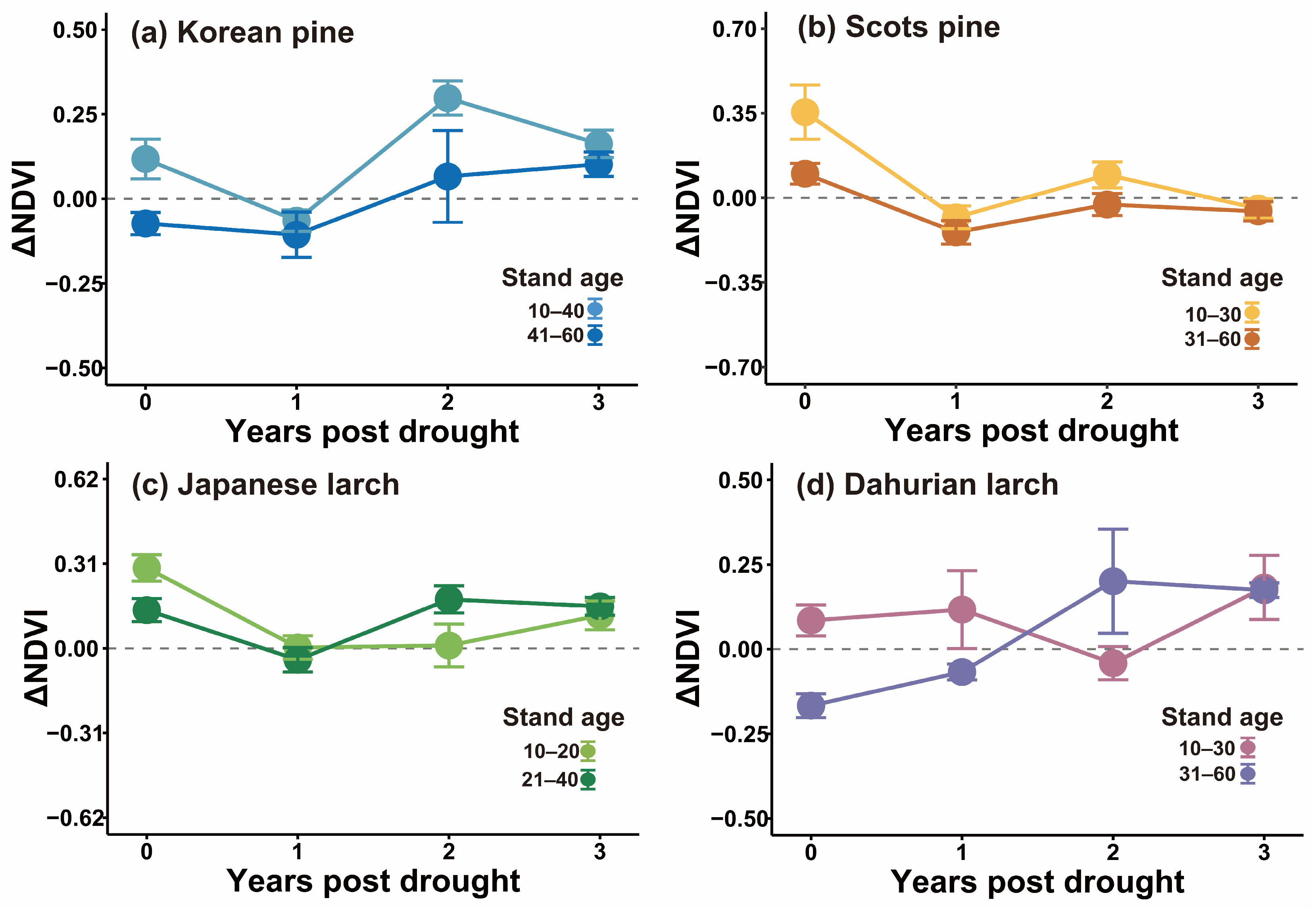
At the species level, ΔNDVI was negative in the first year post-drought for Korean pine and Japanese larch stands, and the drought legacies lasted for about one year (Figure 5). In contrast, ΔNDVI was negative for Dahurian larch stands immediately in the year of drought, and the legacies also lasted for one year. The longest drought legacy duration was for Scots pine stands, with a negative ΔNDVI until the third year post-drought. The magnitude of the first-year drought legacy was the largest for Scots pine, followed by Japanese larch, Korean pine, and then Dahurian larch.
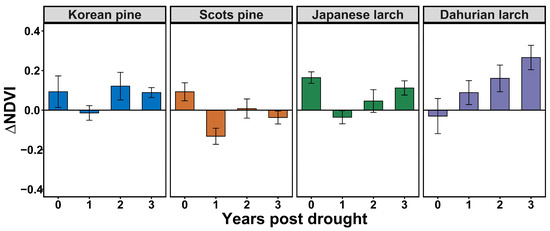
Figure 5.
Tree-species-specific drought legacies indicated by negative average residuals of growing-season NDVI during the year of drought and one to three years post-drought (mean ± standard error) in plantation forest stands in northeast China.
3.3. Stand Age Modified Drought Legacy
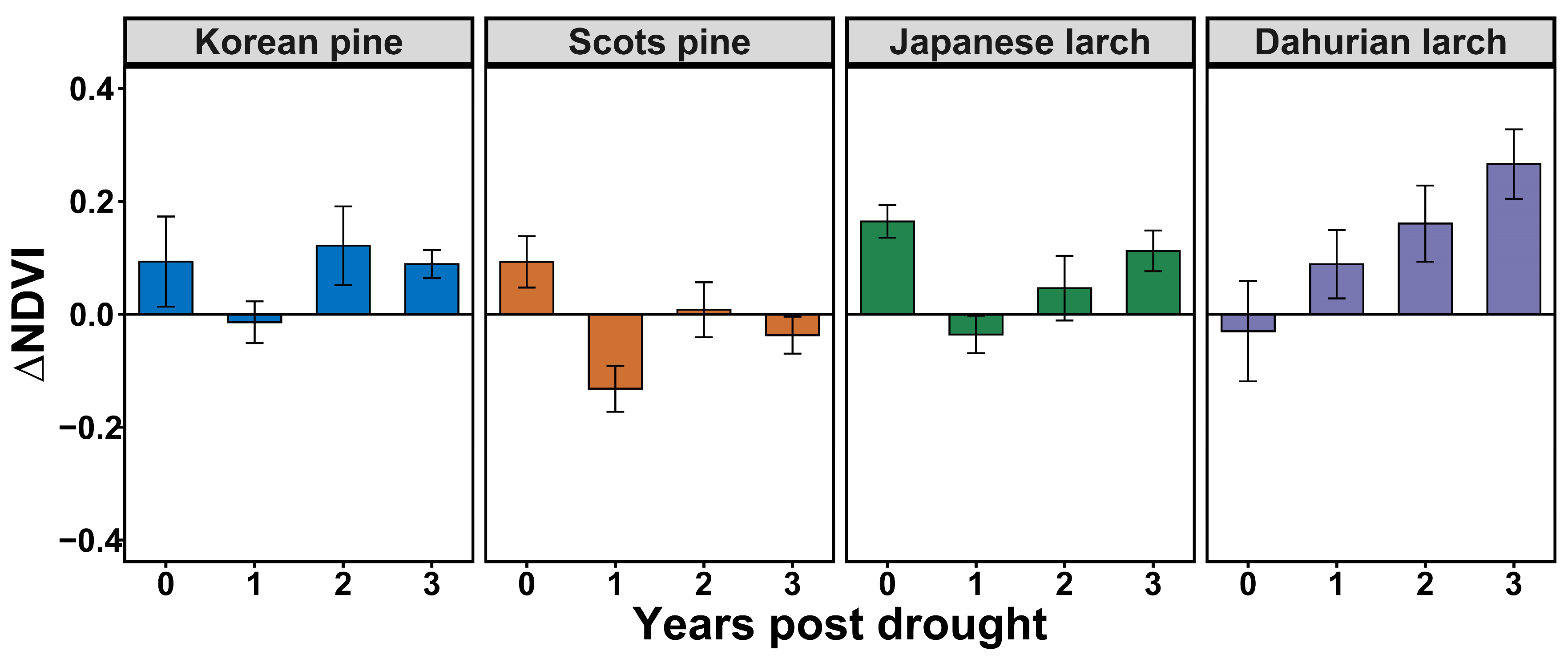
The duration and temporal patterns of drought legacy effects on the forest stands were dependent either on stand age, time since drought, or their interaction (Table 4, Figure 6). The drought legacy for Korean pine lasted only for one year for both old and young stands, but the drought impact in the year of drought was stronger for the old stands (Figure 6a). Age modified the duration of drought legacy effects on Scots pine (p = 0.02), with a longer duration for old Scots pine stands (aged 31–60 years) (>3 years) than for the younger stands (aged 10–30 years) (ca. 1 year) (Figure 6b). Both the time since drought, and the interaction between stand age and time since drought affected Japanese larch, suggesting that the extent of the drought legacy differed between age groups and varied over time (Figure 6c). The drought legacy was shorter for young Japanese larch stands (less than one year) than for old stands (more than one year). Further, drought legacies for Dahurian larch differed between stand age groups (p = 0.03), with a longer duration of the drought legacy in old stands than in young conspecifics, and tree age also modified the temporal pattern of the drought legacy (Figure 6d). Drought caused a growth reduction in the drought year and the first year post-drought in the relatively old stands, whereas for young stands, growth was only reduced in the second year post-drought.

Table 4.
ANOVA outputs of general linear mixed model analyses of response patterns of four tree species since drought (drought, 1, 2, and 3 years) and stand age group (Korean pine: 10–40 years and 41–60 years; Scots pine: 10–30 years and 31–60 years; Japanese larch: 10–20 years and 21–40 years; Dahurian larch: 10–30 years and 31–60 years).
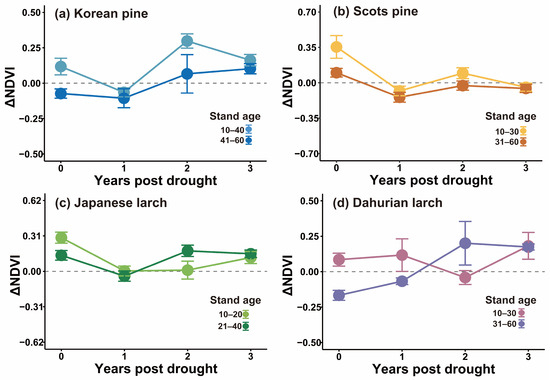
Figure 6.
Drought legacy effects on growing-season NDVI (mean ± standard error) of different tree species in young and old stands of (a) Korean pine; (b) Scots pine; (c) Japanese larch; and (d) Dahurian larch.
4. Discussion
This study quantified how tree species and stand age modified tree-growth–climate sensitivity and the direction, duration, and magnitude of the drought legacy for plantation forests in northeast China. The results showed that the tree-growth–climate sensitivity and drought legacy differed among the four conifer species. Dahurian larch and Japanese larch growth was mainly controlled by temperature, whereas Korean pine and Scots pine growth was mainly affected by precipitation. Moreover, the drought legacy lasted one year for Korean pine, Japanese larch, and Dahurian larch and over three years for Scots pine. The magnitude of the drought legacy for Scots pine was stronger in the first year than that for the other tree species. Thus, our first hypothesis (“Tree growth-climate sensitivity and drought legacy on tree growth vary among tree species.”) can be accepted. Furthermore, the stand age modified tree-growth–climate sensitivity and the duration and magnitude of drought legacy effects. Old Japanese larch (21–40 years) and Dahurian larch trees (31–60 years) were more sensitive to climate than young conspecifics, whereas old Korean pine (41–60 years) and Scots pine (31–60 years) were less sensitive to climate than young conspecifics. The drought legacy for old Scots pine, Japanese larch, and Dahurian larch lasted significantly longer than for young conspecifics. Age-related differences in the temporal pattern of the drought legacy were also evident for Dahurian larch stands, with the drought legacy in young stands found only in the second year after drought and the magnitude of the drought legacy being lower than that in older stands. Therefore, our second hypothesis (“drought legacy is modified by stand age and that drought legacy will increase with tree age”) can be partially accepted.
4.1. Tree-Growth–Climate Sensitivity and Drought Legacy Varied among Species
The growing-season NDVIs in the forest stands of all four species were significantly affected by temperature, precipitation, and their interaction (p < 0.05). Scots pine and Korean pine were more water-dependent than other species, while temperature strongly determined the growth of Japanese larch and Dahurian larch (Figure S1, Table 3 and Table S1). This shows that tree-growth–climate sensitivity is species-specific but may also be influenced by divergent water-use strategies and by the location of the stand. This is because larch species differ from other conifers in that they shed their leaves, which may alter their water-use efficiency compared to other conifers. Scots pine stands were generally in dry areas with an annual mean precipitation of 554 mm, and the growth of Scots pine declined with decreasing precipitation input. This is in agreement with Feichtinger et al. [44], who reported that reduced growing-season precipitation limits tree growth and photosynthetic activity. Korean pine stands were generally in relatively moist areas with an annual mean precipitation of 714 mm on average, but apparently, its growth was also determined primarily by precipitation. As shown by Lyu et al. [45], Korean pine species are sensitive to low water supply. On the other hand, the growth of both larch species was controlled by the growing-season temperature. A high temperature is more likely to enhance photosynthesis in generally cold regions (i.e., the winter season is approximately 4–5 months), as in the study area, and extend the growing period [39,40].
In about 70% of the stands, tree growth generally decreased in the first year after drought, suggesting a strong legacy effect (with negative ΔNDVI). In the second and third years post-drought, the drought effect was diminished (Figure 4). Overall, the growth of the forest stands returned to pre-drought levels within two years. A relatively short legacy duration for tree species is in agreement with previous studies [46,47]. The apparent carry-over effect of drought is due to alterations in the active xylem area, root function, access to deep soil moisture, canopy size, and the amounts or availability of stored non-structural carbohydrates (NSCs) [10].
At the species level, the drought legacies of Korean pine, Japanese larch, and Dahurian larch were about one year, whereas it was approximately three years for Scots pine (Figure 5). Scots pine had a longer duration and a greater magnitude of the drought legacy in the first year after drought than the other three coniferous species (Figure 5). This is largely consistent with the pattern reported by Bose et al. [48] for Scots pine trees growing in Europe (from southern Spain to northern Germany). There are several possible reasons for the differences in the drought legacy among tree species. Firstly, this is likely due to the differences in the NSC concentration and photosynthetic rate [49]. Generally, species with greater NSC concentrations are less affected by drought. If the dry climate persists and a negative whole-plant carbon balance is reached, the stored NSC pool will be used to maintain metabolic processes [50]. Hence, the ability of trees to recover from drought stress would be determined by the depletion rate of NSCs [51]. Scots pine, in particular, has an isohydric behavior that controls stomata to avoid excessive transpiration during drought, thus avoiding hydraulic failure but limiting carbon assimilation [52]. The reduced photosynthetic activity would result in a greater reduction in the stored NSC pool, which may lead to a more severe impact of drought and a stronger legacy effect. Secondly, variability in the total leaf area may modulate the drought legacy [53]. A recent study showed that even under severe drought conditions, newly developed needles of Scots pine may represent a large part of the total foliage [54]. The formation of new needles could exacerbate the depletion of stored NSCs and thereby influence the recovery of trees [55,56]. Moreover, Scots pine, with a greater change in canopy size (i.e., dropping more needles) during drought, had a more negative response to drought than trees with a smaller change in canopy size [54]. Lastly, Scots pine is more vulnerable to drought-induced xylem embolism than other coniferous species [57]. This suggests that Scots pine will require a longer time to recover from drought compared to other tree species. We showed that the magnitude of the drought legacy was greater for Japanese larch than for Korean pine, although the duration of the drought legacy was comparable (Figure 5). This is likely because the size, location, or mobility of transient NSC stores during drought are lower in Korean pine than in Japanese larch. We also showed that ΔNDVI values in Korean pine and Scots pine stands were higher in the second year than in the third year post-drought. This can be partially due to a compensation effect that ensures that these trees can quickly recover from drought [58].
Apart from the differences in functional traits among tree species described above, the variation is also likely due to differences in mean annual precipitation, as it has been reported that the extent of the drought legacy effect was enhanced with increasing water stress [59]. Sites with Scots pine are generally drier (annual mean precipitation of 554 mm) than sites with Dahurian larch, Korean pine, and Japanese larch (annual mean precipitation of 619, 714, and 821 mm). Thus, drought stress is likely to be greater for Scots pine than for the other three species.
4.2. Stand Age Modified Tree-Growth–Climate Sensitivity and Drought Legacy
Our results showed that the tree-growth–climate sensitivity varied not only with the tree species but also with stand age (Figure 3). The sensitivity of Japanese larch and Dahurian larch growth to climate was greater in old than in young stands, while older Korean pine and Scots pine had lower climate sensitivity than young stands. A few studies found that tree sensitivity increases with age [19,60], but this is not a universal effect. For example, Au et al. [61] found in Mediterranean, temperate and alpine/boreal regions that the upper canopies of young trees had greater climate sensitivity than those of older trees, with a greater reduction in productivity during and after drought. We showed that the response of trees to climate differs between old and young trees, which supports the hypothesis of Peltier and Ogle [17] that the climatic sensitivity of tree growth is not stationarity. As mentioned above, this may be due to differences in the production and distribution of NSCs, as well as in the total leaf area.
Our study also confirmed that stand age can modify the magnitude and duration of drought legacies for some tree species (Figure 6). Specifically, old Scots pine and Dahurian larch had longer drought legacies than young stands (Figure 6b–d), whereas this was not the case for Korean pine (Figure 6a). Thus, young Scots pine, Japanese larch, and Dahurian larch might not be as vulnerable to extreme climate events as we initially speculated, but older trees are likely to be severely damaged by drought and require an extended period of time to recover. This is likely because a larger tree has higher amounts of non-photosynthetic biomass, which requires a greater investment for defense and maintenance [62]. This suggests that compared to young Scots pine and Dahurian larch, older trees may have lower photosynthetic efficiency and thus not be able to maintain normal physiological activity when exposed to severe drought. Moreover, there is a positive relationship between stand age and canopy size [63]. Older trees tend to have greater water demand due to their larger canopy sizes and higher transpiration rates, thereby leading to a higher risk of hydraulic failure during drought. Additionally, older trees have significantly lower water-use efficiency than young trees [64]. Hence, recovery from drought in older trees can take a long time, given the high maintenance and growth demands of repairing their hydraulic systems and acquiring the nutrients needed for growth [48,65]. We also found that the immediate impact in the year of drought was stronger for the old stands. This again proves that drought resilience differed between age groups, which may be due to the greater nutrient losses in older trees during the drought year [66]. The drought legacy effect of Korean pine was relatively transient (ca. one year). This was likely because the extent of the depletion of stored NSCs was small. But it is still unclear and requires further studies to quantify whether the stored NSC content differs between old and young Scots pine, Korean pine, and Japanese larch trees under varying dry conditions.
We also found that the temporal pattern of the drought legacy differed between young and old Dahurian larch stands (Figure 6d). Compared to older trees, young Dahurian larch showed a positive ΔNDVI in the drought year and the first year post-drought, suggesting a delay in the drought legacy effect on younger trees. This is interesting and has not been reported in other studies. Possible explanations might be related to pre-drought climatic conditions, the timing of drought, the difference in relocating NSCs to plant organs, and the strategy used by young trees to continuously develop new needles after and even during drought [62,67,68]. On the other hand, drought reduced old Dahurian larch growth in the year of drought, and then the legacy effect gradually diminished. This may be due to leaf abscission to reduce transpiration rates in response to drought. Drought-induced damage would then be repaired over time, thereby making the drought legacy less apparent. Therefore, the replacement of older trees with young ones in Dahurian larch and Scots pine plantations might help increase tree resistance to drought. This is particularly vital for plantation forest management with increased episodes of drought in a warming future.
5. Conclusions
This study showed that tree-growth–climate sensitivity and drought legacy effects on plantation forests in northeast China differed among tree species and age groups. Scots pine and Korean pine were more water-dependent than other tree species, and the old trees of the two species were less climate-sensitive than the young conspecifics. On the other hand, Dahurian larch and Japanese larch growth was primarily determined by temperature, but old trees were more climate-sensitive than young conspecifics. Moreover, the drought legacy lasted one year for Korean pine, Japanese larch, and Dahurian larch and over three years for Scots pine. Scots pine was more vulnerable to prolonged drought than the other species, with a greater magnitude and exceptionally long duration of the drought legacy. Stand age was a key factor in regulating the drought legacy effect on tree growth. Relatively old trees were more severely affected by drought, particularly Scots pine and Dahurian larch. This is because the duration of the drought legacy was longer in relatively old trees than in young conspecifics. These findings combined highlight the role of stand age in determining the climate sensitivity and post-drought recovery of different tree species in plantation forests. Information on the differential drought resilience and recovery of tree species at varying ages is particularly useful to better inform plantation forest management and practices in a warm and dry future.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/rs16020281/s1, Figure S1: The absolute slopes from general linear regression models for precipitation and growing-season NDVI at given air temperature (averaged July–August temperatures) of each tree species; Table S1: Summary of the 85 sites’ information [69,70,71,72,73,74,75,76,77,78].
Author Contributions
Conceptualization, T.L. and Q.S.; Methodology, T.L., Q.S. and P.M.; Investigation, T.L.; Writing—Original Draft Preparation, T.L.; Writing—Review and Editing, Q.S., H.Z. and P.M.; Supervision, Q.S. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Heilongjiang Province of China, grant number YQ2021C002; the Fundamental Research Funds for the Central Universities of China, grant number 2572021DT05; and Postdoctoral Science Foundation of Heilongjiang Province of China, grant number LBH-Q20063.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J.; et al. Climate-driven risks to the climate mitigation potential of forests. Science 2020, 368, eaaz7005. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Field, C.B. Changes in Ecologically Critical Terrestrial Climate Conditions. Science 2013, 341, 486–492. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Anderegg, W.R.L.; Michalak, A.M.; Fisher, J.B.; Biondi, F.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Wolf, A.; et al. Global patterns of drought recovery. Nature 2017, 548, 202–205. [Google Scholar] [CrossRef]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martinez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef]
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.M.; Bragg, D.C.; D’Amato, A.W.; Davis, F.W.; Hersh, M.H.; Ibanez, I.; et al. The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob. Chang. Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef]
- Brando, P.M.; Paolucci, L.; Ummenhofer, C.C.; Ordway, E.M.; Hartmann, H.; Cattau, M.E.; Rattis, L.; Medjibe, V.; Coe, M.T.; Balch, J. Droughts, Wildfires, and Forest Carbon Cycling: A Pantropical Synthesis. Annu. Rev. Earth Planet. Sci. 2019, 47, 555–581. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Kim, J.B.; Trugman, A.T.; Kim, Y.; Still, C.J. Linking tree physiological constraints with predictions of carbon and water fluxes at an old-growth coniferous forest. Ecosphere 2019, 10, e02692. [Google Scholar] [CrossRef]
- Ogaya, R.; Barbeta, A.; Basnou, C.; Penuelas, J. Satellite data as indicators of tree biomass growth and forest dieback in a Mediterranean holm oak forest. Ann. For. Sci. 2015, 72, 135–144. [Google Scholar] [CrossRef]
- Zhou, L.M.; Tian, Y.H.; Myneni, R.B.; Ciais, P.; Saatchi, S.; Liu, Y.Y.; Piao, S.L.; Chen, H.S.; Vermote, E.F.; Song, C.H.; et al. Widespread decline of Congo rainforest greenness in the past decade. Nature 2014, 509, 86–90. [Google Scholar] [CrossRef]
- Muller, L.M.; Bahn, M. Drought legacies and ecosystem responses to subsequent drought. Glob. Chang. Biol. 2022, 28, 5086–5103. [Google Scholar] [CrossRef]
- Huang, M.T.; Wang, X.H.; Keenan, T.F.; Piao, S.L. Drought timing influences the legacy of tree growth recovery. Glob. Chang. Biol. 2018, 24, 3546–3559. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Connolly, S.R.; Baird, A.H.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Jacobson, M.; Liu, G.; et al. Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat. Clim. Chang. 2019, 9, 40–43. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Mencuccini, M.; Martinez-Vilalta, J. Changes in tree resistance, recovery and resilience across three successive extreme droughts in the northeast Iberian Peninsula. Oecologia 2018, 187, 343–354. [Google Scholar] [CrossRef]
- Manrique-Alba, A.; Begueria, S.; Camarero, J.J. Long-term effects of forest management on post-drought growth resilience: An analytical framework. Sci. Total Environ. 2022, 810, 152374. [Google Scholar] [CrossRef] [PubMed]
- Canning, C.M.; Mood, B.J.; Bonsal, B.; Howat, B.; Laroque, C.P. Comparison of tree-growth drought legacies of three shelterbelt species in the Canadian Prairies. Agric. For. Meteorol. 2023, 330, 109317. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, J.; Kang, M.; Cho, S.; Park, J.; Lee, M.; Lee, H.; Kim, D.; Kim, H.S. The resilience of the carbon cycles of temperate coniferous and broadleaved forests to drought. For. Ecol. Manag. 2021, 491, 119178. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Ogle, K. Tree growth sensitivity to climate is temporally variable. Ecol. Lett. 2020, 23, 1561–1572. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Gazol, A.; Piovesan, G.; Borghetti, M.; Baliva, M.; Gentilesca, T.; Rita, A.; Schettino, A.; Ripullone, F. Mediterranean old-growth forests exhibit resistance to climate warming. Sci. Total Environ. 2021, 801, 149684. [Google Scholar] [CrossRef]
- Fang, K.Y.; Chen, D.; Gou, X.H.; D’Arrigo, R.; Davi, N. Influence of non-climatic factors on the relationships between tree growth and climate over the Chinese Loess Plateau. Glob. Planet. Chang. 2015, 132, 54–63. [Google Scholar] [CrossRef]
- Xu, L.L.; Meng, P.; Tong, X.J.; Zhang, J.S.; Li, J.; Wang, X.; Xie, H.; Liu, P.R. Productivity and water use efficiency of Pinus tabulaeformis responses to climate change in the temperate monsoon region. Agric. For. Meteorol. 2022, 327, 109188. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Palmer, P.I.; Liu, Y.; Fang, S.X.; Bosch, H.; O’Dell, C.W.; Tang, X.P.; Yang, D.X.; Liu, L.X.; et al. Large Chinese land carbon sink estimated from atmospheric carbon dioxide data. Nature 2020, 586, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, J.J.; Zhang, M.; Yan, Q.L.; Sun, O.J. Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: A comparison between natural secondary forest and larch plantation. J. Plant Ecol. 2010, 3, 175–182. [Google Scholar] [CrossRef]
- Faria, J.; Sanchez-Oliver, J.S.; Beja, P.; Moreira, F.; Catry, I.; Vasconcelos, S.; Pina, S.; Rotenberry, J.T.; Reino, L.; Santana, J. Contrasting effects of eucalyptus, pine and oak plantations on nest predation risk in Mediterranean grasslands. For. Ecol. Manag. 2022, 511, 120116. [Google Scholar] [CrossRef]
- Hua, F.Y.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.R.; Wang, W.Y.; McEvoy, C.; Pena-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, A.; Camarero, J.J.; Aspizua, R.; Sanchez-Gonzalez, M.; Gil, L.; Montes, F. Abiotic factors modulate post-drought growth resilience of Scots pine plantations and rear-edge Scots pine and oak forests. Dendrochronologia 2018, 51, 54–65. [Google Scholar] [CrossRef]
- Steckel, M.; del Rio, M.; Heym, M.; Aldea, J.; Bielak, K.; Brazaitis, G.; Cerny, J.; Coll, L.; Collet, C.; Ehbrecht, M.; et al. Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.)—Site water supply and fertility modify the mixing effect. For. Ecol. Manag. 2020, 461, 117908. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yu, P.; Wang, D.Z.; Xu, Z.Q. Density- and age- dependent influences of droughts and intrinsic water use efficiency on growth in temperate plantations. Agric. For. Meteorol. 2022, 325, 109134. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 31 October 2022).
- GB/T 38590-2020; Technical Regulations for Continuous Forest Inventory. State Administration for Market Regulation: Beijing, China; National Standardization Administration: Beijing, China, 2020; 56p.
- Tucker, C.J.; Vanpraet, C.L.; Sharman, M.J.; Van Ittersum, G. Satellite remote sensing of total herbaceous biomass production in the senegalese sahel: 1980–1984. Remote Sens. Environ. 1985, 17, 233–249. [Google Scholar] [CrossRef]
- Heumann, B.W.; Seaquist, J.W.; Eklundh, L.; Jönsson, P. AVHRR derived phenological change in the Sahel and Soudan, Africa, 1982–2005. Remote Sens. Environ. 2007, 108, 385–392. [Google Scholar] [CrossRef]
- Li, P.L.; Zhu, D.; Wang, Y.L.; Liu, D. Elevation dependence of drought legacy effects on vegetation greenness over the Tibetan Plateau. Agric. For. Meteorol. 2020, 295, 108190. [Google Scholar] [CrossRef]
- Dong, B.G.; Yu, Y.; Pereira, P. Non-growing season drought legacy effects on vegetation growth in southwestern China. Sci. Total Environ. 2022, 846, 157334. [Google Scholar] [CrossRef] [PubMed]
- Schowengerdt, R.A. Remote Sensing: Models and Methods for Image Processing; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Abatzoglou, J.T.; Dobrowski, S.Z.; Parks, S.A.; Hegewisch, K.C. Data Descriptor: TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data 2018, 5, 170191. [Google Scholar] [CrossRef]
- Chu, H.S.; Venevsky, S.; Wu, C.; Wang, M.H. NDVI-based vegetation dynamics and its response to climate changes at Amur-Heilongjiang River Basin from 1982 to 2015. Sci. Total Environ. 2019, 650, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Azen, R.; Budescu, D.V. The dominance analysis approach for comparing predictors in multiple regression. Psychol. Methods 2003, 8, 129–148. [Google Scholar] [CrossRef]
- Li, W.Q.; Jiang, Y.; Dong, M.Y.; Du, E.Z.; Wu, F.; Zhao, S.D.; Xu, H. Species-specific growth-climate responses of Dahurian larch (Larix gmelinii) and Mongolian pine (Pinus sylvestris var. mongolica) in the Greater Khingan Range, northeast China. Dendrochronologia 2021, 65, 125803. [Google Scholar] [CrossRef]
- Fu, Y.H.; Piao, S.; Delpierre, N.; Hao, F.; Hänninen, H.; Liu, Y.; Sun, W.; Janssens, I.A.; Campioli, M. Larger temperature response of autumn leaf senescence than spring leaf-out phenology. Glob. Chang. Biol. 2018, 24, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Jeong, S.; Park, C.E.; Xu, H.; Li, L.Z.X.; Wang, T.; Gentine, P.; Penuelas, J.; Piao, S.L. Biophysical impacts of northern vegetation changes on seasonal warming patterns. Nat. Commun. 2022, 13, 3925. [Google Scholar] [CrossRef]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W.W. The Impact of Levene’s Test of Equality of Variances on Statistical Theory and Practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Feichtinger, L.M.; Eilmann, B.; Buchmann, N.; Rigling, A. Growth adjustments of conifers to drought and to century-long irrigation. For. Ecol. Manag. 2014, 334, 96–105. [Google Scholar] [CrossRef]
- Lyu, S.N.; Wang, X.C.; Zhang, Y.D.; Li, Z.S. Different responses of Korean pine (Pinus koraiensis) and Mongolia oak (Quercus mongolica) growth to recent climate warming in northeast China. Dendrochronologia 2017, 45, 113–122. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Kannenberg, S.A.; Schwalm, C.R.; Anderegg, W.R.L. Ghosts of the past: How drought legacy effects shape forest functioning and carbon cycling. Ecol. Lett. 2020, 23, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.K.; Gessler, A.; Bolte, A.; Bottero, A.; Buras, A.; Cailleret, M.; Camarero, J.J.; Haeni, M.; Heres, A.M.; Hevia, A.; et al. Growth and resilience responses of Scots pine to extreme droughts across Europe depend on predrought growth conditions. Glob. Chang. Biol. 2020, 26, 4521–4537. [Google Scholar] [CrossRef] [PubMed]
- Peltier, D.M.P.; Guo, J.; Nguyen, P.; Bangs, M.; Wilson, M.; Samuels-Crow, K.; Yocom, L.L.; Liu, Y.; Fell, M.K.; Shaw, J.D.; et al. Temperature memory and non-structural carbohydrates mediate legacies of a hot drought in trees across the southwestern USA. Tree Physiol. 2022, 42, 71–85. [Google Scholar] [CrossRef]
- Martin-Gomez, P.; Aguilera, M.; Peman, J.; Gil-Pelegrin, E.; Ferrio, J.P. Contrasting ecophysiological strategies related to drought: The case of a mixed stand of Scots pine (Pinus sylvestris) and a submediterranean oak (Quercus subpyrenaica). Tree Physiol. 2017, 37, 1478–1492. [Google Scholar] [CrossRef]
- Rowland, L.; da Costa, A.C.L.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.R.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Manzoni, S.; Vico, G.; Thompson, S.; Beyer, F.; Weih, M. Contrasting leaf phenological strategies optimize carbon gain under droughts of different duration. Adv. Water Resour. 2015, 84, 37–51. [Google Scholar] [CrossRef]
- Sanguesa-Barreda, G.; Gazol, A.; Camarero, J.J. Drops in needle production are early-warning signals of drought-triggered dieback in Scots pine. Trees-Struct. Funct. 2023, 37, 1137–1151. [Google Scholar] [CrossRef]
- He, W.Q.; Liu, H.Y.; Qi, Y.; Liu, F.; Zhu, X.R. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Chang. Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.N.; Gessler, A.; Saurer, M.; Hagedorn, F.; Gao, D.C.; Wang, X.Y.; Schaub, M.; Li, M.H.; Shen, W.J.; Schonbeck, L. Root carbon and nutrient homeostasis determines downy oak sapling survival and recovery from drought. Tree Physiol. 2021, 41, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salguero, R.; Navarro-Cerrillo, R.M.; Camarero, J.J.; Fernandez-Cancio, A. Selective drought-induced decline of pine species in southeastern Spain. Clim. Chang. 2012, 113, 767–785. [Google Scholar] [CrossRef]
- Gessler, A.; Bottero, A.; Marshall, J.; Arend, M. The way back: Recovery of trees from drought and its implication for acclimation. New Phytol. 2020, 228, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schwalm, C.R.; Samuels-Crow, K.E.; Ogle, K. Ecological memory of daily carbon exchange across the globe and its importance in drylands. Ecol. Lett. 2019, 22, 1806–1816. [Google Scholar] [CrossRef]
- Primicia, I.; Camarero, J.J.; Janda, P.; Cada, V.; Morrissey, R.C.; Trotsiuk, V.; Bace, R.; Teodosiu, M.; Svoboda, M. Age, competition, disturbance and elevation effects on tree and stand growth response of primary Picea abies forest to climate. For. Ecol. Manag. 2015, 354, 77–86. [Google Scholar] [CrossRef]
- Au, T.F.; Maxwell, J.T.; Robeson, S.M.; Li, J.B.; Siani, S.M.O.; Novick, K.A.; Dannenberg, M.P.; Phillips, R.P.; Li, T.; Chen, Z.J.; et al. Younger trees in the upper canopy are more sensitive but also more resilient to drought. Nat. Clim. Chang. 2022, 12, 1168–1174. [Google Scholar] [CrossRef]
- Scholz, F.G.; Phillips, N.G.; Bucci, S.J.; Meinzer, F.C.; Goldstein, G. Hydraulic Capacitance: Biophysics and Functional Significance of Internal Water Sources in Relation to Tree Size. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 341–361. [Google Scholar] [CrossRef]
- Feraandez-Martinez, M.; Garbulsky, M.; Penuelas, J.; Peguero, G.; Espelta, J.M. Temporal trends in the enhanced vegetation index and spring weather predict seed production in Mediterranean oaks. Plant Ecol. 2015, 216, 1061–1072. [Google Scholar] [CrossRef]
- Ouyang, L.; Wu, J.; Zhao, P.; Zhu, L.W.; Ni, G.Y. Stand age rather than soil moisture gradient mainly regulates the compromise between plant growth and water use of Eucalyptus urophylla in hilly South China. Land Degrad. Dev. 2021, 32, 2423–2436. [Google Scholar] [CrossRef]
- Olson, M.E.; Soriano, D.; Rosell, J.A.; Anfodillo, T.; Donoghue, M.J.; Edwards, E.J.; Leon-Gomez, C.; Dawson, T.; Martinez, J.J.C.; Castorena, M.; et al. Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. USA 2018, 115, 7551–7556. [Google Scholar] [CrossRef] [PubMed]
- Maclauchlan, L. Quantification of Dryocoetes confusus-caused mortality in subalpine fir forests of southern British Columbia. For. Ecol. Manag. 2016, 359, 210–220. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Sanchez-Salguero, R.; Vicente-Serrano, S.M.; Serra-Maluquer, X.; Gutierrez, E.; de Luis, M.; Sanguesa-Barreda, G.; Novak, K.; Rozas, V.; et al. Drought legacies are short, prevail in dry conifer forests and depend on growth variability. J. Ecol. 2020, 108, 2473–2484. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, H.Y.; Piao, S.L.; Ciais, P.; Wu, X.C.; Yin, Y.; Wang, H.Y. Enhanced growth after extreme wetness compensates for post-drought carbon loss in dry forests. Nat. Commun. 2019, 10, 195. [Google Scholar] [CrossRef]
- Zhou, Y. The Research of Two Species of Sawfly Damaged on the Coniferous Plantations Influence Carbon Sinks. Master’s Thesis, Northeast Forestry University, Harbin, China, 2014. [Google Scholar]
- Wang, C. Study on the Relationship between Pine Needles of Pinus koraiensis Plantation and Soil Nutrients under Different Terrain Conditions. Master’s Thesis, Northeast Forestry University, Harbin, China, 2014. [Google Scholar]
- Xu, T.; Zhu, J.; Yu, L.; Wang, R.; Zhang, J. Physical and Chemical Properties of Stemflow in Different Forest Types of a Secondary Forest Ecosystem in Montane Regions of Eastern Liaoning Province, China. Acta Ecol. Sin. 2013, 33, 3415–3424. [Google Scholar]
- Wang, A. Spatial Distribution Pattern and Interspecific Relationship of Dominant Species in Typical Forest Community in Mao’er Mountain. Shandong For. Sci. Technol. 2020, 50, 1–9+16. [Google Scholar]
- Xie, J.; Yan, Q.; Zhang, T. Temporal Effects of Thinning on the Composition and Growth of Regenerated Woody Plants in Larix Kaempferi Plantations. Chin. J. Appl. Ecol. 2020, 31, 2481–2490. [Google Scholar]
- Wu, M. Growth and Climatic Response of Pinus sylvestris var. mongolica in Sandy Land of Zhang-Gu-Tai, Liaoning Province. Master Thesis, Beijing Forestry University, Harbin, China, 2019. [Google Scholar]
- Chen, M. Water Uptake of Pinus sylvestris var. mongolica Plantation in Horqin Sandy Land. Master’s Thesis, Beijing Forestry University, Harbin, China, 2020. [Google Scholar]
- Lu, X. Study on the Radial Growth and Spatial Climate Response of Pinus sylvestris var. mongolica in Plantations in Jilin Province. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2018. [Google Scholar]
- Liu, B.; Wang, S.; Dao, R. Effect of Afforestation in Sandy Land on Soil Bulk Density and Particle-Size Composition in Duolun Count. Inn. Mong. For. Sci. Technol. 2013, 39, 11–14. [Google Scholar]
- Tang, S.; Gao, B.; Yu, B.; Wang, X.; Yin, S.; Shan, S.; Han, X.; Cao, L. Temperature Changes and Main Gas Release Characteristics of Larix Gmelinii Plantations During Smoldering in Daxing’anling Mountain Region of Heilongjiang Province, Northeastern China. J. Beijing For. Univ. 2022, 44, 1–7. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).