Proximal Sensing for Characterising Seaweed Aquaculture Crop Conditions: Optical Detection of Ice-Ice Disease
Abstract
1. Introduction
2. Methodology
2.1. In Situ Data Acquisition
2.2. Experimental Scenarios
3. Results
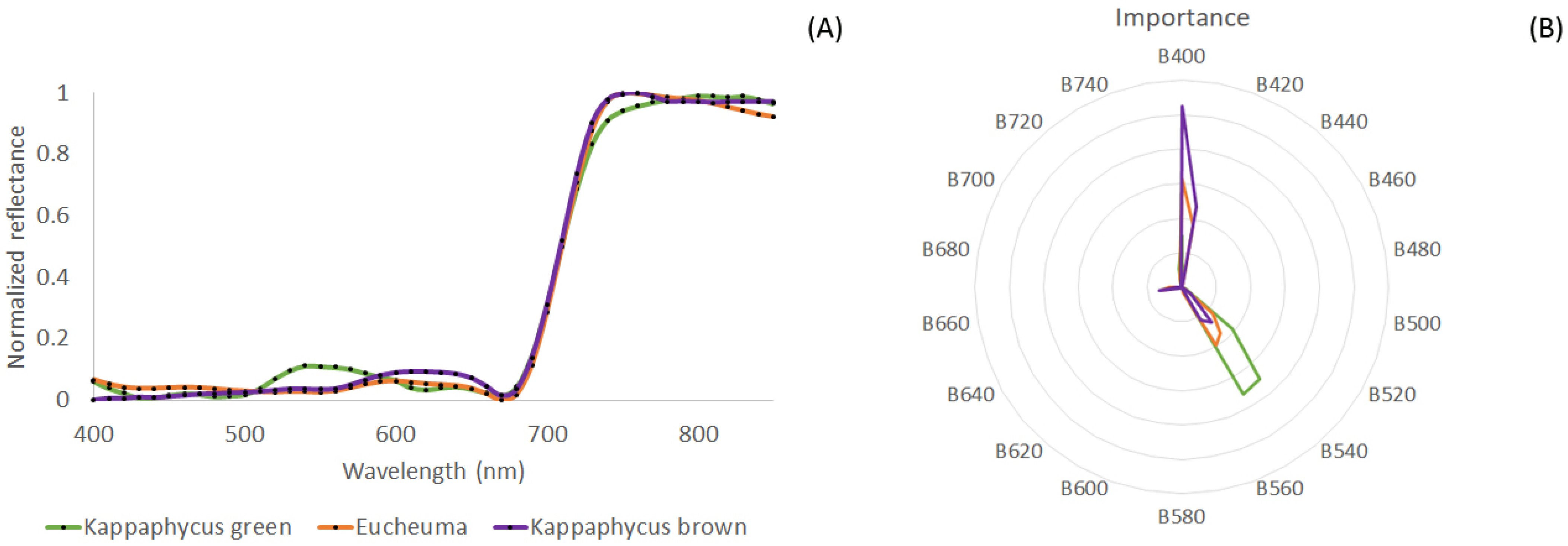
3.1. Spectral Library Classification
3.2. Hyperspectral Data
3.3. Multispectral Data
3.4. Image-Based Classification
4. Discussion
4.1. Species Discrimination
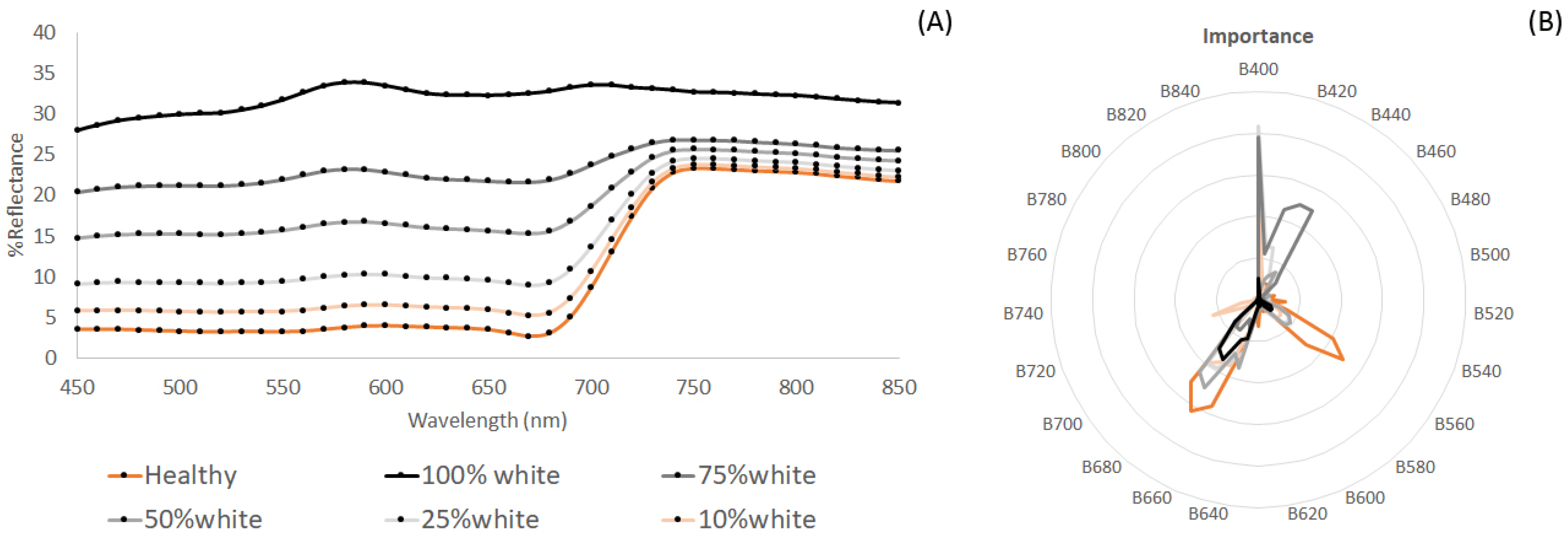
4.2. Detecting and Quantifying Thallus Depigmentation
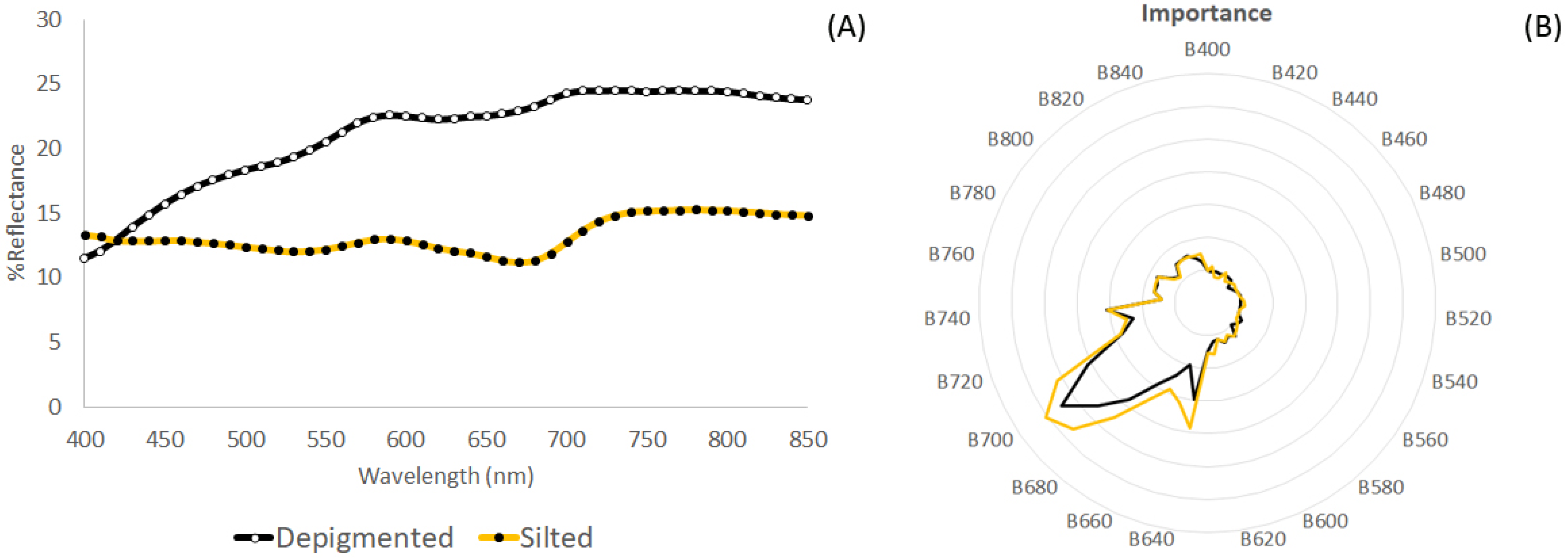
4.3. Discriminating Thallus Covered by Silt Particles
4.4. Spectral Constraints
4.5. Drone Crop Condition Monitoring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| PREDICTION | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy | 10% White | 25% White | 50% White | 75% White | 100% White PA% | |||
| OBSERVATION | Healthy | 15 | 1 | 1 | 0 | 0 | 0 | 88.2 |
| 10% White | 6 | 13 | 1 | 0 | 0 | 0 | 65 | |
| 25% White | 0 | 1 | 14 | 1 | 0 | 0 | 87.5 | |
| 50% White | 0 | 0 | 0 | 19 | 0 | 0 | 100 | |
| 75% White | 0 | 0 | 0 | 0 | 16 | 0 | 100 | |
| 100% White | 0 | 0 | 2 | 1 | 3 | 7 | 53.8 | |
| UA% | 71.4 | 86.7 | 77.8 | 90.5 | 84.2 | 100 | 83.2 | |
| PREDICTION | ||||||||
|---|---|---|---|---|---|---|---|---|
| OBSERVATION | Healthy | 10% White | 25% White | 50% White | 75% White | 100% White PA% | ||
| Healthy | 17 | 0 | 0 | 0 | 0 | 0 | 100 | |
| 10% White | 2 | 18 | 0 | 0 | 0 | 0 | 90 | |
| 25% White | 0 | 0 | 16 | 0 | 0 | 0 | 100 | |
| 50% White | 0 | 0 | 0 | 19 | 0 | 0 | 100 | |
| 75% White | 0 | 0 | 0 | 0 | 16 | 0 | 100 | |
| 100% White | 1 | 1 | 0 | 0 | 0 | 9 | 81.8 | |
| UA% | 85 | 94.7 | 100 | 100 | 100 | 100 | 95.9 | |
| PREDICTION | |||||
|---|---|---|---|---|---|
| Kappaphycus green | Eucheuma | Kappaphycus brown | PA% | ||
| OBSERVATION | Kappaphycus green | 17 | 0 | 1 | 94 |
| Eucheuma | 0 | 17 | 0 | 100 | |
| Kappaphycus brown | 0 | 0 | 16 | 100 | |
| UA% | 100 | 100 | 94.1 | ||
| OBSERVATION | PREDICTION | |||
| 100% White | Silted | PA% | ||
| 100% White | 10 | 3 | 76.9 | |
| Silted | 2 | 21 | 91.3 | |
| UA% | 83.3 | 87.5 | 86.1 | |
| OBSERVATION | PREDICTION | |||||||
| Healthy | 10% White | 25% White | 50% White | 75% White | 100% White | PA% | ||
| Healthy | 15 | 1 | 1 | 0 | 0 | 0 | 88.2 | |
| 10% White | 6 | 12 | 2 | 0 | 0 | 0 | 60 | |
| 25% White | 0 | 2 | 13 | 1 | 0 | 0 | 81.3 | |
| 50% White | 0 | 0 | 0 | 19 | 0 | 0 | 100 | |
| 75% White | 0 | 0 | 0 | 0 | 16 | 0 | 100 | |
| 100% White | 0 | 0 | 2 | 0 | 0 | 11 | 84.6 | |
| UA% | 71.4 | 80 | 72.2 | 95 | 100 | 100 | 85.1 | |
| PREDICTION | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy | 10% White | 25% White | 50% White | 75% White | 100% White | PA% | ||
| OBSERVATION | Healthy | 16 | 1 | 0 | 0 | 0 | 0 | 94.1 |
| 10% White | 0 | 20 | 0 | 0 | 0 | 0 | 100 | |
| 25% White | 0 | 0 | 16 | 0 | 0 | 0 | 100 | |
| 50% White | 0 | 0 | 0 | 19 | 0 | 0 | 100 | |
| 75% White | 0 | 0 | 0 | 0 | 16 | 0 | 100 | |
| 100% White | 0 | 0 | 0 | 0 | 0 | 13 | 100 | |
| UA% | 100 | 95.2 | 100 | 100 | 100 | 100 | 99.0 | |
| PREDICTION | ||||||||
|---|---|---|---|---|---|---|---|---|
| OBSERVATION | Healthy | 10% White | 25% White | 50% White | 75% White | 100% White | PA% | |
| Healthy | 17 | 0 | 0 | 0 | 0 | 0 | 100 | |
| 10% White | 2 | 18 | 0 | 0 | 0 | 0 | 90 | |
| 25% White | 0 | 0 | 16 | 0 | 0 | 0 | 100 | |
| 50% White | 0 | 0 | 0 | 19 | 0 | 0 | 100 | |
| 75% White | 0 | 0 | 0 | 0 | 16 | 0 | 100 | |
| 100% White | 1 | 1 | 0 | 0 | 0 | 9 | 81.8 | |
| UA% | 85 | 94.7 | 100 | 100 | 100 | 100 | 96 | |
| PREDICTION | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy | 10% White | 25% White | 50% White | 75% White | 100% White | PA% | ||
| OBSERVATION | Healthy | 16 | 1 | 0 | 0 | 0 | 0 | 94.1 |
| 10% White | 0 | 20 | 0 | 0 | 0 | 0 | 100 | |
| 25% White | 0 | 0 | 16 | 0 | 0 | 0 | 100 | |
| 50% White | 0 | 0 | 0 | 19 | 0 | 0 | 100 | |
| 75% White | 0 | 0 | 0 | 0 | 16 | 0 | 100 | |
| 100% White | 0 | 0 | 0 | 0 | 0 | 11 | 100 | |
| UA% | 100 | 95.2 | 100 | 100 | 100 | 100 | 98.9 | |
| PREDICTION | |||||
|---|---|---|---|---|---|
| Kappaphycus Green | Eucheuma | Kappaphycus Brown | PA% | ||
| OBSERVATION | Kappaphycus green | 20 | 0 | 0 | 100 |
| Eucheuma | 1 | 15 | 2 | 83 | |
| Kappaphycus brown | 3 | 2 | 15 | 75 | |
| UA% | 83.3 | 88.2 | 88.2 | 86.2 | |
| PREDICTION | ||||
|---|---|---|---|---|
| 100% White | Silted | PA% | ||
| OBSERVATION | 100% White | 10 | 3 | 76.9 |
| Silted | 2 | 21 | 91.3 | |
| UA% | 83.3 | 87.5 | 86.1 | |
| PREDICTION | ||||
|---|---|---|---|---|
| 100% White | Silted | PA% | ||
| OBSERVATION | 100% White | 13 | 0 | 100 |
| Silted | 2 | 21 | 91.3 | |
| UA% | 86.7 | 100 | 94.4 | |
| PREDICTIONS | |||||||
|---|---|---|---|---|---|---|---|
| Healthy | Silted | 100% White | Back | Shade | PA% | ||
| OBSERVATIONS | Healthy | 29 | 0 | 0 | 0 | 2 | 93.5 |
| Silted | 6 | 11 | 0 | 0 | 0 | 64.7 | |
| 100% White | 0 | 0 | 11 | 0 | 1 | 91.7 | |
| Back | 0 | 0 | 0 | 11 | 0 | 100 | |
| Shade | 0 | 0 | 0 | 0 | 29 | 100 | |
| UA% | 82.8 | 100 | 100 | 100 | 90.6 | 91 | |
| PREDICTIONS | |||||||
|---|---|---|---|---|---|---|---|
| Healthy | Silted | 100% White | Back | Shade | PA% | ||
| OBSERVATIONS | Healthy | 27 | 1 | 0 | 0 | 1 | 93.1 |
| Silted | 0 | 16 | 0 | 0 | 0 | 100 | |
| 100% White | 0 | 0 | 11 | 0 | 0 | 100 | |
| Back | 0 | 0 | 0 | 11 | 0 | 100 | |
| Shade | 0 | 0 | 0 | 0 | 31 | 100 | |
| UA% | 100 | 94.1 | 100 | 100 | 96.9 | 97.9 | |
References
- Cai, J. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO Fisheries and Aquaculture Circular; FAO: Rome, Italy, 2021; ISBN 978-92-5-134710-2. [Google Scholar]
- Campbell, R.; Hotchkiss, S. Carrageenan Industry Market Overview. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Developments in Applied Phycology; Springer International Publishing: Cham, Switzerland, 2017; pp. 193–205. ISBN 978-3-319-63498-2. [Google Scholar]
- Bell, T.W.; Nidzieko, N.J.; Siegel, D.A.; Miller, R.J.; Cavanaugh, K.C.; Nelson, N.B.; Reed, D.C.; Fedorov, D.; Moran, C.; Snyder, J.N.; et al. The Utility of Satellites and Autonomous Remote Sensing Platforms for Monitoring Offshore Aquaculture Farms: A Case Study for Canopy Forming Kelps. Front. Mar. Sci. 2020, 7, 520223. [Google Scholar] [CrossRef]
- Langford, A.; Waldron, S.; Sulfahri; Saleh, H. Monitoring the COVID-19-Affected Indonesian Seaweed Industry Using Remote Sensing Data. Mar. Policy 2021, 127, 104431. [Google Scholar] [CrossRef]
- Meng, D.; Yang, X.; Wang, Z.; Liu, Y.; Zhang, J.; Liu, X.; Liu, B. Spatial Distribution and Differentiation Analysis of Coastal Aquaculture in China Based on Remote Sensing Monitoring. Remote Sens. 2024, 16, 1585. [Google Scholar] [CrossRef]
- Pratama, I.; Albasri, H. Mapping and Estimating Harvest Potential of Seaweed Culture Using Worldview-2 Satellite Images: A Case Study in Nusa Lembongan, Bali − Indonesia. Aquat. Living Resour. 2021, 34, 15. [Google Scholar] [CrossRef]
- Setyawidati, N.; Liabot, P.O.; Perrot, T.; Radiarta, N.; Deslandes, E.; Bourgougnon, N.; Rossi, N.; Stiger-Pouvreau, V. In Situ Variability of Carrageenan Content and Biomass in the Cultivated Red Macroalga Kappaphycus alvarezii with an Estimation of Its Carrageenan Stock at the Scale of the Malasoro Bay (Indonesia) Using Satellite Image Processing. J. Appl. Phycol. 2017, 29, 2307–2321. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Critchley, A.T.; Neish, I.C. (Eds.) Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-63497-5. [Google Scholar]
- Nurdin, N.; Alevizos, E.; Syamsuddin, R.; Asis, H.; Zainuddin, E.N.; Aris, A.; Oiry, S.; Brunier, G.; Komatsu, T.; Barillé, L. Precision Aquaculture Drone Mapping of the Spatial Distribution of Kappaphycus alvarezii Biomass and Carrageenan. Remote Sens. 2023, 15, 3674. [Google Scholar] [CrossRef]
- Ubina, N.A.; Cheng, S.-C. A Review of Unmanned System Technologies with Its Application to Aquaculture Farm Monitoring and Management. Drones 2022, 6, 12. [Google Scholar] [CrossRef]
- Kumar, Y.N.; Poong, S.-W.; Gachon, C.; Brodie, J.; Sade, A.; Lim, P.-E. Impact of Elevated Temperature on the Physiological and Biochemical Responses of Kappaphycus alvarezii (Rhodophyta). PLoS ONE 2020, 15, e0239097. [Google Scholar] [CrossRef]
- Neish, I.C. Monograph No. HB2F 1008 V3 GAP; SEAPlant.net: Kuala Lumpur, Malaysia, 2008. [Google Scholar]
- Largo, D.B.; Chung, I.K.; Phang, S.-M.; Gerung, G.S.; Sondak, C.F.A. Impacts of Climate Change on Eucheuma-Kappaphycus Farming. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Developments in Applied Phycology; Springer International Publishing: Cham, Switzerland, 2017; pp. 121–129. ISBN 978-3-319-63498-2. [Google Scholar]
- Pang, T.; Liu, J.; Liu, Q.; Li, H.; Li, J. Observations on Pests and Diseases Affecting a Eucheumatoid Farm in China. J. Appl. Phycol. 2015, 27, 1975–1984. [Google Scholar] [CrossRef]
- Ward, G.M.; Faisan Jr, J.P.; Cottier-Cook, E.J.; Gachon, C.; Hurtado, A.Q.; Lim, P.E.; Matoju, I.; Msuya, F.E.; Bass, D.; Brodie, J. A Review of Reported Seaweed Diseases and Pests in Aquaculture in Asia. J. World Aquac. Soc. 2020, 51, 815–828. [Google Scholar] [CrossRef]
- Hayashi, L.; Reis, R.P.; dos Santos, A.A.; Castelar, B.; Robledo, D.; de Vega, G.B.; Msuya, F.E.; Eswaran, K.; Yasir, S.M.; Ali, M.K.M.; et al. The Cultivation of Kappaphycus and Eucheuma in Tropical and Sub-Tropical Waters. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Developments in Applied Phycology; Springer International Publishing: Cham, Switzerland, 2017; pp. 55–90. ISBN 978-3-319-63498-2. [Google Scholar]
- Loureiro, R.R.; Hurtado, A.Q.; Critchley, A.T. Impacts of AMPEP on Epiphytes and Diseases in Kappaphycus and Eucheuma Cultivation. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Developments in Applied Phycology; Springer International Publishing: Cham, Switzerland, 2017; pp. 111–119. ISBN 978-3-319-63498-2. [Google Scholar]
- Kambey, C.S.B.; Campbell, I.; Sondak, C.F.A.; Nor, A.R.M.; Lim, P.E.; Cottier-Cook, E.J. An Analysis of the Current Status and Future of Biosecurity Frameworks for the Indonesian Seaweed Industry. J. Appl. Phycol. 2020, 32, 2147–2160. [Google Scholar] [CrossRef]
- Cottier-Cook, E.J.; Cabarubias, J.P.; Brakel, J.; Brodie, J.; Buschmann, A.H.; Campbell, I.; Critchley, A.T.; Hewitt, C.L.; Huang, J.; Hurtado, A.Q.; et al. A New Progressive Management Pathway for Improving Seaweed Biosecurity. Nat. Commun. 2022, 13, 7401. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Du, G.; Wang, N.; He, K.; Mo, Z.; Sun, B.; Chen, Y.; Cao, Y.; Wang, J.; Mao, Y. Biomass Estimation of Cultivated Red Algae Pyropia Using Unmanned Aerial Platform Based Multispectral Imaging. Plant Methods 2021, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Solvang, T.; Bale, E.S.; Broch, O.J.; Handå, A.; Alver, M.O. Automation Concepts for Industrial-Scale Production of Seaweed. Front. Mar. Sci. 2021, 8, 613093. [Google Scholar] [CrossRef]
- Xing, Q.; An, D.; Zheng, X.; Wei, Z.; Wang, X.; Li, L.; Tian, L.; Chen, J. Monitoring Seaweed Aquaculture in the Yellow Sea with Multiple Sensors for Managing the Disaster of Macroalgal Blooms. Remote Sens. Environ. 2019, 231, 111279. [Google Scholar] [CrossRef]
- Tait, L.; Bind, J.; Charan-Dixon, H.; Hawes, I.; Pirker, J.; Schiel, D. Unmanned Aerial Vehicles (UAVs) for Monitoring Macroalgal Biodiversity: Comparison of RGB and Multispectral Imaging Sensors for Biodiversity Assessments. Remote Sens. 2019, 11, 2332. [Google Scholar] [CrossRef]
- Fidai, Y.A.; Botelho Machado, C.; Dominguez Almela, V.; Oxenford, H.A.; Jayson-Quashigah, P.-N.; Tonon, T.; Dash, J. Innovative Spectral Characterisation of Beached Pelagic Sargassum towards Remote Estimation of Biochemical and Phenotypic Properties. Sci. Total Environ. 2024, 914, 169789. [Google Scholar] [CrossRef]
- Chandler, C.J.; Ávila-Mosqueda, S.V.; Salas-Acosta, E.R.; Magaña-Gallegos, E.; Mancera, E.E.; Reali, M.A.G.; de la Barreda-Bautista, B.; Boyd, D.S.; Metcalfe, S.E.; Sjogersten, S.; et al. Spectral Characteristics of Beached Sargassum in Response to Drying and Decay over Time. Remote Sens. 2023, 15, 4336. [Google Scholar] [CrossRef]
- Casal, G.; Kutser, T.; Domínguez-Gómez, J.A.; Sánchez-Carnero, N.; Freire, J. Assessment of the Hyperspectral Sensor CASI-2 for Macroalgal Discrimination on the Ría de Vigo Coast (NW Spain) Using Field Spectroscopy and Modelled Spectral Libraries. Cont. Shelf Res. 2013, 55, 129–140. [Google Scholar] [CrossRef]
- Chao Rodríguez, Y.; Domínguez Gómez, J.A.; Sánchez-Carnero, N.; Rodríguez-Pérez, D. A Comparison of Spectral Macroalgae Taxa Separability Methods Using an Extensive Spectral Library. Algal Res. 2017, 26, 463–473. [Google Scholar] [CrossRef]
- Douay, F.; Verpoorter, C.; Duong, G.; Spilmont, N.; Gevaert, F. New Hyperspectral Procedure to Discriminate Intertidal Macroalgae. Remote Sens. 2022, 14, 346. [Google Scholar] [CrossRef]
- Fuller, K.; Martin, R.E.; Asner, G.P. Spectral Signatures of Macroalgae on Hawaiian Reefs. Remote Sens. 2024, 16, 1140. [Google Scholar] [CrossRef]
- Olmedo-Masat, O.M.; Raffo, M.P.; Rodríguez-Pérez, D.; Arijón, M.; Sánchez-Carnero, N. How Far Can We Classify Macroalgae Remotely? An Example Using a New Spectral Library of Species from the South West Atlantic (Argentine Patagonia). Remote Sens. 2020, 12, 3870. [Google Scholar] [CrossRef]
- Davies, B.F.R.; Gernez, P.; Geraud, A.; Oiry, S.; Rosa, P.; Zoffoli, M.L.; Barillé, L. Multi- and Hyperspectral Classification of Soft-Bottom Intertidal Vegetation Using a Spectral Library for Coastal Biodiversity Remote Sensing. Remote Sens. Environ. 2023, 290, 113554. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random Forest in Remote Sensing: A Review of Applications and Future Directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Brunier, G.; Oiry, S.; Gruet, Y.; Dubois, S.F.; Barillé, L. Topographic Analysis of Intertidal Polychaete Reefs (Sabellaria alveolata) at a Very High Spatial Resolution. Remote Sens. 2022, 14, 307. [Google Scholar] [CrossRef]
- Oiry, S.; Barillé, L. Using Sentinel-2 Satellite Imagery to Develop Microphytobenthos-Based Water Quality Indices in Estuaries. Ecol. Indic. 2021, 121, 107184. [Google Scholar] [CrossRef]
- Stephens, D.; Diesing, M. A Comparison of Supervised Classification Methods for the Prediction of Substrate Type Using Multibeam Acoustic and Legacy Grain-Size Data. PLoS ONE 2014, 9, e93950. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, R.; Kim, K.; Zhang, J.; Cui, T. A Random Forest-Based Algorithm to Distinguish Ulva Prolifera and Sargassum From Multispectral Satellite Images. IEEE Trans. Geosci. Remote Sens. 2022, 60, 4201515. [Google Scholar] [CrossRef]
- Zhang, C. Applying Data Fusion Techniques for Benthic Habitat Mapping and Monitoring in a Coral Reef Ecosystem. ISPRS J. Photogramm. Remote Sens. 2015, 104, 213–223. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.-L.; Kneib, T.; Augustin, T.; Zeileis, A. Conditional Variable Importance for Random Forests. BMC Bioinformatics 2008, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Escadafal, R. Soil Spectral Properties and Their Relationships with Environmental Parameters—Examples from Arid Regions. In Imaging Spectrometry—A Tool for Environmental Observations; Hill, J., Mégier, J., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 71–87. ISBN 978-0-585-33173-7. [Google Scholar]
- Cavanaugh, K.C.; Cavanaugh, K.C.; Bell, T.W.; Hockridge, E.G. An Automated Method for Mapping Giant Kelp Canopy Dynamics from UAV. Front. Environ. Sci. 2021, 8, 587354. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially Located Platform and Aerial Photography for Documentation of Grazing Impacts on Wheat. Geocarto Int. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and Model Inversion Methods with Narrowband Optical Indices for Chlorophyll Content Estimation in Closed Forest Canopies with Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Slaton, M.R.; Raymond Hunt, E., Jr.; Smith, W.K. Estimating Near-Infrared Leaf Reflectance from Leaf Structural Characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef]
- Hayashi, L.; Hurtado, A.Q.; Msuya, F.E.; Bleicher-Lhonneur, G.; Critchley, A.T. A Review of Kappaphycus Farming: Prospects and Constraints. In Seaweeds and their Role in Globally Changing Environments; Seckbach, J., Einav, R., Israel, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 251–283. ISBN 978-90-481-8569-6. [Google Scholar]
- Dawes, C.J. Irradiance Acclimation of the Cultured Philippine Seaweeds, Kappaphycus alvarezii and Eucheuma denticulatum. Bot. Mar. 1992, 35, 189–196. [Google Scholar] [CrossRef]
- Mateo, J.P.; Campbell, I.; Cottier-Cook, E.J.; Luhan, M.R.J.; Ferriols, V.M.E.N.; Hurtado, A.Q. Analysis of Biosecurity-Related Policies Governing the Seaweed Industry of the Philippines. J. Appl. Phycol. 2020, 32, 2009–2022. [Google Scholar] [CrossRef]
- Hu, C. A Novel Ocean Color Index to Detect Floating Algae in the Global Oceans. Remote Sens. Environ. 2009, 113, 2118–2129. [Google Scholar] [CrossRef]
- Silva, T.S.F.; Costa, M.P.F.; Melack, J.M.; Novo, E.M.L.M. Remote Sensing of Aquatic Vegetation: Theory and Applications. Environ. Monit. Assess. 2008, 140, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S. Development of Novel Image Analysis Approaches for Seaweed Discrimination—Species Level Study Using Field Spectroscopy and UAV Multispectral Remote Sensing. Ph.D. Thesis, Auckland University of Technology, Auckland, New Zealand, 2021. [Google Scholar]
- Vahtmäe, E.; Kutser, T.; Martin, G.; Kotta, J. Feasibility of Hyperspectral Remote Sensing for Mapping Benthic Macroalgal Cover in Turbid Coastal Waters—A Baltic Sea Case Study. Remote Sens. Environ. 2006, 101, 342–351. [Google Scholar] [CrossRef]
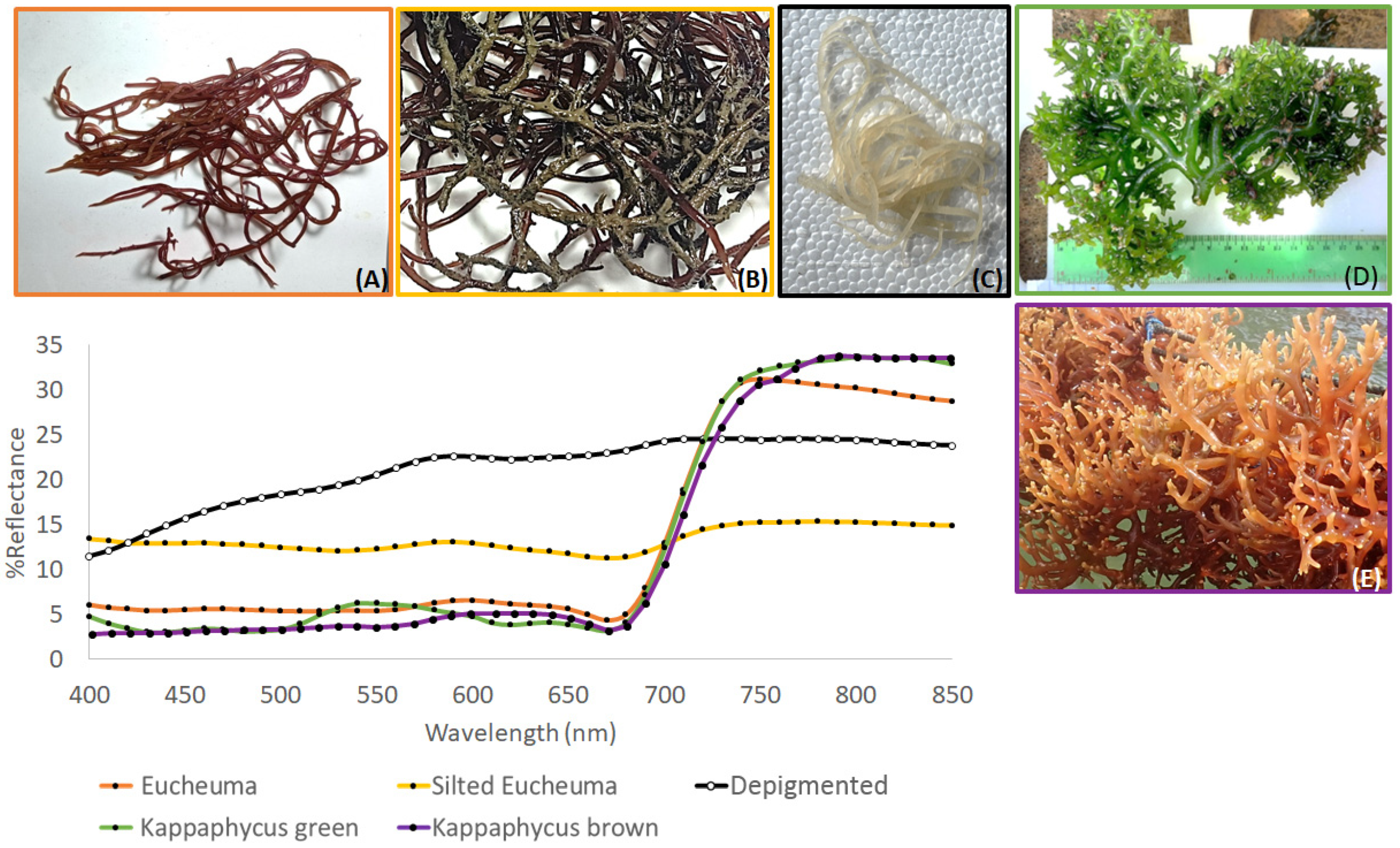

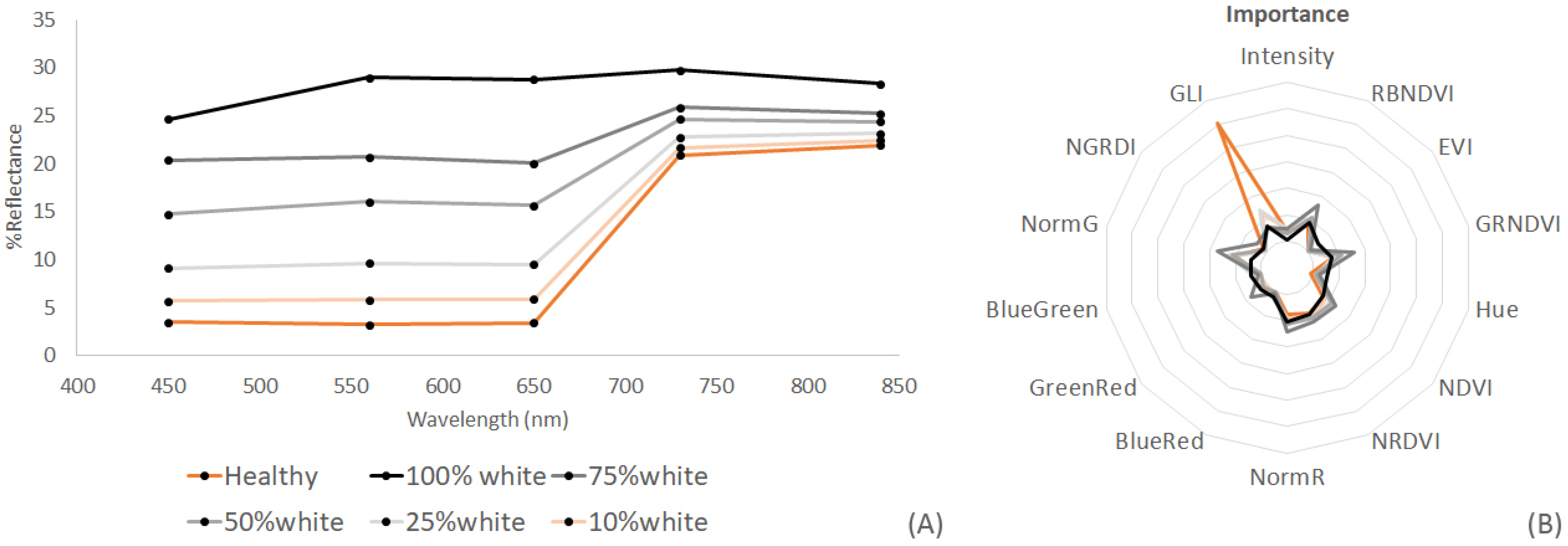
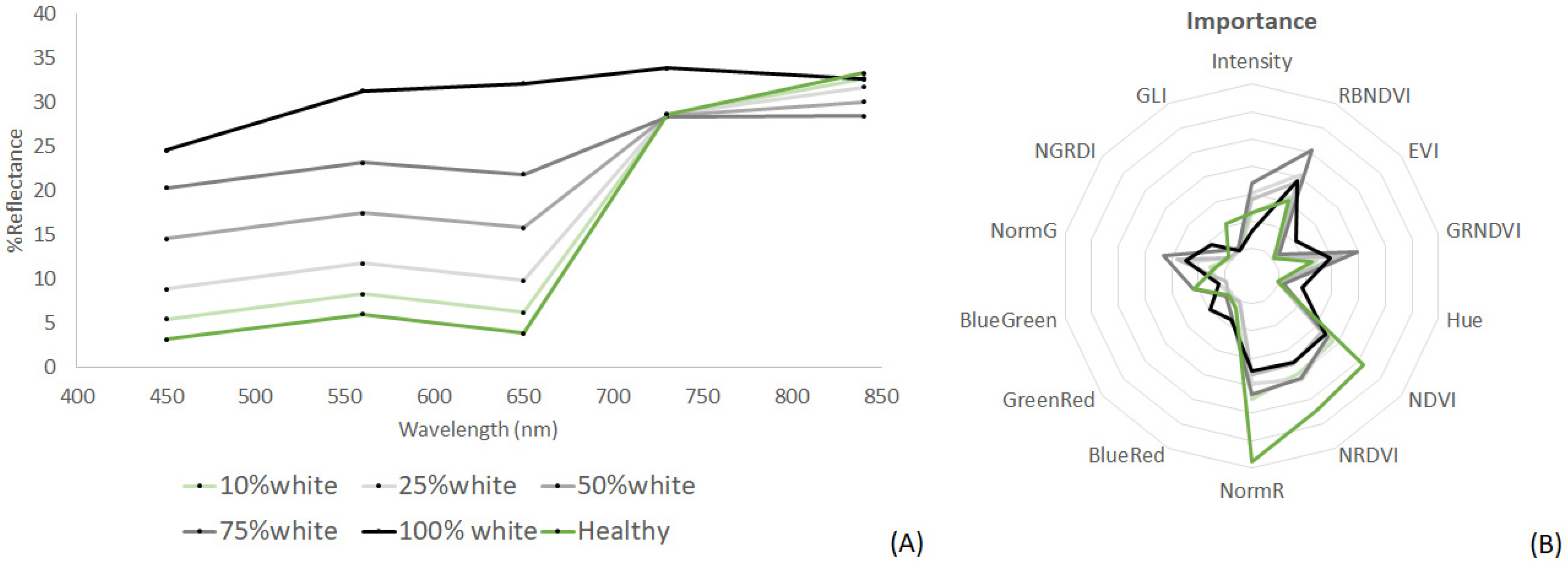
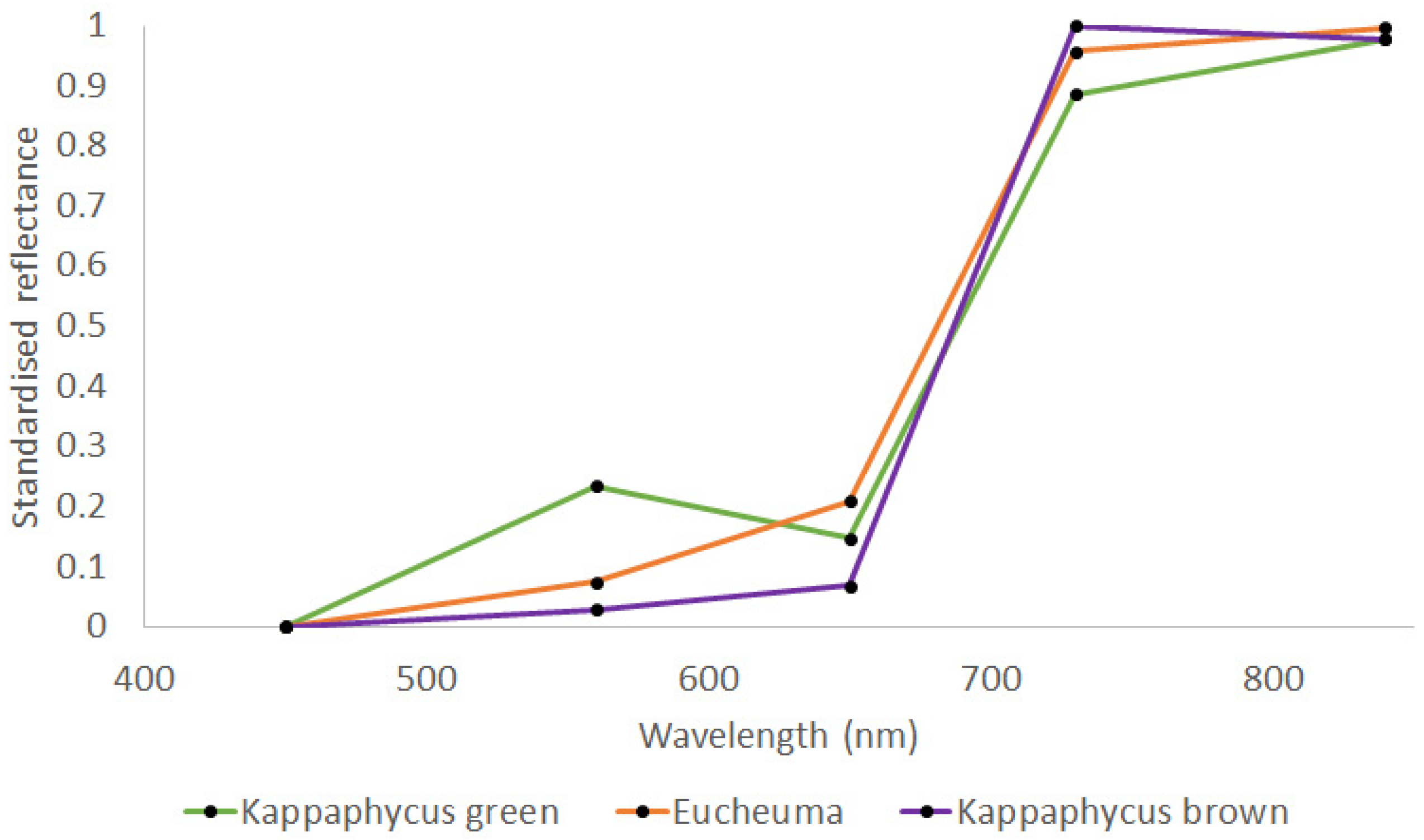
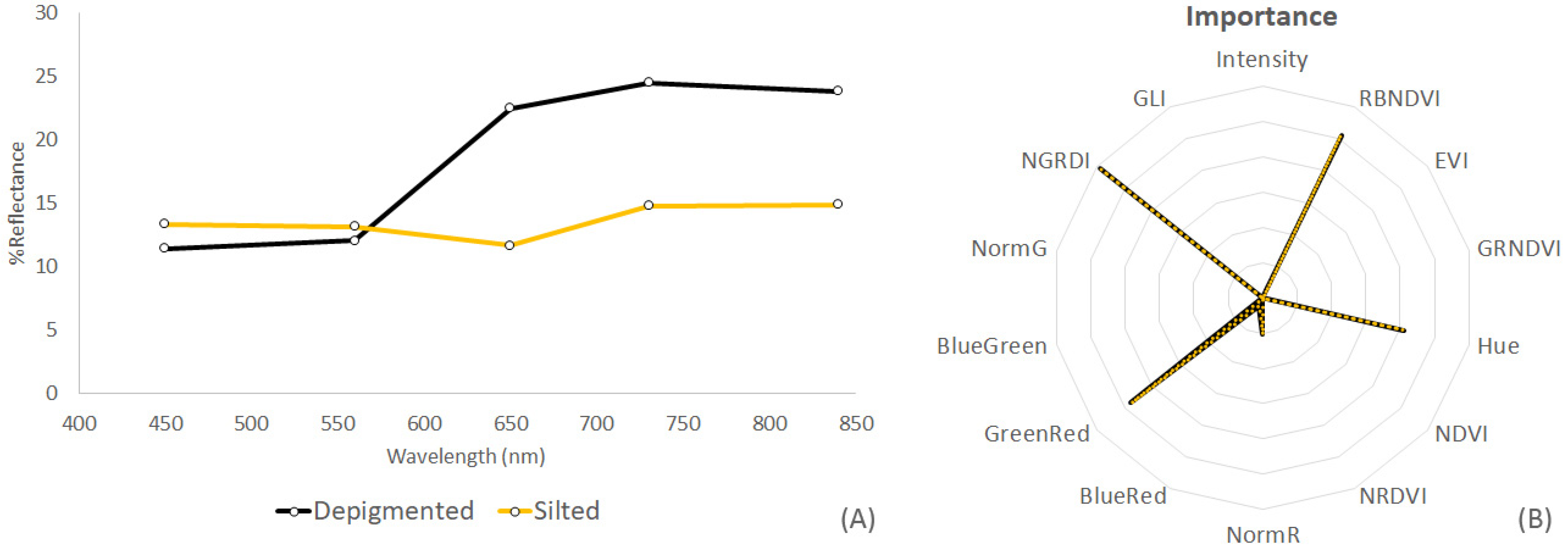

| Thallus Conditions | Spectral Resolution | Training Samples | Validation Samples |
|---|---|---|---|
| Eucheuma/Kappaphycus green/brown | Hyperspectral | 75 | 51 |
| Eucheuma (H, mixed, W) | Hyperspectral | 141 | 101 |
| Kappaphycus (H, mixed, W) | Hyperspectral | 143 | 99 |
| Eucheuma (S, W) | Hyperspectral | 36 | 36 |
| Eucheuma (H, mixed, W) | Multispectral | 141 | 101 |
| Indices Eucheuma (H, mixed, W) | Multispectral | 141 | 101 |
| Kappaphycus (H, mixed, W) | Multispectral | 143 | 99 |
| Indices Kappaphycus (H, mixed, W) | Multispectral | 143 | 99 |
| Eucheuma/Kappaphycus green/brown) | Multispectral | 70 | 58 |
| Eucheuma (S, W) | Multispectral | 36 | 36 |
| Indices Eucheuma (S, W) | Multispectral | 36 | 36 |
| Eucheuma (H, S, W) * | Multispectral | 303 | 100 |
| Indices Eucheuma (H, S, W) * | Multispectral | 303 | 98 |
| Index Name | Formula | Reference |
|---|---|---|
| Intensity | (R + G + B)/30.5 | [40] |
| Hue | arctan[(G − B) × (2 × R − G − B/30.5)] | [40] |
| Blue–Red ratio | B/R | [41] |
| Green–Red ratio | G/R | |
| Blue–Green ratio | B/G | |
| Norm Red | R/(NIR + R + G) | |
| Norm Green | G/(NIR + R + G) | |
| Normalised Difference Vegetation Index (NDVI) | (NIR − R)/(NIR + R) | [42] |
| Normalised Ratio Vegetation Index (NRVI) | [(R/NIR) − 1]/[(R/NIR) + 1] | [32] |
| Normalized Difference Green–Red Index (NGRDI) | (G − R)/(G + R) | [32] |
| Green Leaf Index (GLI) | (2 × − R − B)/(2 × G + R + B) | [43] |
| Green–Red NDVI (GRNDVI) | [NIR − (G + R)]/[NIR + (G + R)] | [44] |
| Enhanced Vegetation Index (EVI) | [2.5 × (NIR − R)]/[(NIR + 6 × R − 7.5 × B) + 1] | [45] |
| Red–Blue NDVI (RBNDVI) | [REDEDGE − (R − B)]/[REDEDGE + (R + B)] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alevizos, E.; Nurdin, N.; Aris, A.; Barillé, L. Proximal Sensing for Characterising Seaweed Aquaculture Crop Conditions: Optical Detection of Ice-Ice Disease. Remote Sens. 2024, 16, 3502. https://doi.org/10.3390/rs16183502
Alevizos E, Nurdin N, Aris A, Barillé L. Proximal Sensing for Characterising Seaweed Aquaculture Crop Conditions: Optical Detection of Ice-Ice Disease. Remote Sensing. 2024; 16(18):3502. https://doi.org/10.3390/rs16183502
Chicago/Turabian StyleAlevizos, Evangelos, Nurjannah Nurdin, Agus Aris, and Laurent Barillé. 2024. "Proximal Sensing for Characterising Seaweed Aquaculture Crop Conditions: Optical Detection of Ice-Ice Disease" Remote Sensing 16, no. 18: 3502. https://doi.org/10.3390/rs16183502
APA StyleAlevizos, E., Nurdin, N., Aris, A., & Barillé, L. (2024). Proximal Sensing for Characterising Seaweed Aquaculture Crop Conditions: Optical Detection of Ice-Ice Disease. Remote Sensing, 16(18), 3502. https://doi.org/10.3390/rs16183502









