Abstract
Remote sensing for water quality evaluation has advanced, with more satellites providing longer data series. Validations of remote sensing-derived data for water quality characteristics, such as chlorophyll-a, Secchi depth, and turbidity, have often remained restricted to small numbers of water bodies and have included local calibration. Here, we present an evaluation of > 100 water bodies in Germany covering different sizes, maximum depths, and trophic states. Data from Sentinel-2 MSI and Sentinel-3 OLCI were analyzed by two processing chains. Our work focuses on analysis of the accuracy of remote sensing products by comparing them to a large in situ data set from governmental monitoring from 13 federal states in Germany and, hence, achieves a national scale assessment. We quantified the fit between the remote sensing data and in situ data among processing chains, satellite instruments, and our three target water quality variables. In general, overall regressions between in situ data and remote sensing data followed the 1:1 regression. Remote sensing may, thus, be regarded as a valuable tool for complementing in situ monitoring by useful information on higher spatial and temporal scales in order to support water management, e.g., for the European Water Framework Directive (WFD) and the Bathing Water Directive (BWD).
1. Introduction
Remote sensing for water quality evaluation has been applied in many water bodies. Different sensors were used under different conditions, both in freshwater and in the marine environment [1,2,3,4,5,6,7,8,9,10,11]. Water quality characteristics that can be evaluated by remote sensing encompass temperature, turbidity, Secchi depth, chlorophyll-a concentration, organic matter absorption, and cyanobacteria occurrence. Many of the previous studies focused on the general calibration, parameterization, and validation of a geographically and/or temporally limited data set of such water quality characteristics [4,12] and are not transferable to other water bodies. They aimed to find the best algorithms to achieve the best correlation between water quality characteristics measured in situ and evaluated from remote sensing and provide a number of solutions for their specific target. However, it remained questionable to what extent such locally optimized applications can really be implemented at large scales to support decision-making. Also, to realise a return of investment in the several satellite missions generally requires applications at large scales and in governmentally relevant environmental policies to allow for exploiting the synoptical investigation of water quality in larger ensembles of waterbodies (lakes and reservoirs) exhibiting varying characteristics.
In governmental monitoring, however, such localized calibration and fitting between in situ and remote sensing data is neither doable nor wanted. Instead, well characterised and transferable methods that can be applied to various water bodies without changing parameterisations are required (compare projects in Supplementary Information SI1; [13]). In order to be economically feasible, monitoring must be performed with as few resources as possible and at the same time must provide detailed information on the status and dynamics of water quality. In that respect, remote sensing is a promising method that may complement existing in situ measurements [14,15,16]. However, the crucial question remains as to how well the remote sensing data really reproduce in situ data at a larger scale and in highly diverse water bodies.
Online interactive tools for visualizing and analysing remote sensing data and for comparing them with in situ data were developed in the academic environment [11,17,18]. These can often only be used by specifically trained experts, however, and cannot easily be implemented by authorities where the limited personnel are confronted with the implementation of national and international water policies, such as the European Union Water Framework Directive (WFD; [19]), and the Bathing Water Directive (BWD; [20]). Public applications of remote sensing products rather have to be realized in a ready-to-use, naïve, and fully reproducible way, i.e., without having to decide on multiple parametrizations or algorithms. In Germany and several other European countries, commercial but science-based products are, therefore, important for initiating an efficient governmental use of satellite-based products. For our study, we investigated the operational services from two companies providing web-based interactive tools for data processing and easy-to-use data visualisation and intuitive data analysis: CyanoAlert® (Brockmann Consult GmbH, Hamburg, Germany; BC from hereon) and eoapp AQUA® (EOMAP GmbH & Co. KG, Seefeld, Germany; EOMAP from hereon). Both processing chains allow users to quickly derive multiple water quality variables of water bodies with well-established algorithms and additionally allow for an intuitive and effortless data analysis. In this study, we focused on comparing in situ data collected by German authorities on more than 100 lakes and reservoirs with the company-provided remote sensing data to allow a comparison with fit for purpose data.
Beyond the governmental status assessment of water bodies, e.g., within EU WFD [19], satellite observations also offer extended information for water resources management in water bodies (lakes and reservoirs) targeting the effects of land cover and nutrient loading [21], the detection of algal blooms [22,23], regime shifts [24], or responses to extreme events and climate change [5,25,26]. Given the spatial and temporal resolution of satellites, their global coverage, and their relatively low costs for end users, such applications can contribute highly valuable information that cannot easily be realized by classical in situ samplings and, thus, complement in situ monitoring in this respect [15].
Moreover, tracking unprecedented changes requires more intense observation. In some cases, it is important to retrospectively track when a development, e.g., deterioration, began. The required field studies are not possible in hindsight, but satellite archives reach back over many years—in the case of the optical ESA Sentinel satellites, the archives reach back to 2016. Irani Rahaghi et al. [27], for example, convincingly demonstrated that remote sensing observations contribute to understanding the emergence of an algal bloom in Lake Geneva.
The application of satellite monitoring products in governmental water policies and decision-making appears promising, e.g., the monitoring of chlorophyll-a or trophic state by Copernicus satellites was proposed as a future extension of European-scale water quality monitoring [28]. In Finland, remote sensing data have already been used to comply with requirements from the WFD [14,29]. Such applications demonstrate the emerging synergies by including satellite-derived products in water quality assessment but also point to the importance of proper validation of these data against in situ observation and a clarification of uncertainties, which are the major research objectives in this study that focuses on the national scale of Germany. This task of broad-scale validation is not easy because in situ monitoring is often realized at a very low temporal resolution (multi-year intervals; 1–6 years between campaigns with monthly sampling frequencies) and temporal matching of sampling and satellite overpass is problematic (compare [30]). Also, disturbing effects from clouds, sunglint, or mixed pixels reduce the availability of remote sensing data. This leads to the fact that evaluation data sets are often much smaller than the data obtained from overpasses, which is another reason for often weak validation. Therefore, we invested effort into generating a nation-wide data set of more than 100 water bodies for validation. Using data from water authorities meant, however, that the in situ database was heterogeneous.
Currently, relevant satellite sensors for inland water quality include Landsat 8 and 9 (OLI; “Operational Land Imager”) from USGS, MODIS (“Moderate Resolution Imaging Spectroradiometer”), and VIIRS (“Visible Infrared Imaging Radiometer Suite”) from the NASA satellite programme, and Sentinel-2 MSI (“MultiSpectral Instruments”) and -3 OLCI (“Ocean and Land Colour Instruments”) from the European Copernicus programme. The two Sentinel-2 satellites (A and B) were launched in 2015 and 2017, respectively, and carry multispectral instruments (MSI) on board. Sentinel-2A and Sentinel-2B each have a repeat cycle of 10 days. The imagery is obtained at spatial resolutions of 10 m, 20 m, and 60 m, depending on the spectral band [31,32]. In total, 13 spectral bands are measured, covering the visible to shortwave infrared wavelengths [33]. Also, the Sentinel-3 mission consists of two satellites, A and B, with daily repeat cycles over Middle Europe. They were launched in 2016 and 2017, respectively [34]. A total of 21 spectral bands are measured by the OLCI, at a spatial resolution of 300 m.
By the operation of two sensors at a time, the repetition rate is doubled for Sentinel satellites [33,34]. Over Germany, depending on the exact location of the waterbody, the return rates are 1–2 days for Sentinel-3 [34] and 2– 5 days for Sentinel-2 [33], while Landsat 8 and 9 pass every 16 days, respectively (8 days combined, since Landsat 8 and 9 carry the same sensors and can, therefore, be treated as equivalent) [35]. Given this better temporal resolution, in this study, we focus on remote sensing data from the Copernicus program (i.e., Sentinel-2/-3). As target variables, we chose chlorophyll-a concentration, turbidity, and Secchi depth, because these variables are usually available as in situ data in governmental monitoring programs.
The ambition of this study is to generate trust in remote sensing products for decision-making and environmental observation as a complement to existing in situ monitoring. Our research aims are focused on (a) establishing a nationwide data collection of in situ water quality monitoring data of more than 100 waterbodies, (b) quantifying the accuracy and robustness of using remote sensing data to derive water quality, here, in terms of chlorophyll-a, turbidity, and Secchi depth, without site-specific parameter optimization, and (c) analyzing for potential differences in accuracy/robustness with respect to satellites and processing chains. Based on these analyses, we hope to draw conclusions on the opportunities and limits of remotely sensed water quality products in governmental or public contexts.
2. Materials and Methods
2.1. In Situ Data
We collected in situ data for turbidity, chlorophyll-a, and Secchi depth (see also Table 1) from 112 waterbodies (Figure 1, Table S1) obtained from 13 German Federal authorities (for more details refer to Supplementary Information SI1 and SI2). They comprised stations where regular monitoring for bathing water quality and/or within WFD-monitoring were performed. In situ data were used from 1 January 2016 until 31 December 2020.

Table 1.
Exclusion criteria for in situ data. In situ and satellite-derived data were only used within the ranges detailed in this table.
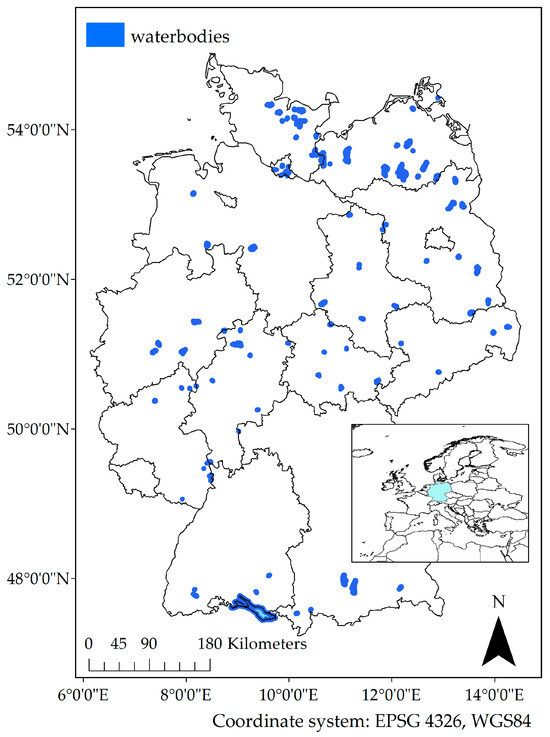
Figure 1.
Investigated waterbodies (dark blue) in Germany within the federal states. Coordinate system EPSG 4326, WGS84. Inset map: situation of Germany within Europe.
2.2. Chemical and Physical Properties: Chlorophyll, Secchi Depth, and Turbidity
Measurements were conducted according to national and EU standards [36,37] by accredited laboratories mostly following WFD requirements. We excluded unlikely values by defining realistic data ranges according to Table 1, both from the in situ and satellite data, because such values probably arose due to measurement or data maintenance errors. All data providers use accredited and fully certified laboratories, and the methodological procedures are according to EU standards accepted among member states. Accordingly, the analytical standards are at high levels, and analytical accuracy represents the state of the art. Note also that water quality monitoring in Germany is implemented at the federal state level and, therefore, slight differences in sampling frequency, sampling depths, or analytical methods (e.g., chlorophyll-a by photometry or by HPLC) emerge when data from several states are merged.
Satellite measurements are restricted to the surface layer, and the relevant depth range may be approximated by the Secchi depth [5]. However, WFD prescribes depth-integrated samples, i.e., a sample mixing the whole water column down to the thermocline. In order not to lose such samples, we considered all depth-integrated samples which started from the surface (i.e., only epilimnion samples; no hypolimnion samples), even if the thermocline was deeper than the Secchi depth. In contrast, where discrete samples had been taken, i.e., 0.1 m, 0.5 m, 1 m, etc., for practical reasons, we averaged all available in situ values between 0–2 m depth at a given sampling site and date. The Secchi depths in the in situ data ranged from 0.1 to 14.7 m, with a mean of 2.8 and a median of 2 m (SI4, Figure S2). Therefore, we considered restricting discrete sampling depths to down to 2 m as appropriate for comparison with the remote sensing data. This very simple approach worked very well and assures that the upper meters of the waterbody (lake or reservoir) are representative when compared to satellite observations. Problems may arise in cases of heavy scum formation, i.e., when the satellite’s view is facing extremely high chlorophyll concentrations, which are not represented in the in situ data (unless a real surface sample was taken).
2.3. Remote Sensing Data Processing and Extraction
For the generic approach we followed here, we focused on two recommended workflows. The aim here was to enable monitoring, which requires fixed algorithms that are not adapted to the individual characteristics of each waterbody.
Evaluations were performed on data for chlorophyll-a, Secchi depth, and turbidity from Sentinel-2 MSI and Sentinel-3 OLCI. For the differences between the instruments on the two satellites please refer to Supplementary Information SI3. All waterbodies were large enough to be evaluated by Sentinel-2 MSI. For a waterbody to be evaluated with Sentinel-3 OLCI, we checked for each waterbody whether at least a 5 × 5 contiguous macropixel would cover the waterbody. However, a pixel touching the shoreline might have been disturbed by land and might, thus, return values representative of land instead of water constituents. Therefore, we made sure that each of the 25 pixels did not touch the shoreline and was not disturbed by land. The choice of a 5 × 5 megapixel is supported by the findings of Schröder et al. (2024) [30]. This resulted in 38 of the waterbodies being fit for Sentinel-3 OLCI (see column “S3” in Table S1). In detail, a central point was calculated, which was the furthest point from the entire shoreline. A buffer of 750 m was calculated around this central point, and this buffer zone was encased by a quadrat with an edge length of 1500 m. All waterbodies for which the shape file of this encasing quadrat overlapped or cut the shoreline shape were excluded from further analysis with Sentinel-3 data.
We used data for the years 2016–2020, processed by two alternative commercially available processing chains that use science-based approaches that are fully published in the international literature. These two processing chains are (i) the EOMAP Processor (online processing platform eoapp AQUA®, https://aqua.eoapp.de/, accessed on 15 August 2023), referred to as eoapp AQUA from hereon in); and (ii) the Brockmann Consult Processor (online processing platform Calvalus and here the processing chain dedicated for CyanoAlert®, https://www.brockmann-consult.de/calbigfe/calvalus.jsp, accessed on 15 August 2023, version 2.22; referred to as CyanoAlert from hereon in). Note that the underlying signal processing in both cases follows fully published procedures, but the software implementation in a high-performance environment is the intellectual property of the companies and is available for scientific or consulting purposes.
CyanoAlert products are generated by different processing steps. The atmospheric correction and in-water retrieval is performed by the C2RCC processor for the respective sensors (Case 2 Regional Coast Colour [38,39]), while special treatment for cyanobacteria detection is performed with the MPH algorithm (maximum peak height [40,41]). For pixel identification (e.g., cloud detection), the IdePix processor is applied for each sensor [42,43]. These processing elements are available in the open source software SNAP (SeNtinel Application Platform; https://github.com/senbox-org/snap-engine/blob/master/README.md; accessed on 15 February 2024). Turbidity is retrieved following the algorithm developed by Nechad et al. [44,45]. Although these processing steps could in principle also be conducted independently of CyanoAlert as all software components are open source and free to use for all, the implementation in CyanoAlert provides high performance, easier application, and approved workflows. Compared to a rather work-intense implementation of several open source software blocks, state authorities usually prefer a full-fledged, proprietary implementation, as provided by CyanoAlert.
The eoapp AQUA procedure is deriving water quality data through a radiative transfer model by a purely physics-based approach [46]. The processor underlying eoapp AQUA is called MIP (Modular Inversion and Processing System) and consists of a sensor-independent suite of algorithms and databases to derive atmospheric and in-water properties by taking absorbing and scattering properties of atmosphere and water constituents into account [47,48]. The model performs all relevant processing steps and applies necessary corrections, such as surface type detection (land/water/cloud), adjacency, sunglint, and atmospheric correction. Water quality variables are retrieved by modelling the influence of their respective optically active components on the measured radiances [47,48].
The workflows based on the products derived by eoapp AQUA and CyanoAlert and for the in situ data are shown in Figure 2. Chlorophyll values derived from the remote sensing data were interpreted as chlorophyll-a.
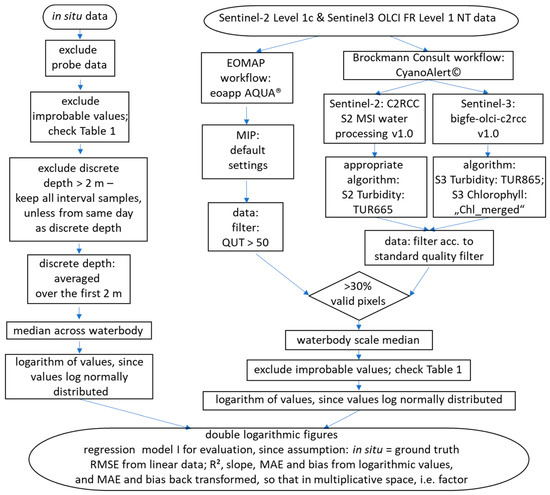
Figure 2.
Workflow of preparing and combining in situ and satellite data. QUT is the quality indicator in the eoapp AQUA workflow (see text); TUR = turbidity in FNU.
Quality filtering is essential when working with satellite data. Both processors employ filtering strategies, and we followed their respective recommendations. In the eoapp AQUA workflow, pixels are assigned a quality indicator which ranks their quality on a scale from 0 to 100 based on illumination, satellite view angles, atmospheric properties and concentration ranges. Pixels with a quality below 50 were excluded. Quality filtering in the CyanoAlert workflow was performed based on the standard quality filter band, which is either 0 or 1, and is also derived from different aspects related to the image acquisition conditions. All pixels which lacked quality according to this band were excluded. Note that whenever one processor filter excludes more or less pixels than the other, the comparison may end up being based on different data sets. In order to smooth over the strengths and weaknesses of both algorithms, like Kauer et al. [49], we averaged the results from the two algorithms in a combined approach assuming that the average of two different processors is better than single estimates. We finally want to stress that no further calibration of the algorithms on the available in situ data was performed and that the processors followed a naïve approach for retrieving water quality.
Regarding temporal mismatch, we restricted the evaluation procedure to measurements from the same day (in situ and satellite-derived data had to be acquired on the same day). An even smaller time window would have been preferable, but since the exact sampling time is not always known for most in situ measurements, this was not possible. Regarding the spatial scale, we evaluated all valid remote sensing pixels for the whole waterbody in order to have a waterbody-wide representative value. Previous work [30] showed that the waterbody-scale evaluation of the satellite data represented observation data as adequately as local macropixels. Macropixels are, e.g., 3 × 3, 5 × 5 pixels, i.e., 9 or 15 pixels, around the 1 pixel which covers a sampling site, and ensure that single pixel outliers did not compromise the result.
Some waterbodies are sampled in situ at more than one site. In such cases, only one in situ sampling site per waterbody was chosen in order to avoid giving waterbodies with more than one in situ sampling site too much weight. This in situ sampling site was chosen to be as representative of the whole waterbody as possible, i.e., far from the shore, close to the center of the waterbody, and close to the deepest point. Remote sensing-derived values were only considered when at least 30% of the pixels within the waterbody extent were valid.
The CyanoAlert procedure yields two different results for turbidity measurements for both Sentinel satellites (-2 and -3), namely turbidity at 665 nm and turbidity at 865 nm; and two types of chlorophyll-a results for Sentinel-3 [50], while the eoapp AQUA procedure gives one sensor-independent result for chlorophyll-a and turbidity, respectively. For the results from CyanoAlert, we decided to use the turbidity algorithm centered around 665 nm for Sentinel-2, and the one centered around 865 nm for Sentinel-3 after visual inspection of the ranges of the results. For chlorophyll-a from Sentinel-3 OLCI, we used the “chl_merged” algorithm, referred to as “C15-M10” in Schaeffer et al. [50]. It relies on the “chl_conc” algorithm from C2RCC, but also allows for higher concentrations of chlorophyll-a than the original C2RCC algorithm for chlorophyll-a, and deals better with elevated cyanobacteria concentrations, both being relevant for many inland waters.
2.4. Statistical Analyses
As a first step, we analyzed results from each processing chain separately and compared them with in situ values. In the second step, we averaged values from the two processing chains and analyzed how this combined value fits with the respective in situ measurements.
The variability of all remote sensing-based target variables tend to increase with the respective variable values when compared to in situ data. For instance, variability in chlorophyll is usually much higher in waterbodies where high chlorophyll concentrations were encountered. This pattern is typical in many environmental variables and points to underlying exponential processes so that a log-transformation leads to homoscedasticity. Therefore, we used log-transformed target variables for both in situ and satellite-based data. Thus, the coefficient of determination (R2), slope, bias, and mean absolute error (MAE) were given for the logarithmically transformed data. This is also the recommended procedure according to Seegers et al. [51]. In contrast, for direct comparability with other studies, we presented the RMSE on the untransformed, i.e., linear, data as this was used by many other researchers (e.g., [52]) and because it retains the units of the measurements, e.g., µg/L for chlorophyll-a. The RMSE is not appropriate for non-Gaussian error distributions [53] and is sensitive to outliers, both of which are common features in deriving water properties [51]. The mean absolute error (MAE), in contrast, particularly in the transformed form, is less sensitive to outliers, is appropriate for non-Gaussian error distributions, and is, therefore, recommended over the RMSE [51]. The mean bias (MB), from hereon in only called bias, is a measure of systematic errors [51], i.e., identifies if satellite-derived data are systematically lower or higher than the in situ data. In contrast to the RMSE, deviances in both the negative and positive direction may cancel out, which means that the bias is usually smaller than the RMSE [46]. It complements the RMSE and MAE, which are insensitive to systematic errors. In summary, the following error metrics were calculated:
In Equations (1)–(5) is the value of the satellite measurement predicted in the log-log-linear model and satellitei is the i-th observed satellite value. All statistical analyses and plots were performed using R version 4.2.2 [54].
3. Results
Error measures for the target variables chlorophyll-a, turbidity, and Secchi depth varied between the two satellites and processing chains. The errors in the single processing chain evaluations were in many cases—but not in all—higher than the errors in the combined approach (Figure 3, Figure 4 and Figure 5, Table 2). Therefore, merging the results from both processing chains into a combined value often improved the error metrices, indicating that averaging over processing chains makes the observations more robust. As an example, the waterbody-scale evaluation of chlorophyll-a for S3-OLCI nicely documents this statement as the MAE values in the combined case were lower than the ones from separate processing chains (1.9 in combined versus 2.2 in CyanoAlert and 3.2 in eoapp AQUA) (Figure 4, Table 2).
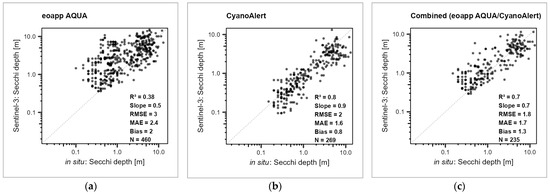
Figure 3.
Regression between in situ (x axis) and Sentinel-3 OLCI remote sensing Secchi depth values (y axis) from the waterbody-scale evaluation, values from the same day, on a logarithmic scale, dots transparent black. (a) eoapp AQUA processing chain, (b) CyanoAlert processing chain, and (c) combined approach. R2 = determination measure, Slope = regression line slope, MAE = mean average error, Bias = mean bias; all four based on log transformed data, RMSE = root mean square error, N = number of measurements. For the results for Sentinel-2, refer to Supplementary Information SI5.
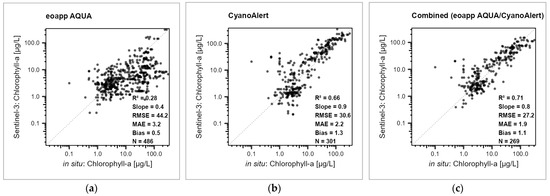
Figure 4.
Regression between in situ (x axis) and Sentinel-3 OLCI remote sensing chlorophyll-a values (y axis) from the waterbody-scale evaluation, values from the same day, on a logarithmic scale, dots transparent black. (a) eoapp AQUA processing chain, (b) CyanoAlert processing chain, and (c) combined approach. R2 = determination measure, Slope = regression line slope, MAE = mean average error, Bias = mean bias; all four based on log transformed data, RMSE = root mean square error, N = number of measurements. For the results for Sentinel-2, refer to Supplementary Information SI5.
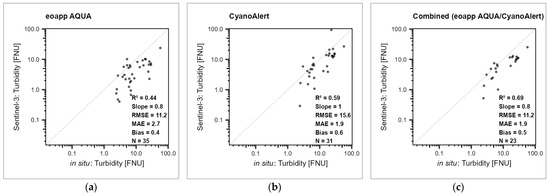
Figure 5.
Regression between in situ (x axis) and Sentinel-3 OLCI remote sensing turbidity values (y axis) from the waterbody-scale evaluation, values from the same day, on a logarithmic scale, dots transparent black. (a) eoapp AQUA processing chain, (b) CyanoAlert processing chain, and (c) combined approach. R2 = determination measure, Slope = regression line slope, MAE = mean average error, Bias = mean bias; all four based on log transformed data, RMSE = root mean square error, N = number of measurements. For the results for Sentinel-2, refer to Supplementary Information SI5.

Table 2.
Error measures of the fits between in situ and satellite data from the two processing chains and the combined version of both (column Combined), for the three variables and the two satellites, on the waterbody scale. CyanoAlert = CyanoAlert workflow, eoapp AQUA = eoapp AQUA workflow; Combined = combination of the CyanoAlert and eoapp AQUA workflows. chl = chlorophyll-a; tur = turbidity; secchi = Secchi depth. Slope = double logarithmic regression line slope; R2 = determination measure of the double logarithmic regression line; RMSE = root mean square error of the linear data; MAE = mean average error in the logarithmic form; MB = mean bias in the logarithmic form, N = number of measurements.
Nevertheless, in the combined approach, the different error indices for the target water quality variables also appeared to be rather high. For example, the RSME for Secchi depth was about 2 m (1.8 for Sentinel-3 and 2.3 for Sentinel-2) and for chlorophyll-a it was around 20 µg/L. Error quantification for turbidity was not meaningful due to the low sample sizes. The high error terms characterize the limited overall accuracy of remote sensing products when applied in a broad scale application and naïve processing, i.e., without local optimization and based on the diverse sources of in situ data. But at the same time, the regression analysis also indicated that a basic status assessment of a given waterbody is possible. For example, whether a given waterbody is more oligotrophic (i.e., high Secchi depth and low chlorophyll-a) or (vice versa) eutrophic is generally determinable in a reliable way by remote sensing.
The RMSE for the chlorophyll-a data comparison ranged between 20.9 µg/L (CyanoAlert processing chain; Sentinel-2; Figure 4b; Table 2) and 44.2 µg/L (eoapp AQUA processing chain; Sentinel-3; Figure 4a; Table 2) and was 20.7 and 27.2 µg/L in the combined versions for Sentinel-2 and Sentinel-3 OLCI, respectively (Figure 4c; Table 2). Except for the slopes and the mean bias, for the chlorophyll-a data, the combined versions were always better (Figure 4 and Figure S4; Table 2).
In situ turbidity was only available mainly from reservoirs. Therefore, the number of in situ observations was much lower than the number of observations from chlorophyll-a and Secchi depth. The R2 values were mostly lower than those for the other two variables, and again improved in the combined version. The RMSE between remote sensing-derived turbidity from Sentinel-2 data and in situ-measured turbidity was 4.3 FNU in the CyanoAlert processing chain and 14.1 FNU in the eoapp AQUA processing chain (Table 2; Figure S2a,b), and decreased to 2.5 FNU when combining the data from the two processing chains, while for the other error measures, combining did not offer any improvement over the fit from the CyanoAlert processing chain. For Sentinel-3 values, error measures from the combined approach largely reflected the error measures from eoapp AQUA (Figure 5 and Figure S2c,d; Table 2). Note that in all cases, the calculated error metrices for the different approaches are (partly) based on considerably different numbers of results (N).
Sentinel-2 and Sentinel-3 values from the same day were largely comparable within a workflow and variable (Figures S6–S8). The combined approach overall did not yield better results.
4. Discussion
Statistical fit quality between in situ and remote sensing data is sometimes lower than desirable, due to a range of factors which have been discussed in other contributions (e.g., [55,56]). In order to address the question of whether the match could still be sufficient for regular monitoring purposes, the aim was, thus, not to address the hindrances in evaluating the use of remote sensing for inland water quality assessment per se in detail, but to test whether satellite data would prove sufficiently robust in validation or evaluation, as a basis for a harmonized monitoring strategy, e.g., for federal states in Germany. In situ and remote sensing will always have to be used in a complementary way in order to benefit from the specific advantages of both strategies [57], e.g., high spatial and temporal coverage by satellites and analytical accuracy in laboratory-based analysis of in situ samples.
4.1. Atmospheric Correction, Retrieval of Water Constituents, and Contrasting Approaches in Signal Processing
Using remote sensing data for water quality assessment requires atmospheric correction because the atmospheric influences can contribute up to 90% of the measured signal at the satellite sensor [53]. The atmospheric correction algorithms and also further algorithms for the optical reactivity of the waterbody differ in the two workflows and are, thus, a source of deviation in the results from the two workflows.
While eoapp AQUA follows a fully physical approach of radiative transfer modelling with sensor-agnostic retrieval of the water constituents in the MIP algorithm [46,47], CyanoAlert uses artificial neural networks that invert simulated spectra derived from radiative transfer modelling by using the C2RCC algorithm [37,38], while for turbidity for specific satellites a semi-empirical algorithm is used. Contrasting elements for both approaches are accordingly different in the applicability ranges from in principle unrestricted fully physics-based models to the trained data ranges of neural networks (C2RCC) and the empirical Nechad algorithm for “coastal” or moderate to turbid conditions. This contrast can also be seen when we look at the satellite applicability: the retrieval in MIP/eoapp AQUA is applied in a harmonized way to all investigated sensors, while CyanoAlert uses sensor-specific versions of the neural networks (see Figure 2).
These contrasting approaches, among other factors, contribute to variability and explain why the same satellite scene, i.e., exactly the same raw data of a given water body, result in two different values of the final target variable, e.g., chlorophyll or turbidity. Nevertheless, both approaches also have similarities, as the underlying model of radiative transfer are following the physics of radiative transfer, with differences in the implementation level (e.g., sensor-generic adjacency correction is implemented in MIP). Our results indicate that it is difficult to decide which of the two processing approaches is the better one, as this depends on the target variables and even differs among water bodies. Therefore, we believe that it is not wise to give a generalized recommendation for one specific processor but rather want to demonstrate how EO-derived water quality variables compare to in situ data and how the derived information can be used for supporting water quality monitoring. For this we used the combined approach where values from both processing lines are averaged. A deeper analysis of the different processing chains would require analyzing results at the individual waterbody scale in order to identify where deviations from in situ values are high or low and whether systematic over- or underestimation occurs. This is the next step in our research but goes beyond the scope of this paper, where the general performance is addressed.
4.2. Prerequisites for Validation
For validation to be tractable and reproducible, it should follow protocols, i.e., a standard of procedure, covering all parts of the validation, on the remote sensing side and the in situ data side, covering, e.g., macropixel formation [58] and error measures [51]. To agree on such overarching protocols is an iterative process, initiated, e.g., by the Working Group on Calibration & Validation (WGCV; https://ceos.org/ourwork/workinggroups/wgcv/, accessed on 15 August 2023) within the international CEOS (Committee on Earth Observation Satellites; https://ceos.org/, accessed on 15 August 2023). They define terms of references [59] and rules for calibration and validation, see, e.g., Pahlevan et al. [52]. Further validations for aquatic products have been written up, e.g., in EUMETSAT [60,61] or Simis et al. [62].
If a variable, such as chlorophyll-a, is validated for a specific set of algorithms within a specific workflow, such validation is not universal. The validation will have to be repeated for every new satellite instrument and new workflow [63]. However, there is an imbalance in the availability of water quality data compared with, e.g., data on water quantity [55]. This leads to the validation of water quality products lagging behind that of water quantity.
The major prerequisite for validating remote sensing processed data is the in situ data. To achieve a meaningful validation which is valid for, e.g., waterbodies from different biogeographical and climatic zones, altitudes, water types etc., a high number of in situ data from diverse waterbodies is necessary. The in situ data have to be as homogeneous as possible in terms of sampling procedure and methods [64]. To achieve such a bespoke data set (in radiometry referred to as fiducial reference data [65]) is costly and is usually only collected for a limited number of waterbodies, if at all. Instead, monitoring data lend themselves to such a task (a global compilation for inland waters, which, however, does not cover Germany well, is, e.g., Lehmann et al. [66]). Here, we used in situ data that were compiled by authorities according to international standards as ruled by, e.g., the European Water Framework Directive [19]. The variation and insecurities, error margins, etc., of the in situ methods are well known and well described in the national and international standards, e.g., for aquatic chlorophyll-a in the German national standard DIN 38409-60:2019-12 [37] or ISO 10260 [67] as the international standard (but see below for a discussion on water quality methods).
4.3. Comparisons between In Situ and Satellite Data
Considering the difficulties in generalizing remote sensing for different waterbodies, it is hardly surprising that, in general, the fits shown here between in situ and satellite data were lower than those known from similar studies that focused on either a single or a small suite of waterbodies by adjusting the algorithms to the respective in situ data [4,11,12,68,69]. The difficulties in generalizing remote sensing for different waterbodies are one reason why in situ and remote sensing measurements will always be used in a complementary way [57].
The goodness of fit, R2, in the present study showed a wide range (between 0.18 and 0.81), and the error measures (RMSE, MAE, and bias) were relatively high for both Sentinel-2 and Sentinel-3 for all investigated characteristics, e.g., the RMSE between in situ and satellite-derived chlorophyll-a extracted as the combined result between the two workflows, was up to 58.3 µg/L (Sentinel-2). This is tenfold higher than what Domínguez Gómez et al. [70] deemed acceptable when comparing the average of 5 samples within 200 m squares with MERIS in the 100 largest reservoirs in Spain, and higher than what, e.g., Dörnhöfer et al. [4] found for Lake Kummerow (5.1 µg/L). In the current study, data from over 100 waterbodies within different regions and in different environments were used. Thus, the RMSE in the current study had to be expected to be higher. RMSE from two variables (Secchi depth and turbidity), and MAE and bias from all variables (Chlorophyll-a, Secchi depth and turbidity), were larger than anticipated from studies that focused mostly on older satellites which have provided longer time series, such as, e.g., MODIS for which RMSE for chlorophyll-a in Lake Erie was between 1.36 and 14.52 [71]. One such reason is the differences in recording times between in situ and remote sensing. This is especially true when and where water quality characteristics change quickly locally on short time scales, mostly brought about by meteorological dynamics, such as wind influencing the flows, but also the vertical migration of phytoplankton. Even if the effects are only local, they do impact on either the in situ site or the whole waterbody median, or both, but not necessarily in the same way. Such heterogeneity over space and time may lead to the waterbody scale evaluation of remote sensing data fitting better to in situ data, at least considering the MAE [30]. The waterbody scale integrates over such small-scale changes that this may lead to a 3 × 3 macropixel being not representative for a sampling site. Keeping in mind that we compare an area of at least 100 m2 (10 m resolution of Sentinel-2) with a water sample of about 1 to 2 L at one position, the representativity of water samples is low in the case of small-scale changes in the waterbody. Furthermore, the data set comprised waterbodies from different regions, with different trophic states, different sizes, different depths, and where especially chlorophyll-a was measured with different in situ methods.
Another reason for differences between in situ and satellite data may be pixels covering shallow areas. The signal from the bottom of shallow waterbodies or shallow zones, e.g., along the shoreline, is not separated from the signal from water contents [72]. Thus, shallow water depth, unless the algal blooms or other water contents restrict visibility, cannot be appropriately evaluated with remote sensing [72]. For a strict procedure, all shallow areas must be excluded. However, in authorities’ monitoring, such shallow areas are not necessarily known in detail for a given waterbody. Therefore, a workflow applicable to monitoring of a large number of waterbodies must remain generic or naïve. As mentioned above, here, the aim was not to find the best individual fit for every single waterbody, but to check whether a generic approach would yield results of sufficient quality for monitoring according to regulations.
A further reason for a lack in quality fit is the sun angle. The lower the sun elevation for a region, the less radiation reaches into the water, and the less radiation is returned to the instruments onboard the satellites [2]. Thus, zones near the poles, and winter data from zones with a high seasonality in radiation, are not optimal for remote sensing evaluations in winter months. This applies to the northern waterbodies in the present study more than to the southern ones (Table S1). However, since the approach we followed was supposed to be generic, such individual filtering of scenes per season was not implemented here. Further measures to improve the fit between in situ and remote sensing data are discussed below.
4.4. Limitations in the In Situ Data Which Become Apparent When Comparing Them to Remote Sensing Data
Sampling and measuring chlorophyll-a is a complex challenge and carried out using different methods in the laboratory which usually follow standardized procedures. As an example, and to illustrate the complexity of the method, there is a known result dependence on algae taxa. It is easier to extract chlorophyll-a from some taxa than from others [73,74,75]. The different methods result either in total chlorophyll (chlorophyll-a and chlorophyllides; when not acidifying, and when not using HPLC), or chlorophyll-a separate from other derivates, such as divinyl chlorophyll-a derivates, or in differentiating accessory pigments (e.g., chlorophyll b and c), or degradation products [37]. Interlaboratory comparisons have resulted in differences in chlorophyll-a measurements of up to 68% (summarized in Seegers et al. [76]). For the purpose of the approach taken here, i.e., not adapting/calibrating based on prior knowledge, we assumed that all the chlorophyll-a methods were comparable to a reasonable degree, and that they are precise and accurate. However, round robins showed that this is not necessarily the case [77].
Remote sensing for estimating chlorophyll-a does not resolve into taxonomies of molecules but derives chlorophyll-a values based on data from the radiation measured in the spectral bands of the sensors onboard of the satellites. The results from remote sensing depend on the algorithms though, including atmospheric correction, like the laboratory analysis of chlorophyll depends on the reagents, the instruments, and the skillful handling. On the other hand, because of the differences in in situ methods and their respective focus and accuracy, the comparison between in situ and a generic, not adjusted, remote sensing-derived chlorophyll-a value cannot be expected to be more precise than within a relatively broad margin.
Several in situ methods exist for measuring turbidity. The results are then either on the FNU (formazin nephelometric unit) or NTU (nephelometric turbidity unit) scale. While each specific method is straightforward, their intercomparability is low. Thus, it is not easily possible to create homogenous in situ data for turbidity when different methods have been applied. A further difficulty when comparing such in situ data to remote sensing data is that different remote sensing data workflows result in different units, e.g., the CyanoAlert workflow resulted in two variables: FNU at 665 nm (used here for Sentinel-2) and FNU at 865 nm (used here for Sentinel-3), while the eoapp AQUA workflow resulted in one variable: FTU at 550 nm. Therefore, comparability between any field method and the remote sensing-derived method is lower than for Secchi depth, and this is most likely one reason why the error measures were higher for turbidity than for Secchi depth.
4.5. Improving the Fit between In Situ and Satellite Data
Steps are taken to improve the fit between remote sensing data and in situ data, but most of them are not generalizable [1]. The fit between in situ and remote sensing data can be improved by several measures that are beyond the scope of this publication: (1) excluding shore areas and shallow water zones where signal might be disturbed; (2) improved or dedicated atmospheric correction [52]; (3) manual filtering of scenes where, e.g., sunglint might not have been recognized by the work flow; (4) excluding scenes from seasons with low light (i.e., winter); (5) excluding scenes from waterbodies of a certain water quality which might not lead to good results; (6) improving water retrieval algorithms [77]; (7) excluding values below the detection limit [12,56,78]; (8) using algorithms individually for each optical water type [17,79,80].
In addition to practical approaches (like points 1 to 5 above), it needs to be noted that new algorithms are constantly being developed and existing ones are further improved. Such algorithm adaptations focus, e.g., on specific environmental conditions and lake inherent characteristics, such as water types that have so far not been addressed adequately [81]. Depending on how the algorithms were developed, i.e., physically or empirically, algorithms are better adapted to some circumstances than others. Thus, new developments should take more circumstances into consideration, in a more appropriate way, e.g., including sub routines for more water types.
Further measures to improve the fit between remote sensing data and in situ data include excluding values below a level that might be a determination limit. As examples, studies for specific waterbodies indicate a certain limit of quantification, e.g., Palmer et al. [82] excluded chlorophyll-a values below 10 µg/L from further analysis with the fluorescence line height (FLH) algorithm from MERIS data because it was found that the fit was not sufficient for low chlorophyll-a values. Ogashawara [12] found that remote sensing algorithms did not perform well for pigment concentrations that were lower than 50 µg/L, and Dörnhöfer et al. [78] stated that a chlorophyll-a value of around 1 µg/L is too low for a solid retrieval.
Further approaches that will not be discussed here include dividing the in situ data sets according to (a) measurement method (e.g., fluorescence versus HPLC versus spectrophotometric chlorophyll-a determination [1]), (b) the physical and hydrographic characteristics of the waterbodies [14], (c) the “ecoregions” from the WFD [70], the (d) trophic classes [1], or by (e) region [10], with regions, e.g., being characterized by different rainfall patterns [83]. All these approaches are applied on a case-by-case basis and cannot be used for a universal recommendation for authorities concerned with water quality as for how to use remote sensing for the regular monitoring of waterbodies.
Based on the discussed consequences of using algorithms independent from prior knowledge of the waterbody, without calibration and further measures to improve the fit between remote sensing and in situ data, we considered the satellite data in this generic way to be sufficiently accurate, both for Sentinel-2 and -3, by the resulting error indices. In some cases, the accuracy was lower than expected, and we discussed possible reasons. However, the advantages and complementarity of satellite data for spatial information and higher temporal resolution provides great added value. Thus, the information can also be included in thematic maps, which are useful for local and regional governments [66] and show spatial patterns within lakes and among different lakes in a region.
5. Conclusions
We showed that Sentinel-2 and -3 optic properties as processed by the two processing chains can be used in regular monitoring towards fulfilling, e.g., the requirements by the Water Framework Directive (WFD; [19]), or the requirements by the bathing water directory (BWD; [20]). The disadvantages of the Sentinel-2 MSI (low spectral resolution and low radiometric sensitivity for clear water) and Sentinel-3 OLCI instruments (low spatial resolution with 300 × 300 m) are outweighed by the advantages of enabling the monitoring of a large number of waterbodies with a higher frequency than is currently possible by in situ sampling alone. Ready-to-use data on water quality in terms of Secchi depth, turbidity, and chlorophyll concentration which result from the workflows of two companies may thus enable authorities to use them complementary to in situ data without having to invest in personnel or machinery. A good strategy for integrating satellite data in the monitoring activities would enable authorities to use the advantages and assets of both techniques for a comprehensive monitoring program.
Authorities concerned with water quality must decide whether the accuracy is sufficient, depending on the intended purpose on where and how to use the data. The general approaches that we described here could be applied in further and future endeavors on a case-by-case basis.
The adoption of remote sensing methods as a permissible analysis tool in the relevant guidelines would certainly help to simplify the use and implementation of remote sensing for official end users in authorities concerned with water quality. Future missions with, e.g., higher spatio-temporal resolutions, will improve estimating water quality further.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/rs16183416/s1, Table S1: Water body characteristics. O = oligotrophic, M = mesotrophic, E = eutrophic, P = polytrophic. Waterbodies with an “x” in the column “S3” are large enough to be evaluated with Sentinel-3 OLCI. See main text for the derivation. In brief, a 5 × 5 contiguous macro pixel, i.e., 1500 × 1500 m2, can fit into waterbodies with an “x” in this column. Figure S1. Overview over the two satellites used and their specifications, along with two example images from Lindau, Lake Constance, Germany. MSI = Multispectral Instrument; OLCI = Ocean and Land Color Imager. Figure S2. Boxplot of the Secchi depths in the in situ data. The upper whisker extends here from the upper line of the box to the largest value, with the whisker not being further than 1.5 times the inter-quartile range from the upper line of the box. The inter-quartile range is the distance between the first and third quartiles. The lower whisker extends to the smallest value which is maximum 1.5 times the inter-quartile range. Outliers are points outside of the inter-quartile range. They are plotted as separate points. Figure S3. Regression between in situ (x axis) and Sentinel-2 MSI remote sensing Secchi depth values (y axis) from the whole water body, values from the same day, on a logarithmic scale. (a), eoapp AQUA processing chain, (b) CyanoAlert processing chain, (c) combined approach. R² = Determination measure, Slope = regression line slope, MAE = Mean average error, all three based on log transformed data, RMSE = root mean square error, Bias =mean bias from the untransformed data, N = number of measurements. For the results for Sentinel-3, refer to Figure 3 of the main paper. Figure S4. Regression between in situ (x axis) and Sentinel-2 remote sensing chlorophyll-a values (y axis) from the whole water body, values from the same day, on a logarithmic scale. (a) eoapp AQUA processing chain, (b) CyanoAlert processing chain, (c) combined approach. R² = Determination measure, Slope = regression line slope, MAE = Mean average error, all three based on log transformed data, RMSE = root mean square error, Bias =mean bias from the untransformed data, N = number of measurements. For the results for Sentinel-3, refer to Figure 4 of the main paper. Figure S5. Regression between in situ (x axis) and Sentinel-2 remote sensing turbidity values (y axis) from the whole water body, values from the same day, on a logarithmic scale. (a) eoapp AQUA processing chain, (b) CyanoAlert processing chain, (c) combined approach. R² = Determination measure, Slope = regression line slope, MAE = Mean average error, all three based on log transformed data, RMSE = root mean square error, Bias =mean bias from the untransformed data, N = number of measurements. For the results for Sentinel-3, refer to Figure 5 of the main paper. Figure S6: Regression between remote sensing Secchi depth values from the waterbody-scale Sentinel-2 (x axis) and Sentinel-3 (y axis), values from the same day, on a logarithmic scale. (a) eoapp AQUA processing chain, (b) CyanoAlert processing chain, (c) combined approach. R² = Determination measure, Slope = regression line slope, MAE = Mean average error, Bias = mean bias; all four based on log transformed data, RMSE = root mean square error, N = number of measurements. Figure S7. Regression between remote sensing chlorophyll-a values from the waterbody-scale Sentinel-2 (x axis) and Sentinel-3 (y axis), values from the same day, on a logarithmic scale. (a) eoapp AQUA processing chain, (b) CyanoAlert processing chain, (c) combined approach. R² = Determination measure, Slope = regression line slope, MAE = Mean average error, Bias = mean bias; all four based on log transformed data, RMSE = root mean square error, N = number of measurements. Figure S8. Regression between remote sensing turbidity values from the waterbody-scale Sentinel-2 (x axis) and Sentinel-3 (y axis), values from the same day, on a logarithmic scale. (a) eoapp AQUA processing chain, (b) CyanoAlert processing chain, (c) combined approach. R² = Determination measure, Slope = regression line slope, MAE = Mean average error, Bias = mean bias; all four based on log transformed data, RMSE = root mean square error, N = number of measurements.
Author Contributions
Conceptualization, K.R., K.F., R.D.K., T.S., and S.I.S.; methodology, H.B., K.S., R.D.K., T.S., and S.I.S.; software, H.B. and K.S.; validation, T.S. and S.I.S.; formal analysis, S.I.S. and T.S.; resources, K.R., K.F., H.B., and K.S.; data curation, S.I.S. and T.S.; writing—original draft preparation, S.I.S.; writing—review and editing, K.R., T.S., K.F., P.L., R.D.K., S.I.S., K.S., and H.B.; visualization, S.I.S. and T.S.; supervision, K.R. and K.F.; project administration, K.R. and K.F.; funding acquisition, K.R. and K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by German Space Agency within DLR, on behalf of the Federal Ministry for Digital and Transport: 50EW2101A.
Data Availability Statement
All data are available upon reasonable request.
Acknowledgments
Water quality agencies from 13 federal states provided in situ data for which we are thankful (see Supplementary Information SI1). Discussions with Daniela Gurlin proved immensely helpful.
Conflicts of Interest
Author Bernert, H. was employed by the company EOMAP GmbH & Co. KG. Author Stelzer, K. was employed by the company Brockmann Consult GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alikas, K.; Kangro, K.; Kõks, K.-L.; Tamm, M.; Freiberg, R.; Laas, A. Consistency of Six in situ, in Vitro and Satellite-Based Methods to Derive Chlorophyll a in Two Optically Different Lakes. Front. Environ. Sci. 2023, 10, 989671. [Google Scholar] [CrossRef]
- Attila, J.; Koponen, S.; Kallio, K.; Lindfors, A.; Kaitala, S.; Ylöstalo, P. MERIS Case II Water Processor Comparison on Coastal Sites of the Northern Baltic Sea. Remote Sens. Environ. 2013, 128, 138–149. [Google Scholar] [CrossRef]
- Dekker, A.G.; Malthus, T.J.; Wijnen, M.M.; Seyhan, E. Remote Sensing as a Tool for Assessing Water Quality in Loosdrecht Lakes. Hydrobiologia 1992, 233, 137–159. [Google Scholar] [CrossRef]
- Dörnhöfer, K.; Klinger, P.; Heege, T.; Oppelt, N. Multi-Sensor Satellite and In situ Monitoring of Phytoplankton Development in a Eutrophic-Mesotrophic Lake. Sci. Total Environ. 2018, 612, 1200–1214. [Google Scholar] [CrossRef]
- Dörnhöfer, K.; Oppelt, N. Remote Sensing for Lake Research and Monitoring—Recent Advances. Ecol. Indic. 2016, 64, 105–122. [Google Scholar] [CrossRef]
- Gurlin, D.; Gitelson, A.A.; Moses, W.J. Remote Estimation of Chl-a Concentration in Turbid Productive Waters—Return to a Simple Two-Band NIR-Red Model? Remote Sens. Environ. 2011, 115, 3479–3490. [Google Scholar] [CrossRef]
- Kutser, T.; Paavel, B.; Verpoorter, C.; Ligi, M.; Soomets, T.; Toming, K.; Casal, G. Remote Sensing of Black Lakes and Using 810 Nm Reflectance Peak for Retrieving Water Quality Parameters of Optically Complex Waters. Remote Sens. 2016, 8, 497. [Google Scholar] [CrossRef]
- Lehmann, M.K.; Schütt, E.M.; Hieronymi, M.; Dare, J.; Krasemann, H. Analysis of Recurring Patchiness in Satellite-Derived Chlorophyll a to Aid the Selection of Representative Sites for Lake Water Quality Monitoring. Int. J. Appl. Earth Obs. Geoinf. 2021, 104, 102547. [Google Scholar] [CrossRef]
- Oppelt, N.; Schulze, F.; Bartsch, I.; Doernhoefer, K.; Eisenhardt, I. Hyperspectral Classification Approaches for Intertidal Macroalgae Habitat Mapping: A Case Study in Heligoland. Opt. Eng. 2012, 51, 111703. [Google Scholar] [CrossRef]
- Pahlevan, N.; Smith, B.; Alikas, K.; Anstee, J.; Barbosa, C.; Binding, C.; Bresciani, M.; Cremella, B.; Giardino, C.; Gurlin, D.; et al. Simultaneous Retrieval of Selected Optical Water Quality Indicators from Landsat-8, Sentinel-2, and Sentinel-3. Remote Sens. Environ. 2022, 270, 112860. [Google Scholar] [CrossRef]
- Werther, M.; Odermatt, D.; Simis, S.G.H.; Gurlin, D.; Jorge, D.S.F.; Loisel, H.; Hunter, P.D.; Tyler, A.N.; Spyrakos, E. Characterising Retrieval Uncertainty of Chlorophyll-a Algorithms in Oligotrophic and Mesotrophic Lakes and Reservoirs. ISPRS J. Photogramm. Remote Sens. 2022, 190, 279–300. [Google Scholar] [CrossRef]
- Ogashawara, I. The Use of Sentinel-3 Imagery to Monitor Cyanobacterial Blooms. Environments 2019, 6, 60. [Google Scholar] [CrossRef]
- Kirstein, V.; Bitterich, L.; Drost, S.; Vogt, A.; Pakzad, K. Copernicus Dienste Für Die Wasserwirtschaft—Ergebnisse Aus Dem Projekt WaCoDiS. Kw Korresp. Wasserwirtsch. 2021, 14, 486. [Google Scholar]
- Attila, J.; Kauppila, P.; Kallio, K.Y.; Alasalmi, H.; Keto, V.; Bruun, E.; Koponen, S. Applicability of Earth Observation Chlorophyll-a Data in Assessment of Water Status via MERIS—With Implications for the Use of OLCI Sensors. Remote Sens. Environ. 2018, 212, 273–287. [Google Scholar] [CrossRef]
- Papathanasopoulou, E.; Simis, S.; Alikas, K.; Ansper, A.; Anttila, S.; Attila, J.; Barillé, A.-L.; Barillé, L.; Brando, V.; Bresciani, M.; et al. [White Paper] Satellite-Assisted Monitoring of Water Quality to Support the Implementation of the Water Framework Directive; Zenodo: Geneva, Switzerland, 2019. [Google Scholar] [CrossRef]
- Rinke, K.; Globevnik, L.; Šubelj, G.; Snoj, L. Satellite-Based Monitoring of Cyanobacteria in Bathing Waters; European Topic Centre on Inland, Coastal and Marine Waters (ETC/ICM): Magdeburg, Germany, 2022; p. 35. [Google Scholar]
- Neil, C.; Spyrakos, E.; Hunter, P.D.; Tyler, A.N. A Global Approach for Chlorophyll-a Retrieval across Optically Complex Inland Waters Based on Optical Water Types. Remote Sens. Environ. 2019, 229, 159–178. [Google Scholar] [CrossRef]
- Spyrakos, E.; Hunter, P.; Simis, S.; Neil, C.; Riddick, C.; Wang, S.; Varley, A.; Blake, M.; Groom, S.; Palenzuela, J.T.; et al. Moving towards Global Satellite Based Products for Monitoring of Inland and Coastal Waters. Regional Examples from Europe and South America. In Proceedings of the 2020 IEEE Latin American GRSS & ISPRS Remote Sensing Conference (LAGIRS), Santiago, Chile, 22–26 March 2020. [Google Scholar]
- European Parliament; Council of the European Union. Directive 2000/60/EC of the European Parliament and of the Council of the 23 October 2000: Establishing Framework for the Community Action in the Field of Water Policy (WFD). Off. J. Eur. Comm. 2000, L327, 321–371. [Google Scholar]
- European Parliament; Council of the European Union. Bathing Water Directive (BWD). Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC. Off. J. Eur. Union 2006, L64, 37–51. [Google Scholar]
- Politi, E.; Cutler, M.E.J.; Carvalho, L.; Rowan, J.S. A Global Typological Approach to Classify Lakes Based on Their Eutrophication Risk. Aquat. Sci. 2024, 86, 52. [Google Scholar] [CrossRef]
- Binding, C.E.; Pizzolato, L.; Zeng, C. EOLakeWatch; Delivering a Comprehensive Suite of Remote Sensing Algal Bloom Indices for Enhanced Monitoring of Canadian Eutrophic Lakes. Ecol. Indic. 2021, 121, 106999. [Google Scholar] [CrossRef]
- Ho, J.; Michalak, A.M.; Pahlevan, N. Widespread Global Increase in Intense Lake Phytoplankton Blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Gilarranz, L.J.; Narwani, A.; Odermatt, D.; Siber, R.; Dakos, V. Regime Shifts, Trends, and Variability of Lake Productivity at a Global Scale. Proc. Natl. Acad. Sci. USA 2022, 119, e2116413119. [Google Scholar] [CrossRef] [PubMed]
- Chawla, I.; Karthikeyan, L.; Mishra, A.K. A Review of Remote Sensing Applications for Water Security: Quantity, Quality, and Extremes. J. Hydrol. 2020, 585, 124826. [Google Scholar] [CrossRef]
- Woolway, R.I.; Kraemer, B.M.; Lenters, J.D.; Merchant, C.J.; O’Reilly, C.M.; Sharma, S. Global Lake Responses to Climate Change. Nat. Rev. Earth Environ. 2020, 1, 388–403. [Google Scholar] [CrossRef]
- Irani Rahaghi, A.; Odermatt, D.; Anneville, O.; Sepúlveda Steiner, O.; Reiss, R.S.; Amadori, M.; Toffolon, M.; Jacquet, S.; Harmel, T.; Werther, M.; et al. Combined Earth Observations Reveal the Sequence of Conditions Leading to a Large Algal Bloom in Lake Geneva. Commun. Earth Environ. 2024, 5, 229. [Google Scholar] [CrossRef]
- Carvalho, L.; Mackay, E.B.; Cardoso, A.C.; Baattrup-Pedersen, A.; Birk, S.; Blackstock, K.L.; Borics, G.; Borja, A.; Feld, C.K.; Ferreira, M.T.; et al. Protecting and Restoring Europe’s Waters: An Analysis of the Future Development Needs of the Water Framework Directive. Sci. Total Environ. 2019, 658, 1228–1238. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Hallikainen, M. Water Quality Monitoring Using Remote Sensing in Support of the EU Water Framework Directive (WFD): A Case Study in the Gulf of Finland. Environ. Monit. Assess. 2007, 124, 157–166. [Google Scholar] [CrossRef]
- Schröder, T.; Schmidt, S.I.; Kutzner, R.D.; Bernert, H.; Stelzer, K.; Friese, K.; Rinke, K. Exploring Spatial Aggregations and Temporal Windows for Water Quality Match-up Analysis Using Sentinel-2 MSI and Sentinel-3 OLCI Data. Remote Sens. 2024, 16, 2798. [Google Scholar] [CrossRef]
- ESA S2 Mission—Overview of Sentinel-2 Mission. 2024. Available online: https://sentiwiki.copernicus.eu/web/s2-mission (accessed on 15 August 2024).
- Chambrelan, A. Sentinel-2 S2 MPC Level-2A Algorithm Theoretical Basis Document; Ref. S2-PDGS-MPC-ATBD-L2A. 2023. Available online: https://step.esa.int/thirdparties/sen2cor/2.10.0/docs/S2-PDGS-MPC-L2A-ATBD-V2.10.0.pdf (accessed on 15 August 2023).
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Donlon, C.; Berruti, B.; Buongiorno, A.; Ferreira, M.-H.; Féménias, P.; Frerick, J.; Goryl, P.; Klein, U.; Laur, H.; Mavrocordatos, C.; et al. The Global Monitoring for Environment and Security (GMES) Sentinel-3 Mission. Remote Sens. Environ. 2012, 120, 37–57. [Google Scholar] [CrossRef]
- Runge, A.; Grosse, G. Mosaicking Landsat and Sentinel-2 Data to Enhance Landtrendr Time Series Analysis in Northern High Latitude Permafrost Regions. Remote Sens. 2020, 12, 2471. [Google Scholar] [CrossRef]
- DIN EN ISO 7027-1:2016-11; Water Quality—Determination of Turbidity—Part 1: Quantitative Methods (ISO 7027-1:2016); German Version EN ISO 7027-1:2016. Beuth Verlag GmbH: Berlin, Germany, 2016.
- DIN 38409-60:2019-12; German Standard Methods for the Examination of Water, Waste Water and Sludge—Parameters Characterizing Effects and Substances (Group H)—Part 60: Spectrometric Determination of the Chlorophyll-a Concentration in Water (H 60). Beuth Verlag GmbH: Berlin, Germany, 2020.
- Brockmann, C.; Doerffer, R.; Peters, M.; Stelzer, K.; Embacher, S.; Ruescas, A. Evolution of the C2RCC Neural Network for Sentinel 2 and 3 for the Retrieval of Ocean Colour Products in Normal and Extreme Optically Complex Waters. In Proceedings of the ‘Living Planet Symposium 2016’, Prague, Czech Republic, 9–13 May 2016. ESA-SP 740. [Google Scholar]
- Doerffer, R.; Schiller, H. The MERIS Case 2 Water Algorithm. Int. J. Remote Sens. 2007, 28, 517–535. [Google Scholar] [CrossRef]
- Matthews, M.W.; Odermatt, D. Improved Algorithm for Routine Monitoring of Cyanobacteria and Eutrophication in Inland and Near-Coastal Waters. Remote Sens. Environ. 2015, 156, 374–382. [Google Scholar] [CrossRef]
- Pitarch, J.; Volpe, G.; Colella, S.; Krasemann, H.; Santoleri, R. Remote Sensing of Chlorophyll in the Baltic Sea at Basin Scale from 1997 to 2012 Using Merged Multi-Sensor Data. Ocean Sci. 2016, 12, 379–389. [Google Scholar] [CrossRef]
- Wevers, J.; Müller, D.; Kirches, G.; Quast, R.; Brockmann, C. IdePix for Sentinel-3 OLCI Algorithm Theoretical Basis Document; Zenodo: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- Wevers, J.; Müller, D.; Scholze, J.; Kirches, G.; Quast, R.; Brockmann, C. IdePix for Sentinel-2 MSI Algorithm Theoretical Basis Document; Zenodo: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- Dogliotti, A.I.; Ruddick, K.G.; Nechad, B.; Doxaran, D.; Knaeps, E. A Single Algorithm to Retrieve Turbidity from Remotely-Sensed Data in All Coastal and Estuarine Waters. Remote Sens. Environ. 2015, 156, 157–168. [Google Scholar] [CrossRef]
- Nechad, B.; Dogliotti, A.I.; Ruddick, K.G.; Doxaran, D. Particulate Backscattering and Suspended Matter Concentration Retrieval from Remote-Sensed Turbidity in Various Coastal and Riverine Turbid Waters. In Proceedings of the ESA Living Planet Symposium, Prague, Czech Republic, 9–13 May 2016. ESA-SP 740. [Google Scholar]
- Heege, T.; Fischer, J. Mapping of Water Constituents in Lake Constance Using Multispectral Airborne Scanner Data and a Physically Based Processing Scheme. Can. J. Remote Sens. 2004, 30, 77–86. [Google Scholar] [CrossRef]
- Bresciani, M.; Giardino, C.; Stroppiana, D.; Dessena, M.A.; Buscarinu, P.; Cabras, L.; Schenk, K.; Heege, T.; Bernet, H.; Bazdanis, G.; et al. Monitoring Water Quality in Two Dammed Reservoirs from Multispectral Satellite Data. Eur. J. Remote Sens. 2019, 52, 113–122. [Google Scholar] [CrossRef]
- Heege, T.; Kiselev, V.; Wettle, M.; Hung, N.N. Operational Multi-Sensor Monitoring of Turbidity for the Entire Mekong Delta. Int. J. Remote Sens. 2014, 35, 2910–2926. [Google Scholar] [CrossRef]
- Kauer, T.; Kutser, T.; Arst, H.; Danckaert, T.; Nõges, T. Modelling Primary Production in Shallow Well Mixed Lakes Based on MERIS Satellite Data. Remote Sens. Environ. 2015, 163, 253–261. [Google Scholar] [CrossRef]
- Schaeffer, B.; Salls, W.; Coffer, M.; Lebreton, C.; Werther, M.; Stelzer, K.; Urquhart, E.; Gurlin, D. Merging of the Case 2 Regional Coast Colour and Maximum-Peak Height Chlorophyll-a Algorithms: Validation and Demonstration of Satellite-Derived Retrievals across US Lakes. Environ. Monit. Assess. 2022, 194, 179. [Google Scholar] [CrossRef]
- Seegers, B.N.; Stumpf, R.P.; Schaeffer, B.A.; Loftin, K.A.; Werdell, P.J. Performance Metrics for the Assessment of Satellite Data Products: An Ocean Color Case Study. Opt. Express 2018, 26, 7404. [Google Scholar] [CrossRef]
- Pahlevan, N.; Mangin, A.; Balasubramanian, S.V.; Smith, B.; Alikas, K.; Arai, K.; Barbosa, C.; Bélanger, S.; Binding, C.; Bresciani, M.; et al. ACIX-Aqua: A Global Assessment of Atmospheric Correction Methods for Landsat-8 and Sentinel-2 over Lakes, Rivers, and Coastal Waters. Remote Sens. Environ. 2021, 258, 112366. [Google Scholar] [CrossRef]
- Chai, T.; Draxler, R.R. Root Mean Square Error (RMSE) or Mean Absolute Error (MAE)?—Arguments against Avoiding RMSE in the Literature. Geosci. Model. Dev. 2014, 7, 1247–1250. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Ellis, E.A.; Allen, G.H.; Riggs, R.M.; Gao, H.; Li, Y.; Carey, C.C. Bridging the Divide between Inland Water Quantity and Quality with Satellite Remote Sensing: An Interdisciplinary Review. WIREs Water 2024, 11, e1725. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Kutser, T.; Hunter, P.D. Remote Sensing of Inland Waters: Challenges, Progress and Future Directions. Remote Sens. Environ. 2015, 157, 1–8. [Google Scholar] [CrossRef]
- Papenfus, M.; Schaeffer, B.; Pollard, A.I.; Loftin, K. Exploring the Potential Value of Satellite Remote Sensing to Monitor Chlorophyll-a for US Lakes and Reservoirs. Environ. Monit. Assess. 2020, 192, 808. [Google Scholar] [CrossRef]
- Bailey, S.W.; Werdell, P.J. A Multi-Sensor Approach for the on-Orbit Validation of Ocean Color Satellite Data Products. Remote Sens. Environ. 2006, 102, 12–23. [Google Scholar] [CrossRef]
- CEOS. Working Group on Calibration and Validation CEOS WGCV Terms of Reference Version 1.1 2022; CEOS: Geneva, Switzerland, 2022.
- EUMETSAT. Sentinel-3 Scientific Validation Team Implementation Plan; EUMETSAT: Darmstadt, Germany, 2015.
- EUMETSAT. Recommendations for Sentinel-3 OLCI Ocean Colour Product Validations in Comparison with In situ Measurements—Matchup Protocols; EUMETSAT: Darmstadt, Germany, 2021.
- Simis, S.; Stelzer, K.; Dagmar Müller; Selmes, N. Copernicus Global Land Operations “Cryosphere and Water” ”CGLOPS-2” Framework Service Contract N° 199496 (JRC) Quality Assessment Report Lake Water Quality 100M Products Version 1.1.0 2020; Copernicus: Brussels, Belgium, 2020. [Google Scholar]
- Doxani, G.; Vermote, E.; Roger, J.-C.; Gascon, F.; Adriaensen, S.; Frantz, D.; Hagolle, O.; Hollstein, A.; Kirches, G.; Li, F.; et al. Atmospheric Correction Inter-Comparison Exercise. Remote Sens. 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Zibordi, G.; Voss, K.J.; Johnson, B.C.; Mueller, J.L. Ocean Optics & Biogeochemistry Protocols for Satellite Ocean Colour Sensor Validation; IOCCG Protocol Series; IOCCG: Dartmouth, NS, Canada, 2019; Volume 3: Protocols for Satellite Ocean Colour Data Validation: In situ Optical Radiometry (v3.0). [Google Scholar]
- Ruddick, K.G.; Voss, K.; Boss, E.; Castagna, A.; Frouin, R.; Gilerson, A.; Hieronymi, M.; Johnson, B.C.; Kuusk, J.; Lee, Z.; et al. Review of Protocols for Fiducial Reference Measurements of Water-Leaving Radiance for Validation of Satellite Remote-Sensing Data over Water. Remote Sens. 2019, 11, 2198. [Google Scholar] [CrossRef]
- Lehmann, M.K.; Gurlin, D.; Pahlevan, N.; Alikas, K.; Anstee, J.; Balasubramanian, S.V.; Barbosa, C.C.F.; Binding, C.; Bracher, A.; Bresciani, M.; et al. GLORIA—A Globally Representative Hyperspectral in situ Dataset for Optical Sensing of Water Quality. Sci. Data 2023, 10, 100. [Google Scholar] [CrossRef]
- ISO 10260; Water Quality—Measurement of Biochemical Parameters—Spectrometric Determination of the Chlorophyll-a Concentration. ISO (International Standardization Organization): Geneva, Switzerland, 1992.
- Cao, Z.; Ma, R.; Duan, H.; Pahlevan, N.; Melack, J.; Shen, M.; Xue, K. A Machine Learning Approach to Estimate Chlorophyll-a from Landsat-8 Measurements in Inland Lakes. Remote Sens. Environ. 2020, 248, 111974. [Google Scholar] [CrossRef]
- Lymburner, L.; Botha, E.; Hestir, E.; Anstee, J.; Sagar, S.; Dekker, A.; Malthus, T. Landsat 8: Providing Continuity and Increased Precision for Measuring Multi-Decadal Time Series of Total Suspended Matter. Remote Sens. Environ. 2016, 185, 108–118. [Google Scholar] [CrossRef]
- Domínguez Gómez, J.A.; Alonso, C.A.; García, A.A. Remote Sensing as a Tool for Monitoring Water Quality Parameters for Mediterranean Lakes of European Union Water Framework Directive (WFD) and as a System of Surveillance of Cyanobacterial Harmful Algae Blooms (SCyanoHABs). Environ. Monit. Assess. 2011, 181, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.A.; Ortiz, J.; Bonini, N.; Shuman, M.; Sydow, C. Application of Aqua MODIS Sensor Data for Estimating Chlorophyll a in the Turbid Case 2 Waters of Lake Erie Using Bio-Optical Models. GIScience Remote Sens. 2016, 53, 483–505. [Google Scholar] [CrossRef]
- Odermatt, D.; Danne, O.; Philipson, P.; Brockmann, C. Diversity II Water Quality Parameters from ENVISAT (2002–2012): A New Global Information Source for Lakes. Earth Syst. Sci. Data 2018, 10, 1527–1549. [Google Scholar] [CrossRef]
- Havskum, H.; Schlüter, L.; Scharek, R.; Berdalet, E.; Jacquet, S. Routine Quantification of Phytoplankton Groups‹Microscopy or Pigment Analyses? Mar. Ecol. Prog. Ser. 2004, 273, 31–42. [Google Scholar] [CrossRef]
- Jacobsen, T.R.; Rai, H. Comparison of Spectrophotometric, Fluorometric and High Performance Liquid Chromatography Methods for Determination of Chlorophyll a in Aquatic Samples: Effects of Solvent and Extraction Procedures. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1990, 75, 207–217. [Google Scholar] [CrossRef]
- Marker, A.F.H. The Use of Acetone and Methanol in the Estimation of Chlorophyll in the Presence of Phaeophytin. Freshw. Biol. 1972, 2, 361–385. [Google Scholar] [CrossRef]
- Seegers, B.N.; Werdell, P.J.; Vandermeulen, R.A.; Salls, W.; Stumpf, R.P.; Schaeffer, B.A.; Owens, T.J.; Bailey, S.W.; Scott, J.P.; Loftin, K.A. Satellites for Long-Term Monitoring of Inland U.S. Lakes: The MERIS Time Series and Application for Chlorophyll-a. Remote Sens. Environ. 2021, 266, 112685. [Google Scholar] [CrossRef]
- Staatliche Betriebsgesellschaft für Umwelt und Landwirtschaft. Auswertung des LÜRV B11 “Chlorophyll Im Oberflächenwasser“. Available online: https://www.bful.sachsen.de/archiv-4400.html?_cp=%7B%22accordion-content-4591%22%3A%7B%222%22%3Atrue%7D%2C%22previousOpen%22%3A%7B%22group%22%3A%22accordion-content-4591%22%2C%22idx%22%3A2%7D%7D. 2020 (accessed on 15 April 2024).
- Niroumand-Jadidi, M.; Bovolo, F.; Bruzzone, L.; Gege, P. Inter-Comparison of Methods for Chlorophyll-a Retrieval: Sentinel-2 Time-Series Analysis in Italian Lakes. Remote Sens. 2021, 13, 2381. [Google Scholar] [CrossRef]
- Dörnhöfer, K.; Göritz, A.; Gege, P.; Pflug, B.; Oppelt, N. Water Constituents and Water Depth Retrieval from Sentinel-2A—A First Evaluation in an Oligotrophic Lake. Remote Sens. 2016, 8, 941. [Google Scholar] [CrossRef]
- Keith, D.J.; Milstead, B.; Walker, H.; Snook, H.; Szykman, J.J.; Wusk, M.; Kagey, L.; Howell, C.; Mellanson, C.; Drueke, C. Trophic Status, Ecological Condition, and Cyanobacteria Risk of New England Lakes and Ponds Based on Aircraft Remote Sensing. J. Appl. Remote Sens. 2012, 6, 063577. [Google Scholar] [CrossRef]
- Spyrakos, E.; O’Donnell, R.; Hunter, P.D.; Miller, C.; Scott, M.; Simis, S.G.H.; Neil, C.; Barbosa, C.C.F.; Binding, C.E.; Bradt, S.; et al. Optical Types of Inland and Coastal Waters. Limnol. Oceanogr. 2018, 63, 846–870. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Odermatt, D.; Hunter, P.D.; Brockmann, C.; Présing, M.; Balzter, H.; Tóth, V.R. Satellite Remote Sensing of Phytoplankton Phenology in Lake Balaton Using 10years of MERIS Observations. Remote Sens. Environ. 2015, 158, 441–452. [Google Scholar] [CrossRef]
- Matthews, M.W. Eutrophication and Cyanobacterial Blooms in South African Inland Waters: 10years of MERIS Observations. Remote Sens. Environ. 2014, 155, 161–177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).