New 3-D Fluorescence Spectral Indices for Multiple Pigment Inversions of Plant Leaves via 3-D Fluorescence Spectra
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Data
2.1.1. Experimental Materials
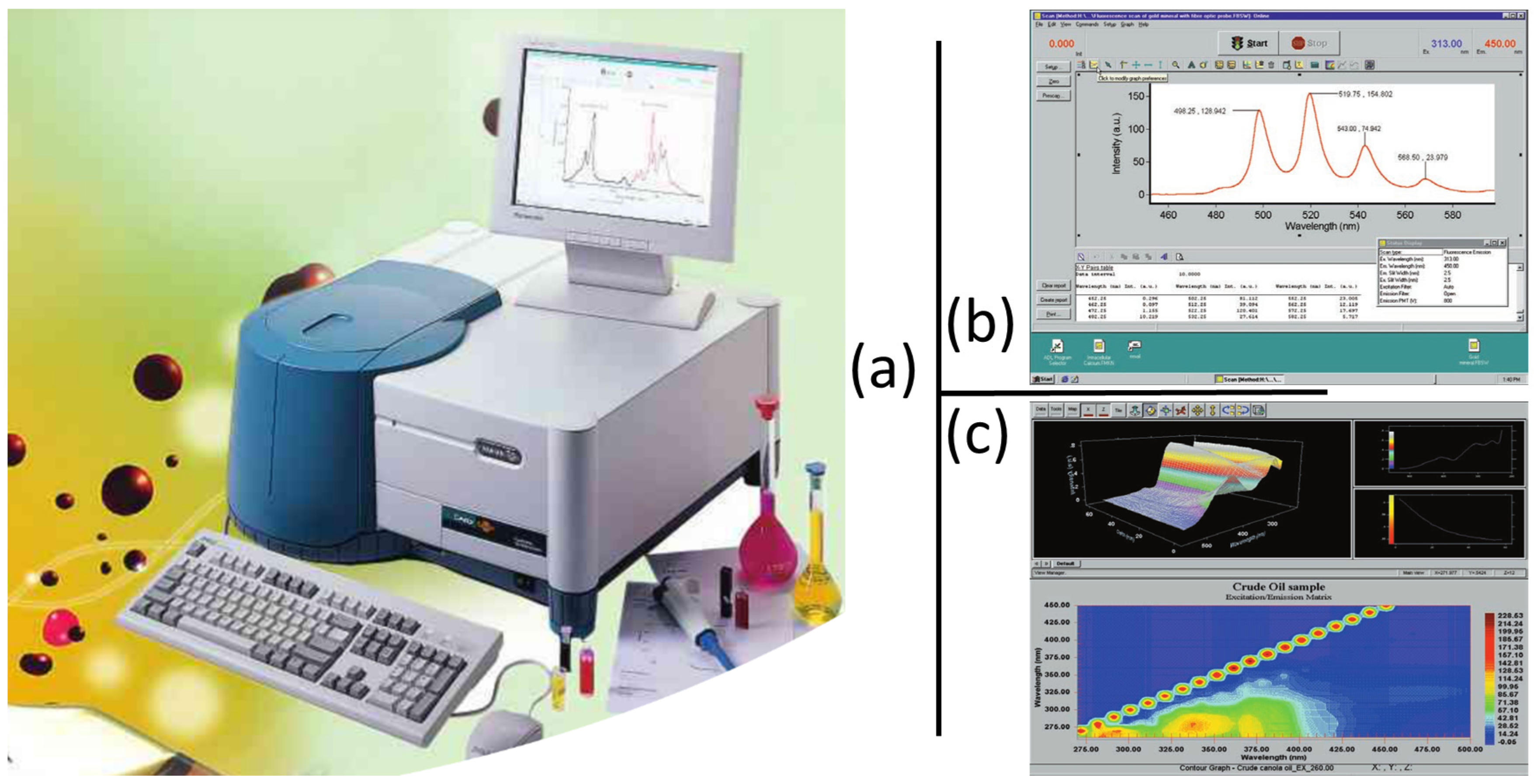
2.1.2. Leaf 3-D Fluorescence Spectrometry
2.1.3. Multiple Pigment Properties of Leaves
2.1.4. Auxiliary Data Acquisition
2.2. Methods
2.2.1. Research Technical Framework
2.2.2. New 3-D Fluorescence Spectral Index
2.2.3. Multiple Pigment Content Inversion Method for Plants via 3-D Fluorescence Spectrum
3. Results and Analysis
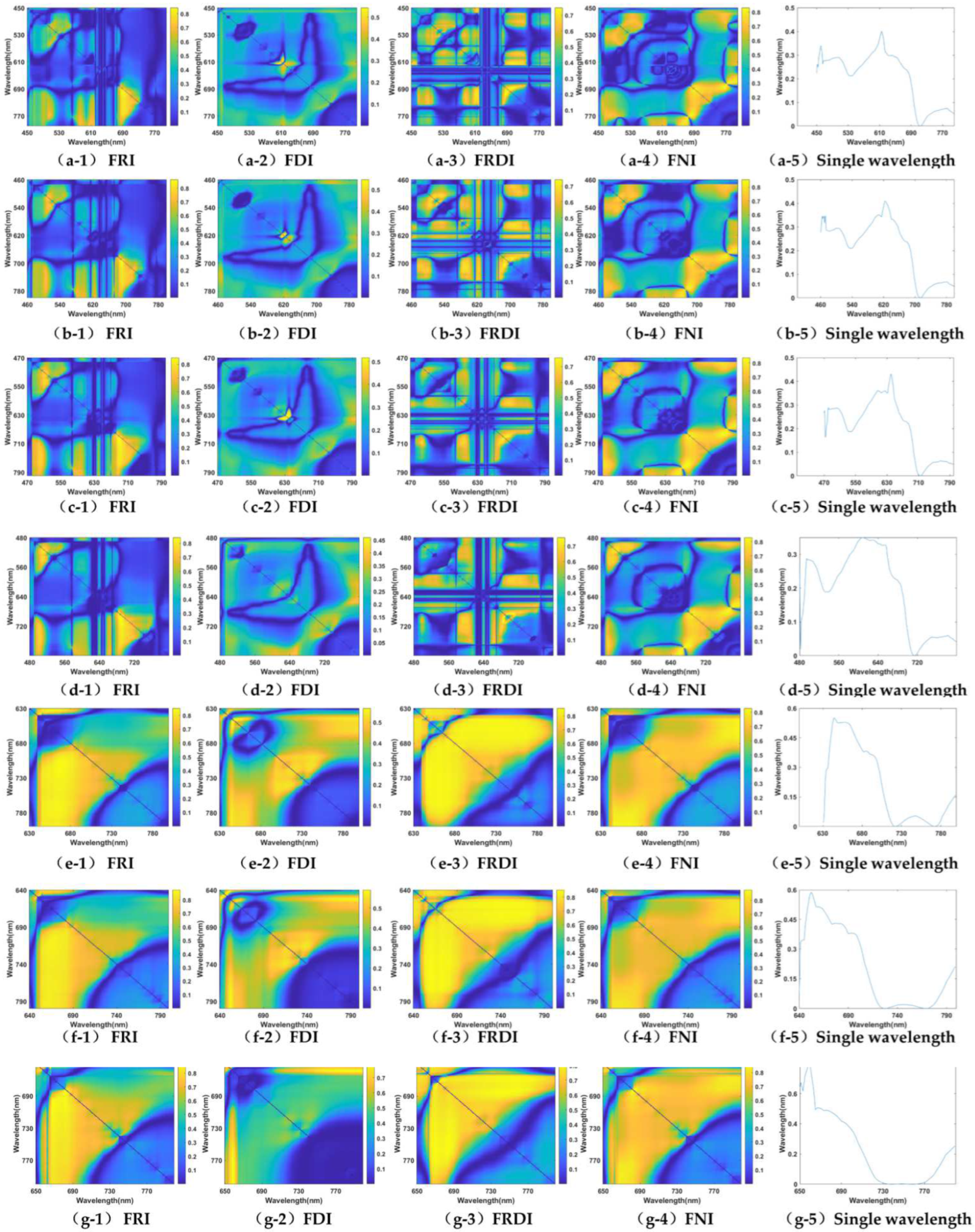
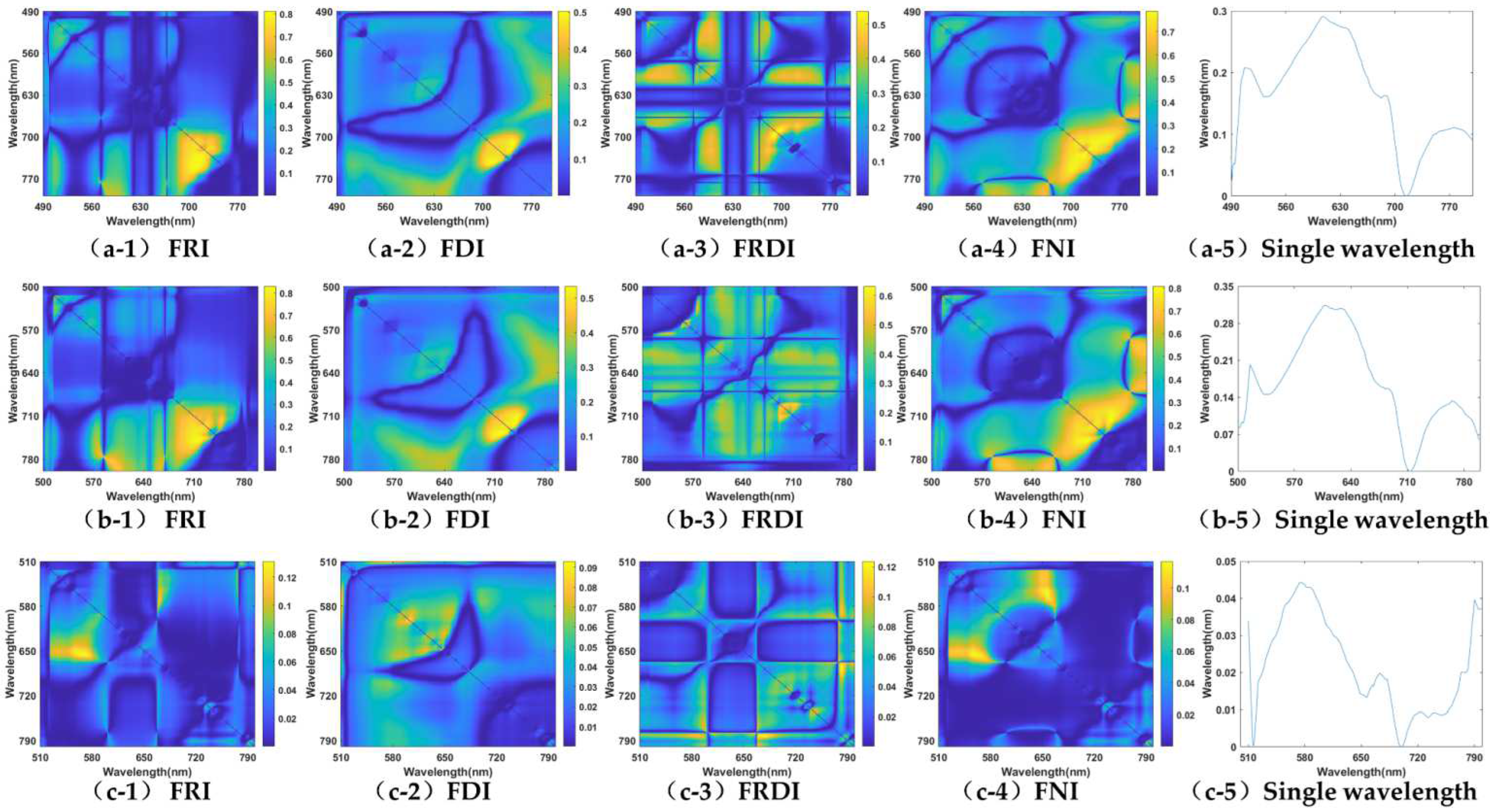
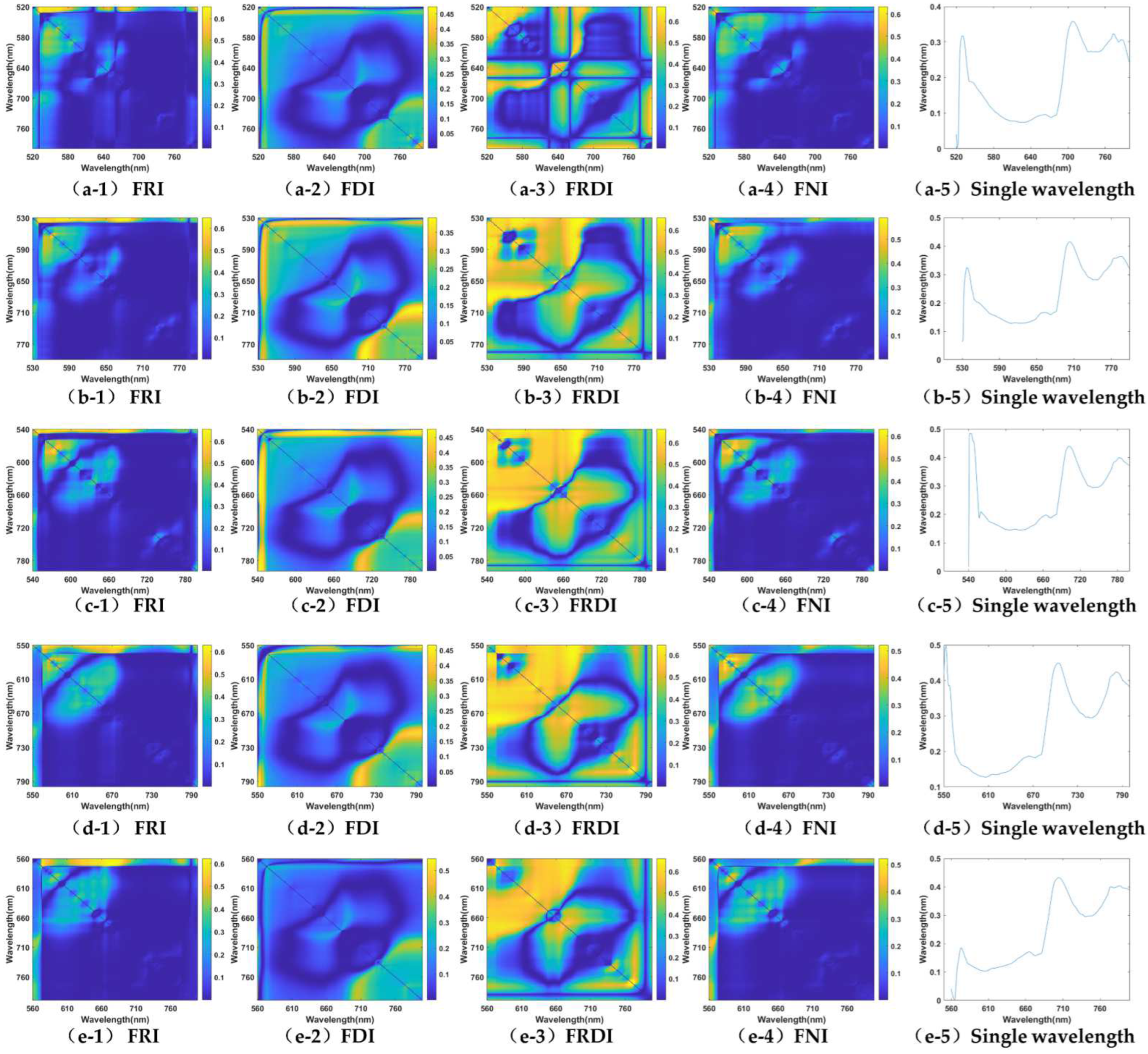
3.1. Band Sensitivity Analysis of 3-D Fluorescence Spectral Index
3.1.1. Chlorophyll a (Chla)
3.1.2. Chlorophyll b (Chlb)
3.1.3. Carotenoids (Cars)
3.1.4. Flavonoids (Flas)
3.1.5. Anthocyanin (Ants)
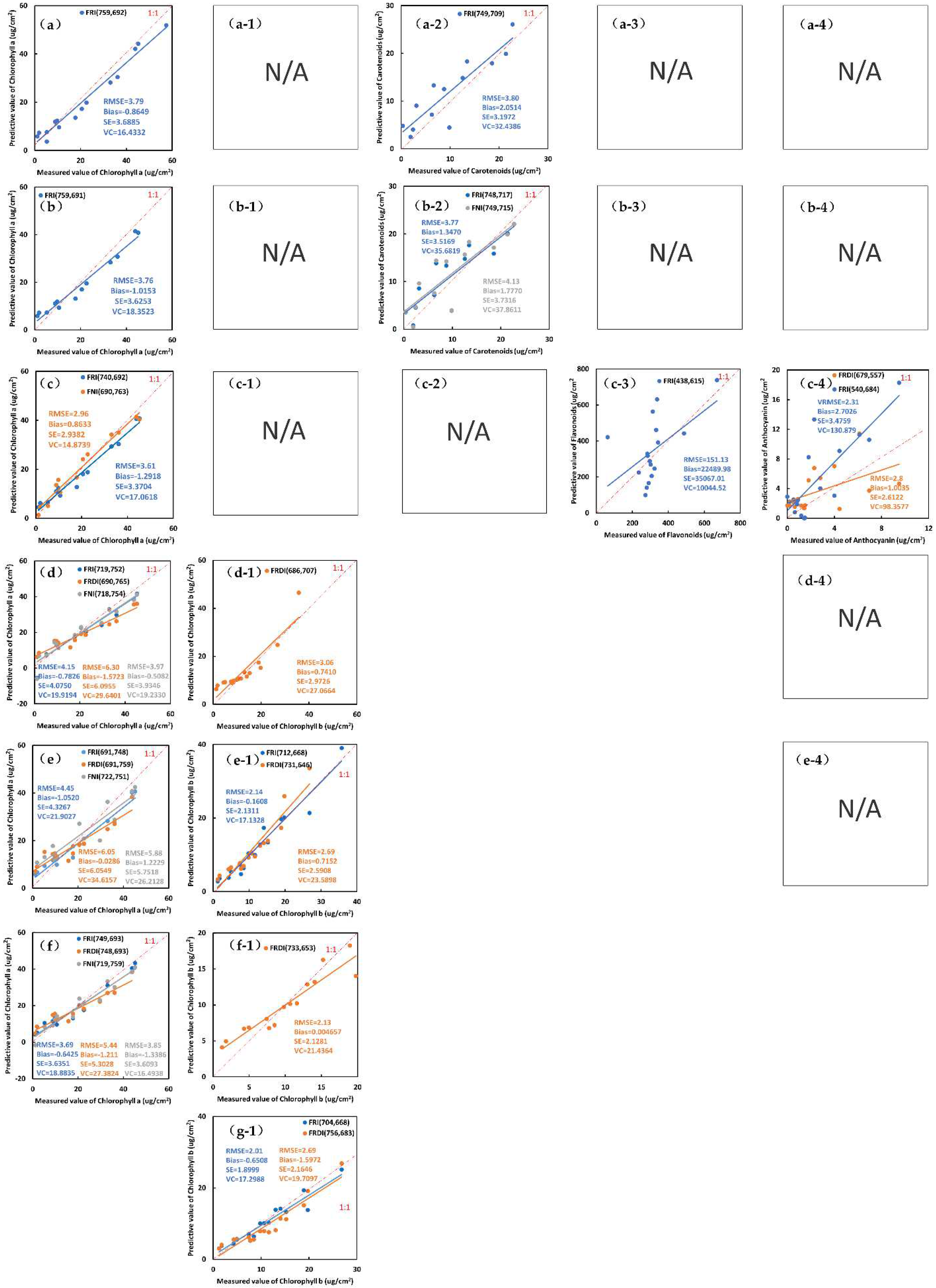
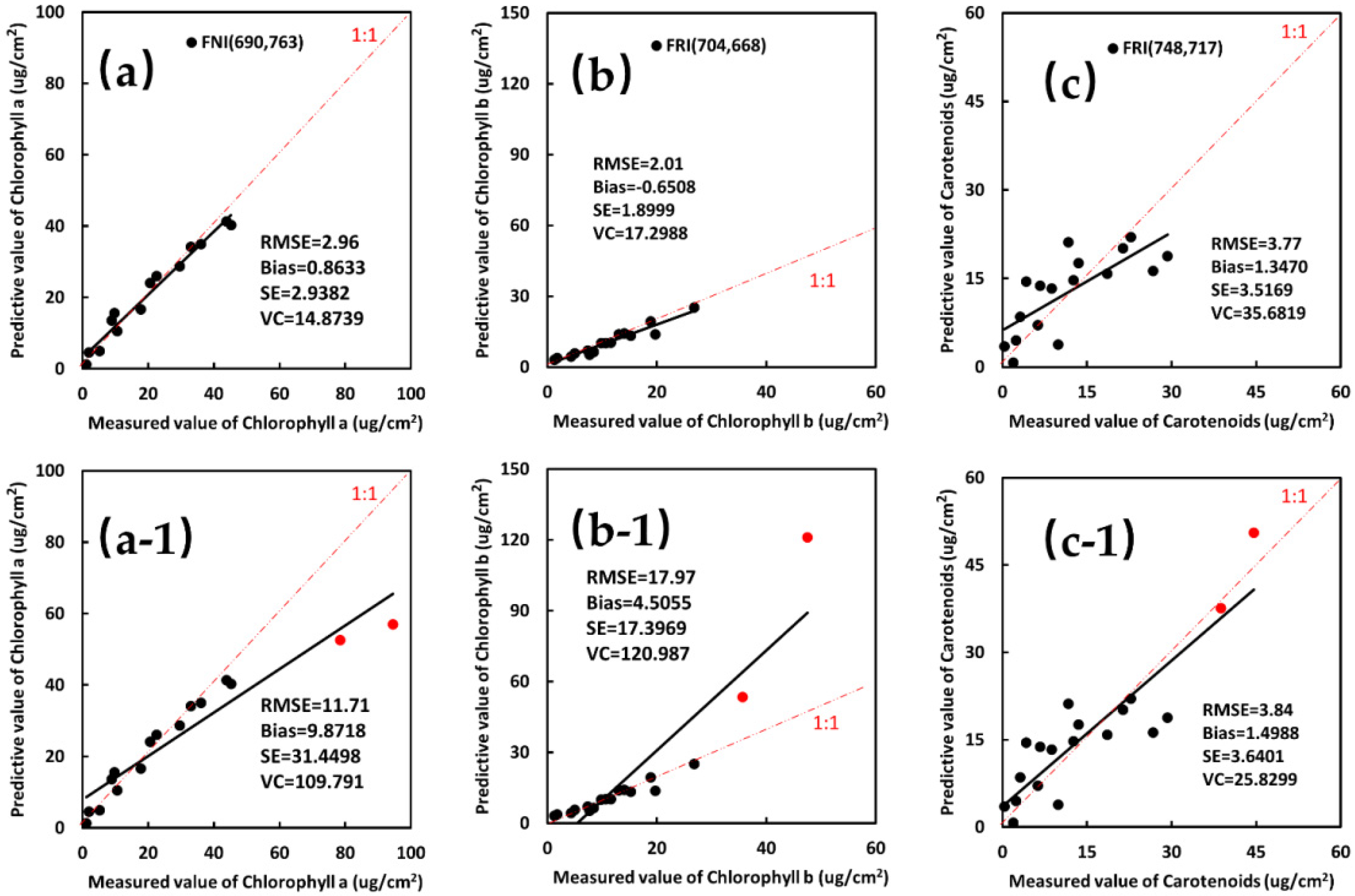
3.2. Modeling and Validation of Multiple Pigment Content Inversion Model of Leaves from 3-D FSIs
4. Discussion
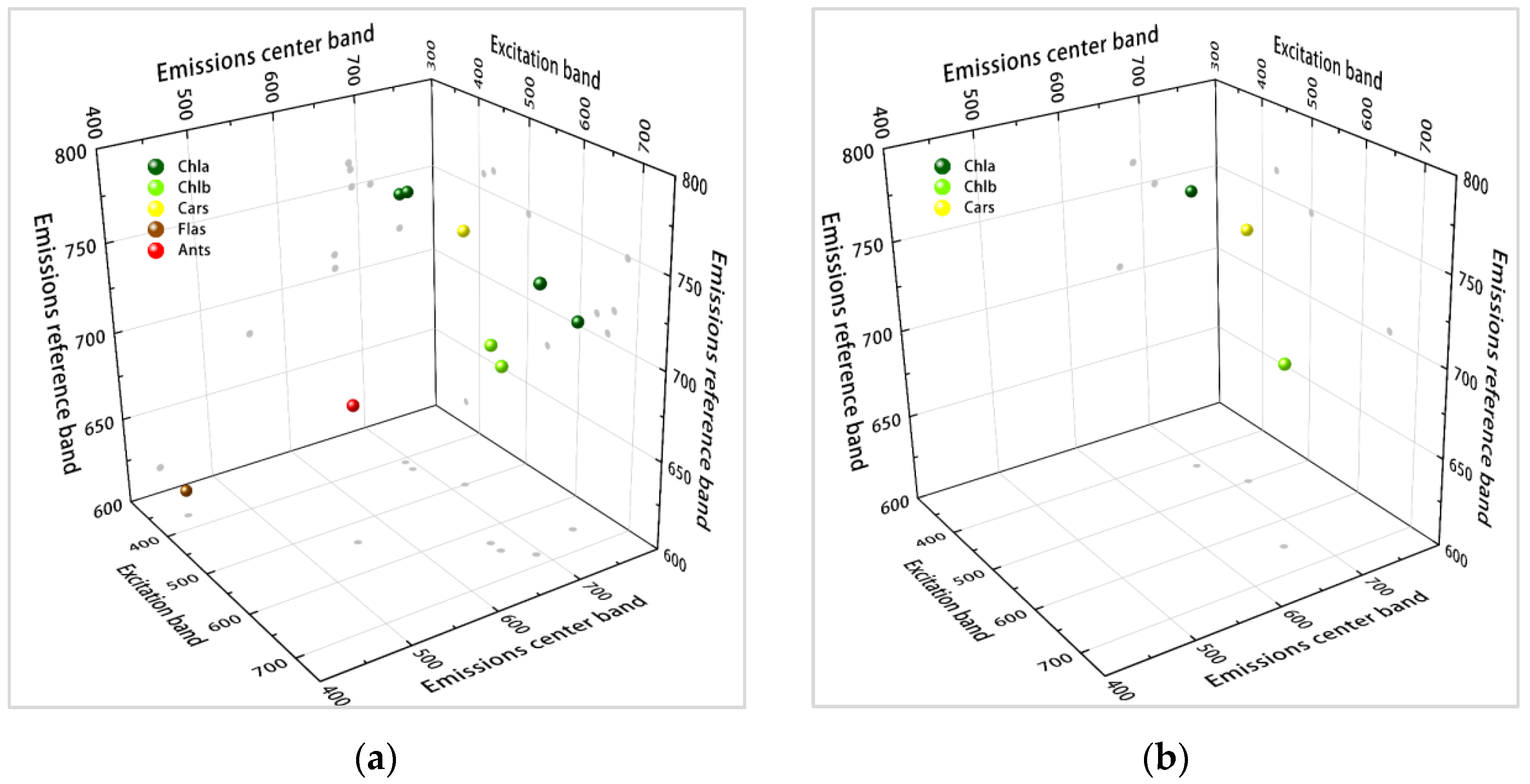
4.1. New 3-D Fluorescence Indices for Multiple Pigment Content Inversions
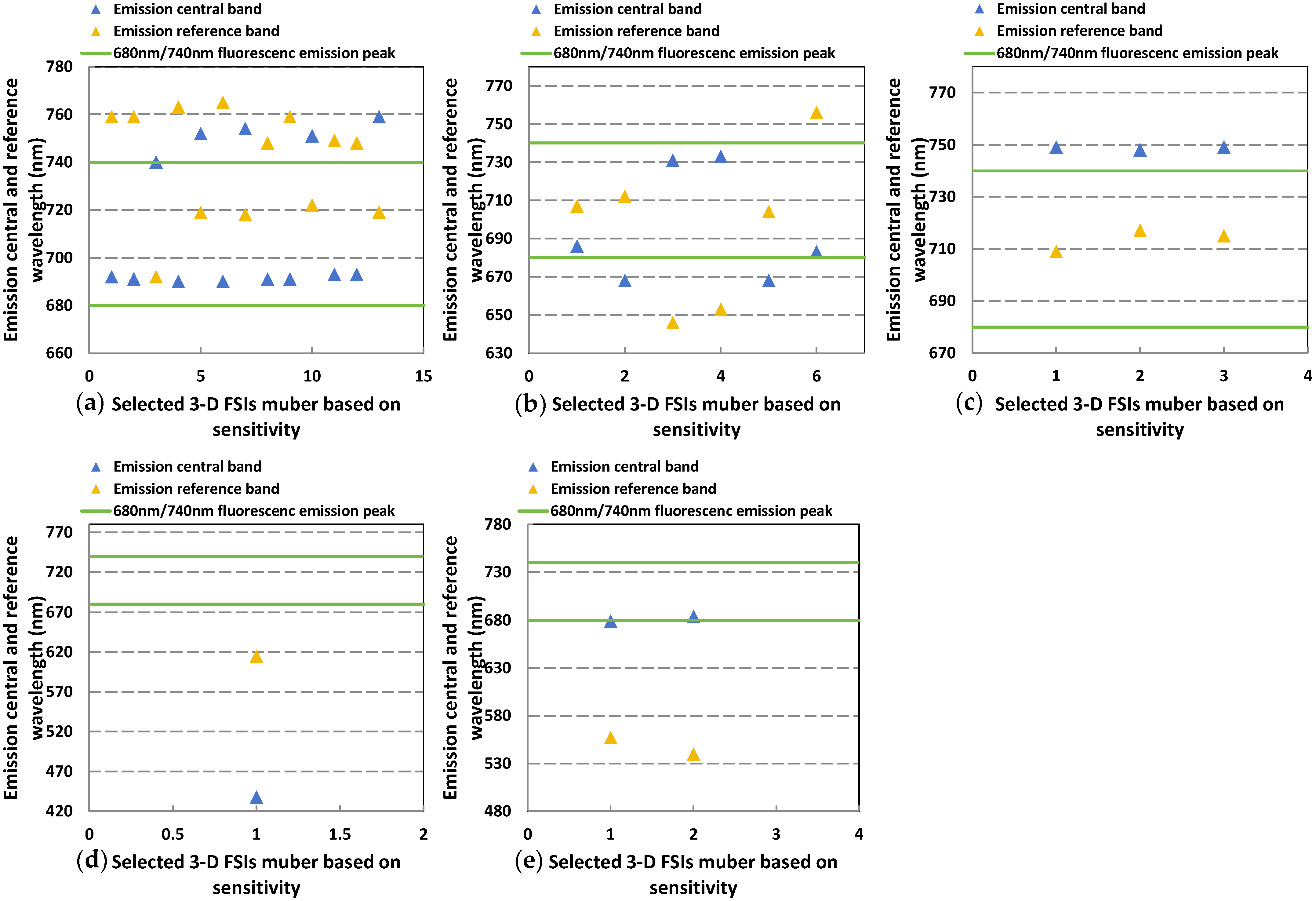
4.2. The 3-D Relationship between the Excitation Band of Excitation Fluorescence and the Central and Reference Bands of Emission Fluorescence in the New 3-D FSI
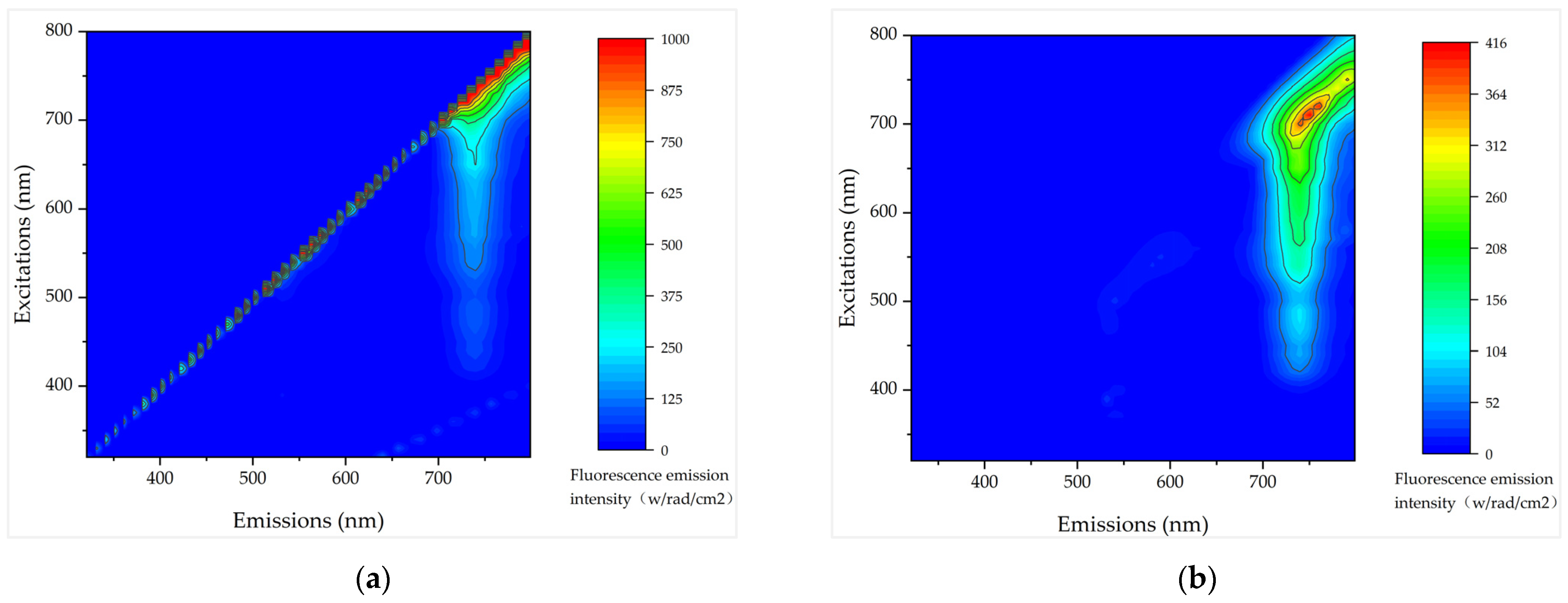
4.3. Fluorescent Oversaturation Characteristics of High Chlorophyll Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


Appendix B

References
- Emerson, R.; Arnold, W. The photochemical reaction in photosynthesis. J. Gen. Physiol. 1932, 16, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Zhu, W. The extraction and exploitation of natural pigment. J. Henan Univ. Ence Technol. 2003, 23, 68–74. [Google Scholar]
- Nishio, J.N. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2010, 23, 539–548. [Google Scholar] [CrossRef]
- Michel, K.P.; Pistorius, E.K. Adaptation of the photosynthetic electron transport chain in cyanobacteria to iron deficiency: The function of IdiA and IsiA. Physiol. Plant 2010, 120, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Van Der Tol, C.; Verhoef, W.; Rosema, A. A model for chlorophyll fluorescence and photosynthesis at leaf scale. Agric. For. Meteorol. 2009, 149, 96–105. [Google Scholar] [CrossRef]
- Zhang, M. Relationship Between Soluble Protein, Chlorophyll and ATP in Drought-Resistant Sweet Potato Under Water Stress. Agric. Sci. China 2002, 1, 1329–1333. [Google Scholar]
- Verhoeven, A.S.; Demmig-Adams, B.; Adams III, W.W. Enhanced Employment of the Xanthophyll Cycle and Thermal Energy Dissipation in Spinach Exposed to High Light and N Stress. Plant Physiol. 1997, 113, 817–824. [Google Scholar] [CrossRef]
- Tattini, M.; Landi, M.; Brunetti, C.; Giordano, C.; Remorini, D.; Gould, K.S.; Guidi, L. Epidermal coumaroyl anthocyanins protect sweet basil against excess light stress: Multiple consequences of light attenuation. Physiol. Plant. 2014, 152, 585–598. [Google Scholar] [CrossRef]
- He, D.; Singh, S.K.; Peng, L.; Kaushal, R.; Vílchez, J.I.; Shao, C.; Wu, X.; Zheng, S.; Morcillo, R.J.L.; Paré, P.W.; et al. Correction to: Flavonoid-attracted Aeromonas sp from the Arabidopsis root microbiome enhances plant dehydration resistance. ISME J. 2022, 16, 2622–2632. [Google Scholar] [CrossRef]
- Blackburn, G.A.; Steele, C.M. Towards the Remote Sensing of Matorral Vegetation Physiology: Relationships between Spectral Reflectance, Pigment, and Biophysical Characteristics of Semiarid Bushland Canopies. Remote Sens. Environ. 1999, 70, 278–292. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.; Kim, S.D. Damage Assessment of Rice Crop after Toluene Exposure Based on the Vegetation Index (VI) and UAV Multispectral Imagery. Remote Sens. 2020, 13, 25. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, P.; Kattawar, G.; Huang, H.-L.A.; Greenwald, T.; Li, J.; Baum, B.A.; Zhou, D.K.; Hu, Y. A fast infrared radiative transfer model based on the adding–doubling method for hyperspectral remote-sensing applications. J. Quant. Spectrosc. Radiat. Transf. 2007, 105, 243–263. [Google Scholar] [CrossRef]
- Mempel, T.R.; Moser, C.; Hutter, J.; Kuebler, W.M.; Krombach, F. Visualization of Leukocyte Transendothelial and Interstitial Migration Using Reflected Light Oblique Transillumination in Intravital Video Microscopy. J. Vasc. Res. 2003, 40, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, Z.; Wang, J.; Ding, J.; Yu, Y.; Li, J.; Xiao, N.; Jiang, L.; Zheng, Y.; Rimmington, G.M. Monitoring plant response to phenanthrene using the red edge of canopy hyperspectral reflectance. Mar. Pollut. Bull. 2014, 86, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Volpati, D.; Spada, E.R.; Plá Cid, C.C.; Sartorelli, M.L.; Aroca, R.F.; Constantino, C.J.L. Exploring copper nanostructures as highly uniform and reproducible substrates for plasmon-enhanced fluorescence. Analyst 2015, 140, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Li, A.; Xu, N.; Liu, T.; Duan, S. Characteristics of fluorescence emission spectrum at 77K of diatom and chrysophyta in Chromaphytae. Chin. J. Ecol. 2006, 25, 1033–1037. [Google Scholar] [CrossRef]
- Chen, D.; Gao, P.; Wu, X.; Gao, Y.; Wen, G.; Wang, Y.; Gao, R.; Zhang, R. Chloroplast Ultrastructure and Emission Fluorescence Spectrum Characteristics for Stems of Phyllostachys pubescens. Chin. Bull. Bot. 2013, 48, 635–642. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, S.; Zhu, Y.; Cui, C.; Pan, J. Application of Three-Dimensional Fluorescence Spectroscopy in Smart Agriculture—Detection of Oil Pollutants in Water. Int. J. Pattern Recognit. Artif. Intell. 2023, 37, 23550042. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, B.; Cai, G.; Shao, M.; Tian, P. Study on the Relationship between Fluorescence Spectra and Chlorophyll Content of fresh tea Leaves. Jiangsu Agric. Sci. 2012, 40, 270–272. [Google Scholar] [CrossRef]
- Ndao, A.S.; Konté, A.; Biaye, M.; Faye, M.E.; Faye, N.A.B.; Wagué, A. Analysis of Chlorophyll Fluorescence Spectra in Some Tropical Plants. J. Fluoresc. 2005, 15, 123–129. [Google Scholar] [CrossRef]
- Swarin, S.J.; Lipari, F. Determination of Formaldehyde and Other Aldehydes by High Performance Liquid Chromatography with Fluorescence Detection. J. Liq. Chromatogr. 2015, 6, 425–444. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef]
- Tubuxin, B.; Rahimzadeh-Bajgiran, P.; Ginnan, Y.; Hosoi, F.; Omasa, K. Estimating chlorophyll content and photochemical yield of photosystem II (ΦPSII) using solar-induced chlorophyll fluorescence measurements at different growing stages of attached leaves. J. Exp. Bot. 2015, 66, 5595–5603. [Google Scholar] [CrossRef]
- Yang, H.; Yu, H.; Zhang, L.; Sui, Y. Detecting of Chlorophyll Content of Cucumber Leaves Based on Laser-induced Fluorescence Spectrum Analysis Technique. Trans. Chin. Soc. Agric. Mach. 2009, 40, 169–172. [Google Scholar]
- Jiang, D.; Li, J.; Xu, J.; Zhang, L. Three-Dimensional Fluorescence Spectra of Dissolved Organic Matter in a Eutrophic River on Chongming Island. J. Hydroecol. 2019, 40, 33–40. [Google Scholar] [CrossRef]
- Suarez-Fernandez, M.; Marhuenda-Egea, F.C.; Lopez-Moya, F.; Arnao, M.B.; Lopez-Llorca, L.V. Chitosan induces plant hormones and defences in tomato root exudates. Front. Plant Sci. 2020, 11, 572087. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, S.; Wang, X.; Wang, L.; Zhu, C. Discrimination of phytoplankton classes using characteristic spectra of 3D fluorescence spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 361–369. [Google Scholar] [CrossRef]
- Yi, Y.; Zhou, Y.; Bridgeman, J.; Kong, W.; Yang, C.; Mao, H.; Wang, Z. Determination of Organic Pollutants in Municipal Sewage by Three-Dimensional Fluorescence Spectroscopy. Adm. Tech. Environ. Monit. 2017, 29, 37–39. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Y. Molecular Spectra of Dissolved Organic Matter Fractions from Corn Straw. J. Hebei Agric. Sci. 2023, 27, 66–74. [Google Scholar]
- Wang, J.; Liu, B.; Du, H.; Liang, Z.; Zhao, Y.; Zhao, Y.; Zhang, M.; Wang, L. New Method for Identification of Dalbergia Cochinchinensis Pierre and Dalbergia Oliveri Prain by Fluorescence Spectroscopy. Spectrosc. Spectr. Anal. 2019, 39, 2182–2189. [Google Scholar]
- Gonsamo, A.; Chen, J.M. Spectral Response Function Comparability Among 21 Satellite Sensors for Vegetation Monitoring. IEEE Trans. Geosci. Remote Sens. 2013, 51, 1319–1335. [Google Scholar] [CrossRef]
- Wang, F.; Huang, J.; Wang, X.; Cheng, L.; Tang, Y. Effects of Band Position and Bandwidth on NDVI Measurements of Rice at Different Growth Stages. J. Remote Sens. 2008, 12, 626–632. [Google Scholar]
- Bartold, M.; Kluczek, M. Estimating of chlorophyll fluorescence parameter Fv/Fm for plant stress detection at peatlands under Ramsar Convention with Sentinel-2 satellite imagery. Ecol. Inform. 2024, 81, 102603. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Huang, Y.; Reddy, K.N.; Zhao, Y.; Molin, W.T. Detection of the onset of glyphosate-induced soybean plant injury through chlorophyll fluorescence signal extraction and measurement. J. Appl. Remote Sens. 2015, 9, 097098. [Google Scholar] [CrossRef]
- Juskowiak, B. Nucleic acid-based fluorescent probes and their analytical potential. Anal. Bioanal. Chem. 2011, 399, 3157–3176. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Huang, J.; Wang, F.; Huang, R.; Lin, H.; Chen, F.; Wu, K. Exploring the Optical Properties of Leaf Photosynthetic and Photo-Protective Pigments In Vivo Based on the Separation of Spectral Overlapping. Remote Sens. 2020, 12, 3615. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, C.; Zhang, K.; Huang, J.; Wang, X. FOLIUM Model and Multi Pigment Leaf Spectral Simulation Method. CN Patent 201610629796.6, 4 January 2017. [Google Scholar]
- Zhang, Y. Hyperspectral Quantitative Remote Sensing Inversion Model and Regieme of Multiple Pigments at Leaf Scale Based on PROSPECT-PLUS Model. Ph.D Thesis, Zhejiang University, Hangzhou, China, 2015. [Google Scholar]
- Hu, J.; Tian, Q.; Lu, X. Relationship between changes of the pigments content and leaf color changing in Liquidambar formosana. J. North West A F Univ. 2007, 35, 219–223. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Y.; Wang, C.; Zheng, W. Uncertainty evaluation of the arsenic in the bush twigs and leaves by atomic fluorescence spectrometry. Strait Pharm. J. 2012, 24, 46–48. [Google Scholar] [CrossRef]
- Guan, Y.; Lin, B.; Ling, B. Interaction effects of light density and nitrogen on maize leaf pigments, chlorophyll fluorescence and energy transition. Plant Nutr. Fertil. Sci. 2000, 6, 152–158. [Google Scholar] [CrossRef]
- Richard Burns, G. Photosynthesis and the Absorption Spectra of Plant Pigments. II. Am. J. Bot. 1938, 24, 257–265. [Google Scholar] [CrossRef]
- Peters, R.D.; Noble, S.D. Spectrographic measurement of plant pigments from 300 to 800 nm. Remote Sens. Environ. 2014, 148, 119–123. [Google Scholar] [CrossRef]
- Zhang, R. Extraction of Fluorescence Characteristics of Photosynthetic Pigments in Plant Leaves and Development of Portable Net Photosynthetic Rate Detection Instrument. Ph.D Thesis, Hangzhou Dianzi University, Hangzhou, China, 2023. [Google Scholar]
- He, Y.; Liu, S.; Liu, Q.; Liu, Z.; Hu, X. Absorption, fluorescence and resonance Rayleigh scattering spectral characteristics of interaction of gold nanoparticle with safranine T. Sci. China 2005, 48, 216–226. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Zhu, J.; Zhang, C.; Zhang, G. Analysis of fluorescent and raman spectra with spatial resolution (depth analysis) method. J. Infrared Millim. Waves 2001, 20, 169–173. [Google Scholar] [CrossRef]
- Zavřel, T.; Sinetova, M.A.; Červený, J. Measurement of Chlorophyll a and Carotenoids Concentration in Cyanobacteria. Bio-Protocol 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Chen, J. A Drying Method for Guava Leaves. CN Patent 201611150134.7, 26 April 2017. [Google Scholar]
- Pekal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Lang, M.; Siffel, P.; Braunová, Z.; Lichtenthaler, H.K. Investigations of the Blue-green Fluorescence Emission of Plant Leaves. Bot. Acta 1992, 105, 435–440. [Google Scholar] [CrossRef]
- Li, M.M.; Chen, Y.T.; Ruan, J.C.; Wang, W.J.; Chen, J.G.; Zhang, Q.F. Structure-activity relationship of dietary flavonoids on pancreatic lipase. Curr. Res. Food Sci. 2023, 6, 100424. [Google Scholar] [CrossRef]
- Udenfriend, S.; Zaltzman, P. Fluorescence characteristics of purines, pyrimidines, and their derivatives: Measurement of guanine in nucleic acid hydrolyzates. Anal. Biochem. 1962, 3, 49–59. [Google Scholar] [CrossRef]
- Yin, H.; Chi, H.; Shang, Z.; Qaitoon, A.; Yu, J.; Meng, Q.; Zhang, Z.; Jia, H.; Zhang, R. Development of a new water-soluble fluorescence probe for hypochlorous acid detection in drinking water. Food Chem. Mol. Sci. 2021, 2, 100027. [Google Scholar] [CrossRef]
- Xu, P.; Chen, B.; Zhang, J.; Xue, L.; Zhu, L.; Qiu, B. Greedy compressive sensing and reconstruction of vegetation spectra for plant physiological and biochemical parameters inversion. Comput. Electron. Agric. 2018, 145, 379–388. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment: An Earth Resource Perspective, 2nd ed.; China Science PRESS: Beijing, China, 2000; pp. 355–408. [Google Scholar]
- Feret, J.-B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.R.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. Leaf chlorophyll fluorescence corrected for re-absorption by means of absorption and reflectance measurements. J. Plant Physiol. 1998, 152, 283–296. [Google Scholar] [CrossRef]
- Moore, D.S.; Craig, B.A.; McCabe, G.P. Introduction to the Practice of Statistics, 7th ed.; W. H. Freeman: New York, NY, USA, 2012; p. 401. [Google Scholar]
- Zhang, Y.; Zhao, C.; Liu, L.; Wang, J.; Ma, Z.; Wang, R. Preliminary study on the effects of water stress on maize leaf physiological status through passive chlorophyll fluorescence detection. Trans. Chin. Soc. Agric. Eng. 2006, 22, 39–43. [Google Scholar] [CrossRef]
- Cheng, T.; Song, R.; Li, D.; Zhou, K.; Zheng, H.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Spectroscopic Estimation of Biomass in Canopy Components of Paddy Rice Using Dry Matter and Chlorophyll Indices. Remote Sens. 2017, 9, 319. [Google Scholar] [CrossRef]
- Barnhart, I.; Rosso, L.H.M.; Secchi, M.A.; Ciampitti, I.A. Evaluating Sorghum Senescence Patterns Using Small Unmanned Aerial Vehicles and Multispectral Imaging. Kans. Agric. Exp. Stn. Res. Rep. 2019, 5. [Google Scholar] [CrossRef]
- Grishina, A.; Sherstneva, O.; Zhavoronkova, A.; Ageyeva, M.; Zdobnova, T.; Lysov, M.; Brilkina, A.; Vodeneev, V. Comparison of the Efficiency of Hyperspectral and Pulse Amplitude Modulation Imaging Methods in Pre-Symptomatic Virus Detection in Tobacco Plants. Plants 2023, 12, 3831. [Google Scholar] [CrossRef]
- Bojović, B.; Stojanović, J. Chlorophyll and carotenoid content in wheat cultivars as a function of mineral nutrition. Arch. Biol. Sci. 2005, 57, 283–290. [Google Scholar] [CrossRef]
- Deng, S.; Chen, D.; Huang, B.; Luo, Z.; Fan, H. Study on Fluorescence Photometric Determination of Flavonoids. Chem. World 1999, 40, 375–379. [Google Scholar]
- Drabent, R.; Pliszka, B.; Olszewska, T. Fluorescence properties of plant anthocyanin pigments. I. Fluorescence of anthocyanins in Brassica oleracea L. extracts. J. Photochem. Photobiol. B Biol. 1999, 50, 53–58. [Google Scholar] [CrossRef]
- Deng, S.; Chen, Q. Overview of vegetation spectral characteristics and vegetation indices. In Proceedings of the 17th China Remote Sensing Conference, Hangzhou, China, 27–31 August 2010. [Google Scholar]
- Yin, G.; Zhao, N.; Hu, L.; Yu, X.; Shi, C.; Xiao, X.; Li, F.; Duan, J.; Gan, T.; Zhang, Y.; et al. Classified Measurement of Phytoplankton Based on Characteristic Fluorescence of Photosynthetic Pigments. Acta Opt. Sin. 2014, 34, 9. [Google Scholar] [CrossRef]
- Yu, L.; Cai, H.; Yao, F.; Zheng, Z.; Wang, J.; Li, Z. Research on the applicability of vegetation index inversion for winter wheat vegetation coverage. Trans. Chin. Soc. Agric. Mach. 2015, 46, 231–249. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M. Signature Analysis of Leaf Reflectance Spectra: Algorithm Development for Remote Sensing of Chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]



| Species No. | Common Name | Species Name | Vegetation Type and Function | The Position of Selected Leaf in Vegetation Canopy | Leaf Sample | Leaf Life Cycle Stage | SPAD Range |
|---|---|---|---|---|---|---|---|
| 1 | Red Robin | Photiniaxfraseri | Evergreen shrub for soil improvement | The 3–5th leaf in the top of new shoots | 2/5 (color/green) | Y, M | 22.3–60.5 |
| 2 | Japan Arrow wood | Viburnum awabukiK.Koch | Evergreen shrub for garden ornament | The 4–6th leaf in the top of new shoots | 0/5 (color/green) | Y, M | 32.6–70.2 |
| 3 | Ginkgo | Ginkgo biloba | Deciduous tree for landscape ornament | The 3rd pair of whorled leaves from the top | 0/5 (color/green) | M, S | 3.8–41.8 |
| 4 | Sweet-scented osmanthus | OsmanthusFragrans | Evergreen tree for garden ornament | The 4–6th leaf in the top of new shoots | 0/5 (color/green) | Y, M | 15.1–51.5 |
| 5 | Mulberry | Morus Alba | Deciduous tree for cash crop | The 2–4th leaf in the top of new shoots | 0/4 (color/green) | Y, M | 13.7–52.5 |
| 6 | Moso Bamboo | Phyllostachysheterocycla (Carr.) | Evergreen tree for garden ornament | The 5–7th leaf in the top of new shoots | 0/4 (color/green) | Y, M, S | 12.3–52.3 |
| 7 | Decipiens | Elaeocarpussylvestris (Lour.) Poir | Deciduous tree for landscape ornament | The 4–6th leaf in the top of new shoots | 3/5 (color/green) | M, S | 1.5–61.0 |
| 8 | Pterostyrax | Pterostyrax corymbosus Sieb. et Zucc | Deciduous tree for garden ornament | Middle leaflet of 5–7th trifoliate compound leaf from the top | 0/5 (color/green) | Y, M, S | 4.3–44.0 |
| 9 | Sapindus | SapindusmukurossiGaertn | Deciduous tree for garden ornament | Middle leaflet of 3–5th pinnately compound leaves from the top | 0/5 (color/green) | M, S | 0.0–42.9 |
| 10 | Sugar Maple | Acer saccharum Marsh | Deciduous tree for landscape ornament | The 2–4th leaf in the top of new shoots | 0/6 (color/green) | M, S, A | 0.0–30.3 |
| 11 | Camphor Tree | Cinnamomumcamphora (L.) Presl. | Evergreen tree for landscape ornament | The 4–6th leaf in the top of new shoots | 0/5 (color/green) | M, S | 4.2–34.7 |
| 12 | Tea Tree | Camellia Sinensis (L.) | Evergreen shrub for cash crop | Choose one from the third pair of leaves from the top | 0/6 (color/green) | Y, M | 34.1–80.4 |
| Leaf Pigment | Maximum | Minimum | Average | Unit |
|---|---|---|---|---|
| Chla | 94.53 | 0.04 | 25.20 | μg/cm2 |
| Chlb | 47.49 | 0.05 | 12.96 | μg/cm2 |
| Cars | 44.55 | 0.24 | 13.32 | μg/cm2 |
| Ants | 47.22 | 0.01 | 4.37 | μg/cm2 |
| Flas | 1064.45 | 61.07 | 399.73 | μg/cm2 |
| Cars A | 44.55 | 0.24 | 16.09 | μg/cm2 |
| Lu | 17.71 | 0.02 | 4.76 | μg/cm2 |
| An | 1.83 | 0.00 | 0.37 | μg/cm2 |
| Ze | 6.99 | 0.02 | 1.06 | μg/cm2 |
| Vi | 4.10 | 0.00 | 0.95 | μg/cm2 |
| Ne | 7.43 | 0.00 | 1.85 | μg/cm2 |
| β-car | 15.33 | 0.02 | 4.10 | μg/cm2 |
| Water content | 73.83 | 11.61 | 52.34 | % |
| Pigment | Absorption Peak Number | Range of Absorption (nm) | Excitation Wavelength (nm) |
|---|---|---|---|
| Chlorophyll a | 1 | 400–434 | 410, 420, 430 |
| 2 | 659–699 | 660, 670, 680 | |
| Chlorophyll b | 1 | 442–495 | 450, 460, 470, 480 |
| 2 | 639–683 | 630, 640, 650 | |
| Carotenoids | 1 | 447–517 | 490, 500, 510 |
| Anthocyanin | 1 | 494–594 | 520, 530, 540, 550, 560 |
| Flavonoids | 1 | 200–285 | —— |
| 2 | 340–380 | 350, 360, 370 |
| Fluorescence Spectral Index | Abbreviation | Calculation Formula |
|---|---|---|
| Fluorescence difference index | FDI | |
| Fluorescence ratio index | FRI | |
| Fluorescence reciprocal difference index | FRDI | |
| Fluorescence normalization index | FNI |
| Excitation Wavelength | 3-D Fluorescence Spectral Index | Single Wavelength | |||
|---|---|---|---|---|---|
| Maximum Value of C2 from FRI | Maximum Value of C2 from FDI | Maximum Value of C2 from FRDI | Maximum Value of C2 from FNI | Maximum Value of C2 | |
| Fex,410nm | 0.8513 *** (Fem,759/Fem,692) | 0.3552 (Fem,607 − Fem,799) | 0.5576 (1/Fem,509 − 1/Fem,511) | 0.7746 * ((Fem,685 − Fem,777)/(Fem,685 + Fem,777)) | 0.2623 (Fem,607nm) |
| Fex,420nm | 0.8783 ** (Fem,759/Fem,691) | 0.5105 (Fem,422 − Fem,740) | 0.5661 (1/Fem,693 − 1/Fem,695) | 0.7974 * ((Fem,689 − Fem,762)/(Fem,689 + Fem,762)) | 0.4495 (Fem,422nm) |
| Fex,430nm | 0.8816 *** (Fem,740/Fem,692) | 0.5414 (Fem,431 − Fem,740) | 0.7076 (1/Fem,692 − 1/Fem,694) | 0.8251 *** ((Fem,690 − Fem,763)/(Fem,690 + Fem,763)) | 0.4700 (Fem,431nm) |
| Fex,660nm | 0.8315 ** (Fem,752/Fem,719) | 0.6970 (Fem,666 − Fem,748) | 0.8119 ** (1/Fem,690 − 1/Fem,765) | 0.8346 ** ((Fem,718 − Fem,754)/(Fem,718 + Fem,754)) | 0.5678 (Fem,667nm) |
| Fex,670nm | 0.8407 *** (Fem,748/Fem,691) | 0.7306 * (Fem,728 − Fem,738) | 0.8246 *** (1/Fem,759 − 1/Fem,691) | 0.8343 *** ((Fem,722 − Fem,751)/(Fem,722 + Fem,751)) | 0.5270 (Fem,675nm) |
| Fex,680nm | 0.8594 *** (Fem,749/Fem,693) | 0.7505 * (Fem,725 − Fem,736) | 0.8304 ** (1/Fem,748 − 1/Fem,693) | 0.8429 *** ((Fem,719 − Fem,759)/(Fem,719 + Fem,759)) | 0.5287 (Fem,680nm) |
| Excitation Wavelength | 3-D Fluorescence Spectral Index | Single Wavelength | |||
|---|---|---|---|---|---|
| Maximum Value of C2 from FRI | Maximum Value of C2 from FDI | Maximum Value of C2 from FRDI | Maximum Value of C2 from FNI | Maximum Value of C2 | |
| Fex450nm | 0.8420 *** (Fem,701/Fem,691) | 0.5426 (Fem,603 − Fem,615) | 0.7475 ** (1/Fem,687 − 1/Fem,702) | 0.8302 *** ((Fem,692 − Fem,690)/(Fem,692 + Fem,690)) | 0.4006 (Fem,615nm) |
| Fex,460nm | 0.8623 *** (Fem,698/Fem,691) | 0.5463 (Fem,622 − Fem,619) | 0.7409 ** (1/Fem,686 − 1/Fem,705) | 0.8540 *** ((Fem,692 − Fem,690)/(Fem,692 + Fem,690)) | 0.4096 (Fem,624nm) |
| Fex,470nm | 0.8473 *** (Fem,701/Fem,691) | 0.5174 (Fem,628 − Fem,641) | 0.7575 ** (1/Fem,694 − 1/Fem,693) | 0.8380 ** ((Fem,691 − Fem,690)/(Fem,691 + Fem,690)) | 0.4314 (Fem,640nm) |
| Fex,480nm | 0.8408 *** (Fem,692/Fem,689) | 0.4596 (Fem,480 − Fem,749) | 0.7537 ** (1/Fem,686 − 1/Fem,707) | 0.8298 *** ((Fem,692 − Fem,689)/(Fem,692 + Fem,689)) | 0.3499 (Fem,607nm) |
| Fex,630nm | 0.8510 *** (Fem,712/Fem,668) | 0.5677 (Fem,651 − Fem,645) | 0.8563 *** (1/Fem,731 − 1/Fem,646) | 0.8289 *** ((Fem,643 − Fem,639)/(Fem,643 + Fem,639)) | 0.5513 (Fem,644nm) |
| Fex,640nm | 0.8741 *** (Fem,650/Fem,648) | 0.5912 (Fem,650 − Fem,643) | 0.8528 ** (1/Fem,733 − 1/Fem,653) | 0.8784 *** ((Fem,650 − Fem,648)/(Fem,650 + Fem,648)) | 0.5872 (Fem,652nm) |
| Fex,650nm | 0.8696 *** (Fem,704/Fem,668) | 0.7933 * (Fem,779 − Fem,659) | 0.8135 ** (1/Fem,756 − 1/Fem,683) | 0.8649 *** ((Fem,683 − Fem,682)/(Fem,683 + Fem,682)) | 0.7971 (Fem,659nm) |
| Excitation Wavelength | 3-D Fluorescence Spectral Index | Single Wavelength | |||
|---|---|---|---|---|---|
| Maximum Value of C2 from FRI | Maximum Value of C2 from FDI | Maximum Value of C2 from FRDI | Maximum Value of C2 from FNI | Maximum Value of C2 | |
| Fex,490nm | 0.8122 ** (Fem,749/Fem,709) | 0.5029 * (Fem,743 − Fem,713) | 0.5390 * (1/Fem,696 − 1/Fem,691) | 0.7874 * ((Fem,730 − Fem,720)/(Fem,730 + Fem,720)) | 0.2915 (Fem,607nm) |
| Fex,500nm | 0.8302 ** (Fem,748/Fem,717) | 0.5339 * (Fem,737 − Fem,721) | 0.6315 * (1/Fem,565 − 1/Fem,555) | 0.8070 ** ((Fem,749 − Fem,715)/(Fem,749 + Fem,715)) | 0.3136 (Fem,607nm) |
| Fex,510nm | 0.1237 (Fem,782/Fem,677) | 0.0931 (Fem,648 − Fem,647) | 0.1237 * (1/Fem,782 − 1/Fem,677) | 0.1169 ((Fem,656 − Fem,557)/(Fem,656 + Fem,557)) | 0.0443 (Fem,574nm) |
| Excitation Wavelength | 3-D Fluorescence Spectral Index | Single Wavelength | |||
|---|---|---|---|---|---|
| Maximum Value of C2 from FRI | Maximum Value of C2 from FDI | Maximum Value of C2 from FRDI | Maximum Value of C2 from FNI | Maximum Value of C2 | |
| Fex,350nm | 0.3736 * (Fem,671/Fem,526) | 0.4967 (Fem,566 − Fem,644) | 0.4632 (1/Fem,523 − 1/Fem,545) | 0.3896 * ((Fem,523 − Fem,541)/(Fem,523 + Fem,541)) | 0.0917 (Fem,523nm) |
| Fex,360nm | 0.4629 * (Fem,392/Fem,521) | 0.4961 * (Fem,581 − Fem,615) | 0.3567 (1/Fem,522 − 1/Fem,770) | 0.3598 ((Fem,551 − Fem,393)/(Fem,551 + Fem,393)) | 0.0863 (Fem,648nm) |
| Fex,370nm | 0.5438 ** (Fem,615/Fem,438) | 0.4966 * (Fem,587 − Fem,603) | 0.5032 * (1/Fem,580 − 1/Fem,370) | 0.4951 * ((Fem,465 − Fem,614)/(Fem,465 + Fem,614)) | 0.4266 (Fem,532nm) |
| Excitation Wavelength | 3-D Fluorescence Spectral Index | Single Wavelength | |||
|---|---|---|---|---|---|
| Maximum Value of R2 from FRI | Maximum Value of R2 from FDI | Maximum Value of R2 from FRDI | Maximum Value of R2 from FNI | Maximum Value of C2 | |
| Fex520nm | 0.6560 ** (Fem,520/Fem,531) | 0.4743 * (Fem,787 − Fem,520) | 0.6703 ** (1/Fem,533 − 1/Fem,532) | 0.6329 ** ((Fem,533 − Fem,531)/(Fem,533 + Fem,531)) | 0.3579 (Fem,708nm) |
| Fex,530nm | 0.6494 * (Fem,531/Fem,677) | 0.3903 * (Fem,799 − Fem,704) | 0.6577 ** (1/Fem,547 − 1/Fem,546) | 0.5897 * ((Fem,540 − Fem,539)/(Fem,540 + Fem,539)) | 0.4147 (Fem,703nm) |
| Fex,540nm | 0.6578 ** (Fem,540/Fem,684) | 0.4781 * (Fem,543 − Fem,542) | 0.6640 *** (1/Fem,679 − 1/Fem,557) | 0.6320 * ((Fem,548 − Fem,547)/(Fem,548 + Fem,547)) | 0.4852 (Fem,542nm) |
| Fex,550nm | 0.6284 * (Fem,550/Fem,682) | 0.4670 * (Fem,551 − Fem,550) | 0.6458 * (1/Fem,682 − 1/Fem,565) | 0.5218 * ((Fem,579 − Fem,573)/(Fem,579 + Fem,573)) | 0.4979 (Fem,553nm) |
| Fex,560nm | 0.6221 * (Fem,569/Fem,676) | 0.5462 * (Fem,798 − Fem,560) | 0.6615 ** (1/Fem,772 − 1/Fem,770) | 0.5231 * ((Fem,574 − Fem,573)/(Fem,574 + Fem,573)) | 0.4322 (Fem,705nm) |
| Pigment | Excitation Band (nm) | 3-D FSI | Modeling (n = 40) | Validating (m = 18) | ||||
|---|---|---|---|---|---|---|---|---|
| Linear Function | R2 | RMSE (µg/cm2) | Bias (µg/cm2) | SEC (µg/cm2) | VC (%) | |||
| Cha | Fex,410 | FRI(Fem,759/Fem,692) | y = 14.192x + 3.8228 | 0.8513 *** | 3.79 *** | −0.8649 ** | 3.6885 *** | 16.4332 *** |
| Fex,420 | FRI(Fem,759/Fem,691) | y = 13.293x + 3.8864 | 0.8783 *** | 3.76 *** | −1.0153 * | 3.6253 *** | 18.3523 *** | |
| Fex,430 | FRI(Fem,740/Fem,692) | y = 7.9658x + 2.2746 | 0.8816 *** | 3.61 *** | −1.2918 * | 3.3704 *** | 17.0618 *** | |
| FNI((Fem,690 − Fem,763)/(Fem,690 + Fem,763)) | y = −32.308x + 26.707 | 0.8251 ** | 2.96 *** | 0.8633 *** | 2.9382 *** | 14.8739 *** | ||
| Fex,660 | FRI(Fem,752/Fem,719) FRDI((1/Fem,690 − 1/Fem,765)) FNI((Fem,718 − Fem,754)/(Fem,718 + Fem,754)) | y = 131.47x − 66.499 y = 1928.3x + 23.275 y = −176.25x + 63.736 | 0.8315 ** 0.8119 * 0.8346 ** | 4.15 * 6.30 * 3.97 *** | −0.7826 ** −1.5723 * −0.5082 ** | 4.0750 ** 6.0955 * 3.9346 *** | 19.9194 *** 29.6401 * 19.2330 *** | |
| Fex,670 | FRI(Fem,748/Fem,691) FRDI(1/Fem,759 − 1/Fem,691) FNI((Fem,751 − Fem,722)/(Fem,751 + Fem,722)) | y = 10.641x + 3.5315 y = −2146x + 19.379 y = 242.53x + 72.021 | 0.8407 ** 0.8246 * 0.8343 * | 4.45 ** 6.05 * 5.88 * | −1.0520 * −0.0286 *** 1.2229 * | 4.3267 ** 6.0549 * 5.7518 * | 21.9027 ** 34.6157 * 26.2128 ** | |
| Fex,680 | FRI(Fem,749/Fem693) FRDI(1/Fem,748 − 1/Fem,693) | y = 18.453x − 0.1245 y = −3669.1x + 18.569 | 0.8594 ** 0.8304 * | 3.69 *** 5.44 * | −0.6425 ** −1.211 * | 3.6351 *** 5.3028 * | 18.8835 *** 27.3824 ** | |
| FNI((Fem,719 − Fem,759)/(Fem,719 + Fem,759)) | y = −212.81x + 100.3 | 0.8429 * | 3.85 *** | −1.3386 * | 3.6093 *** | 16.4938 *** | ||
| Chlb | Fex,480 | FRDI(1/Fem,686 − 1/Fem,707) | y = 117.74x + 9.4286 | 0.7537 * | 3.06 *** | 0.7410 ** | 2.9726 * | 27.0664 ** |
| Fex,630 | FRI(Fem,712/Fem,668) | y = 2.7284x + 1.8016 | 0.8510 ** | 2.14 *** | −0.1608 *** | 2.1311 *** | 17.1328 *** | |
| FRDI((1/Fem,731) − (1/Fem,646)) | y = −513.65x + 7.0899 | 0.8563 ** | 2.69 *** | 0.7152 ** | 2.5908 * | 23.5898 ** | ||
| Fex,640 | FRDI((1/Fem,733) − (1/Fem,653)) | y = −621.23x + 7.7039 | 0.8528 ** | 2.13 *** | 0.004657 *** | 2.1281 * | 21.4364 ** | |
| Fex,650 | FRI(Fem,704/Fem,668) FRDI((1/Fem,756) − (1/Fem,683)) | y = 2.5455x + 2.199 y = −571.93x + 9.2498 | 0.8696 ** 0.8135 * | 2.01 *** 2.69 * | −0.6508 ** −1.5972 * | 1.8999 ** 2.1646 * | 17.2988 *** 19.7097 * | |
| Cars | Fex,490 | FRI(Fem,749/Fem,709) | y = 25.056x − 11.295 | 0.8122 ** | 3.80 *** | 2.0514 * | 3.1972 *** | 32.4386 ** |
| Fex,500 | FRI(Fem,748/Fem,717) | y = 47.655x − 28.052 | 0.8302 ** | 3.77 *** | 1.3470 ** | 3.5169 ** | 35.6819 ** | |
| FNI((Fem,749 − Fem,715)/(Fem,749 + Fem,715)) | y = 69.5x + 19.264 | 0.8070 * | 4.13 ** | 1.7770 * | 3.7316 * | 37.8611 * | ||
| Flas | Fex,370 | FRI(Fem,615/Fem,438) | y = 302.29x + 264.68 | 0.5438 | 151.13 | 22,489.98 | 35,067.01 | 10,044.52 |
| Ants | Fex,540 | FRI(Fem,540/Fem,684) FRDI(1/Fem,679 − 1/Fem,557) | y = 0.0837x + 0.8159 y = 96.778x + 0.7982 | 0.6578 ** 0.6640 ** | 2.31 *** 2.8 ** | 2.7026 * 1.0035 ** | 3.4759 * 2.6122 ** | 130.879 * 98.3577 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Zhang, Y.; Wang, J.; Zhang, R.; Wu, W.; He, Y.; Wu, X.; Sun, W.; Li, D.; Xiao, Y.; et al. New 3-D Fluorescence Spectral Indices for Multiple Pigment Inversions of Plant Leaves via 3-D Fluorescence Spectra. Remote Sens. 2024, 16, 1885. https://doi.org/10.3390/rs16111885
Tian S, Zhang Y, Wang J, Zhang R, Wu W, He Y, Wu X, Sun W, Li D, Xiao Y, et al. New 3-D Fluorescence Spectral Indices for Multiple Pigment Inversions of Plant Leaves via 3-D Fluorescence Spectra. Remote Sensing. 2024; 16(11):1885. https://doi.org/10.3390/rs16111885
Chicago/Turabian StyleTian, Shoupeng, Yao Zhang, Jiaoru Wang, Rongxu Zhang, Weizhi Wu, Yadong He, Xiaobin Wu, Wei Sun, Dong Li, Yixin Xiao, and et al. 2024. "New 3-D Fluorescence Spectral Indices for Multiple Pigment Inversions of Plant Leaves via 3-D Fluorescence Spectra" Remote Sensing 16, no. 11: 1885. https://doi.org/10.3390/rs16111885
APA StyleTian, S., Zhang, Y., Wang, J., Zhang, R., Wu, W., He, Y., Wu, X., Sun, W., Li, D., Xiao, Y., & Wang, F. (2024). New 3-D Fluorescence Spectral Indices for Multiple Pigment Inversions of Plant Leaves via 3-D Fluorescence Spectra. Remote Sensing, 16(11), 1885. https://doi.org/10.3390/rs16111885







