Optimized Transfer Learning for Chlorophyll Content Estimations across Datasets of Different Species Using Sun-Induced Chlorophyll Fluorescence and Reflectance
Abstract
1. Introduction
2. Materials and Methods
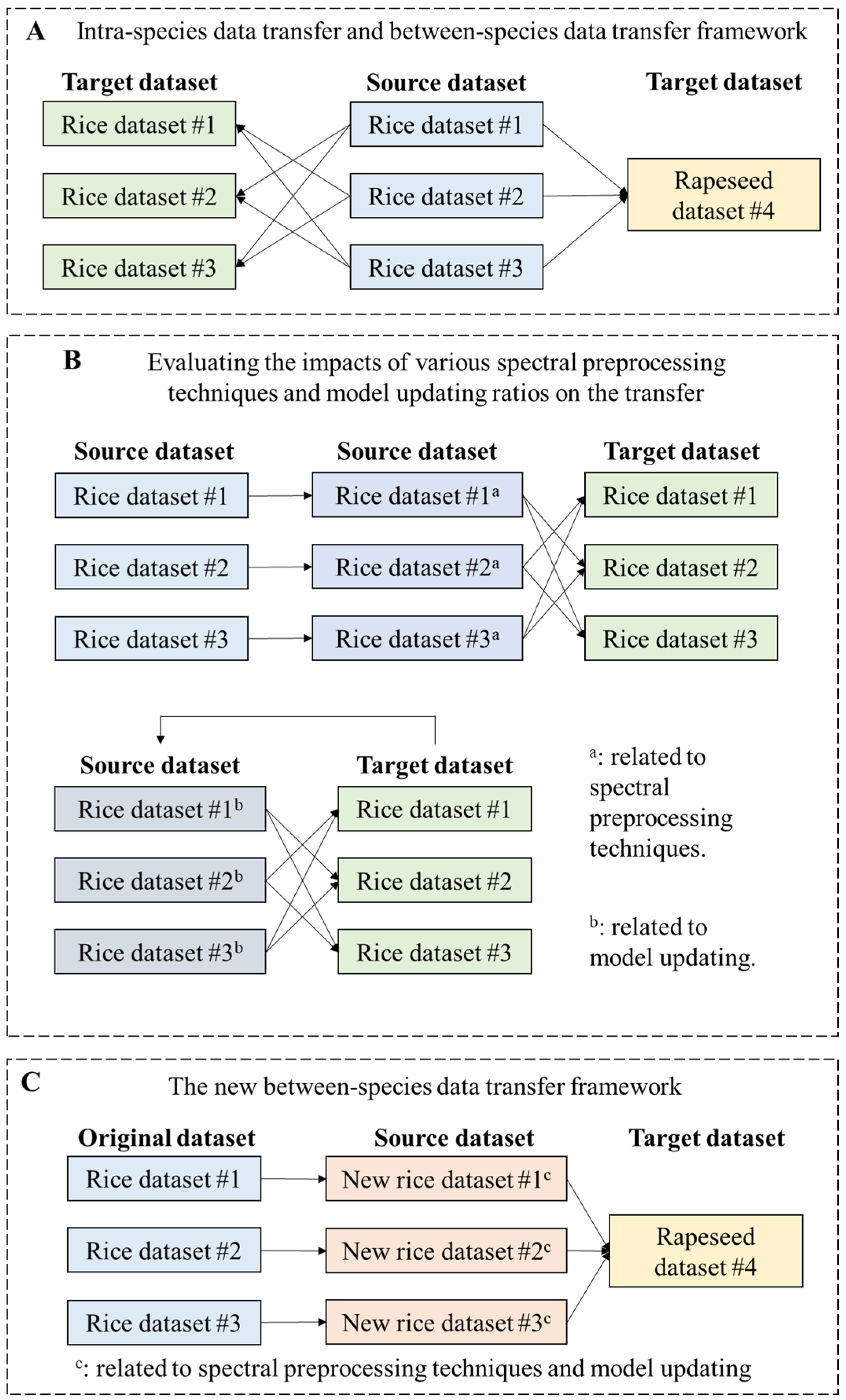
2.1. Experimental Design
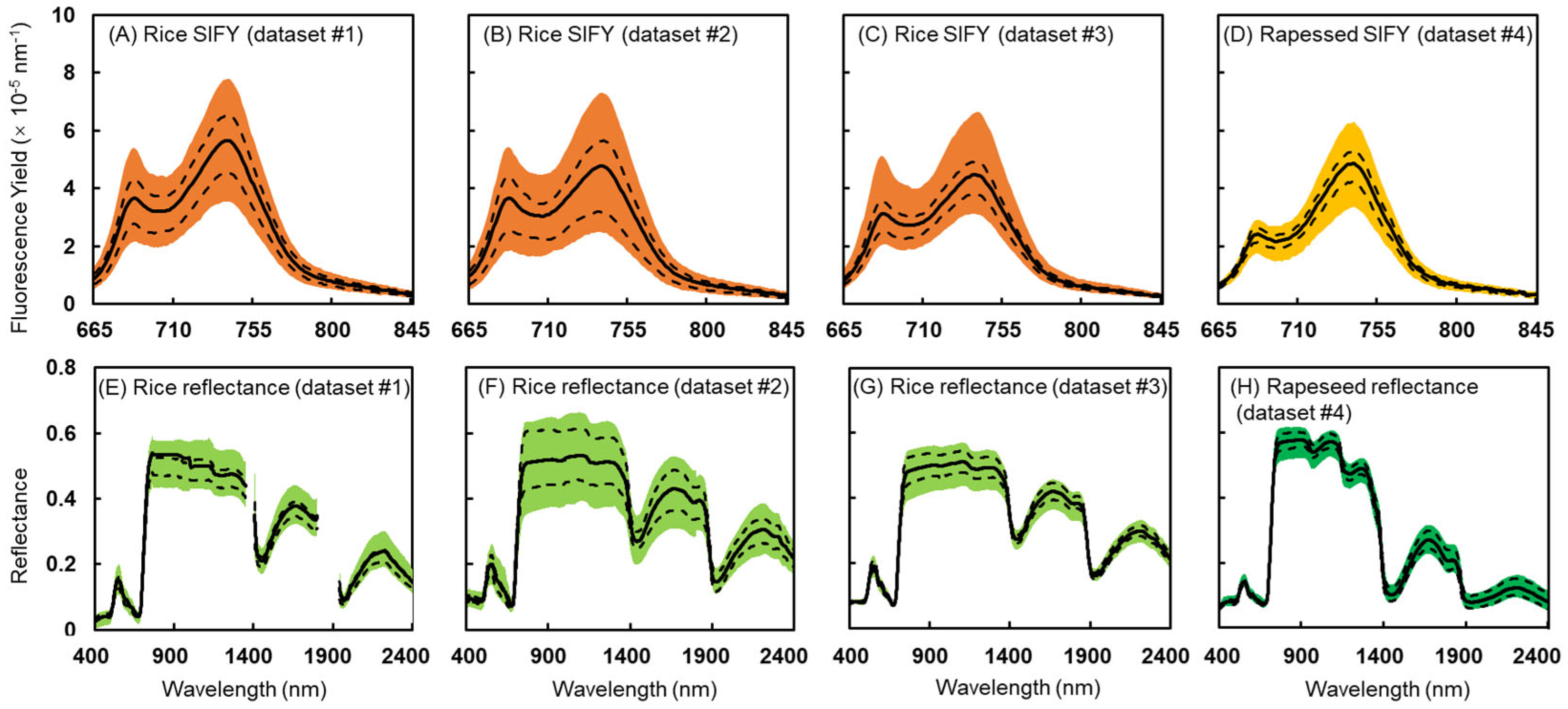
2.2. Leaf Sun-Induced Chlorophyll Fluorescence and Reflectance Measurements
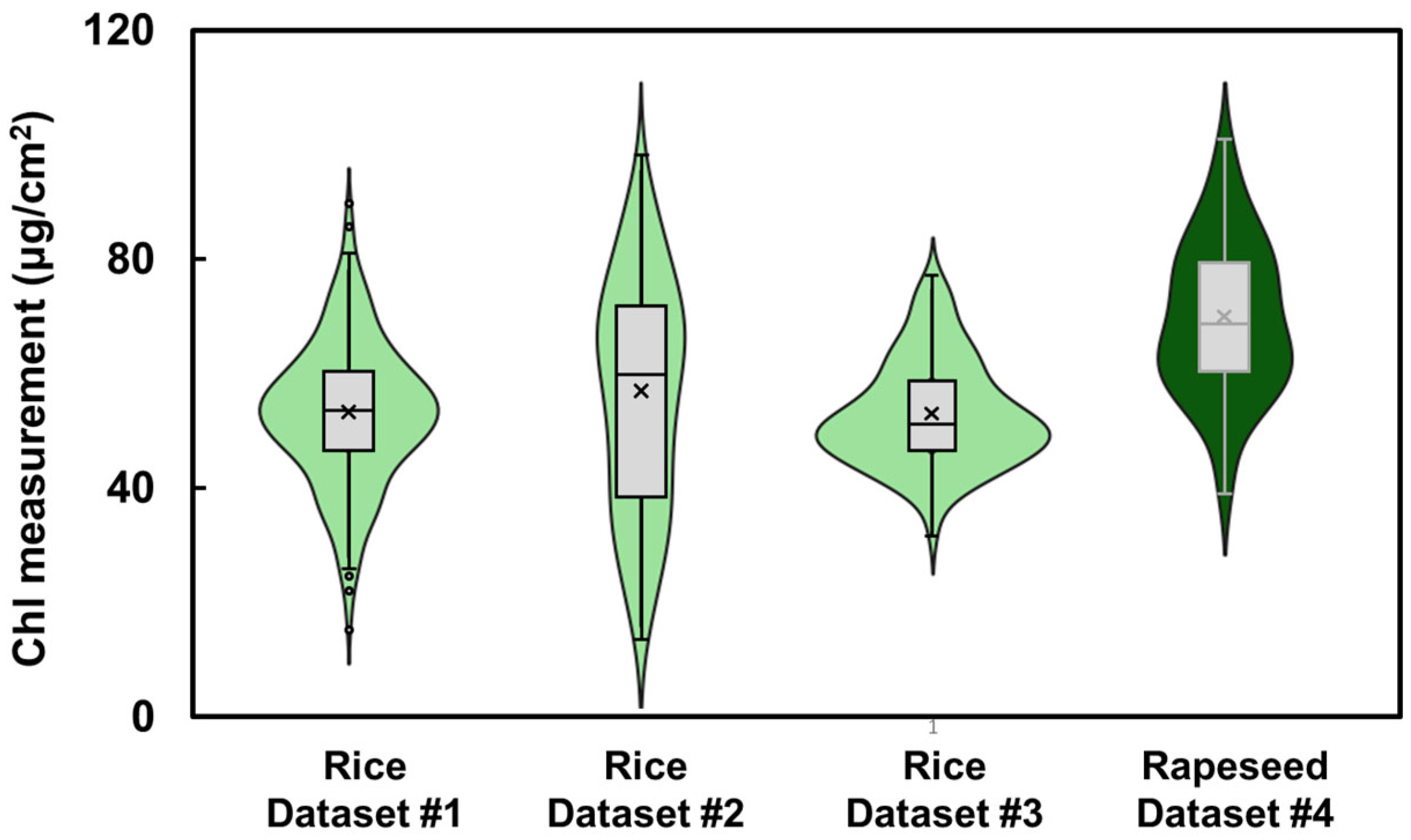
2.3. Leaf Chlorophyll and Carotenoid Measurements
2.4. Modeling Approaches
2.4.1. Regression Models and Model Evaluation
2.4.2. Spectral Preprocessing
2.4.3. Model Updating
3. Results
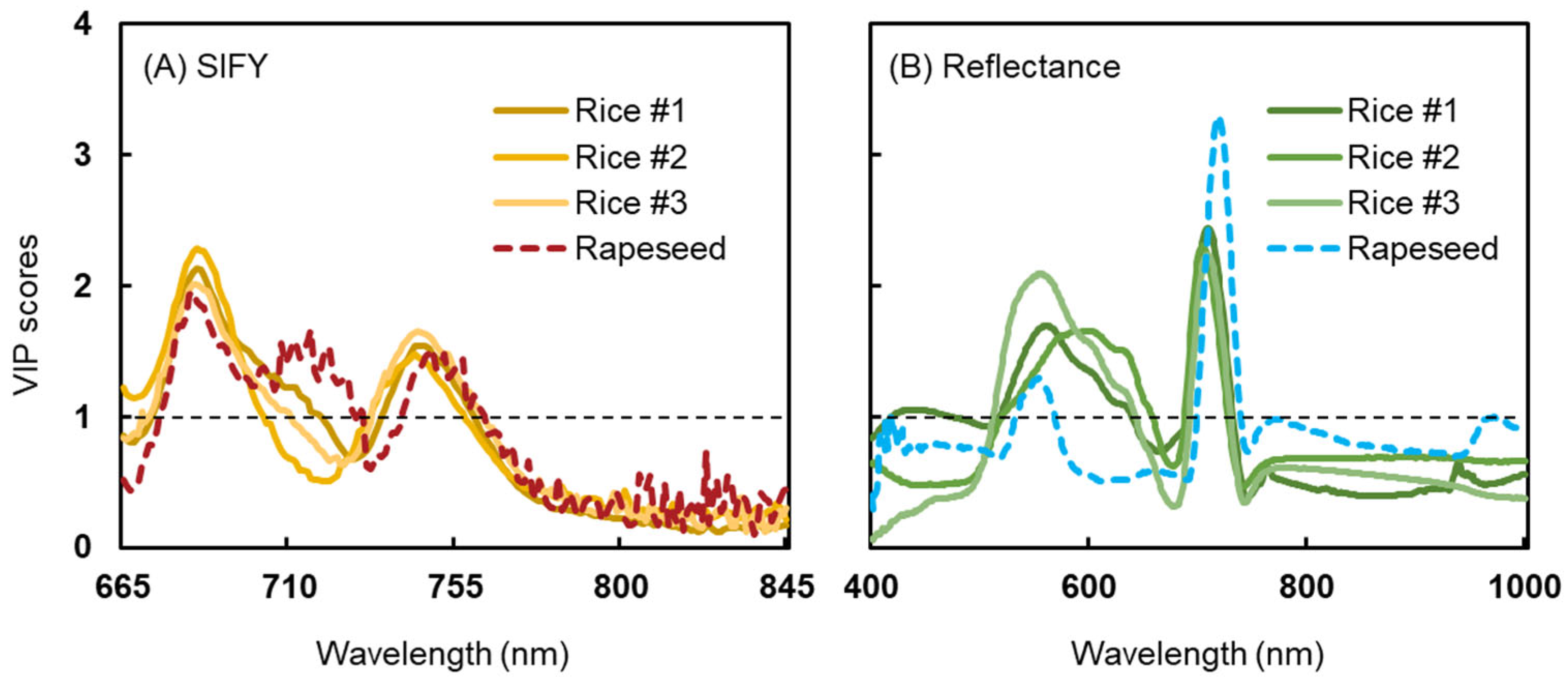
3.1. Spectral Profiles and Distribution of Physiological Parameters
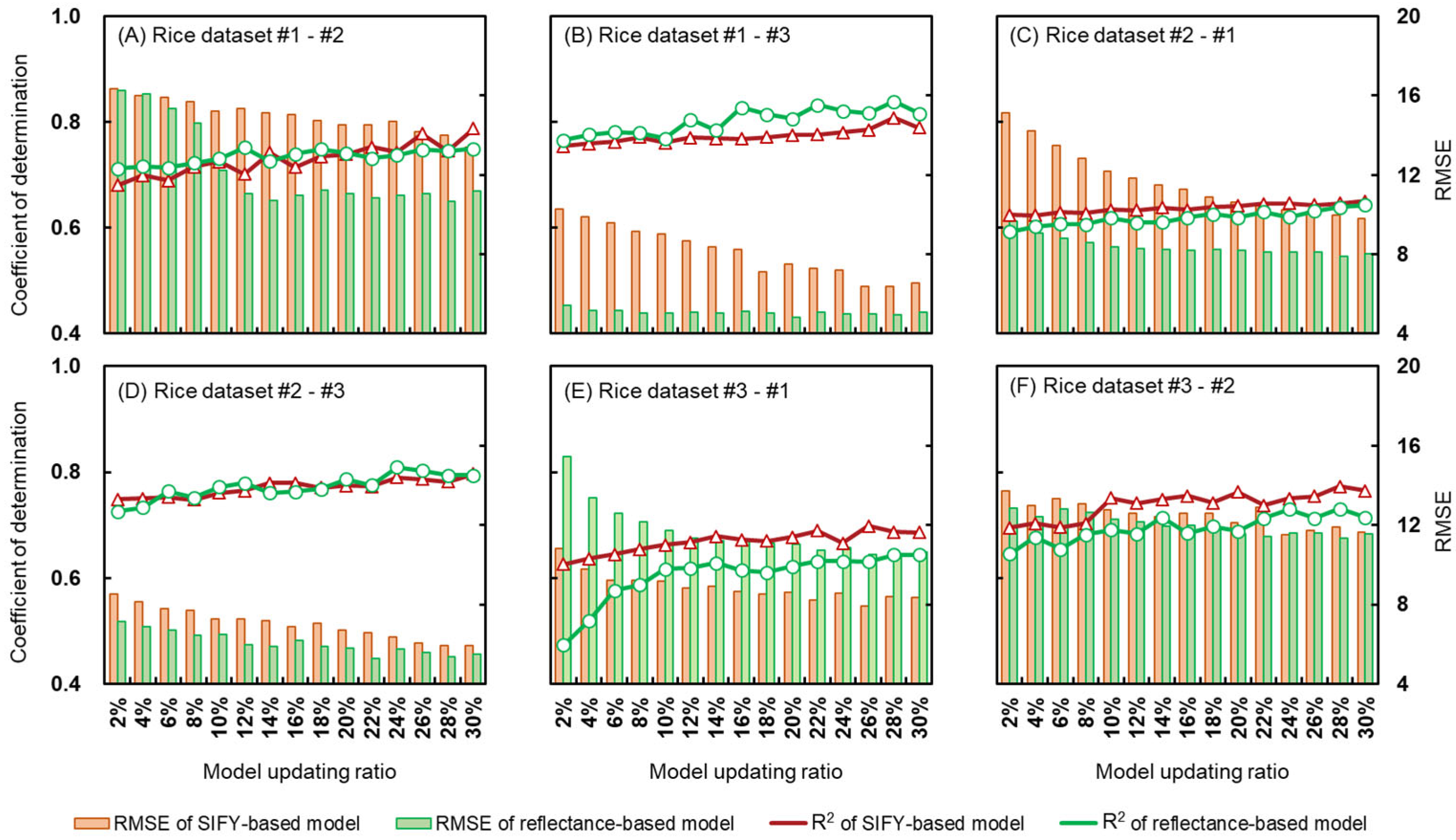
3.2. Model Transferability between Different Rice Datasets
3.2.1. The Direct Transfer Results between Different Rice Datasets
3.2.2. Effects of Different Pretreatments and Model Updating Ratios on Model Transfer
3.3. Between-Species Data Transfer Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gitelson, A.A.; Peng, Y.; Arkebauer, T.J.; Schepers, J. Relationships between gross primary production, green LAI, and canopy chlorophyll content in maize: Implications for remote sensing of primary production. Remote Sens. Environ. 2014, 144, 65–72. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Andrianto, H.; Faizal, A. Measurement of chlorophyll content to determine nutrition deficiency in plants: A systematic literature review. In Proceedings of the 2017 International Conference on Information Technology Systems and Innovation (ICITSI), Bandung, Indonesia, 23–24 October 2017; pp. 392–397. [Google Scholar]
- Kior, A.; Sukhov, V.; Sukhova, E. Application of Reflectance Indices for Remote Sensing of Plants and Revealing Actions of Stressors. Photonics 2021, 8, 582. [Google Scholar] [CrossRef]
- Verrelst, J.; Muñoz, J.; Alonso, L.; Delegido, J.; Rivera, J.P.; Camps-Valls, G.; Moreno, J. Machine learning regression algorithms for biophysical parameter retrieval: Opportunities for Sentinel-2 and -3. Remote Sens. Environ. 2012, 118, 127–139. [Google Scholar] [CrossRef]
- Chang, S.X.; Robison, D.J. Nondestructive and rapid estimation of hardwood foliar nitrogen status using the SPAD-502 chlorophyll meter. For. Ecol. Manag. 2003, 181, 331–338. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Verrelst, J.; Rivera, J.P.; Alonso, L.; Moreno, J.; Samson, R. Gaussian processes retrieval of leaf parameters from a multi-species reflectance, absorbance and fluorescence dataset. J. Photochem. Photobiol. B 2014, 134, 37–48. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Slaton, M.R.; Raymond Hunt, E.; Smith, W.K. Estimating near-infrared leaf reflectance from leaf structural characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L. Effects of leaf structure on reflectance estimates of chlorophyll content. Int. J. Remote Sens. 2010, 29, 5265–5274. [Google Scholar] [CrossRef]
- Esteban, R.; Fernández-Marín, B.; Hernandez, A.; Jiménez, E.T.; León, A.; García-Mauriño, S.; Silva, C.D.; Dolmus, J.R.; Dolmus, C.M.; Molina, M.J.; et al. Salt crystal deposition as a reversible mechanism to enhance photoprotection in black mangrove. Trees 2012, 27, 229–237. [Google Scholar] [CrossRef]
- Feng, L.; Wu, B.; He, Y.; Zhang, C. Hyperspectral Imaging Combined with Deep Transfer Learning for Rice Disease Detection. Front. Plant Sci. 2021, 12, 693521. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Verrelst, J.; Féret, J.-B.; Wang, Z.; Wocher, M.; Strathmann, M.; Danner, M.; Mauser, W.; Hank, T. Crop nitrogen monitoring: Recent progress and principal developments in the context of imaging spectroscopy missions. Remote Sens. Environ. 2020, 242, 111758. [Google Scholar] [CrossRef] [PubMed]
- Verrelst, J.; Malenovsky, Z.; Van der Tol, C.; Camps-Valls, G.; Gastellu-Etchegorry, J.P.; Lewis, P.; North, P.; Moreno, J. Quantifying Vegetation Biophysical Variables from Imaging Spectroscopy Data: A Review on Retrieval Methods. Surv. Geophys. 2019, 40, 589–629. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Struik, P.C.; Goudriaan, J. On the needs for combining physiological principles and mathematics to improve crop models. Field Crops Res. 2021, 271, 108254. [Google Scholar] [CrossRef]
- Brodzicki, A.; Piekarski, M.; Kucharski, D.; Jaworek-Korjakowska, J.; Gorgon, M. Transfer Learning Methods as a New Approach in Computer Vision Tasks with Small Datasets. Found. Comput. Decis. Sci. 2020, 45, 179–193. [Google Scholar] [CrossRef]
- Wan, G.; Yu, A.; Yu, X.; Liu, B. Deep convolutional recurrent neural network with transfer learning for hyperspectral image classification. J. Appl. Remote Sens. 2018, 12, 026028. [Google Scholar] [CrossRef]
- Qiao, L.; Mu, Y.; Lu, B.; Tang, X. Calibration Maintenance Application of Near-infrared Spectrometric Model in Food Analysis. Food Rev. Int. 2021, 39, 1628–1644. [Google Scholar] [CrossRef]
- Xiao, Q.; Tang, W.; Zhang, C.; Zhou, L.; Feng, L.; Shen, J.; Yan, T.; Gao, P.; He, Y.; Wu, N. Spectral Preprocessing Combined with Deep Transfer Learning to Evaluate Chlorophyll Content in Cotton Leaves. Plant Phenomics 2022, 2022, 9813841. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhou, W.; He, Y.; Wanger, T.C.; Cen, H. Combining transfer learning and hyperspectral reflectance analysis to assess leaf nitrogen concentration across different plant species datasets. Remote Sens. Environ. 2022, 269, 112826. [Google Scholar] [CrossRef]
- Meacham-Hensold, K.; Montes, C.M.; Wu, J.; Guan, K.; Fu, P.; Ainsworth, E.A.; Pederson, T.; Moore, C.E.; Brown, K.L.; Raines, C.; et al. High-throughput field phenotyping using hyperspectral reflectance and partial least squares regression (PLSR) reveals genetic modifications to photosynthetic capacity. Remote Sens. Environ. 2019, 231, 111176. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazar, D.; Guo, Y.; Govindjee, G. Photosynthesis: Basics, history and modelling. Ann. Bot. 2020, 126, 511–537. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Berry, J.A.; Zhang, Y.; Joiner, J.; Guanter, L.; Badgley, G.; Lobell, D.B. Improving the monitoring of crop productivity using spaceborne solar-induced fluorescence. Glob. Chang. Biol. 2016, 22, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Tubuxin, B.; Rahimzadeh-Bajgiran, P.; Ginnan, Y.; Hosoi, F.; Omasa, K. Estimating chlorophyll content and photochemical yield of photosystem II (PhiPSII) using solar-induced chlorophyll fluorescence measurements at different growing stages of attached leaves. J. Exp. Bot. 2015, 66, 5595–5603. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhu, J.; Ma, C.; Alonso, L.; Li, D.; Cheng, T.; Tian, Y.; Zhu, Y.; Yao, X.; Cao, W. Difference and Potential of the Upward and Downward Sun-Induced Chlorophyll Fluorescence on Detecting Leaf Nitrogen Concentration in Wheat. Remote Sens. 2018, 10, 1315. [Google Scholar] [CrossRef]
- Fu, P.; Meacham-Hensold, K.; Siebers, M.H.; Bernacchi, C.J. The inverse relationship between solar-induced fluorescence yield and photosynthetic capacity: Benefits for field phenotyping. J. Exp. Bot. 2021, 72, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Magney, T.S.; Frankenberg, C.; Köhler, P.; North, G.; Davis, T.S.; Dold, C.; Dutta, D.; Fisher, J.B.; Grossmann, K.; Harrington, A.; et al. Disentangling Changes in the Spectral Shape of Chlorophyll Fluorescence: Implications for Remote Sensing of Photosynthesis. J. Geophys. Res. Biogeosci. 2019, 124, 1491–1507. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Hermans, I.; Delegido, J.; Veroustraete, F.; Valcke, R.; Moreno, J.; Samson, R. Upward and downward solar-induced chlorophyll fluorescence yield indices of four tree species as indicators of traffic pollution in Valencia. Environ. Pollut. 2013, 173, 29–37. [Google Scholar] [CrossRef]
- Van Wittenberghe, S.; Alonso, L.; Verrelst, J.; Moreno, J.; Samson, R. Bidirectional sun-induced chlorophyll fluorescence emission is influenced by leaf structure and light scattering properties—A bottom-up approach. Remote Sens. Environ. 2015, 158, 169–179. [Google Scholar] [CrossRef]
- Chen, S.; Zhai, L.; Zhou, Y.; Xie, J.; Shao, Y.; Wang, W.; Li, H.; He, Y.; Cen, H. Early diagnosis and mechanistic understanding of citrus Huanglongbing via sun-induced chlorophyll fluorescence. Comput. Electron. Agric. 2023, 215, 108357. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemometrics and Intelligent Laboratory Systems 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Cen, H.; He, Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007, 18, 72–83. [Google Scholar] [CrossRef]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Z.; Han, S.; Chen, Z.; He, X.; Zhao, H.; Ren, S. Exploring the Sensitivity of Solar-Induced Chlorophyll Fluorescence at Different Wavelengths in Response to Drought. Remote Sens. 2023, 15, 1077. [Google Scholar] [CrossRef]
- Zhu, J.; He, W.; Yao, J.; Yu, Q.; Xu, C.; Huang, H.; Mhae, B.; Jandug, C. Spectral Reflectance Characteristics and Chlorophyll Content Estimation Model of Quercus aquifolioides Leaves at Different Altitudes in Sejila Mountain. Appl. Sci. 2020, 10, 3636. [Google Scholar] [CrossRef]
- Féret, J.B.; Gitelson, A.A.; Noble, S.D.; Jacquemoud, S. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef]
- Spafford, L.; le Maire, G.; MacDougall, A.; de Boissieu, F.; Féret, J.-B. Spectral subdomains and prior estimation of leaf structure improves PROSPECT inversion on reflectance or transmittance alone. Remote Sens. Environ. 2021, 252, 112176. [Google Scholar] [CrossRef]
- Geladi, P.; MacDougall, D.; Martens, H. Linearization and Scatter-Correction for Near-Infrared Reflectance Spectra of Meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Isaksson, T.; Næs, T. The effect of multiplicative scatter correction (MSC) and linearity improvement in NIR spectroscopy. Appl. Spectrosc. 1988, 42, 1273–1284. [Google Scholar] [CrossRef]
- Ollinger, S.V. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2011, 189, 375–394. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Malenovsky, Z.; Magney, T.; Van Wittenberghe, S.; Fernandez-Marin, B.; Maignan, F.; Zhang, Y.; Maseyk, K.; Atherton, J.; Albert, L.P.; et al. Chlorophyll a fluorescence illuminates a path connecting plant molecular biology to Earth-system science. Nat. Plants 2021, 7, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.B.; Oja, V.; Eichelmann, H.; Bichele, I.; Dall’Osto, L.; Laisk, A. Fluorescence F 0 of photosystems II and I in developing C3 and C 4 leaves, and implications on regulation of excitation balance. Photosynth. Res. 2014, 122, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Horler, D.; Dockray, M.; Barber, J. The red edge of plant leaf reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.P.; Merz, J.; Townsend, P.A. EcoSIS: A spectral library and the tools to use it. In Proceedings of the AGU Fall Meeting, San Francisco, CA, USA, 9–13 December 2019; p. B11F-2396. [Google Scholar]


| Dataset | Plants | Locations | Number of Samples | Planting Methods | Years |
|---|---|---|---|---|---|
| #1 | 12 rice cultivars and 15 rice materials | CNRRI and WHADP | 356 | Field and potted plants | 2020 |
| #2 | 2 rice cultivars | CNRRI | 119 | Field | 2021 |
| #3 | 2 rice cultivars | CNRRI | 149 | Potted plants | 2022 |
| #4 | 6 rapeseed cultivars | ARSZJU | 49 | Field | 2021–2022 |
| Pretreatment | R2 and RMSE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice Dataset #1–#2 | Rice Dataset #1–#3 | Rice Dataset #2–#1 | Rice Dataset #2–#3 | Rice Dataset #3–#1 | Rice Dataset #3–#2 | |||||||
| None | 0.66 | 17.46 | 0.74 | 9.24 | 0.61 | 16.49 | 0.73 | 9.34 | 0.60 | 12.81 | 0.68 | 13.62 |
| MSC 1 | 0.72 | 17.45 | 0.57 | 8.62 | 0.62 | 16.97 | 0.75 | 7.26 | 0.63 | 10.99 | 0.78 | 11.37 |
| SNV 2 | 0.72 | 16.91 | 0.57 | 8.07 | 0.48 | 18.27 | 0.75 | 7.98 | 0.65 | 12.12 | 0.65 | 12.12 |
| FD 3 | 0.57 | 17.25 | 0.73 | 8.13 | 0.61 | 15.10 | 0.68 | 6.45 | 0.55 | 11.50 | 0.59 | 13.89 |
| MSC + SNV | 0.72 | 16.63 | 0.80 | 10.43 | 0.62 | 18.20 | 0.75 | 6.36 | 0.63 | 12.08 | 0.78 | 11.16 |
| MSC + FD | 0.74 | 12.27 | 0.79 | 6.00 | 0.62 | 9.53 | 0.75 | 12.82 | 0.65 | 8.54 | 0.75 | 11.43 |
| SNV + FD | 0.71 | 17.2 | 0.80 | 8.98 | 0.62 | 18.7 | 0.77 | 9.26 | 0.63 | 11.69 | 0.75 | 11.28 |
| Pretreatment | R2 and RMSE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice Dataset #1–#2 | Rice Dataset #1–#3 | Rice Dataset #2–#1 | Rice Dataset #2–#3 | Rice Dataset #3–#1 | Rice Dataset #3–#2 | |||||||
| None | 0.69 | 16.32 | 0.73 | 5.41 | 0.59 | 11.16 | 0.73 | 7.23 | 0.44 | 26.62 | 0.63 | 13.07 |
| MSC 1 | 0.71 | 14.70 | 0.72 | 6.07 | 0.60 | 12.63 | 0.73 | 7.93 | 0.63 | 13.70 | 0.72 | 12.12 |
| SNV 2 | 0.71 | 14.85 | 0.72 | 5.96 | 0.60 | 12.26 | 0.73 | 7.92 | 0.58 | 13.89 | 0.72 | 12.66 |
| FD 3 | 0.61 | 14.48 | 0.71 | 8.28 | 0.50 | 11.03 | 0.69 | 7.47 | 0.59 | 14.61 | 0.65 | 12.96 |
| MSC + SNV | 0.71 | 14.94 | 0.72 | 5.96 | 0.60 | 12.27 | 0.73 | 7.43 | 0.63 | 12.35 | 0.72 | 12.25 |
| MSC + FD | 0.71 | 13.34 | 0.77 | 5.97 | 0.59 | 13.06 | 0.69 | 6.98 | 0.63 | 10.45 | 0.75 | 11.78 |
| SNV + FD | 0.72 | 13.17 | 0.61 | 6.51 | 0.61 | 13.54 | 0.70 | 7.00 | 0.64 | 13.81 | 0.75 | 11.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-a.; Huang, Z.; Zhou, W.; Cen, H. Optimized Transfer Learning for Chlorophyll Content Estimations across Datasets of Different Species Using Sun-Induced Chlorophyll Fluorescence and Reflectance. Remote Sens. 2024, 16, 1869. https://doi.org/10.3390/rs16111869
Zhou Y-a, Huang Z, Zhou W, Cen H. Optimized Transfer Learning for Chlorophyll Content Estimations across Datasets of Different Species Using Sun-Induced Chlorophyll Fluorescence and Reflectance. Remote Sensing. 2024; 16(11):1869. https://doi.org/10.3390/rs16111869
Chicago/Turabian StyleZhou, Yu-an, Zichen Huang, Weijun Zhou, and Haiyan Cen. 2024. "Optimized Transfer Learning for Chlorophyll Content Estimations across Datasets of Different Species Using Sun-Induced Chlorophyll Fluorescence and Reflectance" Remote Sensing 16, no. 11: 1869. https://doi.org/10.3390/rs16111869
APA StyleZhou, Y.-a., Huang, Z., Zhou, W., & Cen, H. (2024). Optimized Transfer Learning for Chlorophyll Content Estimations across Datasets of Different Species Using Sun-Induced Chlorophyll Fluorescence and Reflectance. Remote Sensing, 16(11), 1869. https://doi.org/10.3390/rs16111869








