Mapping Water Quality in Nearshore Reef Environments Using Airborne Imaging Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Imaging Spectroscopy
2.2. Water Quality Field Measurements
2.3. Water Quality Models
2.4. Performance Evaluation
3. Results
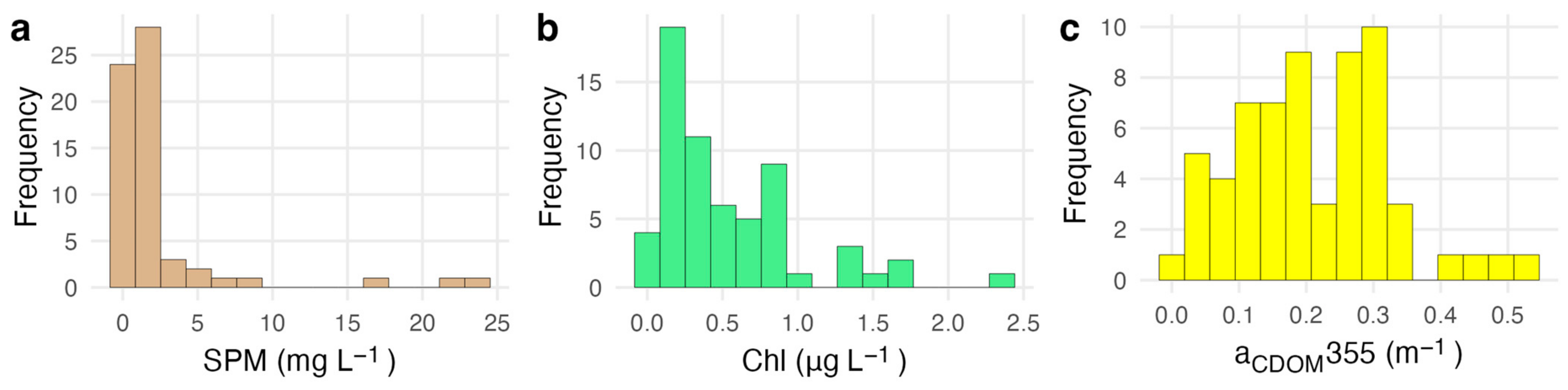
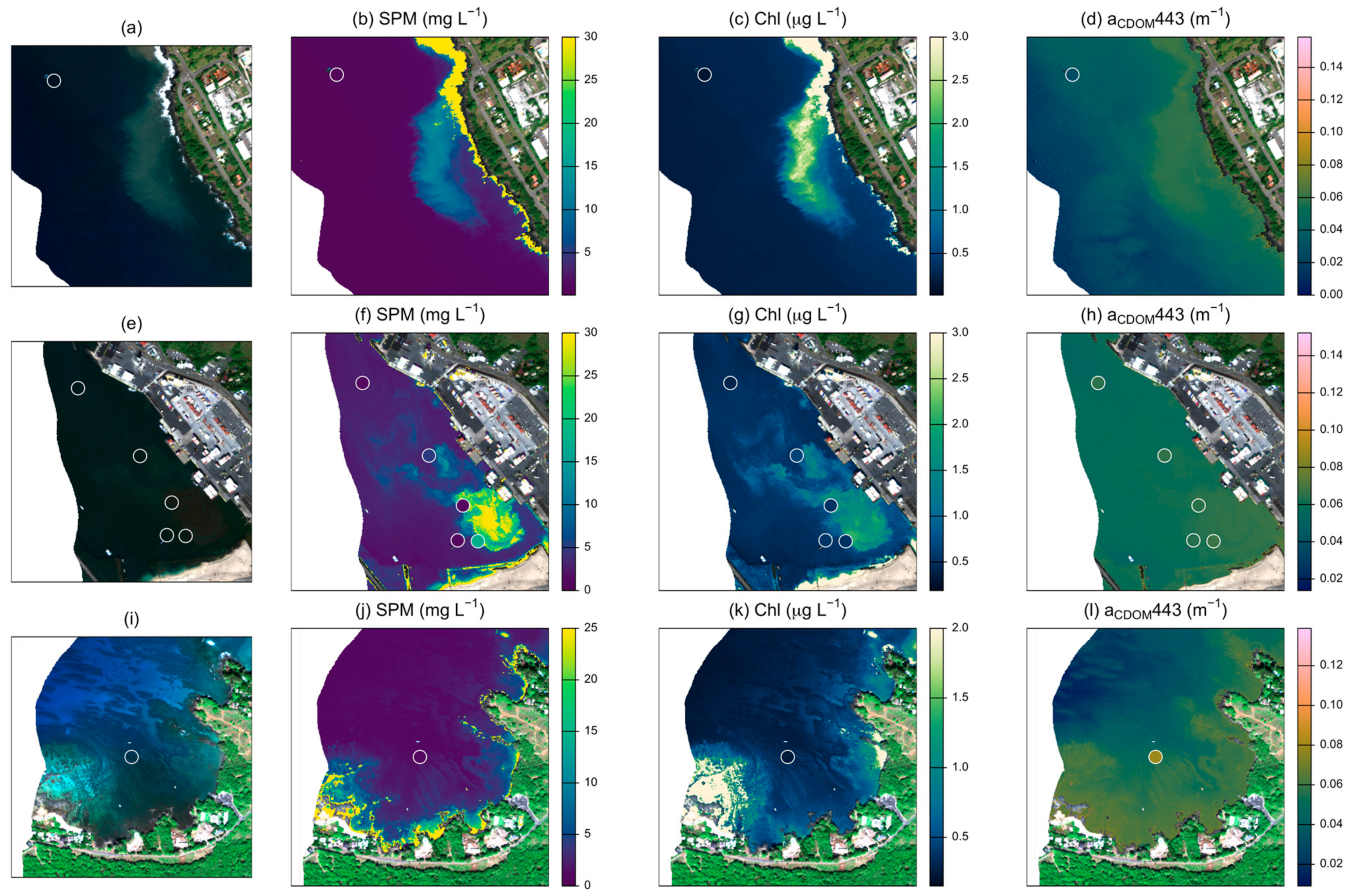
3.1. Field Conditions
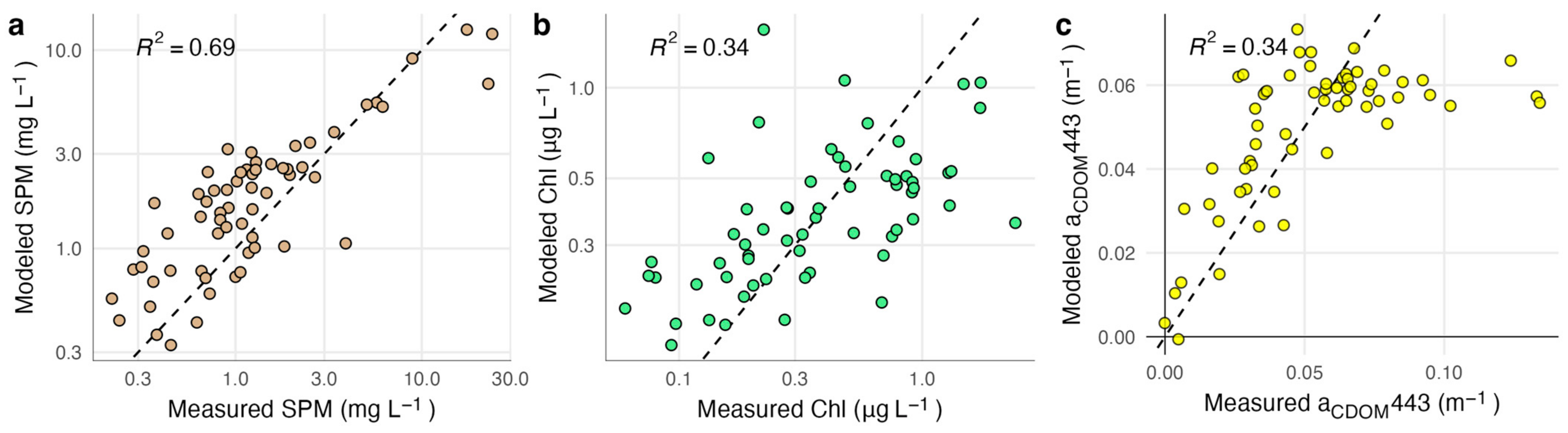
3.2. SPM Model Performance
3.3. Chlorophyll Model Performance
3.4. CDOM Model Performance
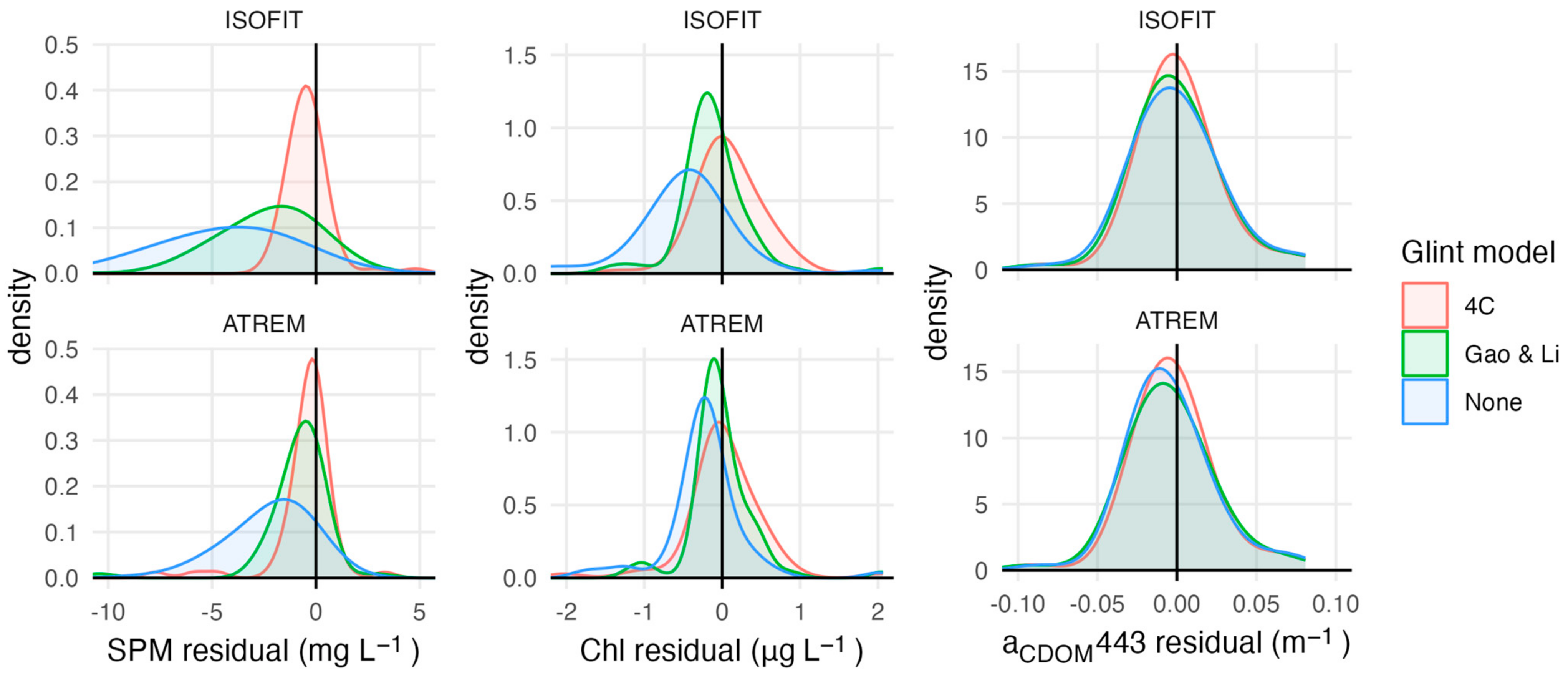
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix
| Parameter | Description | Value | Range |
|---|---|---|---|
| C0 | Concentration of phytoplankton type 1 in µg L−1 | 0 | 0–10 |
| CY | Absorption of CDOM at 440 nm in m−1 | 0 | 0–2 |
| CX | Concentration of type 1 non-algal particles in mg L−1 | 0 | 0–25 |
| CMie | Concentration of type 2 non-algal particles in mg L−1 | 0 | 0–25 |
| F0 | Fractional benthic cover of surface with constant reflectance of 1 | 0 | 0–1 |
| F1 | Fractional benthic cover of sand | 0 | 0–1 |
| F2 | Fractional benthic cover of coral | 0 | 0–1 |
| F3 | Fractional benthic cover of crustose coralline algae | 0 | 0–1 |
| F4 | Fractional benthic cover of macroalgae | 0 | 0–1 |
| ZB | Water depth in m | 10 | 0–1 |
| b*b,phy | Specific backscattering coefficient of phytoplankton at 550 nm in m2 mg−1 | 0.001 | - |
| b*b,X | Specific backscattering coefficient of type 1 non-algal particles in m2 g−1 | 0.0086 | - |
| b*b,Mie | Specific backscattering coefficient of type 2 non-algal particles in m2 g−1 | 0.0042 | - |
| a*NAP | Specific absorption coefficient of non-algal particles at 440 nm in m2 g−1 | 0.041 | - |
| S | Exponent of CDOM absorption | 0.014 | - |
| SNAP | Exponent of non-algal particulate absorption | 0.11 | - |
| η | Ångström exponent of type 2 non-algal particulate backscattering | −1 | - |
References
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and Temporal Patterns of Mass Bleaching of Corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E. Effects of Terrestrial Runoff on the Ecology of Corals and Coral Reefs: Review and Synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Kekuewa, S.A.H.; Courtney, T.A.; Cyronak, T.; Kindeberg, T.; Eyre, B.D.; Stoltenberg, L.; Andersson, A.J. Temporal and Spatial Variabilities of Chemical and Physical Parameters on the Heron Island Coral Reef Platform. Aquat. Geochem. 2021, 27, 241–268. [Google Scholar] [CrossRef]
- Schlaefer, J.A.; Tebbett, S.B.; Bellwood, D.R. The Study of Sediments on Coral Reefs: A Hydrodynamic Perspective. Mar. Pollut. Bull. 2021, 169, 112580. [Google Scholar] [CrossRef] [PubMed]
- Bessell-Browne, P.; Negri, A.P.; Fisher, R.; Clode, P.L.; Jones, R. Cumulative Impacts: Thermally Bleached Corals Have Reduced Capacity to Clear Deposited Sediment. Sci. Rep. 2017, 7, 2716. [Google Scholar] [CrossRef]
- Gove, J.M.; Williams, G.J.; Lecky, J.; Brown, E.; Conklin, E.; Counsell, C.; Davis, G.; Donovan, M.K.; Falinski, K.; Kramer, L.; et al. Coral Reefs Benefit from Reduced Land–Sea Impacts under Ocean Warming. Nature 2023, 621, 536–542. [Google Scholar] [CrossRef]
- Humanes, A.; Ricardo, G.F.; Willis, B.L.; Fabricius, K.E.; Negri, A.P. Cumulative Effects of Suspended Sediments, Organic Nutrients and Temperature Stress on Early Life History Stages of the Coral Acropora Tenuis. Sci. Rep. 2017, 7, 44101. [Google Scholar] [CrossRef]
- Barott, K.L.; Huffmyer, A.S.; Davidson, J.M.; Lenz, E.A.; Matsuda, S.B.; Hancock, J.R.; Innis, T.; Drury, C.; Putnam, H.M.; Gates, R.D. Coral Bleaching Response Is Unaltered Following Acclimatization to Reefs with Distinct Environmental Conditions. Proc. Natl. Acad. Sci. USA 2021, 118, e2025435118. [Google Scholar] [CrossRef]
- Cunning, R.; Silverstein, R.N.; Barnes, B.B.; Baker, A.C. Extensive Coral Mortality and Critical Habitat Loss Following Dredging and Their Association with Remotely-Sensed Sediment Plumes. Mar. Pollut. Bull. 2019, 145, 185–199. [Google Scholar] [CrossRef]
- Foo, S.A.; Asner, G.P. Scaling Up Coral Reef Restoration Using Remote Sensing Technology. Front. Mar. Sci. 2019, 6, 79. [Google Scholar] [CrossRef]
- Zheng, G.; DiGiacomo, P.M. Uncertainties and Applications of Satellite-Derived Coastal Water Quality Products. Prog. Oceanogr. 2017, 159, 45–72. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Ackleson, S.G.; Joyce, K.E.; Hestir, E.L.; Castagna, A.; Lavender, S.; McManus, M.A. Living up to the Hype of Hyperspectral Aquatic Remote Sensing: Science, Resources and Outlook. Front. Environ. Sci. 2021, 9, 649528. [Google Scholar] [CrossRef]
- Giardino, C.; Brando, V.E.; Gege, P.; Pinnel, N.; Hochberg, E.; Knaeps, E.; Reusen, I.; Doerffer, R.; Bresciani, M.; Braga, F.; et al. Imaging Spectrometry of Inland and Coastal Waters: State of the Art, Achievements and Perspectives. Surv. Geophys. 2019, 40, 401–429. [Google Scholar] [CrossRef]
- König, M.; Grunert, B.K.; Hondula, K.; Bohn, N.; Dai, J.; Jamalinia, E.; Vaughn, N.R.; Thompson, D.R.; Asner, G.P. Assessment of Combined Atmospheric and Glint Correction Approaches for Remote Sensing Reflectance Estimation. Remote Sens. Environ. 2024, under review. [Google Scholar]
- Hochberg, E.J.; Peltier, S.A.; Maritorena, S. Trends and Variability in Spectral Diffuse Attenuation of Coral Reef Waters. Coral Reefs 2020, 39, 1377–1389. [Google Scholar] [CrossRef]
- Kutser, T.; Hedley, J.; Giardino, C.; Roelfsema, C.; Brando, V.E. Remote Sensing of Shallow Waters—A 50 Year Retrospective and Future Directions. Remote Sens. Environ. 2020, 240, 111619. [Google Scholar] [CrossRef]
- Bell, T.W.; Okin, G.S.; Cavanaugh, K.C.; Hochberg, E.J. Impact of Water Characteristics on the Discrimination of Benthic Cover in and around Coral Reefs from Imaging Spectrometer Data. Remote Sens. Environ. 2020, 239, 111631. [Google Scholar] [CrossRef]
- Turner, K.J.; Tzortziou, M.; Grunert, B.K.; Goes, J.; Sherman, J. Optical Classification of an Urbanized Estuary Using Hyperspectral Remote Sensing Reflectance. Opt. Express 2022, 30, 41590. [Google Scholar] [CrossRef]
- Mouw, C.B.; Greb, S.; Aurin, D.; DiGiacomo, P.M.; Lee, Z.; Twardowski, M.; Binding, C.; Hu, C.; Ma, R.; Moore, T.; et al. Aquatic Color Radiometry Remote Sensing of Coastal and Inland Waters: Challenges and Recommendations for Future Satellite Missions. Remote Sens. Environ. 2015, 160, 15–30. [Google Scholar] [CrossRef]
- D’Sa, E.J.; Miller, R.L.; McKee, B.A. Suspended Particulate Matter Dynamics in Coastal Waters from Ocean Color: Application to the Northern Gulf of Mexico. Geophys. Res. Lett. 2007, 34, 2007GL031192. [Google Scholar] [CrossRef]
- Novoa, S.; Doxaran, D.; Ody, A.; Vanhellemont, Q.; Lafon, V.; Lubac, B.; Gernez, P. Atmospheric Corrections and Multi-Conditional Algorithm for Multi-Sensor Remote Sensing of Suspended Particulate Matter in Low-to-High Turbidity Levels Coastal Waters. Remote Sens. 2017, 9, 61. [Google Scholar] [CrossRef]
- Hooker, S.; McClain, C.; Mannino, A. NASA Strategic Planning Document: A Comprehensive Plan for the Long-Term Calibra- Tion and Validation of Oceanic Biogeochemical Satellite Data, NASA Special Pub. 2007-214152; National Aeronautics and Space Administration, Goddard Space Flight Center: Greenbelt, MD, USA, 2007. [Google Scholar]
- Rogers, C. Responses of Coral Reefs and Reef Organisms to Sedimentation. Mar. Ecol. Prog. Ser. 1990, 62, 185–202. [Google Scholar] [CrossRef]
- Tuttle, L.J.; Donahue, M.J. Effects of Sediment Exposure on Corals: A Systematic Review of Experimental Studies. Environ. Evid. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, G.F.; Jones, R.J.; Clode, P.L.; Humanes, A.; Negri, A.P. Suspended Sediments Limit Coral Sperm Availability. Sci. Rep. 2015, 5, 18084. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.S.; Ramos-Scharrón, C.E. Assessing Effects of Sediment Delivery to Coral Reefs: A Caribbean Watershed Perspective. Front. Mar. Sci. 2022, 8, 773968. [Google Scholar] [CrossRef]
- Carlson, R.R.; Li, J.; Crowder, L.B.; Asner, G.P. Large-Scale Effects of Turbidity on Coral Bleaching in the Hawaiian Islands. Front. Mar. Sci. 2022, 9, 969472. [Google Scholar] [CrossRef]
- Morgan, K.M.; Perry, C.T.; Smithers, S.G.; Johnson, J.A.; Daniell, J.J. Evidence of Extensive Reef Development and High Coral Cover in Nearshore Environments: Implications for Understanding Coral Adaptation in Turbid Settings. Sci. Rep. 2016, 6, 29616. [Google Scholar] [CrossRef]
- Yu, X.; Lee, Z.; Shen, F.; Wang, M.; Wei, J.; Jiang, L.; Shang, Z. An Empirical Algorithm to Seamlessly Retrieve the Concentration of Suspended Particulate Matter from Water Color across Ocean to Turbid River Mouths. Remote Sens. Environ. 2019, 235, 111491. [Google Scholar] [CrossRef]
- Petus, C.; Chust, G.; Gohin, F.; Doxaran, D.; Froidefond, J.-M.; Sagarminaga, Y. Estimating Turbidity and Total Suspended Matter in the Adour River Plume (South Bay of Biscay) Using MODIS 250-m Imagery. Cont. Shelf Res. 2010, 30, 379–392. [Google Scholar] [CrossRef]
- Restrepo, J.D.; Park, E.; Aquino, S.; Latrubesse, E.M. Coral Reefs Chronically Exposed to River Sediment Plumes in the Southwestern Caribbean: Rosario Islands, Colombia. Sci. Total Environ. 2016, 553, 316–329. [Google Scholar] [CrossRef]
- Jiang, D.; Matsushita, B.; Pahlevan, N.; Gurlin, D.; Lehmann, M.K.; Fichot, C.G.; Schalles, J.; Loisel, H.; Binding, C.; Zhang, Y.; et al. Remotely Estimating Total Suspended Solids Concentration in Clear to Extremely Turbid Waters Using a Novel Semi-Analytical Method. Remote Sens. Environ. 2021, 258, 112386. [Google Scholar] [CrossRef]
- Bell, P.R.F. Eutrophication and Coral Reefs—Some Examples in the Great Barrier Reef Lagoon. Water Res. 1992, 26, 553–568. [Google Scholar] [CrossRef]
- Hu, C.; Feng, L.; Lee, Z.; Franz, B.A.; Bailey, S.W.; Werdell, P.J.; Proctor, C.W. Improving Satellite Global Chlorophyll a Data Products Through Algorithm Refinement and Data Recovery. J. Geophys. Res. Oceans 2019, 124, 1524–1543. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K. Water Quality as a Regional Driver of Coral Biodiversity and Macroalgae on the Great Barrier Reef. Ecol. Appl. 2010, 20, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Adolf, J.E.; Burns, J.; Walker, J.K.; Gamiao, S. Near Shore Distributions of Phytoplankton and Bacteria in Relation to Submarine Groundwater Discharge-Fed Fishponds, Kona Coast, Hawai‘i, USA. Estuar. Coast. Shelf Sci. 2019, 219, 341–353. [Google Scholar] [CrossRef]
- Johnson, E.E.; Wiegner, T.N. Surface Water Metabolism Potential in Groundwater-Fed Coastal Waters of Hawaii Island, USA. Estuaries Coasts 2014, 37, 712–723. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A Review of Ocean Color Remote Sensing Methods and Statistical Techniques for the Detection, Mapping and Analysis of Phytoplankton Blooms in Coastal and Open Oceans. Prog. Oceanogr. 2014, 123, 123–144. [Google Scholar] [CrossRef]
- Moore, T.S.; Campbell, J.W.; Dowell, M.D. A Class-Based Approach to Characterizing and Mapping the Uncertainty of the MODIS Ocean Chlorophyll Product. Remote Sens. Environ. 2009, 113, 2424–2430. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Dall’Olmo, G.; Moses, W.; Rundquist, D.C.; Barrow, T.; Fisher, T.R.; Gurlin, D.; Holz, J. A Simple Semi-Analytical Model for Remote Estimation of Chlorophyll-a in Turbid Waters: Validation. Remote Sens. Environ. 2008, 112, 3582–3593. [Google Scholar] [CrossRef]
- Keith, D.J.; Schaeffer, B.A.; Lunetta, R.S.; Gould, R.W.; Rocha, K.; Cobb, D.J. Remote Sensing of Selected Water-Quality Indicators with the Hyperspectral Imager for the Coastal Ocean (HICO) Sensor. Int. J. Remote Sens. 2014, 35, 2927–2962. [Google Scholar] [CrossRef]
- Ruddick, K.G.; Gons, H.J.; Rijkeboer, M.; Tilstone, G. Optical Remote Sensing of Chlorophyll a in Case 2 Waters by Use of an Adaptive Two-Band Algorithm with Optimal Error Properties. Appl. Opt. 2001, 40, 3575. [Google Scholar] [CrossRef] [PubMed]
- Neil, C.; Spyrakos, E.; Hunter, P.D.; Tyler, A.N. A Global Approach for Chlorophyll-a Retrieval across Optically Complex Inland Waters Based on Optical Water Types. Remote Sens. Environ. 2019, 229, 159–178. [Google Scholar] [CrossRef]
- Russell, B.J.; Dierssen, H.M.; Hochberg, E.J. Water Column Optical Properties of Pacific Coral Reefs Across Geomorphic Zones and in Comparison to Offshore Waters. Remote Sens. 2019, 11, 1757. [Google Scholar] [CrossRef]
- Zepp, R.G.; Shank, G.C.; Stabenau, E.; Patterson, K.W.; Cyterski, M.; Fisher, W.; Bartels, E.; Anderson, S.L. Spatial and Temporal Variability of Solar Ultraviolet Exposure of Coral Assemblages in the Florida Keys: Importance of Colored Dissolved Organic Matter. Limnol. Oceanogr. 2008, 53, 1909–1922. [Google Scholar] [CrossRef]
- Barber-Lluch, E.; Nieto-Cid, M.; Santos-Echeandía, J.; Sánchez-Marín, P. Effect of Dissolved Organic Matter on Copper Bioavailability to a Coastal Dinoflagellate at Environmentally Relevant Concentrations. Sci. Total Environ. 2023, 901, 165989. [Google Scholar] [CrossRef] [PubMed]
- Harringmeyer, J.P.; Kaiser, K.; Thompson, D.R.; Gierach, M.M.; Cash, C.L.; Fichot, C.G. Detection and Sourcing of CDOM in Urban Coastal Waters With UV-Visible Imaging Spectroscopy. Front. Environ. Sci. 2021, 9, 647966. [Google Scholar] [CrossRef]
- Nezlin, N.P.; Beegan, C.; Feit, A.; Gully, J.R.; Latker, A.; McLaughlin, K.; Mengel, M.J.; Robertson, G.L.; Steele, A.; Weisberg, S.B. Colored Dissolved Organic Matter (CDOM) as a Tracer of Effluent Plumes in the Coastal Ocean. Reg. Stud. Mar. Sci. 2020, 35, 101163. [Google Scholar] [CrossRef]
- Asner, G.P.; Knapp, D.E.; Boardman, J.; Green, R.O.; Kennedy-Bowdoin, T.; Eastwood, M.; Martin, R.E.; Anderson, C.; Field, C.B. Carnegie Airborne Observatory-2: Increasing Science Data Dimensionality via High-Fidelity Multi-Sensor Fusion. Remote Sens. Environ. 2012, 124, 454–465. [Google Scholar] [CrossRef]
- Gao, B.-C.; Heidebrecht, K.B.; Goetz, A.F.H. Derivation of Scaled Surface Reflectances from AVIRIS Data. Remote Sens. Environ. 1993, 44, 165–178. [Google Scholar] [CrossRef]
- Thompson, D.R.; Hochberg, E.J.; Asner, G.P.; Green, R.O.; Knapp, D.E.; Gao, B.-C.; Garcia, R.; Gierach, M.; Lee, Z.; Maritorena, S.; et al. Airborne Mapping of Benthic Reflectance Spectra with Bayesian Linear Mixtures. Remote Sens. Environ. 2017, 200, 18–30. [Google Scholar] [CrossRef]
- Thompson, D.R.; Natraj, V.; Green, R.O.; Helmlinger, M.C.; Gao, B.-C.; Eastwood, M.L. Optimal Estimation for Imaging Spectrometer Atmospheric Correction. Remote Sens. Environ. 2018, 216, 355–373. [Google Scholar] [CrossRef]
- Gege, P. Analytic Model for the Direct and Diffuse Components of Downwelling Spectral Irradiance in Water. Appl. Opt. 2012, 51, 1407. [Google Scholar] [CrossRef]
- Gege, P.; Grötsch, P. A Spectral Model for Correcting Sunglint and Skyglint. Proc. Ocean Opt. XXIII 2016, 2016, 1–10. [Google Scholar]
- Göritz, A.; Berger, S.; Gege, P.; Grossart, H.-P.; Nejstgaard, J.; Riedel, S.; Röttgers, R.; Utschig, C. Retrieval of Water Constituents from Hyperspectral In-Situ Measurements under Variable Cloud Cover—A Case Study at Lake Stechlin (Germany). Remote Sens. 2018, 10, 181. [Google Scholar] [CrossRef]
- Groetsch, P.M.M.; Gege, P.; Simis, S.G.H.; Eleveld, M.A.; Peters, S.W.M. Validation of a Spectral Correction Procedure for Sun and Sky Reflections in Above-Water Reflectance Measurements. Opt. Express 2017, 25, A742. [Google Scholar] [CrossRef]
- Gao, B.-C.; Li, R.-R. Correction of Sunglint Effects in High Spatial Resolution Hyperspectral Imagery Using SWIR or NIR Bands and Taking Account of Spectral Variation of Refractive Index of Water. Adv. Environ. Eng. Res. 2021, 2, 3. [Google Scholar] [CrossRef]
- Thompson, D.R.; Cawse-Nicholson, K.; Erickson, Z.; Fichot, C.G.; Frankenberg, C.; Gao, B.-C.; Gierach, M.M.; Green, R.O.; Jensen, D.; Natraj, V.; et al. A Unified Approach to Estimate Land and Water Reflectances with Uncertainties for Coastal Imaging Spectroscopy. Remote Sens. Environ. 2019, 231, 111198. [Google Scholar] [CrossRef]
- Greenberg, E.; Thompson, D.R.; Jensen, D.; Townsend, P.A.; Queally, N.; Chlus, A.; Fichot, C.G.; Harringmeyer, J.P.; Simard, M. An Improved Scheme for Correcting Remote Spectral Surface Reflectance Simultaneously for Terrestrial BRDF and Water-Surface Sunglint in Coastal Environments. J. Geophys. Res. Biogeosciences 2022, 127, e2021JG006712. [Google Scholar] [CrossRef]
- Feyisa, G.L.; Meilby, H.; Fensholt, R.; Proud, S.R. Automated Water Extraction Index: A New Technique for Surface Water Mapping Using Landsat Imagery. Remote Sens. Environ. 2014, 140, 23–35. [Google Scholar] [CrossRef]
- Mannino, A.; Novak, M.G.; Nelson, N.; Belz, M.; Berthon, J.F.; Blough, N.; Boss, E.; Bricaud, A.; Chaves, J.; Del Castillo, C.; et al. Measurement Protocol of Absorption by Chromophoric Dissolved Organic Matter (CDOM) and Other Dissolved Materials; IOCCG Ocean Optics and Biogeochemistry Protocols for Satellite Ocean Colour Sensor Validation; IOCCG: Dartmouth, NS, Canada, 2019; Available online: https://ioccg.org/wp-content/uploads/2019/10/cdom_abs_protocol_public_draft-19oct-2019-sm.pdf (accessed on 15 December 2022).
- Grunert, B.K.; Mouw, C.B.; Ciochetto, A.B. Characterizing CDOM Spectral Variability Across Diverse Regions and Spectral Ranges. Glob. Biogeochem. Cycles 2018, 32, 57–77. [Google Scholar] [CrossRef]
- Loiselle, S.A.; Bracchini, L.; Dattilo, A.M.; Ricci, M.; Tognazzi, A.; Cózar, A.; Rossi, C. The Optical Characterization of Chromophoric Dissolved Organic Matter Using Wavelength Distribution of Absorption Spectral Slopes. Limnol. Oceanogr. 2009, 54, 590–597. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption Spectral Slopes and Slope Ratios as Indicators of Molecular Weight, Source, and Photobleaching of Chromophoric Dissolved Organic Matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Maritorena, S.; Mitchell, B.G.; Siegel, D.A.; Carder, K.L.; Garver, S.A.; Kahru, M.; McClain, C. Ocean Color Chlorophyll Algorithms for SeaWiFS. J. Geophys. Res. Oceans 1998, 103, 24937–24953. [Google Scholar] [CrossRef]
- Bio-Optical Modelling and Remote Sensing of Inland Waters; Mishra, D.R., Ogashawara, I., Gitelson, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-804644-9. [Google Scholar]
- Ficek, D.; Zapadka, T.; Dera, J. Remote sensing reflectance of Pomeranian lakes and the Baltic. Oceanologia 2011, 53, 959–970. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Arnone, R.A. Deriving Inherent Optical Properties from Water Color: A Multiband Quasi-Analytical Algorithm for Optically Deep Waters. Appl. Opt. 2002, 41, 5755. [Google Scholar] [CrossRef]
- Mannino, A.; Russ, M.E.; Hooker, S.B. Algorithm Development and Validation for Satellite-derived Distributions of DOC and CDOM in the U.S. Middle Atlantic Bight. J. Geophys. Res. Oceans 2008, 113, 2007JC004493. [Google Scholar] [CrossRef]
- Albert, A.; Mobley, C. An Analytical Model for Subsurface Irradiance and Remote Sensing Reflectance in Deep and Shallow Case-2 Waters. Opt. Express 2003, 11, 2873. [Google Scholar] [CrossRef]
- König, M.; Noel, P.; Hondula, K.L.; Jamalinia, E.; Dai, J.; Vaughn, N.R.; Asner, G.P. bio_optics python package. Available online: https://github.com/CMLandOcean/bio_optics (accessed on 1 January 2023). [CrossRef]
- Vandermeulen, R.A.; Mannino, A.; Craig, S.E.; Werdell, P.J. 150 Shades of Green: Using the Full Spectrum of Remote Sensing Reflectance to Elucidate Color Shifts in the Ocean. Remote Sens. Environ. 2020, 247, 111900. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Vandermeulen, R.A.; Barnes, B.B.; Castagna, A.; Knaeps, E.; Vanhellemont, Q. QWIP: A Quantitative Metric for Quality Control of Aquatic Reflectance Spectral Shape Using the Apparent Visible Wavelength. Front. Remote Sens. 2022, 3, 869611. [Google Scholar] [CrossRef]
- Balasubramanian, S.V.; Pahlevan, N.; Smith, B.; Binding, C.; Schalles, J.; Loisel, H.; Gurlin, D.; Greb, S.; Alikas, K.; Randla, M.; et al. Robust Algorithm for Estimating Total Suspended Solids (TSS) in Inland and Nearshore Coastal Waters. Remote Sens. Environ. 2020, 246, 111768. [Google Scholar] [CrossRef]
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll a Algorithms for Oligotrophic Oceans: A Novel Approach Based on Three-band Reflectance Difference. J. Geophys. Res. Oceans 2012, 117, 2011JC007395. [Google Scholar] [CrossRef]
- Loisel, H.; Vantrepotte, V.; Dessailly, D.; Mériaux, X. Assessment of the Colored Dissolved Organic Matter in Coastal Waters from Ocean Color Remote Sensing. Opt. Express 2014, 22, 13109. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; McKee, B.A. Using MODIS Terra 250 m Imagery to Map Concentrations of Total Suspended Matter in Coastal Waters. Remote Sens. Environ. 2004, 93, 259–266. [Google Scholar] [CrossRef]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Satellite Estimation of Chlorophyll-a Concentration Using the Red and NIR Bands of MERIS—The Azov Sea Case Study. IEEE Geosci. Remote Sens. Lett. 2009, 6, 845–849. [Google Scholar] [CrossRef]
- Li, J.; Knapp, D.E.; Schill, S.R.; Roelfsema, C.; Phinn, S.; Silman, M.; Mascaro, J.; Asner, G.P. Adaptive Bathymetry Estimation for Shallow Coastal Waters Using Planet Dove Satellites. Remote Sens. Environ. 2019, 232, 111302. [Google Scholar] [CrossRef]
- Gurlin, D.; Gitelson, A.A.; Moses, W.J. Remote Estimation of Chl-a Concentration in Turbid Productive Waters—Return to a Simple Two-Band NIR-Red Model? Remote Sens. Environ. 2011, 115, 3479–3490. [Google Scholar] [CrossRef]
- Potes, M.; Rodrigues, G.; Penha, A.M.; Novais, M.H.; Costa, M.J.; Salgado, R.; Morais, M.M. Use of Sentinel 2–MSI for Water Quality Monitoring at Alqueva Reservoir, Portugal. Proc. Int. Assoc. Hydrol. Sci. 2018, 380, 73–79. [Google Scholar] [CrossRef]
- Menon, H.B.; Adhikari, A. Remote Sensing of Chlorophyll-A in Case II Waters: A Novel Approach With Improved Accuracy Over Widely Implemented Turbid Water Indices. J. Geophys. Res. Oceans 2018, 123, 8138–8158. [Google Scholar] [CrossRef]
- Lee, Z. Update of the Quasi-Analytical Algorithm (QAA_v6) 2014. Available online: https://www.ioccg.org/groups/Software_OCA/QAA_v6_2014209.pdf (accessed on 1 January 2023).
- Zhu, W.; Yu, Q. Inversion of Chromophoric Dissolved Organic Matter From EO-1 Hyperion Imagery for Turbid Estuarine and Coastal Waters. Geosci. Remote Sens. IEEE Trans. On 2013, 51, 3286–3298. [Google Scholar] [CrossRef]
- Kvålseth, T.O. Cautionary Note about R 2. Am. Stat. 1985, 39, 279–285. [Google Scholar] [CrossRef]
- Seegers, B.N.; Stumpf, R.P.; Schaeffer, B.A.; Loftin, K.A.; Werdell, P.J. Performance Metrics for the Assessment of Satellite Data Products: An Ocean Color Case Study. Opt. Express 2018, 26, 7404. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.B.; Garcia, R.; Hu, C.; Lee, Z. Multi-Band Spectral Matching Inversion Algorithm to Derive Water Column Properties in Optically Shallow Waters: An Optimization of Parameterization. Remote Sens. Environ. 2018, 204, 424–438. [Google Scholar] [CrossRef]
- McKinna, L.I.W.; Werdell, P.J. Approach for Identifying Optically Shallow Pixels When Processing Ocean-Color Imagery. Opt. Express 2018, 26, A915. [Google Scholar] [CrossRef] [PubMed]
- Rymszewicz, A.; O’Sullivan, J.J.; Bruen, M.; Turner, J.N.; Lawler, D.M.; Conroy, E.; Kelly-Quinn, M. Measurement Differences between Turbidity Instruments, and Their Implications for Suspended Sediment Concentration and Load Calculations: A Sensor Inter-Comparison Study. J. Environ. Manag. 2017, 199, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Merten, G.H.; Capel, P.D.; Minella, J.P.G. Effects of Suspended Sediment Concentration and Grain Size on Three Optical Turbidity Sensors. J. Soils Sediments 2014, 14, 1235–1241. [Google Scholar] [CrossRef]
- Minella, J.P.G.; Merten, G.H.; Reichert, J.M.; Clarke, R.T. Estimating Suspended Sediment Concentrations from Turbidity Measurements and the Calibration Problem. Hydrol. Process. 2008, 22, 1819–1830. [Google Scholar] [CrossRef]
- Rasmussen, P.; Gray, J.; Glysson, G.D.; Ziegler, A. Guidelines and Procedures for Computing Time-Series Suspended-Sediment Concentrations and Loads from In-Stream Turbidity-Sensor and Streamflow Data; Techniques and Methods; U.S. Geological Survey: Reston, VA, USA, 2009; ISBN 978 1-4113-2410-7. [Google Scholar]
- Jastram, J.D.; Zipper, C.E.; Zelazny, L.W.; Hyer, K.E. Increasing Precision of Turbidity-Based Suspended Sediment Concentration and Load Estimates. J. Environ. Qual. 2010, 39, 1306–1316. [Google Scholar] [CrossRef]
- Roesler, C.; Uitz, J.; Claustre, H.; Boss, E.; Xing, X.; Organelli, E.; Briggs, N.; Bricaud, A.; Schmechtig, C.; Poteau, A.; et al. Recommendations for Obtaining Unbiased Chlorophyll Estimates from in Situ Chlorophyll Fluorometers: A Global Analysis of WET Labs ECO Sensors. Limnol. Oceanogr. Methods 2017, 15, 572–585. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of Marine and Terrestrial DOM in Seawater Using Excitation-Emission Matrix Spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- D’Andrilli, J.; Silverman, V.; Buckley, S.; Rosario-Ortiz, F.L. Inferring Ecosystem Function from Dissolved Organic Matter Optical Properties: A Critical Review. Environ. Sci. Technol. 2022, 56, 11146–11161. [Google Scholar] [CrossRef]
- Cussioli, M.C.; Seeger, D.; Pratt, D.R.; Bryan, K.R.; Bischof, K.; De Lange, W.P.; Bornman, J.F.; Pilditch, C.A. Spectral Differences in the Underwater Light Regime Caused by Sediment Types in New Zealand Estuaries: Implications for Seagrass Photosynthesis. Geo-Mar. Lett. 2020, 40, 217–225. [Google Scholar] [CrossRef]
- Dierssen, H.M. Hyperspectral Measurements, Parameterizations, and Atmospheric Correction of Whitecaps and Foam From Visible to Shortwave Infrared for Ocean Color Remote Sensing. Front. Earth Sci. 2019, 7, 14. [Google Scholar] [CrossRef]
- Bahr, K.D.; Rodgers, K.S.; Jokiel, P.L.; Prouty, N.G.; Storlazzi, C.D. Pulse Sediment Event Does Not Impact the Metabolism of a Mixed Coral Reef Community. Ocean Coast. Manag. 2020, 184, 105007. [Google Scholar] [CrossRef]
- Bartley, R.; Speirs, W.J.; Ellis, T.W.; Waters, D.K. A Review of Sediment and Nutrient Concentration Data from Australia for Use in Catchment Water Quality Models. Mar. Pollut. Bull. 2012, 65, 101–116. [Google Scholar] [CrossRef]
- Storlazzi, C.D.; Norris, B.K.; Rosenberger, K.J. The Influence of Grain Size, Grain Color, and Suspended-Sediment Concentration on Light Attenuation: Why Fine-Grained Terrestrial Sediment Is Bad for Coral Reef Ecosystems. Coral Reefs 2015, 34, 967–975. [Google Scholar] [CrossRef]
| Model | Reference | Model Type | Input Wavelengths | Parameter |
|---|---|---|---|---|
| SPM 1 | Jiang et al. 2021 [32] | Semi-analytical | Rrs443, Rrs490, Rrs560, Rrs620, Rrs665, Rrs754, Rrs865 | SPM |
| SPM 2 | D’Sa et al. 2007 [20] | Empirical | Rrs671, Rrs551 | SPM |
| SPM 3 | Miller and McKee, 2004 [77] | Empirical | Rrs645 | SPM |
| SPM 4 | Petus et al., 2010 [30] | Empirical | Rrs645 | SPM |
| SPM 5 | König et al., 2023 [71] | Analytical inversion | Rrs400–Rrs900 | SPM |
| SPM 6 | Yu et al., 2019 [29] | Empirical | Rrs486, Rrs551, Rrs671, Rrs745, Rrs862 | SPM |
| SPM 7 | Novoa et al., 2017 [21] | Multi-conditional | Rrs561, Rrs665, Rrs865 | SPM |
| Chl 1 | Hu et al., 2012; 2019 [34,75] | Empirical | Rrs443, Rrs555, Rrs670 | Chl |
| Chl 2 | Keith et al., 2014 [41] | Semi-analytical | Rrs686, Rrs703, Rrs735 | Chl |
| Chl 3 | O’Reilly et al., 1998 [65,66] | Empirical | Rrs443, Rrs489, Rrs510, Rrs560 | Chl |
| Chl 4 | Moses et al., 2009 [78] | Empirical | Rrs665, Rrs708 | Chl |
| Chl 5 | Moses et al., 2009 [78] | Empirical | Rrs665, Rrs708, Rrs753 | Chl |
| Chl 6 | Li et al., 2019 [79] | Empirical | Rrs467, Rrs537, Rrs652 | Chl |
| Chl 7 | Gurlin et al., 2009 [80] | Empirical | Rrs665, Rrs708 | Chl |
| Chl 8 | Gurlin et al., 2009 [80] | Empirical | Rrs665, Rrs708, Rrs753 | Chl |
| Chl 9 | Potes et al., 2018 [81] | Empirical | Rrs443, Rrs560 | Chl |
| Chl 10 | Gitelson et al., 2008 [40] | Semi-analytical | Rrs665, Rrs715, Rrs750 | Chl |
| Chl 11 | Menon and Adhikari, 2018 [82] | Semi-analytical | Rrs663, Rrs623 | Chl |
| Chl 12 | König et al., 2023 [71] | Analytical inversion | Rrs400–Rrs900 | Chl |
| CDOM 1 | Mannino et al., 2008 [69] | Empirical | Rrs490, Rrs551 | aCDOM(443) |
| CDOM 2 | Mannino et al., 2008 [69] | Empirical | Rrs490, Rrs551 | aCDOM(355) |
| CDOM 3 | Mannino et al., 2008 [69] | Empirical | Rrs490, Rrs551 | aCDOM(412) |
| CDOM 4 | Loisel et al., 2014 [76] | Semi-empirical | Rrs412, Rrs555 | aCDOM(412) |
| CDOM 5 | Keith et al., 2014 [41] | Empirical | Rrs670, Rrs490 | aCDOM(412) |
| CDOM 6 | Ficek et al., 2011 [67] | Empirical | Rrs570, Rrs675 | aCDOM(440) |
| CDOM 7 | König et al., 2023 [71] | Analytical inversion | Rrs400–Rrs900 | aCDOM(443) |
| CDOM 8 | Lee, 2014 [83] | Quasi-analytical | Rrs412, Rrs443, Rrs490, Rrs555, Rrs640, Rrs670 | aCDOM(440) |
| CDOM 9 | Zhu and Yu, 2013 [84] | Quasi-analytical | Rrs440, Rrs490, Rrs555, Rrs640 | aCDOM(440) |
| Parameter | Median | Mean | SD | Range |
|---|---|---|---|---|
| Chlorophyll a (µg L−1) | 0.35 | 0.54 | 0.48 | 0.06–2.42 |
| aCDOM(355) (m−1) | 0.2 | 0.21 | 0.11 | 0.01–0.54 |
| aCDOM(443) (m−1) | 0.05 | 0.05 | 0.03 | 0–0.13 |
| fDOM (QSU) | 1.83 | 2.24 | 1.73 | 0.13–8.92 |
| SPM (mg L−1) | 1.06 | 2.44 | 4.62 | 0.22–23.89 |
| Salinity (psu) | 35.2 | 34.3 | 2.1 | 27.6–35.8 |
| Sexp | 0.022 | 0.023 | 0.005 | 0.016–0.039 |
| Turbidity (FNU) | 0.79 | 1.77 | 3.33 | 0.04–18.95 |
| RMSE | R2 | MAE | Bias | %wins | Accuracy | Reference | |
|---|---|---|---|---|---|---|---|
| Suspended particulate matter models | |||||||
| SPM 1 | 2.74 | 0.76 | 1.20 | −0.08 | 62% | 93% | [32] |
| SPM 2 | 3.29 | 0.58 | 1.49 | −1.31 | 48% | 72% | [20] |
| SPM 3 | 3.31 | 0.61 | 1.67 | −1.35 | 45% | 94% | [77] |
| SPM 4 | 3.34 | 0.64 | 1.54 | −0.06 | 40% | 92% | [30] |
| SPM 5 | 3.53 | 0.62 | 2.39 | 1.77 | 31% | 77% | [71] |
| SPM 6 | 3.76 | 0.52 | 1.72 | 0.70 | 57% | 91% | [29] |
| SPM 7 | 4.49 | 0.39 | 1.63 | −1.30 | 67% | 50% | [21] |
| Chlorophyll a models | |||||||
| Chl 1 ** | 0.46 | 0.16 | 0.29 | −0.11 | 96% | 62% | This study |
| Colored Dissolved Organic Matter models | |||||||
| CDOM 1 ** | 0.03 | 0.34 | 0.02 | 0.00 | 87% | 50% | This study |
| CDOM 1 | 0.08 | 0.24 | 0.07 | 0.07 | 44% | 60% | [69] |
| CDOM 2 ** | 0.08 | 0.49 | 0.06 | 0.00 | 97% | 50% | This study |
| CDOM 3 | 0.15 | 0.28 | 0.13 | 0.13 | 50% | 58% | [69] |
| CDOM 4 | 0.24 | 0.11 | 0.17 | 0.17 | 47% | 59% | [76] |
| CDOM 5 | 0.29 | 0.18 | 0.16 | 0.14 | 73% | 71% | [41] |
| CDOM 2 | 0.41 | 0.26 | 0.36 | 0.36 | 3% | 55% | [69] |
| CDOM 6 | 0.61 | 0.10 | 0.32 | 0.32 | 74% | 51% | [67] |
| AC | GC | RMSE | R2 | MAE | Bias | |
|---|---|---|---|---|---|---|
| SPM | ATREM | None | 8.72 | 0.48 | 3.96 | 3.58 |
| Gao and Li | 2.95 | 0.70 | 1.40 | 0.77 | ||
| 4C | 2.59 | 0.71 | 1.07 | 0.22 | ||
| ISOFIT | None | 11.8 | 0.48 | 6.26 | 5.98 | |
| Gao and Li | 4.61 | 0.69 | 2.93 | 2.48 | ||
| 4C | 2.74 | 0.76 | 1.20 | −0.08 | ||
| Chl | ATREM | None | 0.82 | 0.21 | 0.48 | 0.32 |
| Gao and Li | 0.55 | 0.17 | 0.30 | 0.06 | ||
| 4C | 0.50 | 0.14 | 0.31 | −0.03 | ||
| ISOFIT | None | 1.12 | 0.18 | 0.74 | 0.61 | |
| Gao and Li | 0.60 | 0.16 | 0.36 | 0.16 | ||
| 4C | 0.46 | 0.16 | 0.29 | −0.11 | ||
| CDOM | ATREM | None | 0.027 | 0.31 | 0.02 | 0.00 |
| Gao and Li | 0.128 | 0.02 | 0.03 | 0.02 | ||
| 4C | 0.025 | 0.34 | 0.02 | 0.00 | ||
| ISOFIT | None | 0.028 | 0.28 | 0.02 | 0.00 | |
| Gao and Li | 0.027 | 0.32 | 0.02 | 0.00 | ||
| 4C | 0.025 | 0.34 | 0.02 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hondula, K.L.; König, M.; Grunert, B.K.; Vaughn, N.R.; Martin, R.E.; Dai, J.; Jamalinia, E.; Asner, G.P. Mapping Water Quality in Nearshore Reef Environments Using Airborne Imaging Spectroscopy. Remote Sens. 2024, 16, 1845. https://doi.org/10.3390/rs16111845
Hondula KL, König M, Grunert BK, Vaughn NR, Martin RE, Dai J, Jamalinia E, Asner GP. Mapping Water Quality in Nearshore Reef Environments Using Airborne Imaging Spectroscopy. Remote Sensing. 2024; 16(11):1845. https://doi.org/10.3390/rs16111845
Chicago/Turabian StyleHondula, Kelly L., Marcel König, Brice K. Grunert, Nicholas R. Vaughn, Roberta E. Martin, Jie Dai, Elahe Jamalinia, and Gregory P. Asner. 2024. "Mapping Water Quality in Nearshore Reef Environments Using Airborne Imaging Spectroscopy" Remote Sensing 16, no. 11: 1845. https://doi.org/10.3390/rs16111845
APA StyleHondula, K. L., König, M., Grunert, B. K., Vaughn, N. R., Martin, R. E., Dai, J., Jamalinia, E., & Asner, G. P. (2024). Mapping Water Quality in Nearshore Reef Environments Using Airborne Imaging Spectroscopy. Remote Sensing, 16(11), 1845. https://doi.org/10.3390/rs16111845







