Above- and Belowground Biomass Carbon Stock and Net Primary Productivity Maps for Tidal Herbaceous Marshes of the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Spatially Explicit Aboveground Carbon Stock
2.2. CONUS Aboveground Turnover Rate
2.3. Spatially Explicit Net Primary Production
3. Results
3.1. Spatially Explicit Total Peak Biomass Carbon Stocks
3.2. Aboveground Turnover Rate and Spatially Explicit Net Primary Production
4. Discussion
4.1. Regional Trends
4.2. Comparisons
4.3. Limitations
4.4. Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Girardin, C.A.; Jenkins, S.; Seddon, N.; Allen, M.; Lewis, S.L.; Wheeler, C.E.; Griscom, B.W.; Malhi, Y. Nature-based solutions can help cool the planet—If we act now. Nature 2021, 593, 191–194. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17, 1111. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Costa, M.D.P.; Atwood, T.B.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Lovelock, C.E.; Serrano, O.; Duarte, C.M. Blue carbon as a natural climate solution. Nat. Rev. Earth Environ. 2021, 2, 826–839. [Google Scholar] [CrossRef]
- Saintilan, N.; Kovalenko, K.E.; Guntenspergen, G.; Rogers, K.; Lynch, J.C.; Cahoon, D.R.; Lovelock, C.E.; Friess, D.A.; Ashe, E.; Krauss, K.W.; et al. Constraints on the adjustment of tidal marshes to accelerating sea level rise. Science 2022, 377, 523–527. [Google Scholar] [CrossRef]
- Buffington, K.J.; Dugger, B.D.; Thorne, K.M. Climate-related variation in plant peak biomass and growth phenology across Pacific Northwest tidal marshes. Estuar. Coast. Shelf. Sci. 2018, 202, 212–221. [Google Scholar] [CrossRef]
- Buffington, K.J.; Goodman, A.C.; Freeman, C.M.; Thorne, K.M. Testing the interactive effects of flooding and salinity on tidal marsh plant productivity. Aquat. Bot. 2020, 164, 103231. [Google Scholar] [CrossRef]
- McKee, K.L.; Mendelssohn, I.A.; Materne, M.D. Acute salt marsh dieback in the Mississippi River deltaic plain: A drought-induced phenomenon? Glob. Ecol. Biogeogr. 2004, 13, 65–73. [Google Scholar] [CrossRef]
- Stagg, C.L.; Osland, M.J.; Moon, J.A.; Feher, L.C.; Laurenzano, C.; Lane, T.C.; Jones, W.R.; Hartley, S.B. Extreme Precipitation and Flooding Contribute to Sudden Vegetation Dieback in a Coastal Salt Marsh. Plants 2021, 10, 1841. [Google Scholar] [CrossRef]
- Borchert, S.M.; Osland, M.J.; Enwright, N.M.; Griffith, K.T. Coastal wetland adaptation to sea level rise: Quantifying potential for landward migration and coastal squeeze. J. Appl. Ecol. 2018, 55, 2876–2887. [Google Scholar] [CrossRef]
- Whigham, D.F.; Baldwin, A.H.; Barendregt, A. Chapter 18: Tidal Freshwater Wetlands. In Coastal Wetlands; Elsevier: Amsterdam, The Netherlands, 2019; pp. 619–640. [Google Scholar]
- U.S. Fish and Wildlife Service (FWS). National Wetlands Inventory Website; U.S. Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 2014.
- Couvillion, B.R.; Beck, H.; Schoolmaster, D.; Fischer, M. Land Area Change in Coastal Louisiana 1932 to 2016; Scientific Investigations Map 3381; U.S. Geological Survey: Liston, VA, USA, 2017; p. 16. [Google Scholar] [CrossRef]
- Crooks, S.; Sutton-Grier, A.E.; Troxler, T.G.; Herold, N.; Bernal, B.; Schile-Beers, L.; Wirth, T. Coastal wetland management as a contribution to the US National Greenhouse Gas Inventory. Nat. Clim. Chang. 2018, 8, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (EPA). Inventory of US Greenhouse Gas Emissions and Sinks: 1990–2020; EPA 430-R-22-003; US Environmental Protection Agency: Washington, DC, USA, 2022. Available online: https://www.epa.gov/ghgemissions/draft-inventory-us-greenhouse-gas-emissions-and-sinks-1990-2020 (accessed on 1 May 2022).
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme’s; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Hayama, Japan, 2006. [Google Scholar]
- IPCC. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; Hiraishi, T., Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M., Troxler, T.G., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., Federici, S., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Kang, X.; Li, Y.; Wang, J.; Yan, L.; Zhang, X.; Wu, H.; Yan, Z.; Zhang, K.; Hao, Y. Precipitation and temperature regulate the carbon allocation process in alpine wetlands: Quantitative simulation. J. Soils. Sediments 2020, 20, 3300–3315. [Google Scholar] [CrossRef]
- Feagin, R.A.; Forbrich, I.; Huff, T.P.; Barr, J.G.; Ruiz-Plancarte, J.; Fuentes, J.D.; Najjar, R.G.; Vargas, R.; Vázquez-Lule, A.; Windham-Myers, L.; et al. Tidal wetland gross primary production across the continental United States, 2000–2019. Global Biogeochem. Cycles 2020, 34, e2019GB006349. [Google Scholar] [CrossRef]
- Byrd, K.B.; Ballanti, L.; Thomas, N.; Nguyen, D.; Holmquist, J.R.; Simard, M.; Windham-Myers, L. A remote sensing-based model of tidal marsh aboveground carbon stocks for the conterminous United States. ISPRS J. Photogramm. 2018, 139, 255–271. [Google Scholar] [CrossRef]
- Byrd, K.B.; Ballanti, L.; Thomas, N.; Nguyen, D.; Holmquist, J.R.; Simard, M.; Windham-Myers, L. Corrigendum to “A remote sensing-based model of tidal marsh aboveground carbon stocks for the conterminous United States. ISPRS J. Photogramm. 2020, 166, 63–67. [Google Scholar] [CrossRef]
- Kurz, W.; Dymond, C.; White, T.; Stinson, G.; Shaw, C.; Rampley, G.; Smyth, C.; Simpson, B.; Neilson, E.; Trofymow, J.; et al. CBM-CFS3: A model of carbon-dynamics in forestry and land-use change implementing IPCC standards. Ecol. Modell. 2009, 220, 480–504. [Google Scholar] [CrossRef]
- Sleeter, B.M.; Frid, L.; Rayfield, B.; Daniel, C.; Zhu, Z.; Marvin, D.C. Operational assessment tool for forest carbon dynamics for the United States: A new spatially explicit approach linking the LUCAS and CBM-CFS3 models. Carbon Balance Manag. 2022, 17, 1–26. [Google Scholar] [CrossRef]
- Mo, Y.; Kearney, M.S.; Riter, J.A.; Zhao, F.; Tilley, D.R. Assessing biomass of diverse coastal marsh ecosystems using statistical and machine learning models. Int. J. Appl. Earth Obs. Geoinf. 2018, 68, 189–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, S. Fusion of multiple gridded biomass datasets for generating a global forest aboveground biomass map. Remote Sens. 2020, 12, 2559. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Song, Z.; Chen, C.; Zhang, Y.; Chen, X.; Chen, W.; Yuan, W.; Wu, X.; Ran, X.; et al. Modelling Aboveground Biomass Carbon Stock of the Bohai Rim Coastal Wetlands by Integrating Remote Sensing, Terrain, and Climate Data. Remote Sens. 2021, 13, 4321. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn 2001, 45, 5–32. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). National Wetland Condition Assessment 2011: A Collaborative Survey of the Nation’s Wetlands; EPA EPA-843-R-15-005; US Environmental Protection Agency: Washington, DC, USA, 2016.
- U.S. Environmental Protection Agency (EPA). Level III Ecoregions of the Continental United States; map scale 1:7,500,000; National Health and Environmental Effects Research Laboratory: Corvallis, OR, USA, 2013. Available online: https://www.epa.gov/eco-research/level-iii-and-iv-ecoregions-continental-united-states (accessed on 2 March 2020).
- Steeves, P.; Nebert, D. 1:250,000-Scale Hydrologic Units of the United States; Open-File Report. 94-0236; U.S. Geological Survey: Reston, VA, USA, 1994. Available online: https://water.usgs.gov/lookup/getspatial?huc250k (accessed on 9 January 2021).
- Wang, F.; Lu, X.; Sanders, C.J.; Tang, J. Tidal wetland resilience to sea level rise increases their carbon sequestration capacity in United States. Nat. Commun. 2019, 10, 5434. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.R.; Windham-Myers, L.; Kirwan, M. Sea-level rise enhances carbon accumulation in United States tidal wetlands. One Earth 2021, 4, 425–433. [Google Scholar] [CrossRef]
- Noe, G.B.; Childers, D.L.; Jones, R.D. Phosphorus biogeochemistry and the impact of phosphorus enrichment: Why is the Everglades so unique? Ecosystems 2001, 4, 603–624. [Google Scholar] [CrossRef]
- Richardson, C.J. The everglades: North America’s subtropical wetland. Wetl. Ecol. Manag. 2010, 18, 517–542. [Google Scholar] [CrossRef]
- Office for Coastal Management. NOAA’s Coastal Change Analysis Program (C-CAP) 2010 Regional Land Cover Data–Coastal United States. 2022. Available online: https://www.fisheries.noaa.gov/inport/item/48335 (accessed on 19 February 2020).
- Holmquist, J.R.; Windham-Myers, L. Relative Tidal Marsh Elevation Maps with Uncertainty for Conterminous USA, 2010; ORNL DAAC: Oak Ridge, TN, USA, 2021. [Google Scholar] [CrossRef]
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; R Core Team; et al. Caret: Classification and Regression Training; R Package Version 6.0-84. 2019. Available online: https://CRAN.R-project.org/package=caret (accessed on 4 November 2021).
- R Core Team. R: A Language and Environment for Statistical Computing (3.6.3); R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 4 November 2021).
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Enclona, E.A.; Ashton, M.S.; Van Der Meer, B. Accuracy assessments of hyperspectral waveband performance for vegetation analysis applications. Remote Sens. Environ. 2004, 91, 354–376. [Google Scholar] [CrossRef]
- Zhang, M.; Ustin, S.L.; Rejmankova, E.; Sanderson, E.W. Monitoring Pacific coast salt marshes using remote sensing. Ecol. Appl. 1997, 7, 1039–1053. [Google Scholar] [CrossRef]
- Byrd, K.B.; Windham-Myers, L.; Leeuw, T.; Downing, B.; Morris, J.T.; Ferner, M.C. Forecasting tidal marsh elevation and habitat change through fusion of Earth observations and a process model. Ecosphere 2016, 7, e01582. [Google Scholar] [CrossRef]
- Google Earth Engine. USGS Landsat 8 Surface Reflectance Tier 1. Earth Engine Data Catalog. Available online: https://developers.google.com/earth-engine/datasets/catalog/LANDSAT_LC08_C01_T1_SR (accessed on 31 December 2021).
- Falgout, J.T.; Gordon, J. USGS Advanced Research Computing, USGS Yeti Supercomputer; U.S. Geological Survey: Reston, VA, USA, 2021. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). Advanced Research Computing. USGS Yeti Supercomputer: U.S. Geological Survey, n.d. Available online: https://www.usgs.gov/advanced-research-computing (accessed on 3 August 2022).
- Turner, R.E. Geographic variations in salt marsh macrophyte production: A review. Contrib. Mar. Sci. 1976, 20, 47–68. [Google Scholar]
- Edwards, K.R.; Mills, K.P. Aboveground and belowground productivity of spartina alterniflora (smooth cordgrass) in natural and created Louisiana salt marshes. Estuaries 2005, 28, 252–265. [Google Scholar] [CrossRef]
- Woltz, V.L.; Stagg, C.L.; Byrd, K.B.; Windham-Myers, L.; Rovai, A.S.; Zhu, Z. Biomass Carbon Stock and Net Primary Productivity in Tidal Herbaceous Wetlands of the Conterminous United States; U.S. Geological Survey: Reston, VA, USA, 2022. [Google Scholar] [CrossRef]
- Dame, R.F.; Kenny, P.D. Variability of Spartina alterniflora primary production in the euhaline North Inlet estuary. Mar. Ecol. Prog. Ser. 1986, 32, 71–80. Available online: http://www.jstor.org/stable/24825479 (accessed on 11 February 2020).
- Schile, L.M.; Callaway, J.C.; Suding, K.N.; Kelly, N.M. Can community structure track sea-level rise? Stress and competitive controls in tidal wetlands. Ecol. Evol. 2017, 7, 1276–1285. [Google Scholar] [CrossRef]
- Smalley, A.E. The Role of Two Invertebrate Populations, Littorina irrorata and Orchelium fificinium, in the Energy Flow of a Salt Marsh Ecosystem. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 1958. [Google Scholar]
- Wiegert, R.G.; Evans, F.C. Primary production and the disappearance of dead vegetation on an old field in southeastern Michigan. Ecology 1964, 45, 49–63. [Google Scholar] [CrossRef]
- Omernik, J.M.; Griffith, G.E. Ecoregions of the conterminous United States: Evolution of a hierarchical spatial framework. Environ. Manag. 2014, 54, 1249–1266. [Google Scholar] [CrossRef]
- Mendelsohn, R. Land Use, Land Use Change, and Forestry: Special Report of the Intergovernmental Panel on Climate Change; Watson, R.T., Noble, I.R., Bolin, B., Ravindranath, N.H., Verardo, D.J., Dokken, D.J., Eds.; Environmental Conservation; Cambridge University Press: Cambridge, UK, 2000; Volume 28, pp. 284–293. [Google Scholar] [CrossRef]
- Erb, K.H.; Fetzel, T.; Plutzar, C.; Kastner, T.; Lauk, C.; Mayer, A.; Niedertscheider, M.; Körner, C.; Haberl, H. Biomass turnover time in terrestrial ecosystems halved by land use. Nat. Geosci. 2016, 9, 674–678. [Google Scholar] [CrossRef]
- Kukal, M.S.; Irmak, S. US agro-climate in 20th century: Growing degree days, first and last frost, growing season length, and impacts on crop yields. Sci. Rep. 2018, 8, 6977. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture (USDA). California Crops Under Climate Change: Impacts and Opportunities for California Agriculture, n.d. Available online: https://www.climatehubs.usda.gov/hubs/california/california-crops-under-climate-change (accessed on 2 February 2023).
- Holmquist, J.R.; Windham-Myers, L. A Conterminous USA-Scale Map of Relative Tidal Marsh Elevation. Estuar. Coast 2022, 45, 1596–1614. [Google Scholar] [CrossRef]
- Smith, S.M. Vegetation change in salt marshes of Cape Cod National Seashore (Massachusetts, USA) between 1984 and 2013. Wetlands 2015, 35, 127–136. [Google Scholar] [CrossRef]
- Stagg, C.L.; Schoolmaster, D.R.; Piazza, S.C.; Snedded, G.; Steyer, G.D.; Fischenich, C.J.; McComas, R.W. A Landscape-scale assessment of above- and belowground primary production in coastal wetlands: Implications for climate change-induced community shifts. Estuaries Coast 2017, 40, 856–879. [Google Scholar] [CrossRef]
- Woo, I.; Takekawa, J.Y. Will inundation and salinity levels associated with projected sea level rise reduce the survival, growth, and reproductive capacity of Sarcocornia pacifica (pickleweed)? Aquat. Bot. 2012, 102, 8–14. [Google Scholar] [CrossRef]
- Wilson, B.J.; Servais, S.; Charles, S.P.; Mazzei, V.; Gaiser, E.; Kominoski, J.S.; Richards, J.H.; Troxler, T.G. Phosphorus alleviation of salinity stress: Effects of saltwater intrusion on an Everglades freshwater peat marsh. Ecology 2019, 100, e02672. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kominoski, J.S.; Kline, M.; Robinson, M.; Roebling, S. Saltwater and nutrient legacies reduce net ecosystem carbon storage despite freshwater restoration: Insights from experimental wetlands. Restor. Ecol. 2021, 30, e13524. [Google Scholar] [CrossRef]
- Charles, S.P.; Kominoski, J.S.; Troxler, T.G.; Gaiser, E.E.; Servais, S.; Wilson, B.J.; Davis, S.E.; Sklar, F.H.; Coronado-Molina, C.; Madden, C.J.; et al. Experimental saltwater intrusion drives rapid soil elevation and carbon loss in freshwater and brackish Everglades marshes. Estuar. Coast. 2019, 42, 1868–1881. [Google Scholar] [CrossRef]
- Slocum, M.G.; Platt, W.J.; Beckage, B.; Panko, B.; Lushine, J.B. Decoupling natural and anthropogenic fire regimes: A case study in Everglades National Park, Florida. Nat. Areas J. 2007, 27, 41–55. [Google Scholar] [CrossRef]
- Wilson, B.J.; Servais, S.; Charles, S.P.; Davis, S.E.; Gaiser, E.E.; Kominoski, J.S.; Richards, J.H.; Troxler, T.G. Declines in plant productivity drive carbon loss from brackish coastal wetland mesocosms exposed to saltwater intrusion. Estuar. Coast 2018, 41, 2147–2158. [Google Scholar] [CrossRef]
- Ishtiaq, K.S.; Troxler, T.G.; Lamb-Wotton, L.; Wilson, B.J.; Charles, S.P.; Davis, S.E.; Kominoski, J.S.; Rudnick, D.T.; Sklar, F.H. Modeling net ecosystem carbon balance and loss in coastal wetlands exposed to sea-level rise and saltwater intrusion. Ecol. Appl. 2022, 32, e2702. [Google Scholar] [CrossRef]
- Tavakol, A.; Rahmani, V.; Harrington, J. Temporal and spatial variations in the frequency of compound hot, dry, and windy events in the central United States. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Withers, K.; Tunnell, J.W.; Judd, F.W. Wind-tidal flats. In The Laguna Madre of Texas and Tamaulipas, 1st ed.; Tunnell, J.W., Judd, F.W., Eds.; Texas A&M University Press: College Town, TX, USA, 2002; pp. 114–126. [Google Scholar]
- Osland, M.J.; Grace, J.B.; Guntenspergen, G.R.; Thorne, K.M.; Carr, J.A.; Feher, L.C. Climatic controls on the distribution of foundation plant species in coastal wetlands of the conterminous United States: Knowledge gaps and emerging research needs. Estuar. Coast 2019, 42, 1991–2003. [Google Scholar] [CrossRef]
- McKee, K.L.; Mendelssohn, I.A. Response of a freshwater marsh plant community to increased salinity and increased water level. Aquat. Bot. 1989, 34, 301–316. [Google Scholar] [CrossRef]
- Willis, J.M.; Hester, M.W. Interactive effects of salinity, flooding, and soil type on Panicum hemitomon. Wetlands 2004, 24, 43–50. [Google Scholar] [CrossRef]
- Baustian, M.M.; Stagg, C.L.; Perry, C.L.; Moss, L.C.; Carruthers, T.J.; Allison, M. Relationships between salinity and short-term soil carbon accumulation rates from marsh types across a landscape in the Mississippi River Delta. Wetlands 2017, 37, 313–324. [Google Scholar] [CrossRef]
- Callaway, J.C.; Josselyn, M.N. The introduction and spread of smooth cordgrass (Spartina alterniflora) in South San Francisco Bay. Estuaries 1992, 15, 218–226. [Google Scholar] [CrossRef]
- Zedler, J.B.; Winfield, T.; Williams, P. Salt marsh productivity with natural and altered tidal circulation. Oecologia 1980, 44, 236–240. [Google Scholar] [CrossRef]
- Ruber, E.; Gillis, G.; Montagna, P.A. Production of dominant emergent vegetation and of pool algae on a northern Massachusetts salt marsh. Bull. Torrey Bot. Club 1981, 108, 180–188. [Google Scholar] [CrossRef]
- Valiela, I.; Teal, J.M.; Sass, W.J. Production and Dynamics of Salt Marsh Vegetation and the Effects of Experimental Treatment with Sewage Sludge: Biomass, Production and Species Composition. J. Appl. Ecol 1975, 12, 973–981. [Google Scholar] [CrossRef]
- Sickels, F.A.; Simpson, R.L. Growth and survival of giant ragweed (Ambrosia trifida L.) in a Delaware River freshwater tidal wetland. Bull. Torrey Bot. Club 1985, 112, 368–375. [Google Scholar] [CrossRef]
- Odum, E.P.; Fanning, M.E. Comparison of the productivity of Spartina alterniflora and S. cynosuroides in Georgia coastal marshes. Bull. Ga. Acad. Sci. 1973, 31, 1–12. [Google Scholar]
- Daoust, R.J.; Childers, D.L. Ecological effects of low-level phosphorus additions on two plant communities in a neotropical freshwater wetland ecosystem. Oecologia 2004, 141, 672–686. [Google Scholar] [CrossRef]
- NASA. MODIS Gross Primary Production (GPP)/Net Primary Production (NPP); MODIS: Moderate Resolution Imaging Spectrometer: Sioux Falls, SD, USA, 2021. Available online: http://modis.gsfc.nasa.gov/data/dataprod/mod17.php (accessed on 19 October 2021).
- Breemen, N.; Buurman, P. Biological processes in soils. In Soil Formation, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 83–121. [Google Scholar]
- Chapman, S. (Villanova University, Villanova, PA, USA). Personal communication, 2021.
- Morris, J.T.; Callaway, J.C. Physical and biological regulation of carbon sequestration in tidal marshes. In A Blue Carbon Primer, 1st ed.; Windham-Myers, L., Crooks, S., Troxler, T.G., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 67–79. [Google Scholar]
- Utari, D.; Kamal, M.; Sidik, F. Above-ground biomass estimation of mangrove forest using WorldView-2 imagery in Perancak Estuary, Bali. In Proceedings of the IOP Conference Series Earth and Environmental Science, West Java, Indonesia, 17–20 September 2019; IOP Publishing: Bristol, UK, 2020; Volume 500, p. 012011. [Google Scholar]
- Bhatti, S.; Ahmad, S.R.; Asif, M. Estimation of aboveground carbon stock using Sentinel-2A data and Random Forest algorithm in scrub forests of the Salt Range, Pakistan. J. For. Res 2022, cpac036. [Google Scholar] [CrossRef]
- Ganju, N.K.; Couvillion, B.R.; Defne, Z.; Ackerman, K.V. Development and Application of Landsat-Based Wetland Vegetation Cover and UnVegetated-Vegetated Marsh Ratio (UVVR) for the Conterminous United States. Estuar. Coast 2022, 45, 1861–1878. [Google Scholar] [CrossRef]
- MultiResolution Land Characteristics (MRLC) Consortium National Land Cover Database (NLCD). Sioux Falls, SD: U.S. Geological Survey. Available online: http://www.mrlc.gov (accessed on 22 July 2020).
- Zeng, J.; Sun, Y.; Cao, P.; Wang, H. A phenology-based vegetation index classification (PVC) algorithm for coastal salt marshes using Landsat 8 images. Int. J. Appl. Earth Obs. Geoinf 2022, 110, 102776. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS). What are the Acquisition Schedules for the Landsat Satellites? USGS: Science for a Changing World, n.d. Available online: https://www.usgs.gov/faqs/what-are-acquisition-schedules-landsat-satellites#:~:text=Each%20satellite%20makes%20a%20complete,scene%20area%20on%20the%20globe (accessed on 12 December 2022).
- Kearney, M.S.; Stutzer, D.; Turpie, K.; Stevenson, J.C. The effects of tidal inundation on the reflectance characteristics of coastal marsh vegetation. J. Coast Res. 2009, 25, 1177–1186. [Google Scholar] [CrossRef]
- Ray, S.S.; Das, G.; Singh, J.P.; Panigrahy, S. Evaluation of hyperspectral indices for LAI estimation and discrimination of potato crop under different irrigation treatments. Int. J. Remote Sens. 2007, 27, 5373–5387. [Google Scholar] [CrossRef]
- Sleeter, R.; Sleeter, B.M.; Williams, B.; Hogan, D.; Hawbaker, T.; Zhu, Z. A carbon balance model for the great dismal swamp ecosystem. Carbon Balance Manag. 2017, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, Y.; White, J.R. Carbon offset market methodologies applicable for coastal wetland restoration and conservation in the United States: A review. Sci. Total Environ. 2020, 701, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Soil adjusted vegetative index (SAVI): |
| 1: SAVI = ((NIR − R)/(NIR + R + 0.5)) × 1.5 |
| Wide dynamic range vegetation index (WDRVI): |
| 2: WDRVI = (0.5 × NIR − R)/(0.5 × NIR + R) |
| Two-band vegetation indices (TBVI): |
| 3: TBVIRG = (R − G)/(R + G) |
| 4: TBVIGB = (G − B)/(G + B) |
| 5: TBVISR = (Swir2 − R)/(Swir2 + R) |
| 6: TBVISN = (Swir2 − NIR)/(Swir2 + NIR) |
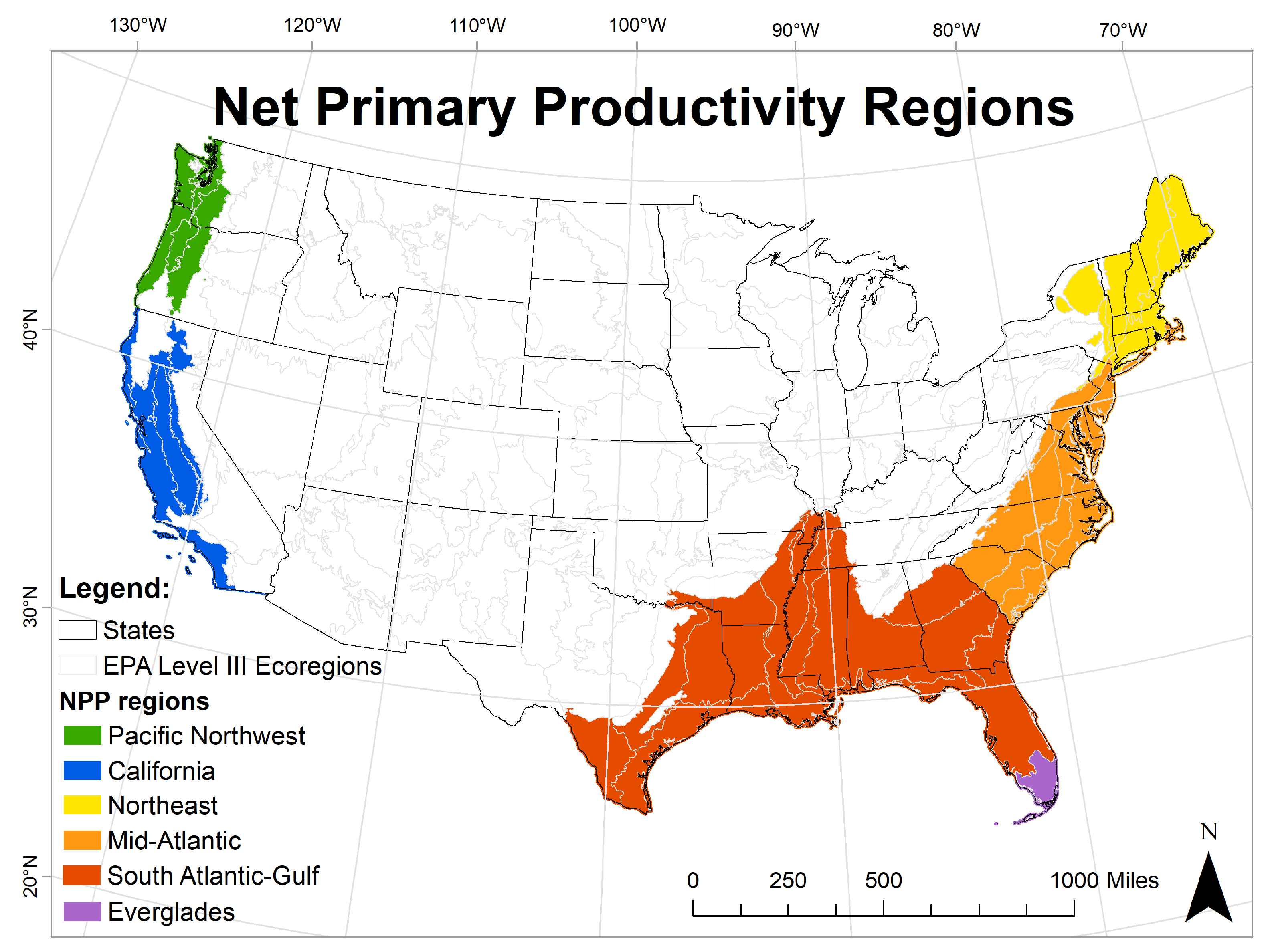
| Region |
| 7: Wetland NPP region * |
| Variable | Importance Score |
|---|---|
| SAVI | 100 |
| nd_swir2_r | 95.59 |
| Everglades | 91.98 |
| San Francisco Bay-freshwater | 80.29 |
| WDRVI5 | 75.17 |
| nd_g_b | 72.45 |
| nd_swir2_nir | 69.2 |
| nd_r_g | 49.75 |
| San Francisco Bay-brackish and saltwater | 38.7 |
| Puget Sound | 13.17 |
| Louisiana | 0.57 |
| Chesapeake | 0 |
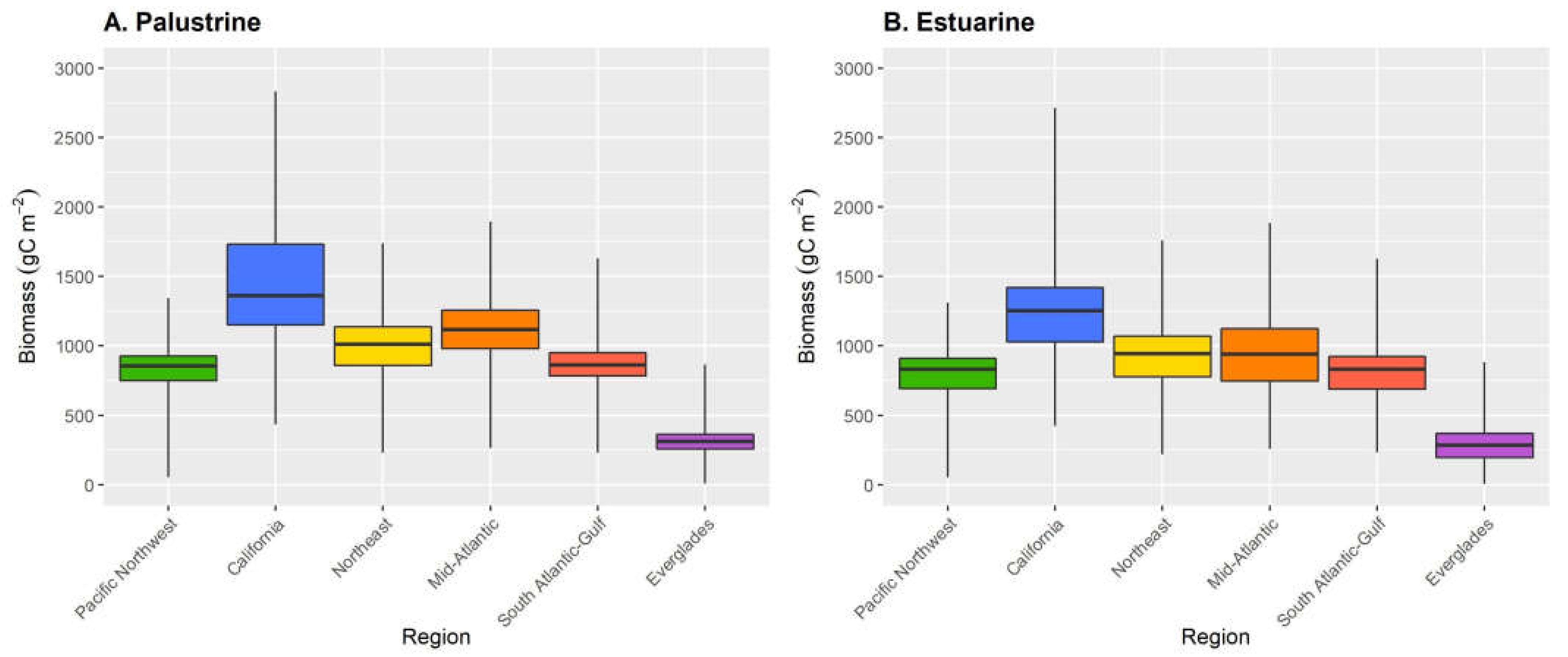
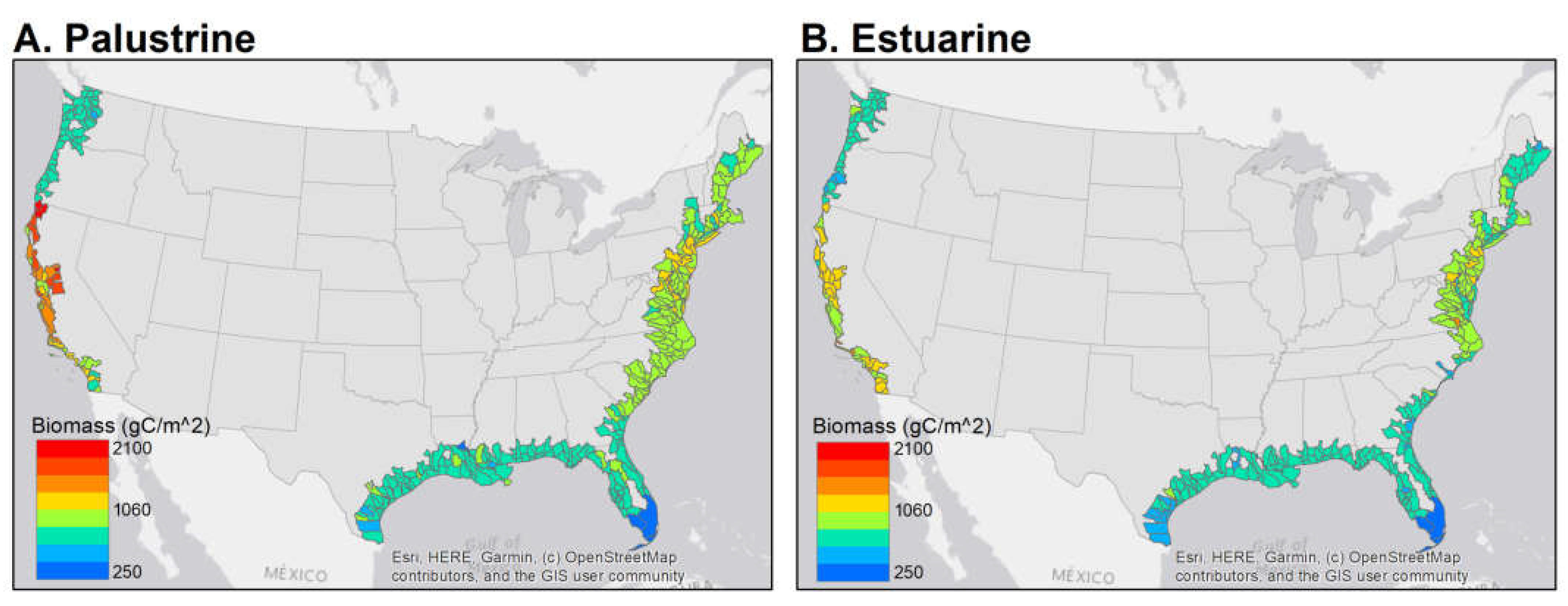
| Marsh Class | Region | Area (km2) | Mean Total Carbon ±SD (g C m−2) |
|---|---|---|---|
| Palustrine | Pacific Northwest | 217 | 838 ± 129 |
| California | 133 | 1441 ± 430 | |
| Northeast | 58 | 998 ± 205 | |
| Mid-Atlantic | 724 | 1118 ± 206 | |
| South Atlantic-Gulf | 4854 | 868 ± 123 | |
| Everglades | 426 | 309 ± 79 | |
| Estuarine | Pacific Northwest | 86 | 801 ± 162 |
| California | 343 | 1223 ± 288 | |
| Northeast | 253 | 923 ± 217 | |
| Mid-Atlantic | 4266 | 935 ± 278 | |
| South Atlantic-Gulf | 10,279 | 806 ± 172 | |
| Everglades | 419 | 283 ± 130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woltz, V.L.; Stagg, C.L.; Byrd, K.B.; Windham-Myers, L.; Rovai, A.S.; Zhu, Z. Above- and Belowground Biomass Carbon Stock and Net Primary Productivity Maps for Tidal Herbaceous Marshes of the United States. Remote Sens. 2023, 15, 1697. https://doi.org/10.3390/rs15061697
Woltz VL, Stagg CL, Byrd KB, Windham-Myers L, Rovai AS, Zhu Z. Above- and Belowground Biomass Carbon Stock and Net Primary Productivity Maps for Tidal Herbaceous Marshes of the United States. Remote Sensing. 2023; 15(6):1697. https://doi.org/10.3390/rs15061697
Chicago/Turabian StyleWoltz, Victoria L., Camille LaFosse Stagg, Kristin B. Byrd, Lisamarie Windham-Myers, Andre S. Rovai, and Zhiliang Zhu. 2023. "Above- and Belowground Biomass Carbon Stock and Net Primary Productivity Maps for Tidal Herbaceous Marshes of the United States" Remote Sensing 15, no. 6: 1697. https://doi.org/10.3390/rs15061697
APA StyleWoltz, V. L., Stagg, C. L., Byrd, K. B., Windham-Myers, L., Rovai, A. S., & Zhu, Z. (2023). Above- and Belowground Biomass Carbon Stock and Net Primary Productivity Maps for Tidal Herbaceous Marshes of the United States. Remote Sensing, 15(6), 1697. https://doi.org/10.3390/rs15061697






