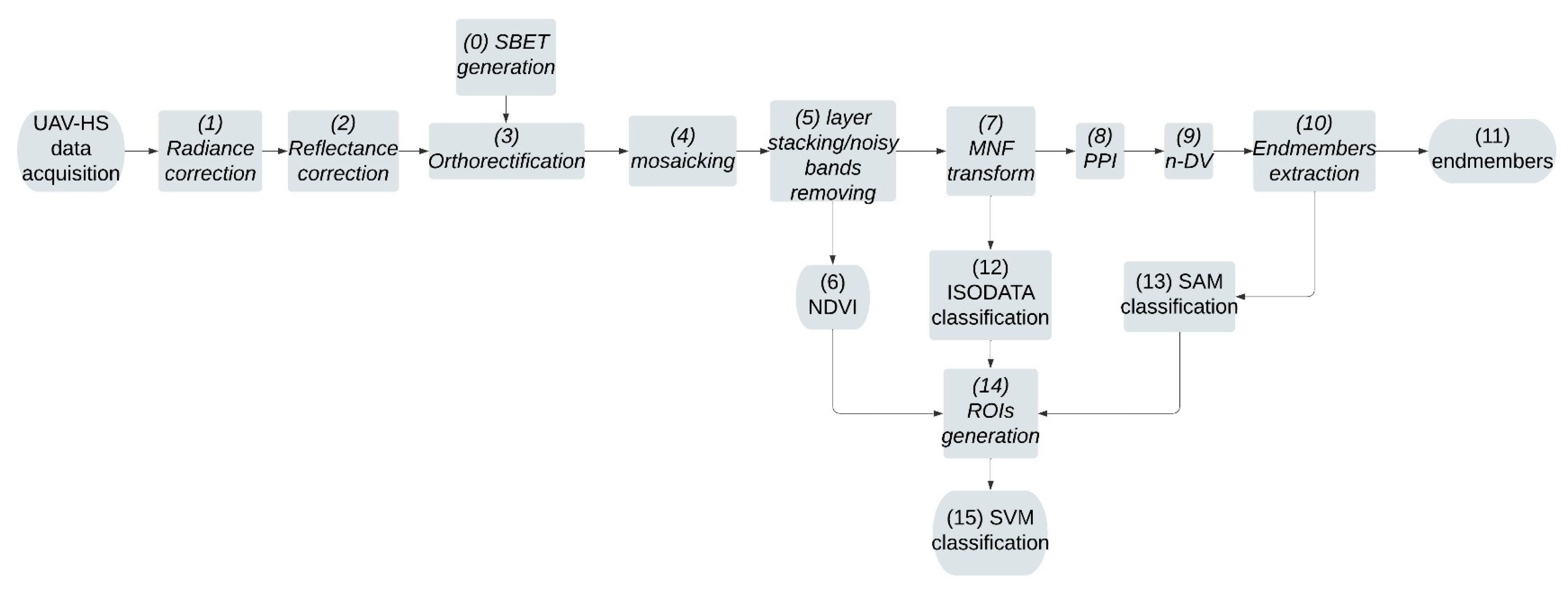
1. Introduction
Salt marshes are ecological transition zones where marine and terrestrial ecosystems interact [
1]. These ecosystems are characterized by a unique and highly specific assemblage of plants and animals [
2] and high primary production, with the plant species being a crucial component of the system dynamics [
3]. They offer numerous recognized ecosystem services; highlights among them are the services of coastal protection and blue carbon sink [
4,
5,
6,
7].
Tidal salt marsh vegetation is typically halophyte and has to tolerate regular periods of immersion/emergence, salinity and anoxia [
8,
9]. To adapt to these stresses, these plants have developed unique morphological, anatomical, and physiological characteristics [
10,
11]. The distribution of the salt marsh plant species follows a typical zonation pattern along the elevation gradient [
12,
13]. This elevation gradient includes gradients in salinity, redox potential, soil N content, soil clay content, and soil organic matter [
2]. However, elevation seems a major determinant for the establishment of all of them.
Unfortunately, increasing human populations have caused an extensive loss, degradation, and fragmentation of coastal ecosystems worldwide [
14]. The main anthropogenic pressures on salt marshes include changes in hydrological and salinity regimes, physical deterioration or removal of coastal features, and urbanisation [
15,
16,
17]. However, the main concern nowadays in any coastal ecosystem is the survival of the particular ecosystem in a climate change scenario [
18]. Although there are numerous examples of modelling these responses in the literature [
19,
20,
21,
22], a major modelling limitation is still the low availability of adequate datasets. Remote sensing (RS) techniques are changing this scenario with the provision of high-resolution spatial data that will support a new generation of computer models [
18].
Sea level rise is probably the major threat to tidal salt marshes [
18]. Changes in sea level are equivalent to changes in elevation. Therefore, our capacity for monitoring changes in elevation and plant species distribution is going to be key for developing early warning management plans. RS techniques are a straightforward and cost-effective way to extract information since they provide recurring datasets in short time scales at affordable prices. Maps and assessments of coastal habitats have both benefited greatly from the use of RS techniques [
1,
23]. For example, the loss and degradation of salt marshes have been successfully evaluated by combining long-term LANDSAT imagery and numerical modelling [
24]. Sentinel-2 and Landsat archives proved to be useful tools for tracking long-term salt marsh extent dynamics [
25]. More recently, deep learning models, powered by Sentinel imagery, have improved the mapping of low and high salt marsh land cover in South Carolina coastal wetlands [
26].
Differences in the biophysical properties of salt marsh plants generate spectral differences that can be detected using hyperspectral (HS) data [
27,
28,
29]. However, airborne or satellite-based HS imaging has a spatial resolution (meter to tens of meters) that is probably not adequate to identify species distribution due to the considerable spatial heterogeneity in salt marshes [
25,
30]. Previous works on HS images from the EO-1 Hyperion satellite (30 m pixels) concluded that 30 m is insufficient spatial resolution to accurately distinguish between species with spectral similarities, such as
Sporobolus maritimus (Curtis) P.M.Peterson & Saarela (previously named
Spartina maritima (Curtis) Fernald) and
Sarcocornia spp. A.J.Scott [
31]. Combinations of Quickbird images (2.4 m resolution in the multispectral mode) with high spectral data from Hyperion (242 narrow bands and 30 m pixel) have been probed to map different salt-marsh species with acceptable accuracies in classification [
32]. Pléiades images provide a robust and consistent global identification of the salt marsh zone. However, the application of its multispectral (MS) 2 m spatial resolution images proved to be insufficient for early assessment of the
Spartina anglica C.E. Hubb. (currently
Sporobolus anglicus (C.E. Hubb.) P.M.Peterson & Saarela) invasion, mainly due to the small size of the patches [
33].
Nowadays, most of the RS techniques have developed integrable sensors into unmanned aerial vehicles (UAVs). For the intertidal zone, UAVs that fly up to 120 m altitude are suitable to identify spatial heterogeneity in microtopography, canopy height or greenness [
34,
35,
36]. In addition, UAVs offer significant operational flexibility and minimal costs [
34,
37], allowing flight dates to be tailored. Therefore, UAVs may provide the necessary spatial and temporal resolution for mapping species distribution and their temporal changes. High-resolution RGB cameras integrated into UAVs have been previously employed in salt marsh environments. Farris et al. [
38] used UAV-LiDAR to track the salt marsh shoreline, while Yan et al. [
39] used UAVs to examine environmental factors influencing the ecological response of
Spartina alterniflora Loisel. (currently
Sporobolus alterniflorus (Loisel.) P.M.Peterson & Saarela). UAV-multispectral (UAV-MS) technology has also shown utility in calculating indices of plant diversity and species richness in wetland communities [
40]. Villoslada et al. [
41] have shown that maps created from UAV-MS images provide useful data for managing plant communities and assessing the effects of climate change on coastal meadows. However, achieving a high-accuracy classification requires the use of a large variety of vegetation indices and the evaluation of the spectral properties of the training samples.
The use of UAV hyperspectral remote sensing (UAV-HS) in salt marshes combines the advantages of high spatial and spectral resolutions to capture the finer scale of spectral and spatial heterogeneity. UAV-HS has previously been used to classify desert steppe species [
42], using spectral transformation to enhance species differences in vegetation indices with an overall accuracy of 87%. UAV-HS is able to detect salt stress in croplands and the accuracy performance of this technique improves in conjunction with other techniques [
43]. Although several salt marsh vegetation species have undergone field hyperspectral investigation through field spectrometer measurements [
28,
44], this is likely the first work using a UAV hyperspectral sensor in a salt marsh environment.
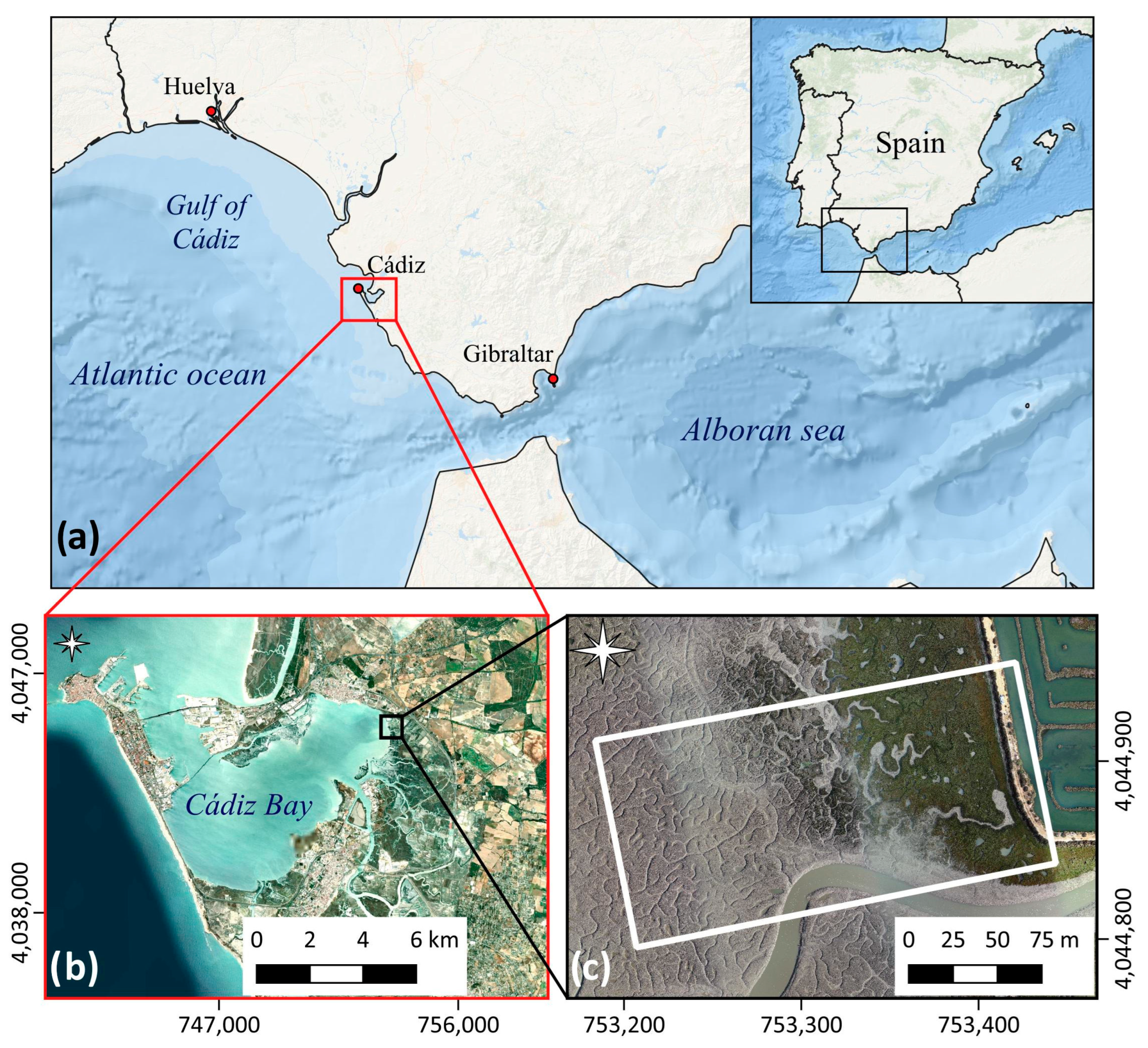
Advances in RS technique applications require adequate study cases. Cadiz Bay offers an excellent system for assessing the capacity of UAV-HS in the discrimination of salt marsh vegetation species distribution. This tidal environment is home to the southernmost tidal salt marshes in Europe and is protected by numerous environmental protection figures at local and international levels. Cadiz Bay was designated a Natural Park in 1989 (Bahía de Cádiz Natural Park, PNBC, [
45]) and RAMSAR site in 2002 (site no. 1265, [
46]). The system is considered an important resting place on the migratory route of birds and is included in the Natura 2000 network (ES0000140, as SCA and SPA). Located between two seas and two tectonic plates, Cadiz Bay is a key place for biodiversity studies [
47,
48,
49,
50].
This work examines the potential of high spatial and spectral resolution UAV-HS data to accurately identify and differentiate the distribution of the salt marsh vegetation at the level of species. The specific goals are (1) to determine which is the appropriate UAV-HS dataset to map salt marsh vegetation; (2) to assess the separability of salt marsh interest classes; and (3) to estimate the elevation ranges of the detected species. These findings are expected to become a starting point for the early assessment of salt marsh degradation and help in the selection of areas for salt marsh rehabilitation or detection of the establishment and spread of alien species.
4. Discussion
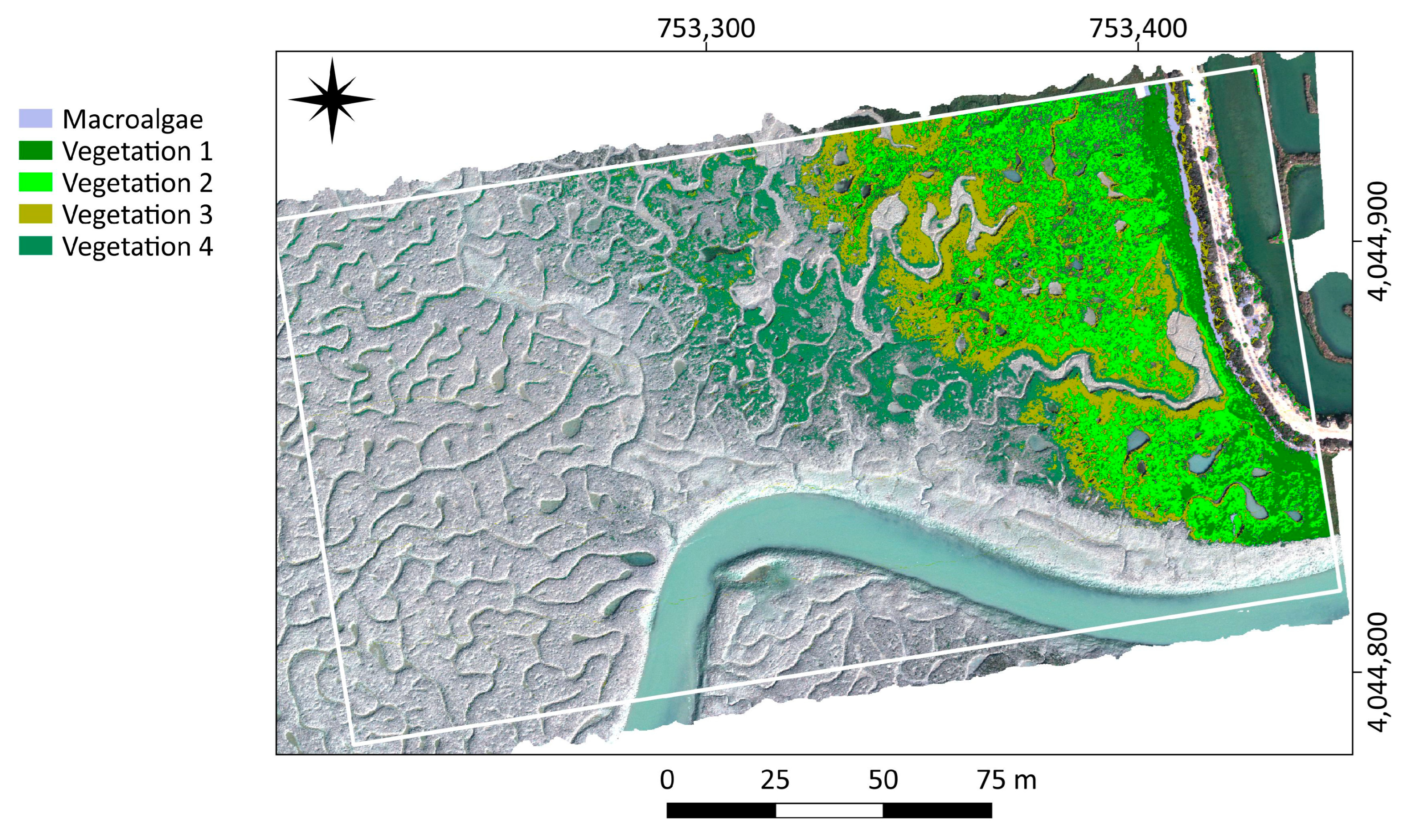
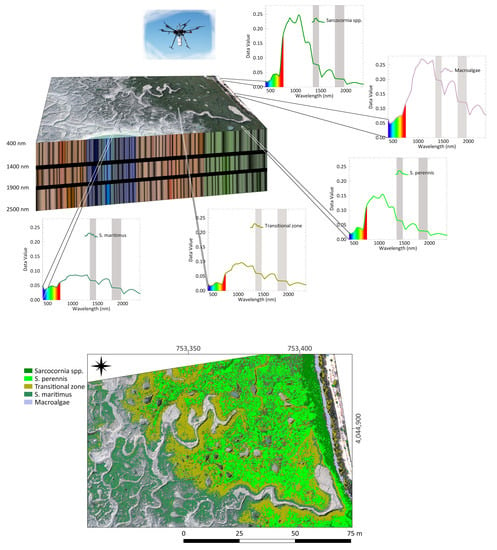
The analyses of hyperspectral datasets from low and medium salt marsh areas in Cadiz Bay are adequate to identify vegetation distribution at the species level. Four plant classes distributed along the horizons of the salt marsh and one class of macrophyte debris were recognised. Three of the plant classes have been associated with monospecific vegetation (
Sarcocornia spp.,
S. perennis, and
S. maritimus), while a fourth class represents the mix of species typical of the convergence of distributions (i.e., transition zone). The results from this study are expected to be extrapolated to other mid-latitude tidal marshes since the low and medium tidal marshes of these latitudes usually present similar structural traits [
75].
The performance of the SAM classification method is limited in areas with several species due to the mix of spectra [
77]. However, this problem can be minimised by using the two supervised classification methods (SAM and SVM) in succession after performing hyperspectral processing procedures. The SVM supervised classification reached up to 98% accuracy, demonstrating its effectiveness in mapping land cover. When looking at the accuracy of individual classes, features such as water bodies and types of soil perfectly match the reference data. Focusing on vegetation classes, the highest accuracy is achieved by vegetation 1, vegetation 4 and macroalgae. The accuracy is lower, although still significant for the remaining classes (
Table A1,
Table A2,
Table A3,
Table A4,
Table A5,
Table A6 and
Table A7). The spectral signature of the transition zone (vegetation 3 class), which is a variable mixture of plant species and, therefore, a variable mix of spectra, makes this class the lowest in accuracy (63.16%). Since the training dataset used to produce the map (i.e., ROIs) determines the quality of the algorithm classification, a single ROI for transition zones is considered insufficient to accurately represent the variability associated with different levels of species mix. Still, the overall accuracy provided by our UAV-HS approach is higher than previous classification attempts. Rasel et al. [
31] obtained a 43% overall accuracy from space-borne hyperspectral data with 30 m spatial resolution. Rajakumari et al. [
28] combined satellite multispectral data and ground spectrometric measurements, achieving 65.8% to 73.55% accuracy for the vegetation and land cover spectral signatures, respectively.
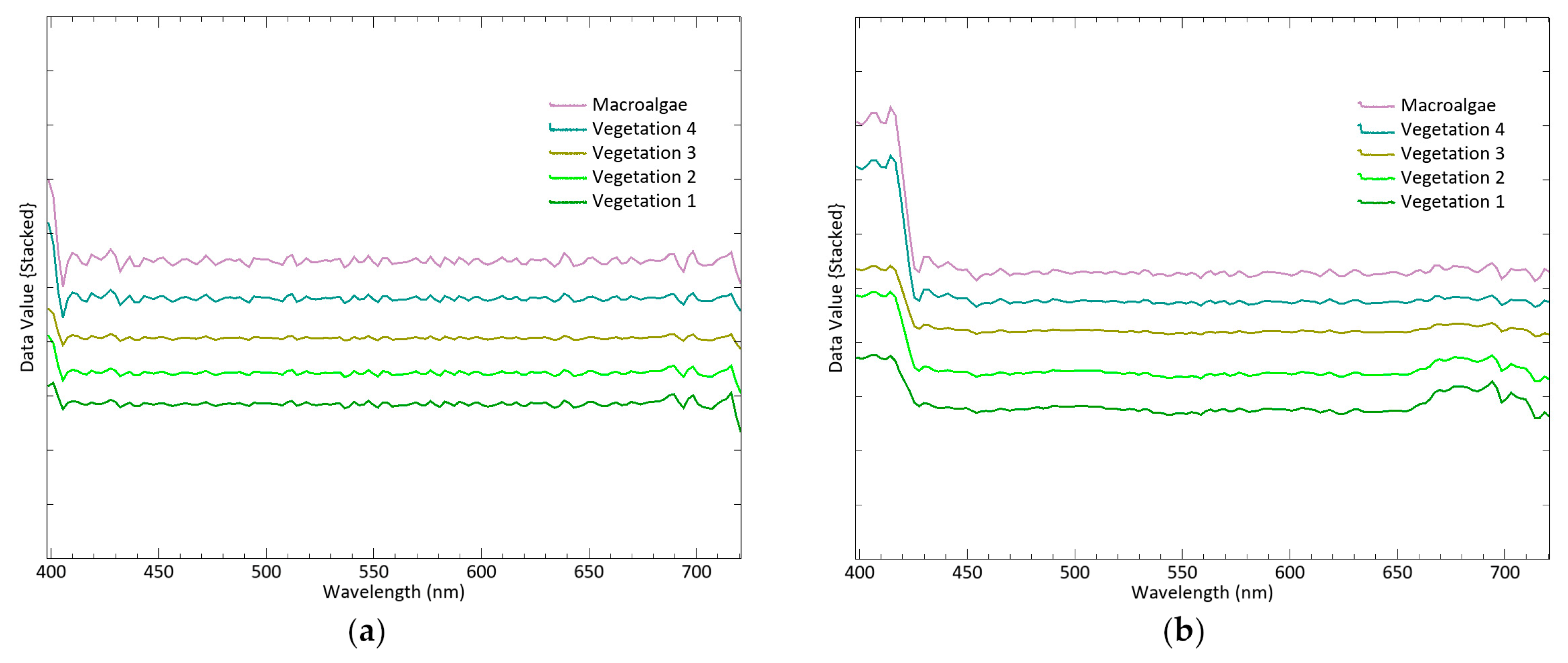
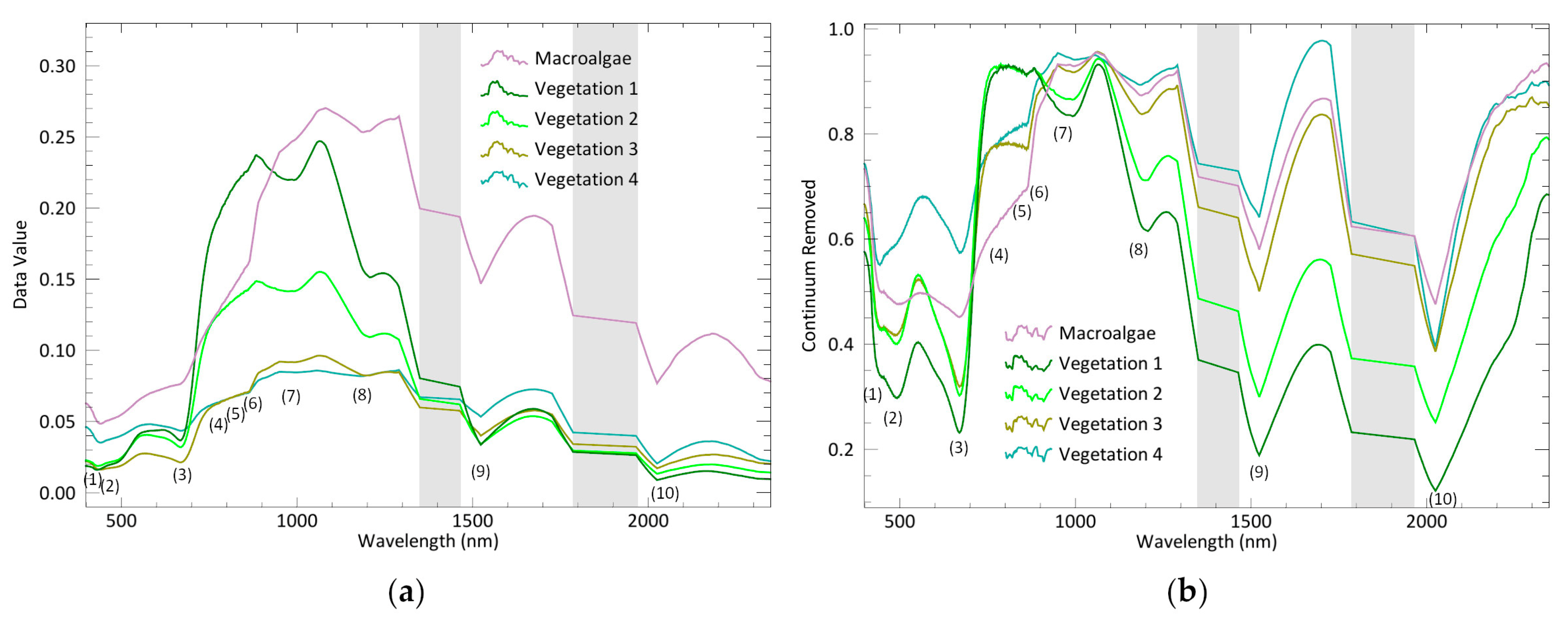
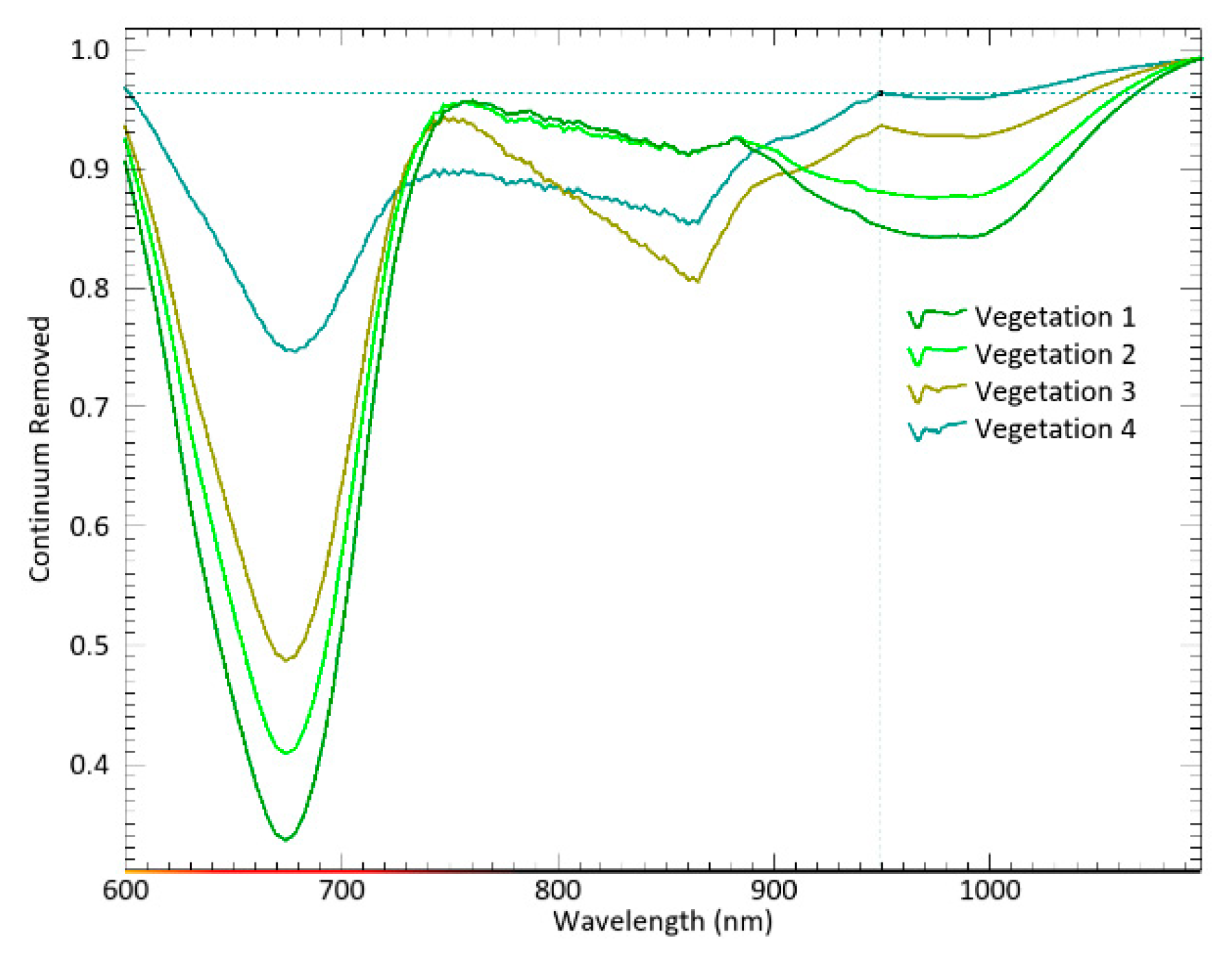
Among the endmembers extracted from Cadiz Bay salt marshes, four exhibit the typical plant pattern, while one shows the macroalgae response with distinct peaks and slopes of the reflectance spectrum. Chlorophyll a (Chl-a), found in both plants and macroalga, determines a typical absorption peak at 669 nm. However, this feature is smoothed in the spectral signature of macroalgae (
Figure 5), maybe due to its yellowish-brown colour related to the fucoxanthin content [
78]. The class of macroalgae in this study corresponds to debris deposited by an extreme flood event at an elevated position far removed from ordinary tidal cycles. This material dries and decomposes, resulting in high reflectance values in the NIR–SWIR, a typical region where water often attenuates spectral signatures [
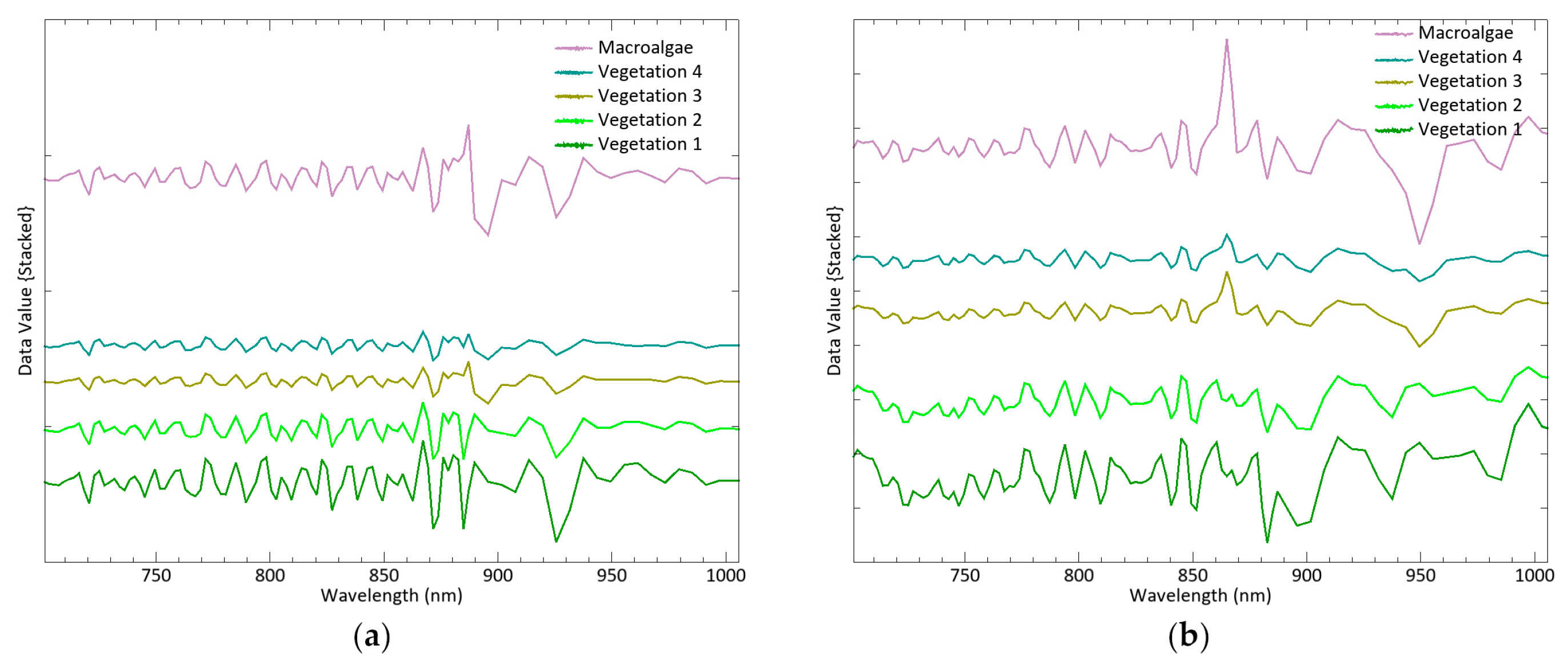
79]. The reflectance curves of the four remaining classes do not differ much from each other, the only significant variations being in the peak intensity. However, the continuum removal transformation enhances different responses in the 740–864 region, with opposite slopes in the curve between 882 and 949 nm (
Figure 7). These findings lead to the acceptance of vegetation 1 and 2 as the spectral signatures of
Sarcocornia species in the medium marsh horizon, and vegetation 3 and 4 as the signatures of species distributed in the transition and the low marsh, respectively.
Different species of
Sarcocornia dominate the medium marsh in Cadiz Bay [
55,
57]. However, because they are morphologically similar to
Salicornia species, it is very challenging to distinguish them in the field when they coexist, and misidentification can occur [
76]. As revealed by the CR spectra, vegetation 1 and 2 differ almost only in the intensity of the peaks, demonstrating optical similarities. However, the 2nd derivative analysis reveals that two groups of halophytes are spectrally distinct (
Figure A1,
Figure A2,
Figure A3 and
Figure A4), with these spectral differences resulting from biochemical variations between salt marsh species [
28]. The pigment content, canopy structure, leaf area and leaf structure all have an impact on the visible region of the reflectance spectrum of plant canopies [
42]. The 2nd derivative of our reflectance curves shows differences in the blue and red regions at wavelengths associated with the chlorophyll and xanthophyll peaks [
74]. However, our work shows that the largest 2nd derivative peaks are in the SWIR region, and many of them coincide with water absorption wavelengths. Water absorption bands are present at 900 and 967 nm (the water band index [
80]), in the 1150–1260 nm region [
81] and in the 1450–1940 nm region [
82]. Salinity in soils and vegetation is also detectable in the SWIR region [
83,
84], and Kumar et al. [
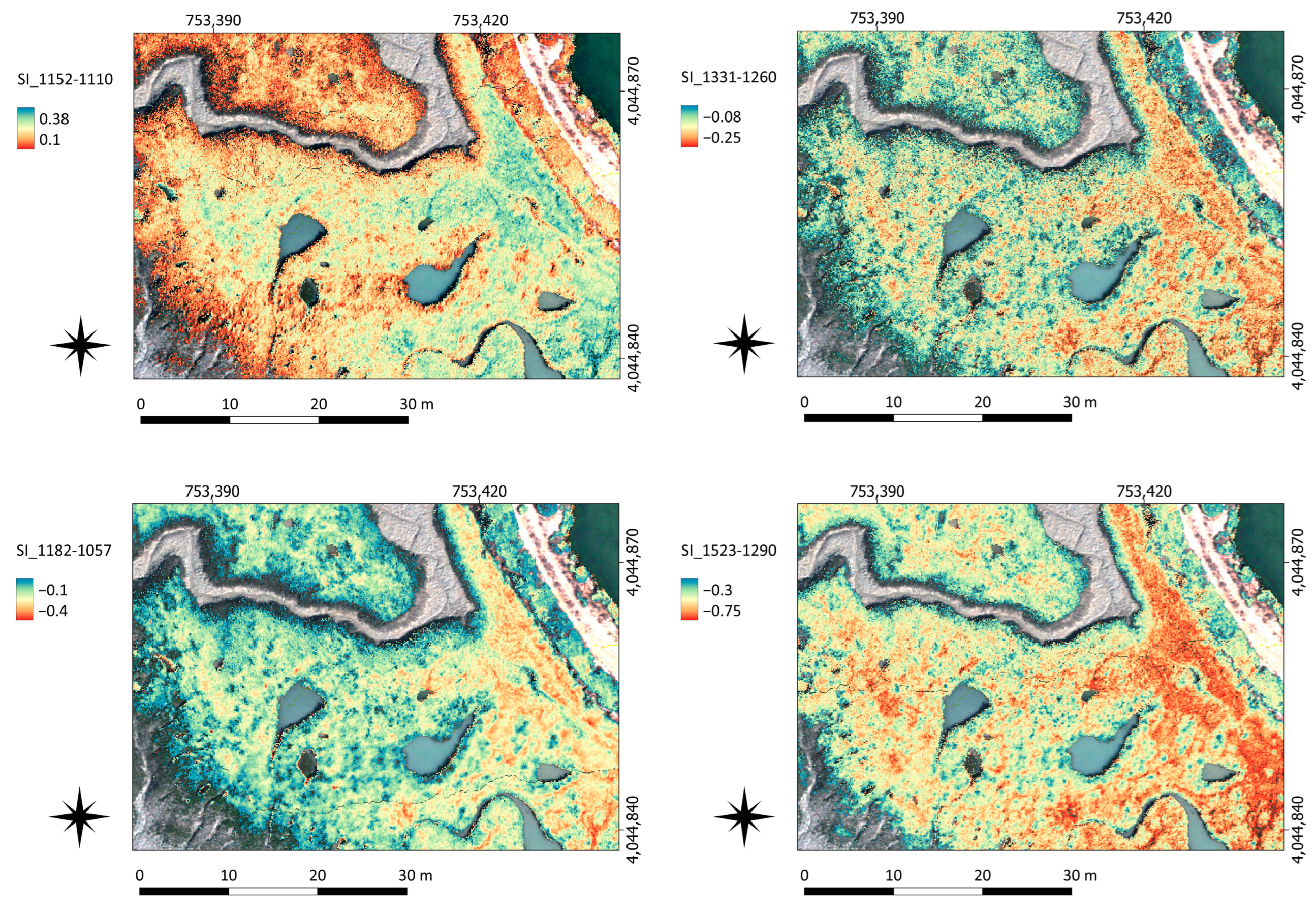
85] proposed a SWIR-based vegetation index to detect changes in vegetation cover from satellites. All these previous works support our conclusion that SWIR, with its highest spectral variability, is a suitable region to discriminate plant species from salt marshes. The SI established here can reveal differences in the canopy cover (
Figure 6), proving that UAV-HS is able to detect variations in canopy cover at the species level. The great advantages of UAVs are the high spatial and temporal resolutions of their products, as well as greater flexibility and lower cost when compared to satellite products. This allows, for example, data collection immediately after an extreme event and then periodically afterwards, providing key data to assess the ability of dynamic systems, such as salt marshes, to return to previous states (or resilience).
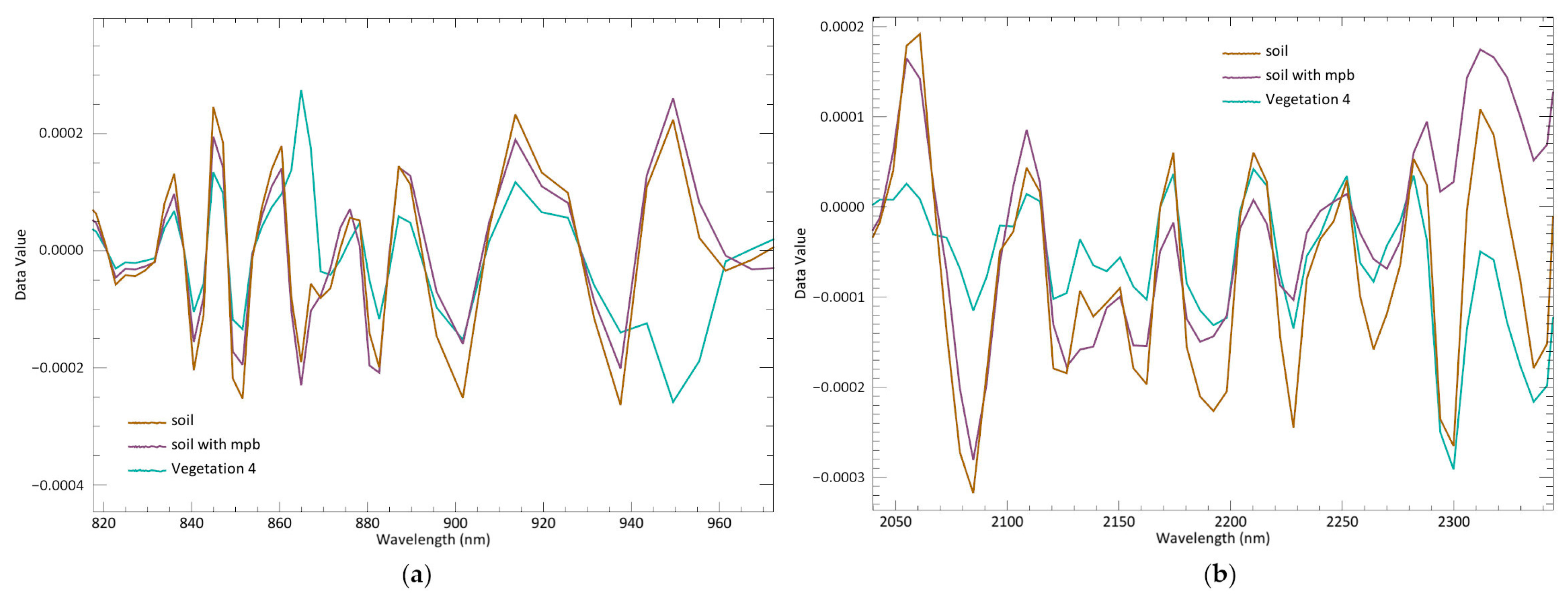
The horizon of the low salt marsh in Cadiz Bay is dominated by
S. maritimus [
55]. Its shoot structure and density allow the soil to be exposed, resulting in a mixed spectrum of soil and plant responses that is very similar to the spectral signature of soil with microphytobenthos. This problem may result in misinterpretations when using low spatial resolution sensors [
1]. The higher spatial resolution (5 cm/pixel) offered by UAVs not only prevents this issue, but also reduces the occurrence of mixed spectral signatures due to the reduced pixel size. The comparison of the spectral responses of the
S. maritimus class (vegetation 4) with the soil classes (
Figure 8) shows that the influence of the soil is inevitable. However,
S. maritimus habitats and soil with microphytobenthos can be distinguished by CR and 2nd derivative transformations in the red-edge and SWIR2 regions (
Figure 9). This demonstrates that these two habitats can be distinguished in UAV-HS datasets, allowing for more precise mapping of
S. maritimus and microphytobenthos soil and minimizing overestimation/underestimation issues for these categories.
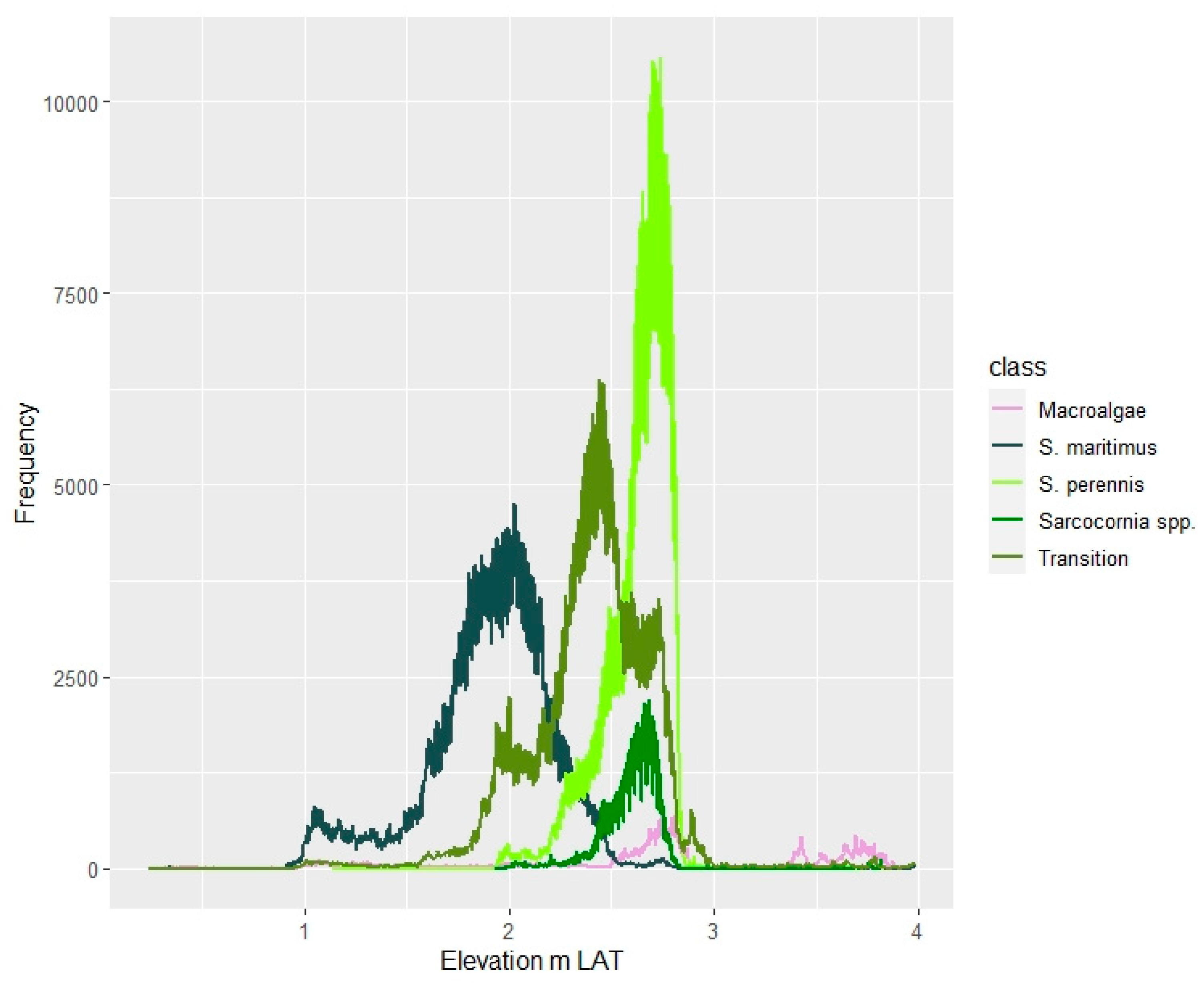
The zonation of salt marsh plant species depends on elevation, tidal regime, and the gradient of environmental variables, such as salinity, redox potential, soil N, clay, and organic matter content, as well as interspecific relationships [
2,
86,
87,
88]. According to Redondo-Gomez et al. [
89], in SW Spain,
S. perennis subsp.
perennis occupies from 2.26 to 2.84 m LAT, and
S. perennis subsp.
alpini from 2.84 to 3.65 m LAT. Our results agree with these findings, showing that
Sarcocornia spp. inhabit the salt marsh horizon between 2.30 m and 2.80 m LAT. Previous studies have described
S. fruticose and
S. perennis as dominant species in the salt marshes of Cadiz Bay [
55]. Unfortunately, the spectral library available in our study area (FAST project, [
75]) does not specify which
Sarcocornia taxa were measured. However, due to differences in the SWIR region, our results suggest that two
Sarcocornia taxa coexist in the medium salt marsh horizon. Differences in this part of the spectrum have previously been related to differences in the salinity [
84], suggesting that soil salinity may be playing a role in the zonation of Cadiz Bay tidal marshes. Although the elevation ranges for vegetation 1 and 2 overlap, suggesting a similar ecological niche, their different mode (2.67 m for vegetation 1 vs 2.79 m for vegetation 2,
Table 3) indicate a shift in the optimum range of environmental conditions between the two groups, supporting the existence of two species. Both histograms are left skewed, indicating that these species can populate lower elevations despite performing better at higher positions. As a result of our findings, some resilience is expected in these habitats under sea level rise scenarios.
Regarding the accumulations of macroalgae, the decomposition of these accumulations of organic matter alters the availability of oxygen and the redox potential in the sediments, which could have negative consequences for multiple trophic levels if their incidence increases significantly [
90,
91]. Understanding the local carbon cycle and the dynamic of the system also requires mapping where macroalgae are deposited [
78,
91]. In Cadiz Bay,
Sarcocornia spp. and
S. maritimus overlap in a narrow area here called the transition zone (vegetation 3). This class has problems with accuracy mainly because of the wide variety of spectral responses due to the different proportions of
Sarcocornia spp. and
S. maritimus. Future studies may include more classes for the transition zone, but they will need careful spectral analysis to investigate the spectral response associated with specific proportions of the dominant species.