Assessing the Efficacy of Phenological Spectral Differences to Detect Invasive Alien Acacia dealbata Using Sentinel-2 Data in Southern Europe
Abstract
1. Introduction
2. Materials and Methods
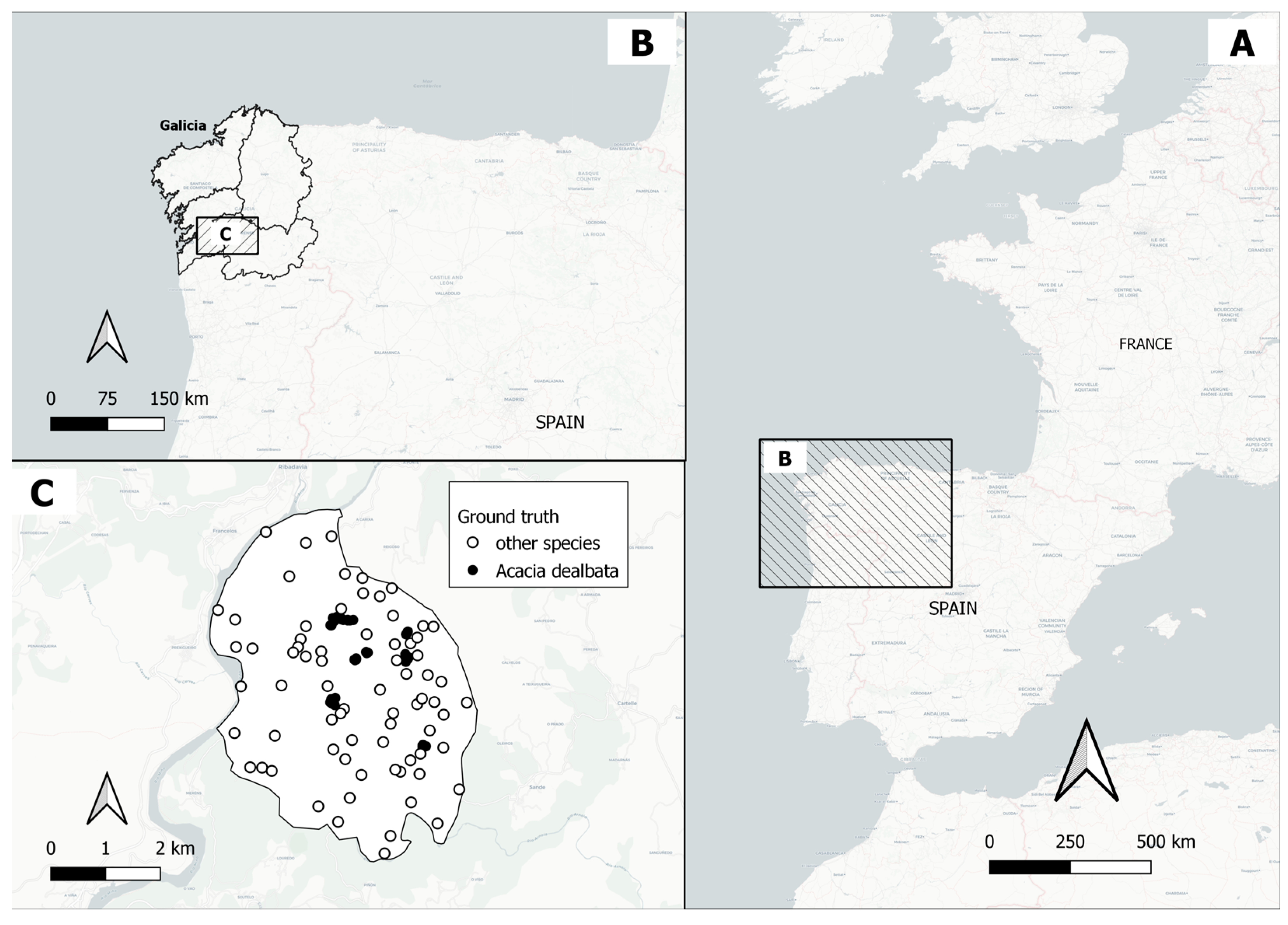
2.1. Study Area
2.2. Field Data Collection
2.3. Sentinel-2 Data Image Collection and Processing
2.4. Identification of Optimal Time for Distinguishing A. dealbata
2.5. Classification of A. dealbata Presence, Model Validation, and Mapping
3. Results
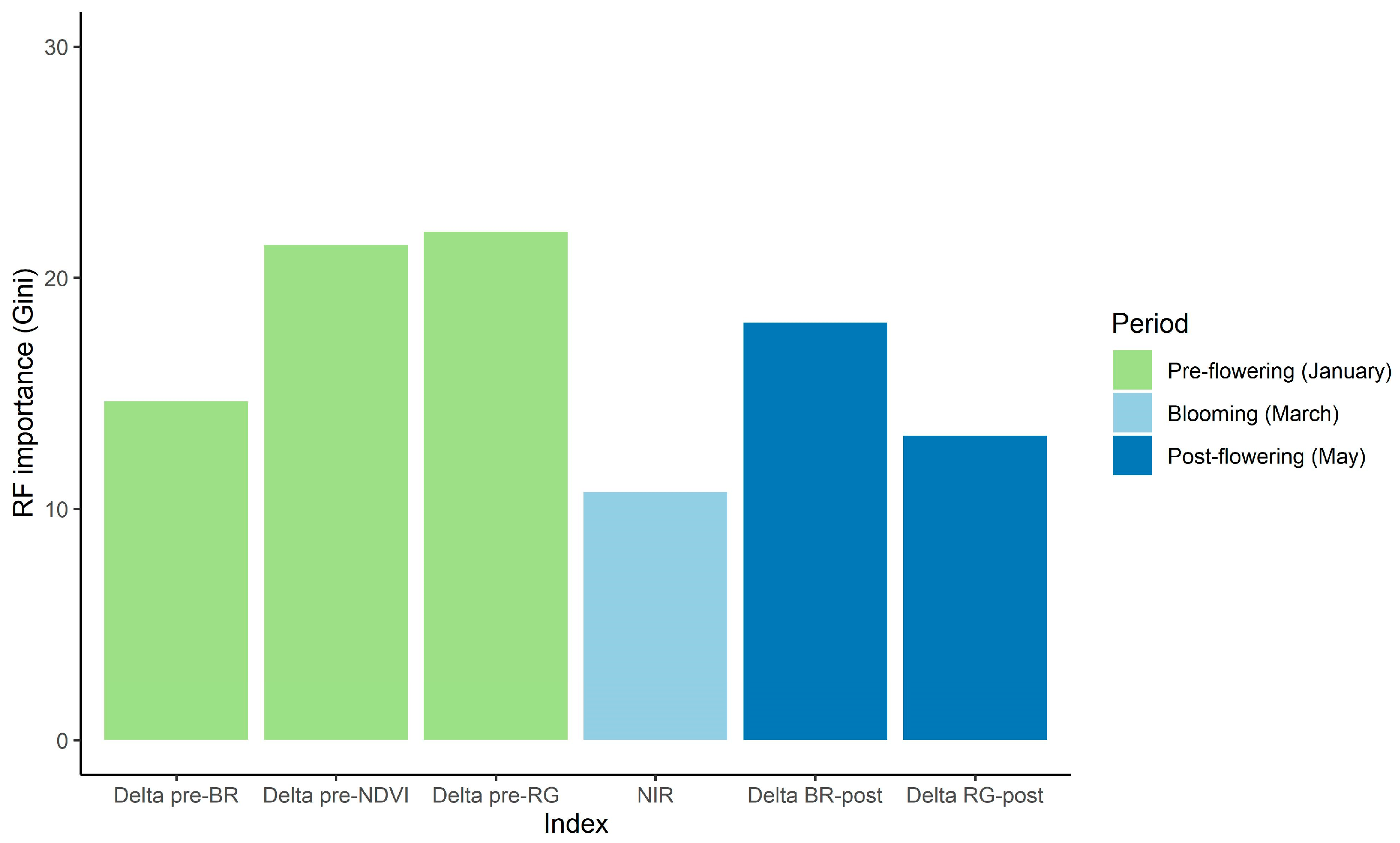
3.1. Determining Critical Periods to Classify A. dealbata

3.2. Classification of A. dealbata Presence
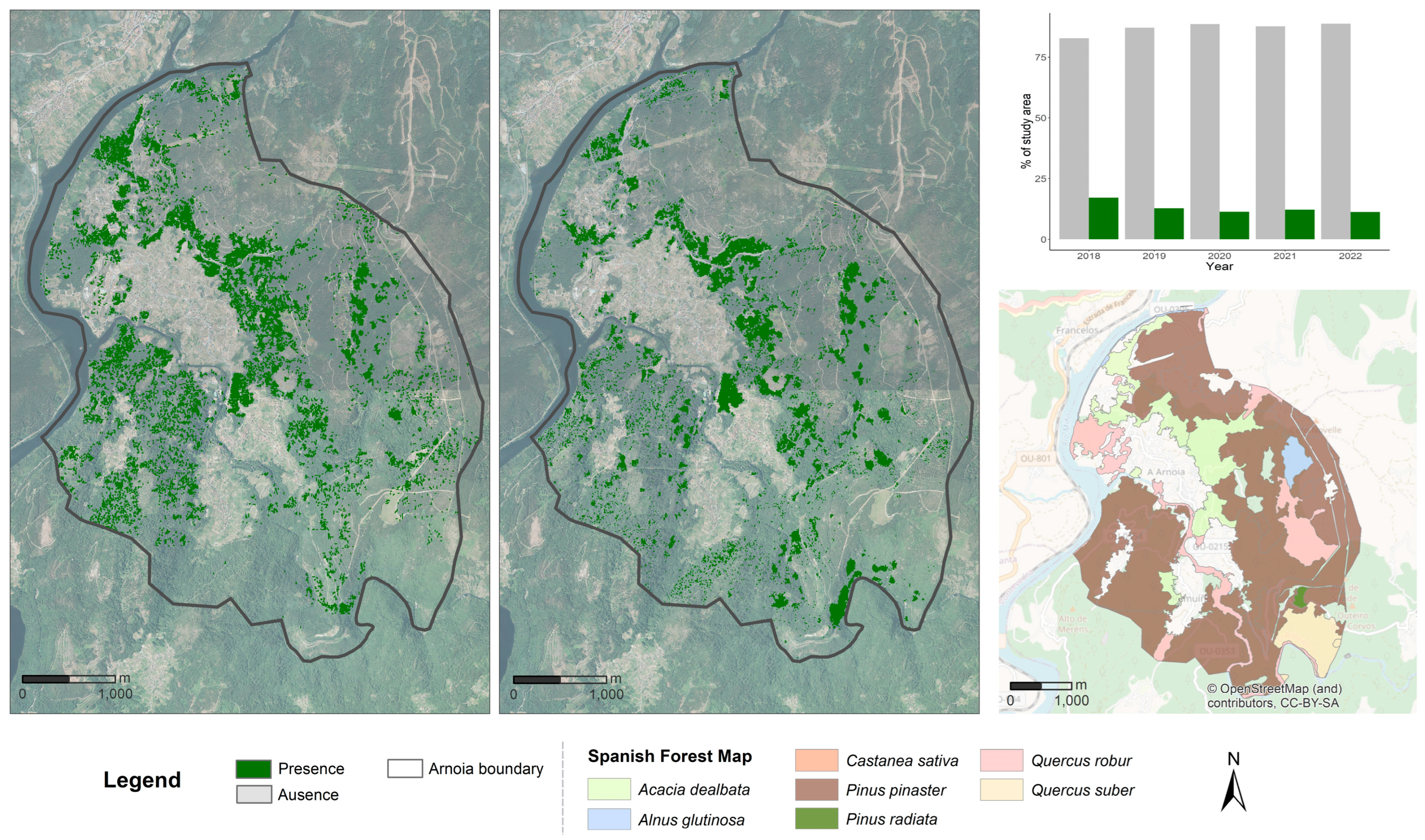
3.3. Mapping of A. dealbata Presence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M. Impacts of Biological Invasions: What’s What and the Way Forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Trade, Transport and Trouble: Managing Invasive Species Pathways in an Era of Globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ Warning on Invasive Alien Species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Kumar Rai, P.; Singh, J.S. Invasive Alien Plant Species: Their Impact on Environment, Ecosystem Services and Human Health. Ecol. Indic. 2020, 111, 106020. [Google Scholar] [CrossRef]
- Lorenzo, P.; González, L.; Reigosa, M.J. The Genus Acacia as Invader: The Characteristic Case of Acacia Dealbata Link in Europe. Ann. For. Sci. 2010, 67, 101. [Google Scholar] [CrossRef]
- Vazquez de la Cueva, A. Case Studies of the Expansion of Acacia Dealbata in the Valley of the River Miño (Galicia, Spain). For. Syst. 2014, 23, 3–14. [Google Scholar] [CrossRef]
- García, L.M.G.; Íñigo, I.L. Catálogo Español de Especies Exóticas Invasoras: 10 Respuestas a 10 Dudas. Quercus 2014, 345, 40–47. [Google Scholar]
- Gómez-García, E. Estimating the Changes in Tree Carbon Stocks in Galician Forests (NW Spain) between 1972 and 2009. For. Ecol. Manag. 2020, 467, 118157. [Google Scholar] [CrossRef]
- Alberdi, I.; Sandoval, V.; Condes, S.; Cañellas, I.; Vallejo, R. El Inventario Forestal Nacional Español, Una Herramienta Para El Conocimiento, La Gestión y La Conservación de Los Ecosistemas Forestales Arbolados. Ecosistemas 2016, 25, 88–97. [Google Scholar] [CrossRef]
- Tecnologías y Servicios Agrarios, S. A Cuarto Inventario Forestal Nacional: Galicia; Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2011; ISBN 978-84-8014-813-9.
- Lorenzo, P.; Palomera-Pérez, A.; Reigosa Roger, M.; González, L. Allelopathic Interference of Invasive Acacia Dealbata Link on the Physiological Parameters of Native Understory Species. Plant Ecol. 2011, 212, 403–412. [Google Scholar] [CrossRef]
- González-Muñoz, N.; Costa-Tenorio, M.; Espigares, T. Invasion of Alien Acacia Dealbata on Spanish Quercus Robur Forests: Impact on Soils and Vegetation. For. Ecol. Manag. 2012, 269, 214–221. [Google Scholar] [CrossRef]
- Rodríguez-Echeverría, S.; Afonso, C.; Correia, M.; Lorenzo, P.; Roiloa, S.R. The Effect of Soil Legacy on Competition and Invasion by Acacia Dealbata Link. Plant Ecol. 2013, 214, 1139–1146. [Google Scholar] [CrossRef]
- Bowd, E.J.; Lindenmayer, D.B.; Banks, S.C.; Blair, D.P. Logging and Fire Regimes Alter Plant Communities. Ecol. Appl. 2018, 28, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Santana, P.; Brandão, T.; Guedes, M. Automatic Detection of Acacia Longifolia Invasive Species Based on UAV-Acquired Aerial Imagery. Inf. Process. Agric. 2022, 9, 276–287. [Google Scholar] [CrossRef]
- Marzialetti, F.; Frate, L.; De Simone, W.; Frattaroli, A.R.; Acosta, A.T.; Carranza, M.L. Unmanned Aerial Vehicle (UAV)-Based Mapping of Acacia Saligna Invasion in the Mediterranean Coast. Remote Sens. 2021, 13, 3361. [Google Scholar] [CrossRef]
- Bombín, R.V. El Mapa Forestal de España Escala 1: 50.000 (MFE50) Como Base Del Tercer Inventario Forestal Nacional. Cuad. La Soc. Española Cienc. For. 2005, 19, 205–210. [Google Scholar]
- Liang, J.; Tsui, V.; Van Abbema, A.; Bao, L.; Barrett, K.; Beresini, M.; Berezhkovskiy, L.; Blair, W.S.; Chang, C.; Driscoll, J.; et al. Lead Identification of Novel and Selective TYK2 Inhibitors. Eur. J. Med. Chem. 2013, 67, 175–187. [Google Scholar] [CrossRef]
- Somers, B.; Asner, G.P. Multi-Temporal Hyperspectral Mixture Analysis and Feature Selection for Invasive Species Mapping in Rainforests. Remote Sens. Environ. 2013, 136, 14–27. [Google Scholar] [CrossRef]
- Lahav-Ginott, S.; Kadmon, R.; Gersani, M. Evaluating the Viability of Acacia Populations in the Negev Desert: A Remote Sensing Approach. Biol. Conserv. 2001, 98, 127–137. [Google Scholar] [CrossRef]
- Wäldchen, J.; Mäder, P. Machine Learning for Image Based Species Identification. Methods Ecol. Evol. 2018, 9, 2216–2225. [Google Scholar] [CrossRef]
- Lary, D.J.; Alavi, A.H.; Gandomi, A.H.; Walker, A.L. Machine Learning in Geosciences and Remote Sensing. Geosci. Front. 2016, 7, 3–10. [Google Scholar] [CrossRef]
- Mohamed, H.; Nadaoka, K.; Nakamura, T. Towards Benthic Habitat 3D Mapping Using Machine Learning Algorithms and Structures from Motion Photogrammetry. Remote Sens. 2020, 12, 127. [Google Scholar] [CrossRef]
- Dubayah, R.O.; Drake, J.B. Lidar Remote Sensing for Forestry. J. For. 2000, 98, 44–46. [Google Scholar]
- Holmgren, J.; Persson, Å. Identifying Species of Individual Trees Using Airborne Laser Scanner. Remote Sens. Environ. 2004, 90, 415–423. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Aschbacher, J. ESA’s Earth Observation Strategy and Copernicus. In Satellite Earth Observations and Their Impact on Society and Policy; Springer: Singapore, 2017; pp. 81–86. [Google Scholar]
- Lawes, R.A.; Wallace, J.F. Monitoring an Invasive Perennial at the Landscape Scale with Remote Sensing. Ecol. Manag. Restor. 2008, 9, 53–59. [Google Scholar] [CrossRef]
- Park, H.-C.; Lim, J.-C.; Lee, J.-H.; Lee, G.-G. Predicting the Potential Distributions of Invasive Species Using the Landsat Imagery and Maxent: Focused On. J. Korean Soc. Environ. Restor. Technol. 2017, 20, 1–12. [Google Scholar] [CrossRef][Green Version]
- Young, K.E.; Abbott, L.B.; Caldwell, C.A.; Schrader, T.S. Estimating Suitable Environments for Invasive Plant Species across Large Landscapes: A Remote Sensing Strategy Using Landsat 7 ETM+. Int. J. Biodivers. Conserv. 2013, 5, 122–134. [Google Scholar]
- Khare, S.; Latifi, H.; Ghosh, S.K. Multi-Scale Assessment of Invasive Plant Species Diversity Using Pléiades 1A, RapidEye and Landsat-8 Data. Geocarto Int. 2018, 33, 681–698. [Google Scholar] [CrossRef]
- Proença, B.; Frappart, F.; Lubac, B.; Marieu, V.; Ygorra, B.; Bombrun, L.; Michalet, R.; Sottolichio, A. Potential of High-Resolution Pléiades Imagery to Monitor Salt Marsh Evolution after Spartina Invasion. Remote Sens. 2019, 11, 968. [Google Scholar] [CrossRef]
- Alvarez-Taboada, F.; Paredes, C.; Julián-Pelaz, J. Mapping of the Invasive Species Hakea Sericea Using Unmanned Aerial Vehicle (UAV) and WorldView-2 Imagery and an Object-Oriented Approach. Remote Sens. 2017, 9, 913. [Google Scholar] [CrossRef]
- Crisigiovanni, E.L.; Figueiredo Filho, A.; Pesck, V.A.; de Lima, V.A. Potential of Machine Learning and WorldView-2 Images for Recognizing Endangered and Invasive Species in the Atlantic Rainforest. Ann. For. Sci. 2021, 78, 54. [Google Scholar] [CrossRef]
- Robinson, T.P.; Wardell-Johnson, G.W.; Pracilio, G.; Brown, C.; Corner, R.; Van Klinken, R.D. Testing the Discrimination and Detection Limits of WorldView-2 Imagery on a Challenging Invasive Plant Target. Int. J. Appl. Earth Obs. Geoinf. 2016, 44, 23–30. [Google Scholar] [CrossRef]
- Sankey, T.; Dickson, B.; Sesnie, S.; Wang, O.; Olsson, A.; Zachmann, L. WorldView-2 High Spatial Resolution Improves Desert Invasive Plant Detection. Photogramm. Eng. Remote Sens. 2014, 80, 885–893. [Google Scholar] [CrossRef]
- Fuller, D.O. Remote Detection of Invasive Melaleuca Trees (Melaleuca Quinquenervia) in South Florida with Multispectral IKONOS Imagery. Int. J. Remote Sens. 2005, 26, 1057–1063. [Google Scholar] [CrossRef]
- Laba, M.; Blair, B.; Downs, R.; Monger, B.; Philpot, W.; Smith, S.; Sullivan, P.; Baveye, P.C. Use of Textural Measurements to Map Invasive Wetland Plants in the Hudson River National Estuarine Research Reserve with IKONOS Satellite Imagery. Remote Sens. Environ. 2010, 114, 876–886. [Google Scholar] [CrossRef]
- Förster, M.; Schmidt, T.; Wolf, R.; Kleinschmit, B.; Fassnacht, F.E.; Cabezas, J.; Kattenborn, T. Detecting the Spread of Invasive Species in Central Chile with a Sentinel-2 Time-Series. In Proceedings of the 2017 9th International Workshop on the Analysis of Multitemporal Remote Sensing Images (MultiTemp), Bruges, Belgium, 27 June 2017; pp. 1–4. [Google Scholar]
- Masemola, C.; Cho, M.A.; Ramoelo, A. Towards a Semi-Automated Mapping of Australia Native Invasive Alien Acacia Trees Using Sentinel-2 and Radiative Transfer Models in South Africa. ISPRS J. Photogramm. Remote Sens. 2020, 166, 153–168. [Google Scholar] [CrossRef]
- Masemola, C.; Cho, M.A.; Ramoelo, A. Sentinel-2 Time Series Based Optimal Features and Time Window for Mapping Invasive Australian Native Acacia Species in KwaZulu Natal, South Africa. Int. J. Appl. Earth Obs. Geoinf. 2020, 93, 102207. [Google Scholar] [CrossRef]
- Kattenborn, T.; Sperlich, M.; Bataua, K.; Koch, B. Automatic Single Tree Detection in Plantations Using UAV-Based Photogrammetric Point Clouds. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2014, XL–3, 139–144. [Google Scholar] [CrossRef]
- Große-Stoltenberg, A.; Hellmann, C.; Werner, C.; Oldeland, J.; Thiele, J. Evaluation of Continuous VNIR-SWIR Spectra versus Narrowband Hyperspectral Indices to Discriminate the Invasive Acacia Longifolia within a Mediterranean Dune Ecosystem. Remote Sens. 2016, 8, 334. [Google Scholar] [CrossRef]
- Große-Stoltenberg, A.; Hellmann, C.; Thiele, J.; Werner, C.; Oldeland, J. Early Detection of GPP-Related Regime Shifts after Plant Invasion by Integrating Imaging Spectroscopy with Airborne LiDAR. Remote Sens. Environ. 2018, 209, 780–792. [Google Scholar] [CrossRef]
- Arasumani, M.; Bunyan, M.; Robin, V.V. Opportunities and Challenges in Using Remote Sensing for Invasive Tree Species Management, and in the Identification of Restoration Sites in Tropical Montane Grasslands. J. Environ. Manag. 2021, 280, 111759. [Google Scholar] [CrossRef] [PubMed]
- Arasumani, M.; Singh, A.; Bunyan, M.; Robin, V.V. Testing the Efficacy of Hyperspectral (AVIRIS-NG), Multispectral (Sentinel-2) and Radar (Sentinel-1) Remote Sensing Images to Detect Native and Invasive Non-Native Trees. Biol. Invasions 2021, 23, 2863–2879. [Google Scholar] [CrossRef]
- Isaacson, S.; Ephrath, J.E.; Rachmilevitch, S.; Maman, S.; Ginat, H.; Blumberg, D.G. Long and Short Term Population Dynamics of Acacia Trees via Remote Sensing and Spatial Analysis: Case Study in the Southern Negev Desert. Remote Sens. Environ. 2017, 198, 95–104. [Google Scholar] [CrossRef]
- Oldeland, J.; Große-Stoltenberg, A.; Naftal, L.; Strohbach, B.J. The Potential of UAV Derived Image Features for Discriminating Savannah Tree Species. In The Roles of Remote Sensing in Nature Conservation; Springer: Cham, Switzerland, 2017; pp. 183–201. [Google Scholar] [CrossRef]
- Paz-Kagan, T.; Silver, M.; Panov, N.; Karnieli, A. Multispectral Approach for Identifying Invasive Plant Species Based on Flowering Phenology Characteristics. Remote Sens. 2019, 11, 953. [Google Scholar] [CrossRef]
- Dixon, D.J.; Callow, J.N.; Duncan, J.M.A.; Setterfield, S.A.; Pauli, N. Satellite Prediction of Forest Flowering Phenology. Remote Sens. Environ. 2021, 255, 112197. [Google Scholar] [CrossRef]
- d’Andrimont, R.; Taymans, M.; Lemoine, G.; Ceglar, A.; Yordanov, M.; van der Velde, M. Detecting Flowering Phenology in Oil Seed Rape Parcels with Sentinel-1 and -2 Time Series. Remote Sens. Environ. 2020, 239, 111660. [Google Scholar] [CrossRef]
- Milicevic, M.; Zubrinic, K.; Grbavac, I.; Obradovic, I. Application of Deep Learning Architectures for Accurate Detection of Olive Tree Flowering Phenophase. Remote Sens. 2020, 12, 120. [Google Scholar] [CrossRef]
- Campbell, T.; Fearns, P. Simple Remote Sensing Detection of Corymbia Calophylla Flowers Using Common 3 –Band Imaging Sensors. Remote Sens. Appl. Soc. Environ. 2018, 11, 51–63. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Wolf, A.F. Using WorldView-2 Vis-NIR Multispectral Imagery to Support Land Mapping and Feature Extraction Using Normalized Difference Index Ratios; SPIE: Bellingham, WA, USA, 2012; Volume 8390, pp. 188–195. [Google Scholar]
- Xu, H. Modification of Normalised Difference Water Index (NDWI) to Enhance Open Water Features in Remotely Sensed Imagery. Null 2006, 27, 3025–3033. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A Commentary Review on the Use of Normalized Difference Vegetation Index (NDVI) in the Era of Popular Remote Sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty Five Years of Remote Sensing in Precision Agriculture: Key Advances and Remaining Knowledge Gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Dube, T.; Gumindoga, W.; Chawira, M. Detection of Land Cover Changes around Lake Mutirikwi, Zimbabwe, Based on Traditional Remote Sensing Image Classification Techniques. Afr. J. Aquat. Sci. 2014, 39, 89–95. [Google Scholar] [CrossRef]
- Rodrigues, M.; De la Riva, J. An Insight into Machine-Learning Algorithms to Model Human-Caused Wildfire Occurrence. Environ. Model. Softw. 2014, 57, 192–201. [Google Scholar] [CrossRef]
- Domingo, D.; Lamelas, M.T.; Montealegre, A.L.; García-Martín, A.; de la Riva, J. Estimation of Total Biomass in Aleppo Pine Forest Stands Applying Parametric and Nonparametric Methods to Low-Density Airborne Laser Scanning Data. Forests 2018, 9, 158. [Google Scholar] [CrossRef]
- García-Gutiérrez, J.; Martínez-Álvarez, F.; Troncoso, A.; Riquelme, J.C. A Comparison of Machine Learning Regression Techniques for LiDAR-Derived Estimation of Forest Variables. Neurocomputing 2015, 167, 24–31. [Google Scholar] [CrossRef]
- Foody, G.M. Explaining the Unsuitability of the Kappa Coefficient in the Assessment and Comparison of the Accuracy of Thematic Maps Obtained by Image Classification. Remote Sens. Environ. 2020, 239, 111630. [Google Scholar] [CrossRef]
- Rodrigues, M.; Jiménez-Ruano, A.; de la Riva, J. Fire Regime Dynamics in Mainland Spain. Part 1: Drivers of Change. Sci. Total Environ. 2020, 721, 135841. [Google Scholar] [CrossRef]
- Cho, M.A.; Ramoelo, A.; Dziba, L. Response of Land Surface Phenology to Variation in Tree Cover during Green-up and Senescence Periods in the Semi-Arid Savanna of Southern Africa. Remote Sens. 2017, 9, 689. [Google Scholar] [CrossRef]
- Key, C. Landscape Assessment (LA): Sampling and Analysis Methods. In FIREMON: Fire Effects Monitoring and Inventory System; Lutes, D., Keane, R., Caratti, J., Key, C., Benson, N., Sutherland, S., Gangi, L., Eds.; General Technical Report RMRS-GTR-164; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; pp. LA1–LA51. [Google Scholar]
- Hernández, L.; Martínez- Fernández, J.; Cañellas, I.; de la Cueva, A.V. Assessing Spatio-Temporal Rates, Patterns and Determinants of Biological Invasions in Forest Ecosystems. The Case of Acacia Species in NW Spain. For. Ecol. Manag. 2014, 329, 206–213. [Google Scholar] [CrossRef]
- Davidson, D.W.; Morton, S.R. Dispersal Adaptations of Some Acacia Species in the Australian Arid Zone. Ecology 1984, 65, 1038–1051. [Google Scholar] [CrossRef]
- Gassó, N.; Thuiller, W.; Pino, J.; Vilà, M. Potential Distribution Range of Invasive Plant Species in Spain. NeoBiota 2012, 12, 25. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingo, D.; Pérez-Rodríguez, F.; Gómez-García, E.; Rodríguez-Puerta, F. Assessing the Efficacy of Phenological Spectral Differences to Detect Invasive Alien Acacia dealbata Using Sentinel-2 Data in Southern Europe. Remote Sens. 2023, 15, 722. https://doi.org/10.3390/rs15030722
Domingo D, Pérez-Rodríguez F, Gómez-García E, Rodríguez-Puerta F. Assessing the Efficacy of Phenological Spectral Differences to Detect Invasive Alien Acacia dealbata Using Sentinel-2 Data in Southern Europe. Remote Sensing. 2023; 15(3):722. https://doi.org/10.3390/rs15030722
Chicago/Turabian StyleDomingo, Dario, Fernando Pérez-Rodríguez, Esteban Gómez-García, and Francisco Rodríguez-Puerta. 2023. "Assessing the Efficacy of Phenological Spectral Differences to Detect Invasive Alien Acacia dealbata Using Sentinel-2 Data in Southern Europe" Remote Sensing 15, no. 3: 722. https://doi.org/10.3390/rs15030722
APA StyleDomingo, D., Pérez-Rodríguez, F., Gómez-García, E., & Rodríguez-Puerta, F. (2023). Assessing the Efficacy of Phenological Spectral Differences to Detect Invasive Alien Acacia dealbata Using Sentinel-2 Data in Southern Europe. Remote Sensing, 15(3), 722. https://doi.org/10.3390/rs15030722








