Characterisation of Banana Plant Growth Using High-Spatiotemporal-Resolution Multispectral UAV Imagery
Abstract
1. Introduction
2. Materials and Methods
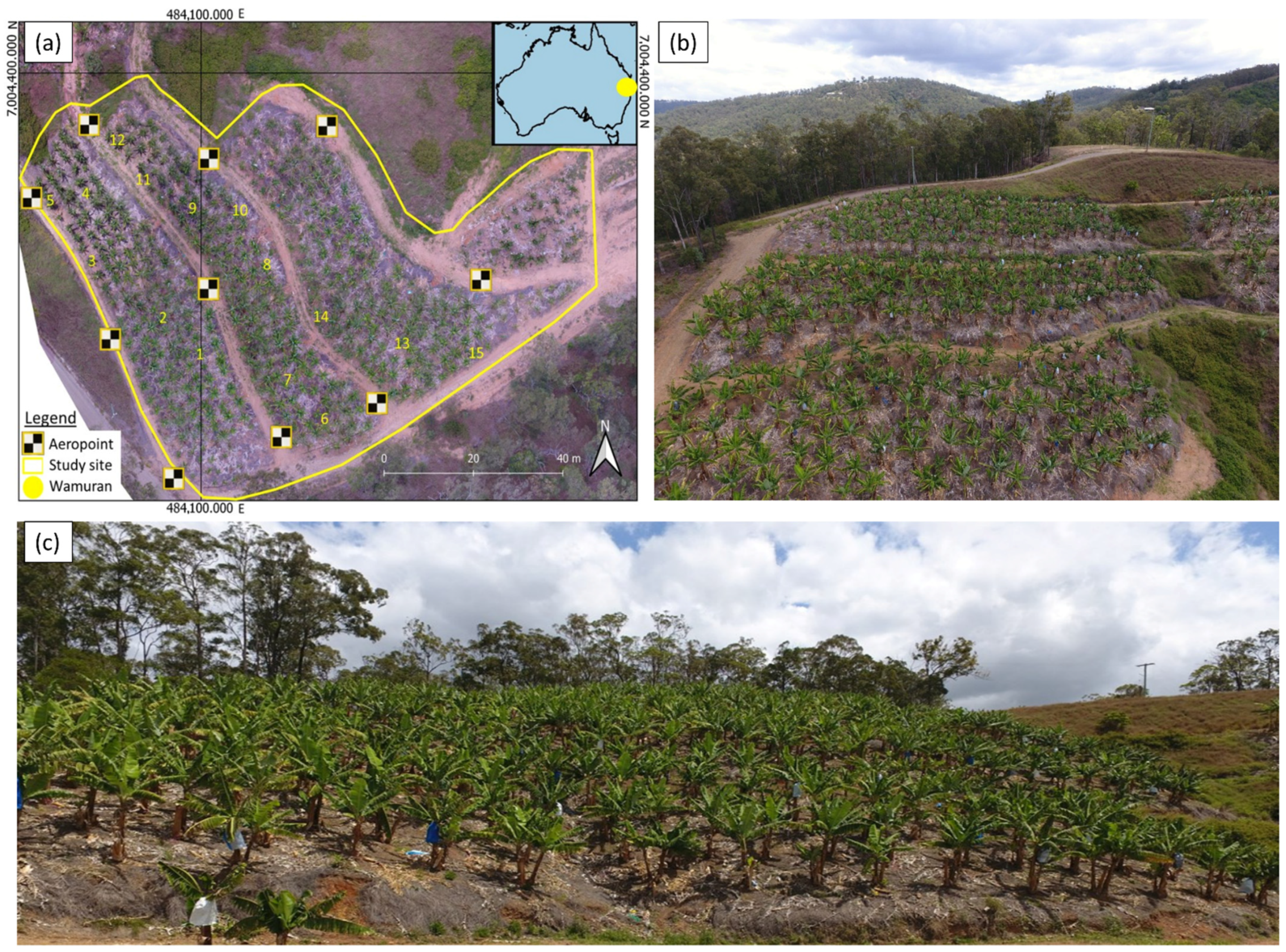
2.1. Study Location
2.2. Field Data Collection and Ground Validation
2.3. UAV Data Collection and Processing
2.4. Individual Plant Crown Delineation, Height, and Crown Spread Estimation
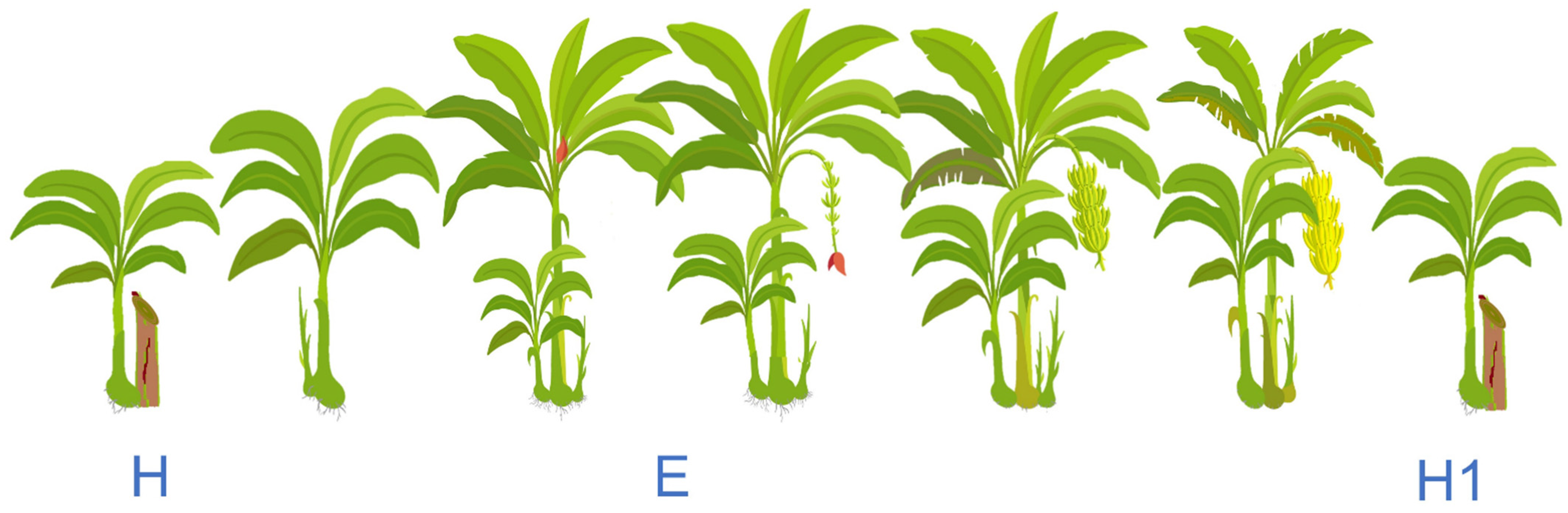
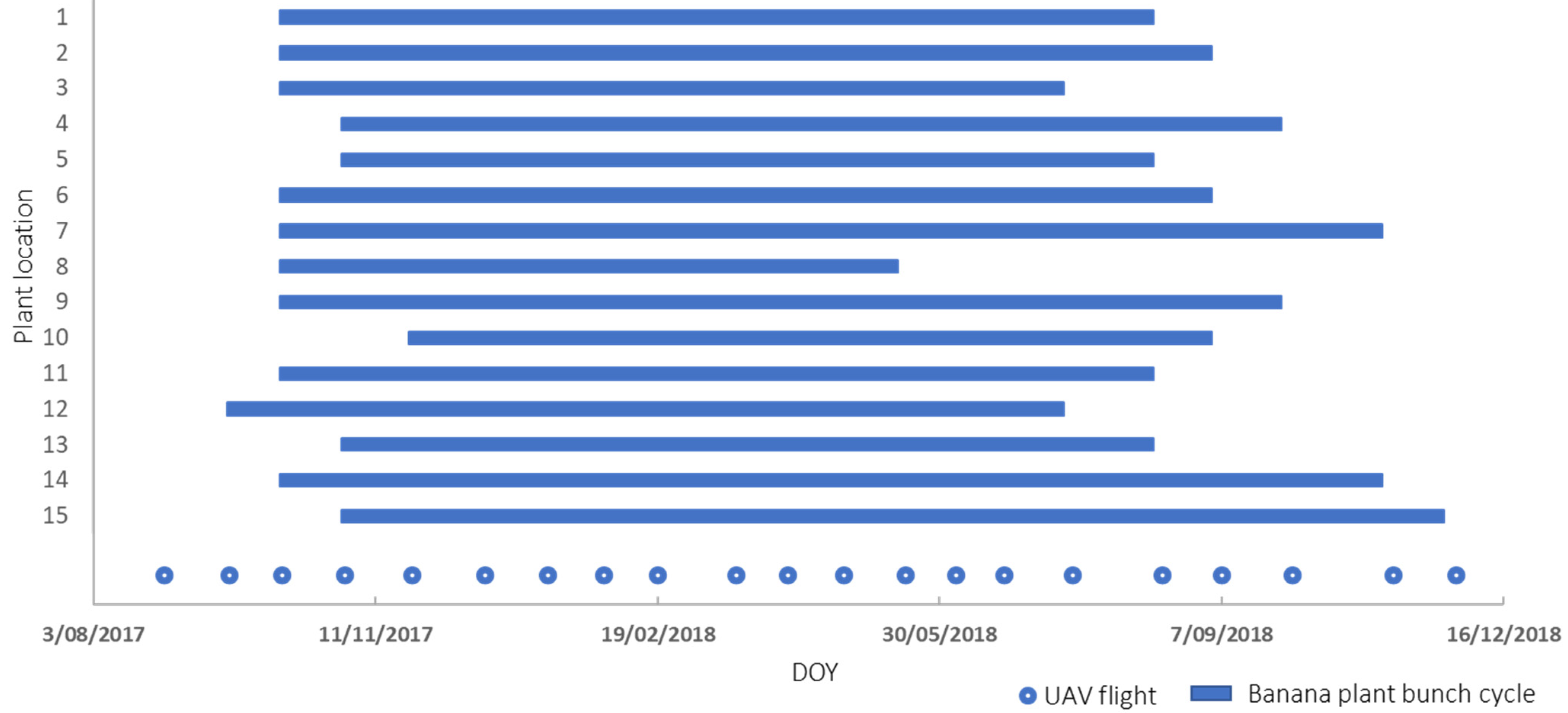
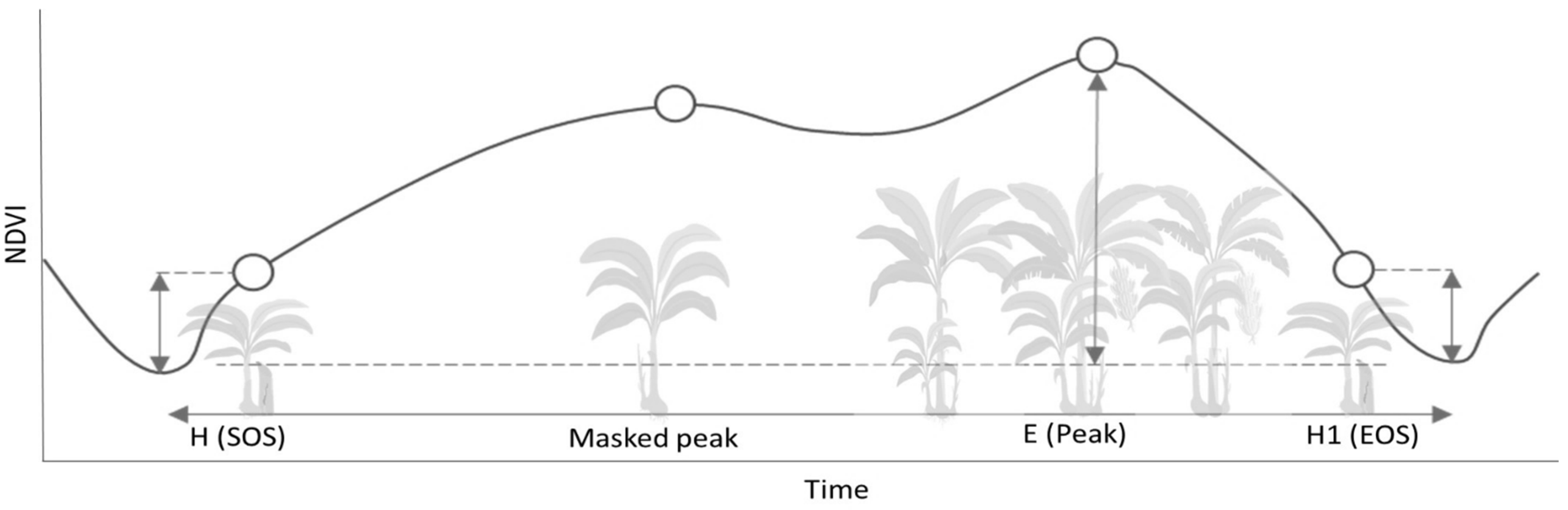
2.5. Phenological Changes (Time Series)
2.6. Yield Relationship to Vegetation Indices and Morphology
3. Results
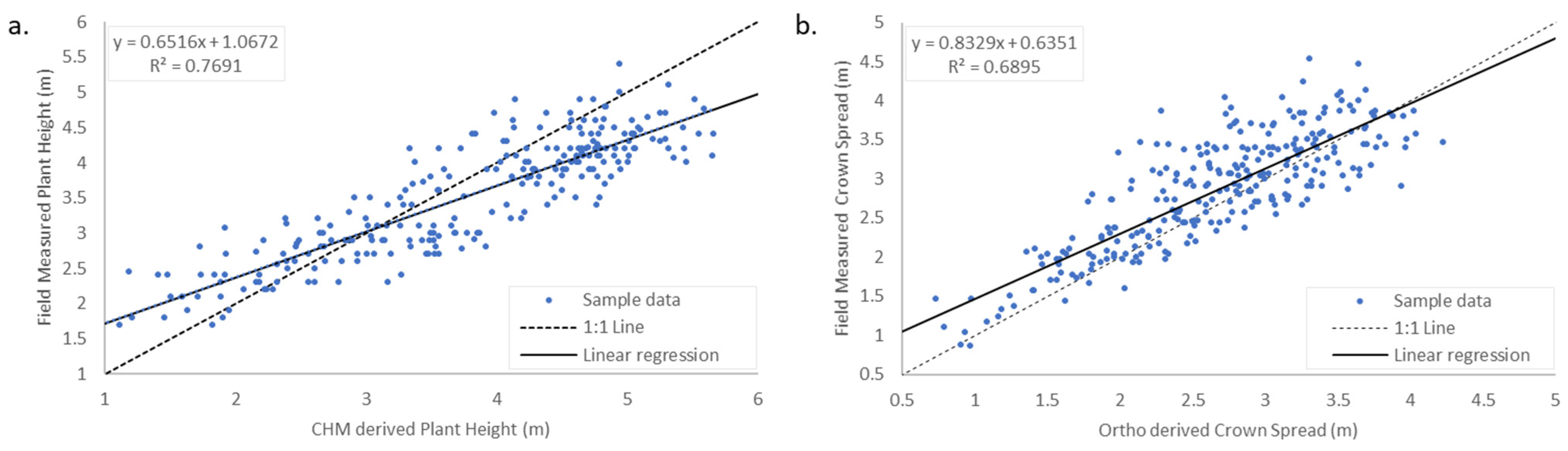
3.1. Banana Plant Morphology Estimation
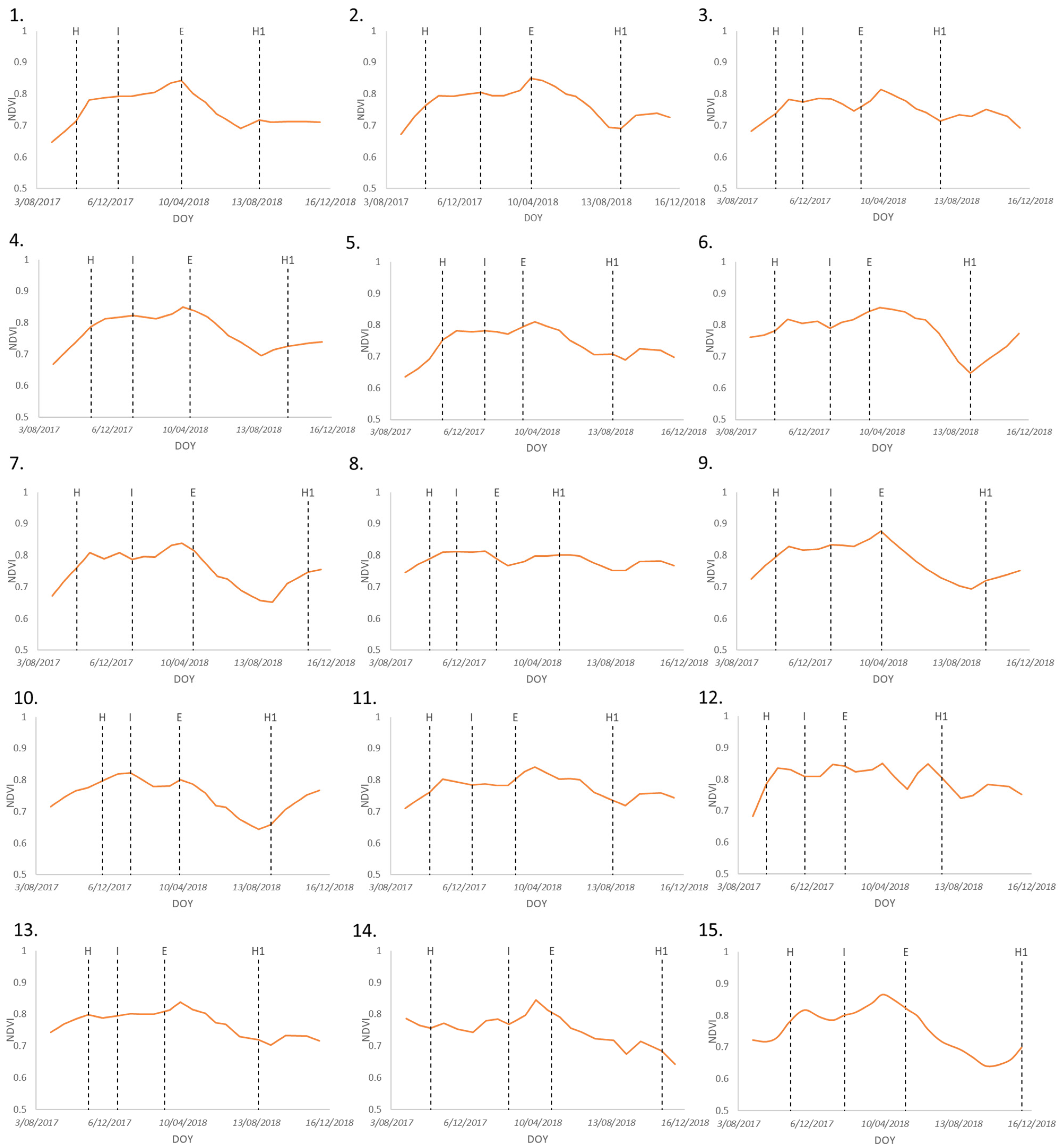
3.2. Banana Plant Phenology Estimation
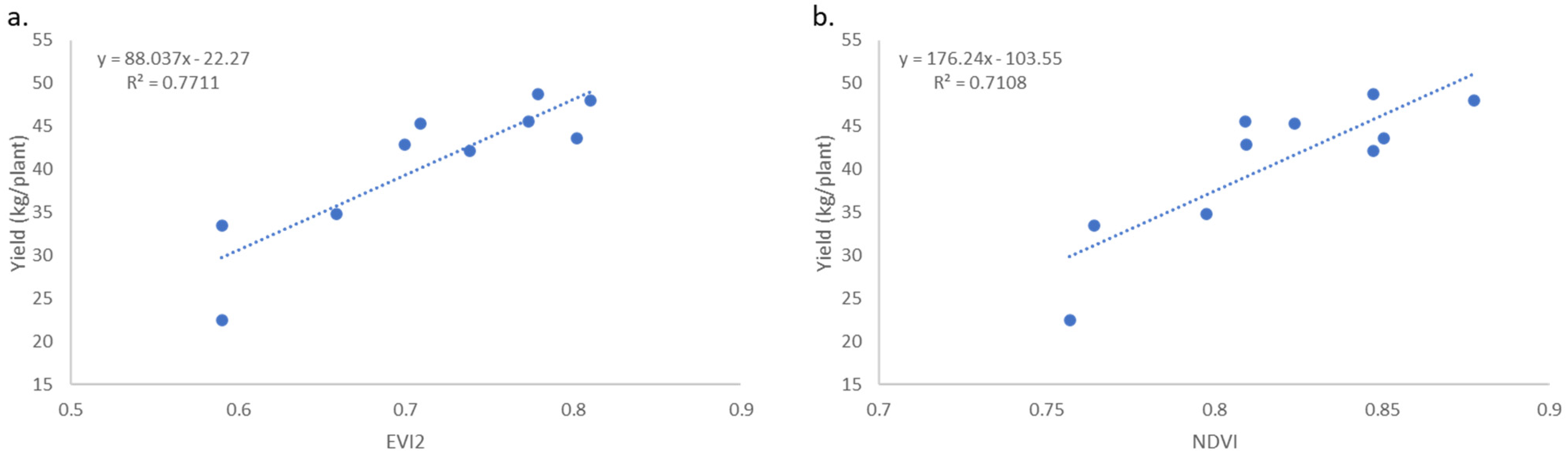
3.3. Yield Relationships to Vegetation Indices and Morphology
4. Discussion
4.1. Individual Crown Delineation and Morphology Estimates
4.2. Phenological Changes (Time Series)
4.3. Yield Relationship to Vegetation Indices and Morphology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brinkhoff, J.; Robson, A.J. Block-level macadamia yield forecasting using spatio-temporal datasets. Agric. For. Meteorol. 2021, 303, 108369. [Google Scholar] [CrossRef]
- Lamour, J.; Le Moguédec, G.; Naud, O.; Lechaudel, M.; Taylor, J.; Tisseyre, B. Evaluating the drivers of banana flowering cycle duration using a stochastic model and on farm production data. Precis. Agric. 2020, 22, 873–896. [Google Scholar] [CrossRef]
- Lindsay, S.; Campagnolo, D.; Daniells, J.; Lemin, C.; Goebel, R.; Pinese, B.; Peterson, R.; Evanas, D.; Pattison, T. Tropical Banana Information Kit; Department of Primary Industries, Queensland Horticulture Institute: Brisbane, QLD, Australia, 1998. [Google Scholar]
- Nyombi, K.; Van Asten, P.J.A.; Leffelaar, P.A.; Corbeels, M.; Kaizzi, C.K.; Giller, K.E. Allometric growth relationships of East Africa highland bananas (Musa AAA-EAHB) cv. Kisansa and Mbwazirume. Ann. Appl. Biol. 2009, 155, 403–418. [Google Scholar] [CrossRef]
- Robinson, J.C.; Saúco, V.G. Bananas and Plantains; Cabi: Wallingford, UK, 2010; Volume 19. [Google Scholar]
- Turner, D.W.; Fortescue, J.A.; Thomas, D.S. Environmental physiology of the bananas (Musa spp.). Braz. J. Plant Physiol. 2007, 19, 463–484. [Google Scholar] [CrossRef]
- Barker, W.G. Growth and Development of the Banana Plant Gross Leaf Emergence. Ann. Bot. 1969, 33, 523–535. [Google Scholar] [CrossRef]
- Gomez Selvaraj, M.; Vergara, A.; Montenegro, F.; Alonso Ruiz, H.; Safari, N.; Raymaekers, D.; Ocimati, W.; Ntamwira, J.; Tits, L.; Omondi, A.B.; et al. Detection of banana plants and their major diseases through aerial images and machine learning methods: A case study in DR Congo and Republic of Benin. ISPRS J. Photogramm. Remote Sens. 2020, 169, 110–124. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring vegetation phenology using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Belda, S.; Pipia, L.; Morcillo-Pallarés, P.; Rivera-Caicedo, J.P.; Amin, E.; De Grave, C.; Verrelst, J. DATimeS: A machine learning time series GUI toolbox for gap-filling and vegetation phenology trends detection. Environ. Model. Softw. 2020, 127, 104666. [Google Scholar] [CrossRef]
- Jönsson, P.; Eklundh, L. TIMESAT—A program for analyzing time-series of satellite sensor data. Comput. Geosci. 2004, 30, 833–845. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Perspectives for remote sensing with unmanned aerial vehicles in precision agriculture. Trends Plant Sci. 2019, 24, 152–164. [Google Scholar] [CrossRef]
- Torres-Sánchez, J.; López-Granados, F.; Serrano, N.; Arquero, O.; Peña, J.M. High-throughput 3-D monitoring of agricultural-tree plantations with unmanned aerial vehicle (UAV) technology. PLoS ONE 2015, 10, e0130479. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-H.; Johansen, K.; Phinn, S.; Robson, A. Measuring Canopy Structure and Condition Using Multi-Spectral UAS Imagery in a Horticultural Environment. Remote Sens. 2019, 11, 269. [Google Scholar] [CrossRef]
- Wu, D.; Johansen, K.; Phinn, S.; Robson, A. Suitability of airborne and terrestrial laser scanning for mapping tree crop structural metrics for improved orchard management. Remote Sens. 2020, 12, 1647. [Google Scholar] [CrossRef]
- Watts, A.C.; Ambrosia, V.G.; Hinkley, E.A. Unmanned Aircraft Systems in Remote Sensing and Scientific Research: Classification and Considerations of Use. Remote Sens. 2012, 4, 1671. [Google Scholar] [CrossRef]
- Wu, D.; Johansen, K.; Phinn, S.; Robson, A.; Tu, Y.-H. Inter-comparison of remote sensing platforms for height estimation of mango and avocado tree crowns. Int. J. Appl. Earth Obs. Geoinf. 2020, 89, 102091. [Google Scholar] [CrossRef]
- Johansen, K.; Raharjo, T.; McCabe, M.F. Using Multi-Spectral UAV Imagery to Extract Tree Crop Structural Properties and Assess Pruning Effects. Remote Sens. 2018, 10, 854. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Bolton, D.K.; Friedl, M.A. Forecasting crop yield using remotely sensed vegetation indices and crop phenology metrics. Agric. For. Meteorol. 2013, 173, 74–84. [Google Scholar] [CrossRef]
- Chlingaryan, A.; Sukkarieh, S.; Whelan, B. Machine learning approaches for crop yield prediction and nitrogen status estimation in precision agriculture: A review. Comput. Electron. Agric. 2018, 151, 61–69. [Google Scholar] [CrossRef]
- Mahlein, A.-K. Plant Disease Detection by Imaging Sensors—Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 2015, 100, 241–251. [Google Scholar] [CrossRef]
- Susič, N.; Žibrat, U.; Širca, S.; Strajnar, P.; Razinger, J.; Knapič, M.; Vončina, A.; Urek, G.; Stare, B.G. Discrimination between abiotic and biotic drought stress in tomatoes using hyperspectral imaging. Sens. Actuators B Chem. 2018, 273, 842–852. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Lamour, J.; Leroux, C.; Le Moguédec, G.; Naud, O.; Léchaudel, M.; Tisseyre, B. Disentangling the sources of chlorophyll-content variability in banana fields. In Precision Agriculture’19; Wageningen Academic Publishers: Waneningen, The Netherlands, 2019; pp. 407–417. [Google Scholar]
- Basso, B.; Fiorentino, C.; Cammarano, D.; Schulthess, U. Variable rate nitrogen fertilizer response in wheat using remote sensing. Precis. Agric. 2016, 17, 168–182. [Google Scholar] [CrossRef]
- Wang, J.; Shen, C.; Liu, N.; Jin, X.; Fan, X.; Dong, C.; Xu, Y. Non-Destructive Evaluation of the Leaf Nitrogen Concentration by In-Field Visible/Near-Infrared Spectroscopy in Pear Orchards. Sensors 2017, 17, 538. [Google Scholar] [CrossRef]
- Joyce Dória Rodrigues, S.; Pasqual, M.; Rodrigues, F.A.; Lacerda, W.S.; Donato, S.L.R.; e Silva, S.D.O.; Paixão, C.A. Correlation between morphological characters and estimated bunch weight of the Tropical banana cultivar. Afr. J. Biotechnol. 2012, 11, 10682. [Google Scholar] [CrossRef]
- Swarupa, V.; Ravishankar, K.V.; Rekha, A. Plant defense response against Fusarium oxysporum and strategies to develop tolerant genotypes in banana. Planta 2014, 239, 735–751. [Google Scholar] [CrossRef]
- Memon, N.; Memon, K.; Shah, Z.-U.-H. Plant Analysis as a Diagnostic Tool for Evaluating Nutritional Requirements of Bananas. Int. J. Agric. Biol. 2005, 7, 824–831. [Google Scholar]
- Barker, W.; Steward, F. Growth and Development of the Banana Plant II. The Transition from the Vegetative to the Floral Shoot in Musa acuminata cv. Gros Michel. Ann. Bot. 1962, 26, 413–423. [Google Scholar] [CrossRef]
- Thomas, D.S.; Turner, D.W. Banana (Musa sp.) leaf gas exchange and chlorophyll fluorescence in response to soil drought, shading and lamina folding. Sci. Hortic. 2001, 90, 93–108. [Google Scholar] [CrossRef]
- Taylor, S.E.; Sexton, O.J. Some Implications of Leaf Tearing in Musaceae. Ecology 1972, 53, 143–149. [Google Scholar] [CrossRef]
- Johansen, K.; Sohlbach, M.; Sullivan, B.; Stringer, S.; Peasley, D.; Phinn, S. Mapping banana plants from high spatial resolution orthophotos to facilitate plant health assessment. Remote Sens. 2014, 6, 8261–8286. [Google Scholar] [CrossRef]
- Stevens, B.; Diels, J.; Vanuytrecht, E.; Brown, A.; Bayo, S.; Rujweka, A.; Richard, E.; Ndakidemi, P.A.; Swennen, R. Canopy cover evolution, diurnal patterns and leaf area index relationships in a Mchare and Cavendish banana cultivar under different soil moisture regimes. Sci. Hortic. 2020, 272, 109328. [Google Scholar] [CrossRef]
- Clark, A.; McKechnie, J. Detecting Banana Plantations in the Wet Tropics, Australia, Using Aerial Photography and U-Net. Appl. Sci. 2020, 10, 2017. [Google Scholar] [CrossRef]
- Campos, B.O.O.; Paredes, F.; Rey, J.C.; Lobo, D.; Galvis-Causil, S. The relationship between the normalized difference vegetation index, rainfall, and potential evapotranspiration in a banana plantation of Venezuela. SAINS TANAH-J. Soil Sci. Agroclimatol. 2021, 18, 58–64. [Google Scholar] [CrossRef]
- Australian Banana Growers Council [ABGC]. Mapping Banana Block Productivity. Available online: https://abgc.org.au/2018/09/12/mapping-banana-block-productivity/ (accessed on 1 October 2021).
- Zou, X.; Mõttus, M. Sensitivity of Common Vegetation Indices to the Canopy Structure of Field Crops. Remote Sens. 2017, 9, 994. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Harto, A.B.; Prastiwi, P.A.D.; Ariadji, F.N.; Suwardhi, D.; Dwivany, F.M.; Nuarsa, I.W.; Wikantika, K. Identification of Banana Plants from Unmanned Aerial Vehicles (UAV) Photos Using Object Based Image Analysis (OBIA) Method (A Case Study in Sayang Village, Jatinangor District, West Java). HAYATI J. Biosci. 2019, 26, 7. [Google Scholar] [CrossRef]
- Handique, B.K.; Goswami, C.; Gupta, C.; Pandit, S.; Gogoi, S.; Jadi, R.; Jena, P.; Borah, G.; Raju, P.L.N. Hierarchical classification for assessment of horticultural crops in mixed cropping pattern using UAV-borne multi-spectral sensor. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2020, XLIII-B3-2020, 67–74. [Google Scholar] [CrossRef]
- Kestur, R.; Angural, A.; Bashir, B.; Omkar, S.N.; Anand, G.; Meenavathi, M.B. Tree Crown Detection, Delineation and Counting in UAV Remote Sensed Images: A Neural Network Based Spectral–Spatial Method. J. Indian Soc. Remote Sens. 2018, 46, 991–1004. [Google Scholar] [CrossRef]
- Neupane, B.; Horanont, T.; Hung, N.D. Deep learning based banana plant detection and counting using high-resolution red-green-blue (RGB) images collected from unmanned aerial vehicle (UAV). PLoS ONE 2019, 14, e0223906. [Google Scholar] [CrossRef]
- Aeberli, A.; Johansen, K.; Robson, A.; Lamb, D.W.; Phinn, S. Detection of banana plants using multi-temporal multispectral uav imagery. Remote Sens. 2021, 13, 2123. [Google Scholar] [CrossRef]
- Rabatel, G.; Lamour, J.; Moura, D.; Naud, O. A multispectral processing chain for chlorophyll content assessment in banana fields by UAV imagery. In Precision Agriculture’19; Wageningen Academic Publishers: Waneningen, The Netherlands, 2019; pp. 109–130. [Google Scholar]
- Calou, V.B.C.; Teixeira, A.d.S.; Moreira, L.C.J.; Lima, C.S.; de Oliveira, J.B.; de Oliveira, M.R.R. The use of UAVs in monitoring yellow sigatoka in banana. Biosyst. Eng. 2020, 193, 115–125. [Google Scholar] [CrossRef]
- Bureau of Meteorology. Climate Statistics for Australian Locations: Beerburrum Weather Station. Available online: http://www.bom.gov.au/climate/averages/tables/cw_040284.shtml (accessed on 18 March 2018).
- Blozan, W. Tree measuring guidelines of the eastern native tree society. Bull. East. Nativ. Tree Soc. 2006, 1, 3–10. [Google Scholar]
- Potdar, M.V.; Pawar, K.R. Non-destructive leaf area estimation in banana. Sci. Hortic. 1991, 45, 251–254. [Google Scholar] [CrossRef]
- Turner, D. Banana plant growth. 2. Dry matter production, leaf area and growth analysis. Aust. J. Exp. Agric. 1972, 12, 216–224. [Google Scholar] [CrossRef]
- Queensland Government. Queensland Spatial Catalogue—QSpatial. Available online: https://qldspatial.information.qld.gov.au/catalogue/custom/search.pag (accessed on 25 March 2021).
- Fawcett, D.; Bennie, J.; Anderson, K. Monitoring spring phenology of individual tree crowns using drone-acquired NDVI data. Remote Sens. Ecol. Conserv. 2021, 7, 227–244. [Google Scholar] [CrossRef]
- Assmann, J.J.; Kerby, J.T.; Cunliffe, A.M.; Myers-Smith, I.H. Vegetation monitoring using multispectral sensors—best practices and lessons learned from high latitudes. J. Unmanned Veh. Syst. 2019, 7, 54–75. [Google Scholar] [CrossRef]
- Tu, Y.-H.; Phinn, S.; Johansen, K.; Robson, A. Assessing Radiometric Correction Approaches for Multi-Spectral UAS Imagery for Horticultural Applications. Remote Sens. 2018, 10, 1684. [Google Scholar] [CrossRef]
- Wang, C.; Myint, S.W. A simplified empirical line method of radiometric calibration for small unmanned aircraft systems-based remote sensing. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 1876–1885. [Google Scholar] [CrossRef]
- JW, R.; Haas, R.; Schell, J.; Deering, D. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third earth resources technology satellite-1 symposium, Washington, DC, USA, 10–14 December 1973; Volume 1, pp. 309–317. [Google Scholar]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Colaizzi, P.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C. Coincident detection of crop water stress, nitrogen status and canopy density using ground-based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Trimble, T. Reference Book eCognition Developer. Available online: https://docs.ecognition.com/v9.5.0/Page%20collection/eCognition%20Suite%20Dev%20RB.htm (accessed on 15 November 2021).
- González-Piqueras, J.; Sánchez, S.; Villodre, J.; López, H.; Calera, A.; Hernández-López, D.; Sánchez, J.M. Radiometric performance of multispectral camera applied to operational precision agriculture. In Proceedings of the IGARSS 2018-2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain, 22–27 July 2018; pp. 3393–3396. [Google Scholar]
- Berra, E.F.; Gaulton, R.; Barr, S. Assessing spring phenology of a temperate woodland: A multiscale comparison of ground, unmanned aerial vehicle and Landsat satellite observations. Remote Sens. Environ. 2019, 223, 229–242. [Google Scholar] [CrossRef]
- Kuenzer, C. Remote Sensing Time Series Revealing Land Surface Dynamics; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Forkel, M.; Wingate, L.; Tomelleri, E.; Morra di Cella, U.; Richardson, A.D. Phenopix: A R package for image-based vegetation phenology. Agric. For. Meteorol. 2016, 220, 141–150. [Google Scholar] [CrossRef]
- Gu, L.; Post, W.M.; Baldocchi, D.D.; Black, T.A.; Suyker, A.E.; Verma, S.B.; Vesala, T.; Wofsy, S.C. Characterizing the Seasonal Dynamics of Plant Community Photosynthesis Across a Range of Vegetation Types. In Phenology of Ecosystem Processes: Applications in Global Change Research; Noormets, A., Ed.; Springer: New York, NY, USA, 2009; pp. 35–58. [Google Scholar]
- Robson, A.; Rahman, M.M.; Muir, J.; Saint, A.; Simpson, C.; Searle, C. Evaluating satellite remote sensing as a method for measuring yield variability in Avocado and Macadamia tree crops. Adv. Anim. Biosci. 2017, 8, 498–504. [Google Scholar] [CrossRef]
- Bohra, P.; Waman, A.A.; Umesha, K.; Sathyanarayana, N.B.; Sreeramu, S.B.; Gangappa, E. Key Phenological Events, their Practical Implications and Effect of Bunch Age on Physico-Chemical and Postharvest Attributes in Ney Poovan Banana (Musa AB). Erwerbs-Obstbau 2015, 57, 13–22. [Google Scholar] [CrossRef]
- Jackson, R.D.; Huete, A.R. Interpreting vegetation indices. Prev. Vet. Med. 1991, 11, 185–200. [Google Scholar] [CrossRef]
- Kuikel, S.; Upadhyay, B.; Aryal, D.; Bista, S.; Awasthi, B.; Shrestha, S. Individual banana tree crown delineation using unmanned aerial vehicle (UAV) images. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2021, XLIII-B3-2021, 581–585. [Google Scholar] [CrossRef]
- Zhen, Z.; Quackenbush, L.J.; Zhang, L. Trends in Automatic Individual Tree Crown Detection and Delineation—Evolution of LiDAR Data. Remote Sens. 2016, 8, 333. [Google Scholar] [CrossRef]
- Damour, G.; Ozier-Lafontaine, H.; Dorel, M. Simulation of the growth of banana (Musa spp.) cultivated on cover-crop with simplified indicators of soil water and nitrogen availability and integrated plant traits. Field Crops Res. 2012, 130, 99–108. [Google Scholar] [CrossRef]
- Burkart, A.; Hecht, V.L.; Kraska, T.; Rascher, U. Phenological analysis of unmanned aerial vehicle based time series of barley imagery with high temporal resolution. Precis. Agric. 2018, 19, 134–146. [Google Scholar] [CrossRef]
- Sofonia, J.; Shendryk, Y.; Phinn, S.; Roelfsema, C.; Kendoul, F.; Skocaj, D. Monitoring sugarcane growth response to varying nitrogen application rates: A comparison of UAV SLAM LiDAR and photogrammetry. Int. J. Appl. Earth Obs. Geoinf. 2019, 82, 101878. [Google Scholar] [CrossRef]
- Guo, Y.; Senthilnath, J.; Wu, W.; Zhang, X.; Zeng, Z.; Huang, H. Radiometric calibration for multispectral camera of different imaging conditions mounted on a UAV platform. Sustainability 2019, 11, 978. [Google Scholar] [CrossRef]
- Olsson, P.-O.; Vivekar, A.; Adler, K.; Garcia Millan, V.E.; Koc, A.; Alamrani, M.; Eklundh, L. Radiometric Correction of Multispectral UAS Images: Evaluating the Accuracy of the Parrot Sequoia Camera and Sunshine Sensor. Remote Sens. 2021, 13, 577. [Google Scholar] [CrossRef]
- Deng, L.; Mao, Z.; Li, X.; Hu, Z.; Duan, F.; Yan, Y. UAV-based multispectral remote sensing for precision agriculture: A comparison between different cameras. ISPRS J. Photogramm. Remote Sens. 2018, 146, 124–136. [Google Scholar] [CrossRef]
- Ban, Y.; Yousif, O. Multitemporal Remote Sensing; Springer: Cham, Switzerland, 2016; Volume 20. [Google Scholar]
- Wu, S.; Wang, J.; Yan, Z.; Song, G.; Chen, Y.; Ma, Q.; Deng, M.; Wu, Y.; Zhao, Y.; Guo, Z.; et al. Monitoring tree-crown scale autumn leaf phenology in a temperate forest with an integration of PlanetScope and drone remote sensing observations. ISPRS J. Photogramm. Remote Sens. 2021, 171, 36–48. [Google Scholar] [CrossRef]
- Qiu, R.; Li, X.; Han, G.; Xiao, J.; Ma, X.; Gong, W. Monitoring drought impacts on crop productivity of the US Midwest with solar-induced fluorescence: GOSIF outperforms GOME-2 SIF and MODIS NDVI, EVI, and NIRv. Agric. For. Meteorol. 2022, 323, 109038. [Google Scholar] [CrossRef]
- Yang, Q.; Shi, L.; Han, J.; Yu, J.; Huang, K. A near real-time deep learning approach for detecting rice phenology based on UAV images. Agric. For. Meteorol. 2020, 287, 107938. [Google Scholar] [CrossRef]
- Machovina, B.L.; Feeley, K.J.; Machovina, B.J. UAV remote sensing of spatial variation in banana production. Crop Pasture Sci. 2016, 67, 1281. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aeberli, A.; Phinn, S.; Johansen, K.; Robson, A.; Lamb, D.W. Characterisation of Banana Plant Growth Using High-Spatiotemporal-Resolution Multispectral UAV Imagery. Remote Sens. 2023, 15, 679. https://doi.org/10.3390/rs15030679
Aeberli A, Phinn S, Johansen K, Robson A, Lamb DW. Characterisation of Banana Plant Growth Using High-Spatiotemporal-Resolution Multispectral UAV Imagery. Remote Sensing. 2023; 15(3):679. https://doi.org/10.3390/rs15030679
Chicago/Turabian StyleAeberli, Aaron, Stuart Phinn, Kasper Johansen, Andrew Robson, and David W. Lamb. 2023. "Characterisation of Banana Plant Growth Using High-Spatiotemporal-Resolution Multispectral UAV Imagery" Remote Sensing 15, no. 3: 679. https://doi.org/10.3390/rs15030679
APA StyleAeberli, A., Phinn, S., Johansen, K., Robson, A., & Lamb, D. W. (2023). Characterisation of Banana Plant Growth Using High-Spatiotemporal-Resolution Multispectral UAV Imagery. Remote Sensing, 15(3), 679. https://doi.org/10.3390/rs15030679






