Multi-Factors Synthetically Contribute to Ulva prolifera Outbreaks in the South Yellow Sea of China
Abstract
:1. Introduction
2. Materials and Methods
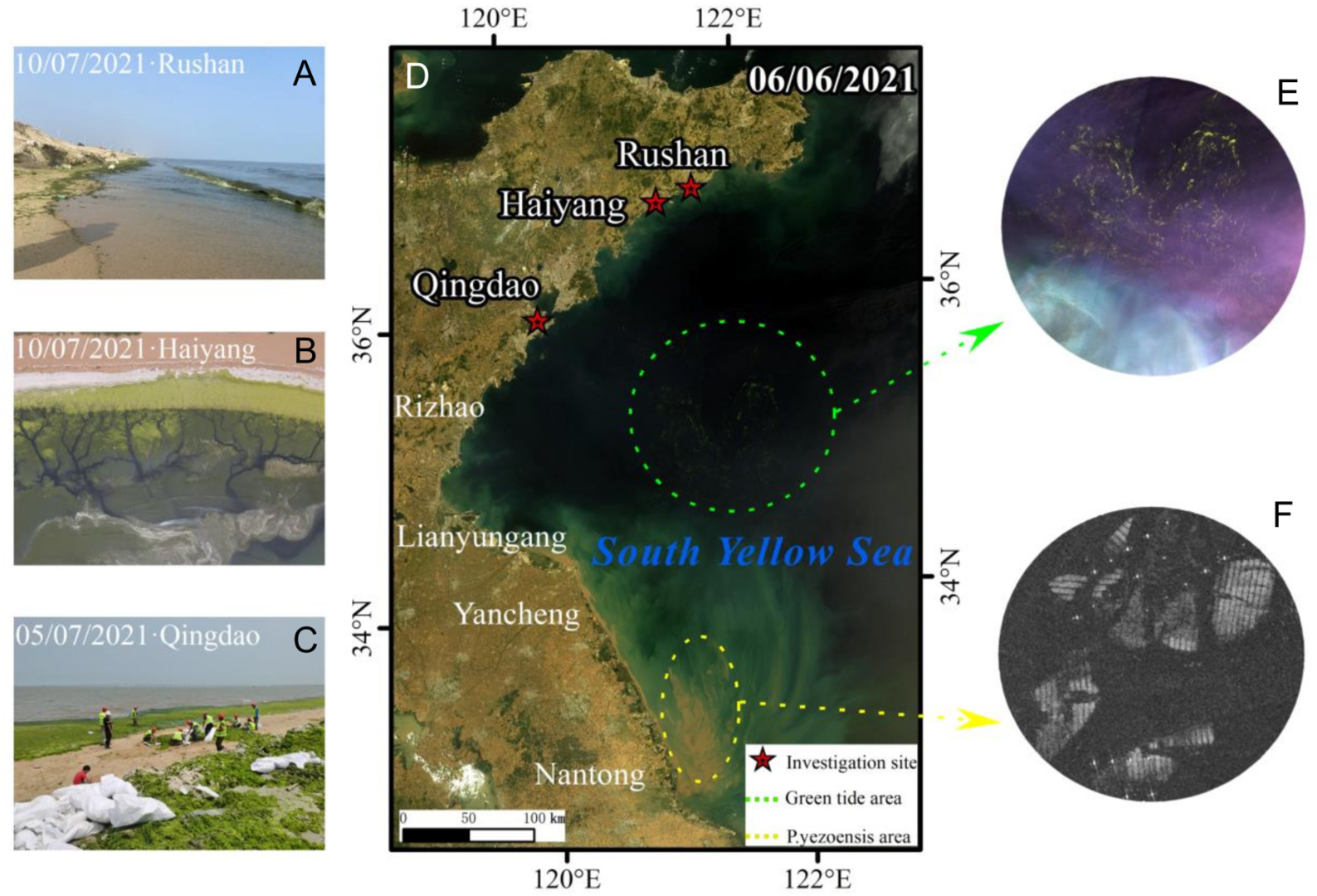
2.1. Study Area
2.2. GEE Data Acquisition and Processing
2.2.1. U. prolifera Information Extraction
2.2.2. Extraction of Information on P. yezoensis Cultivation
2.2.3. Processing of Environmental Factor Data
2.3. Human Footprint Index (HFI) Data Acquisition and Processing
2.4. Influence Factor Processing Methods
3. Results
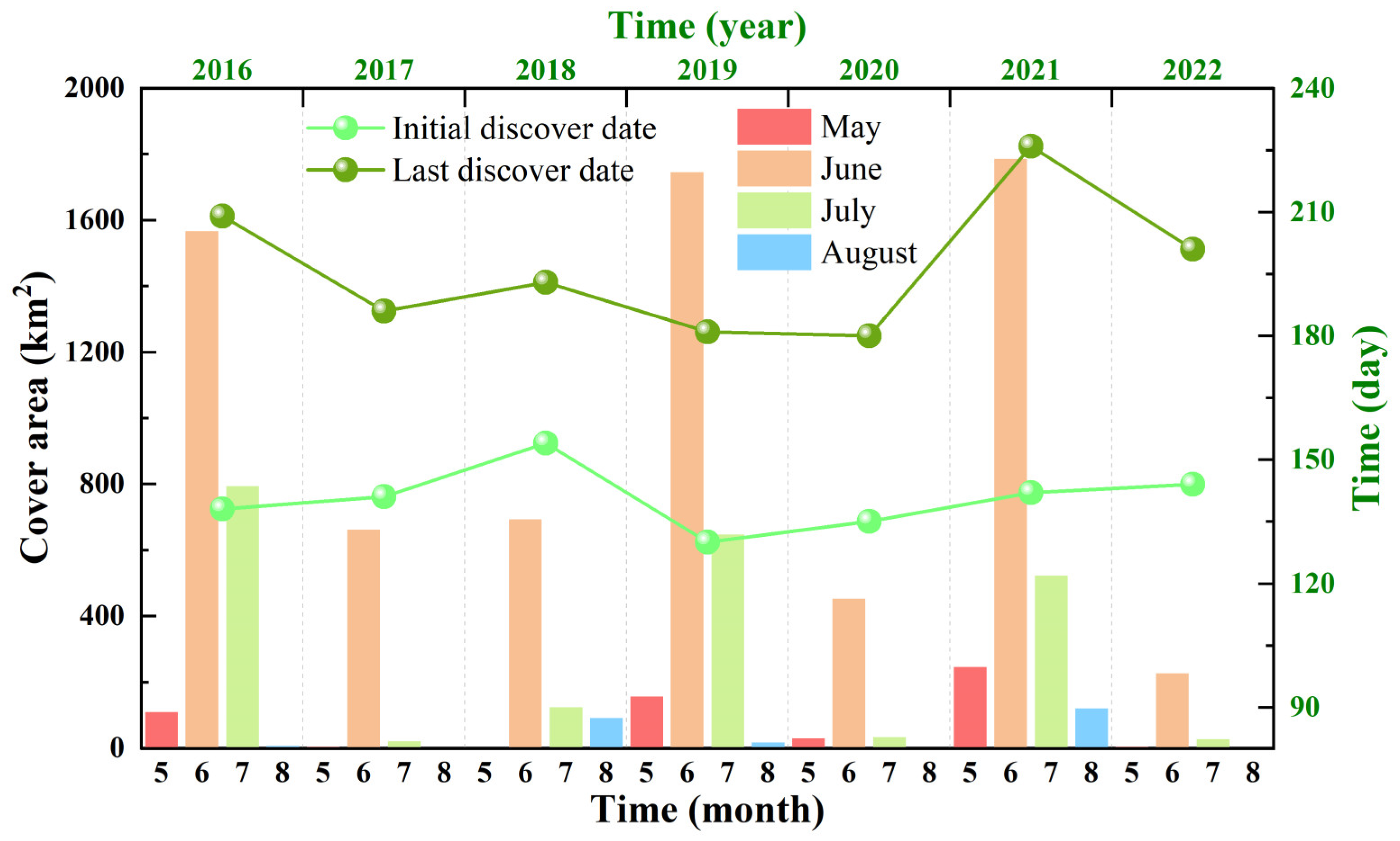
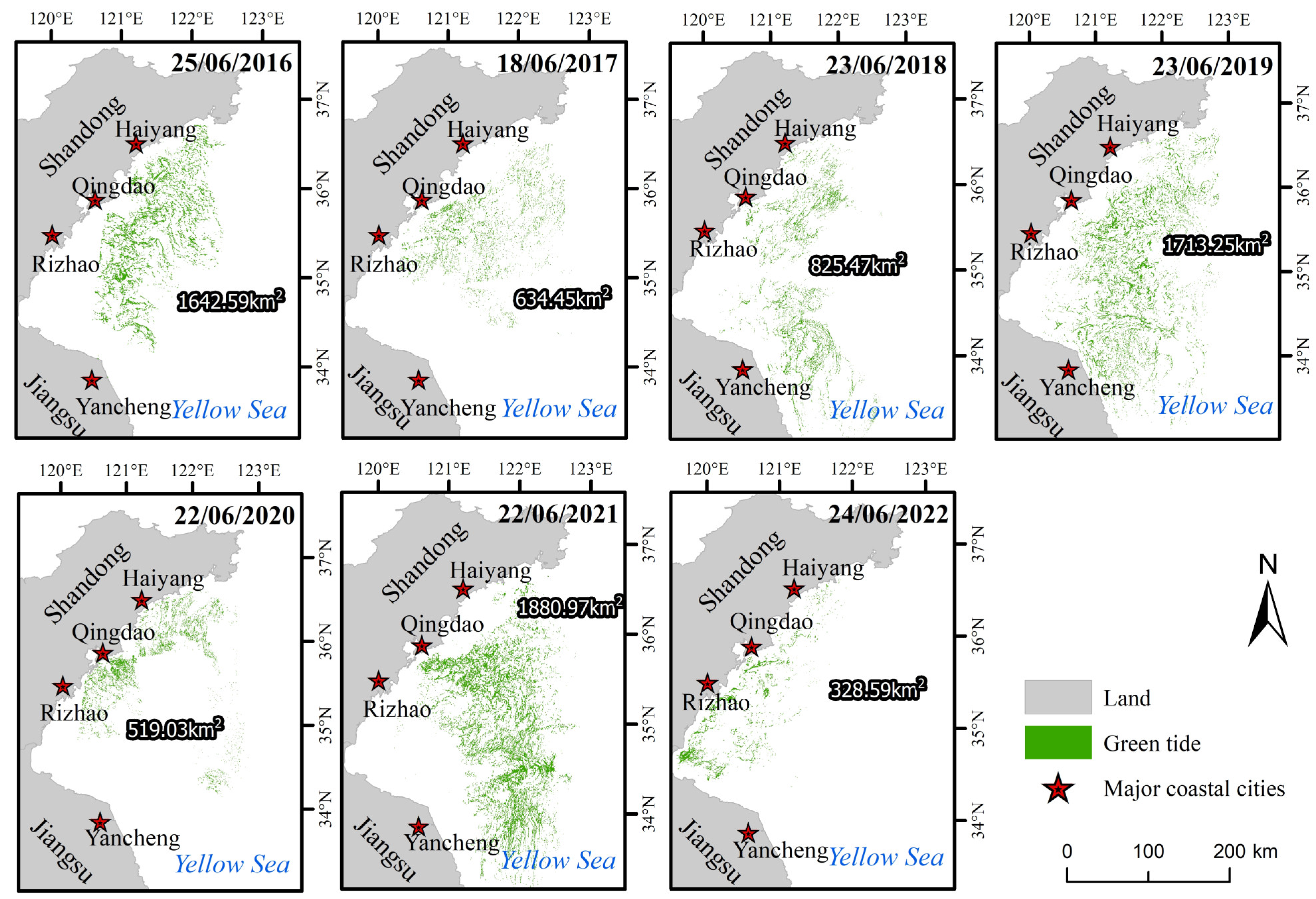
3.1. Variations in the Size and Duration of the Green Tide Phenomenon
3.2. Changes in the Recycle Time of P. yezoensis Cultivation Rafts in the Northern Jiangsu Shoal
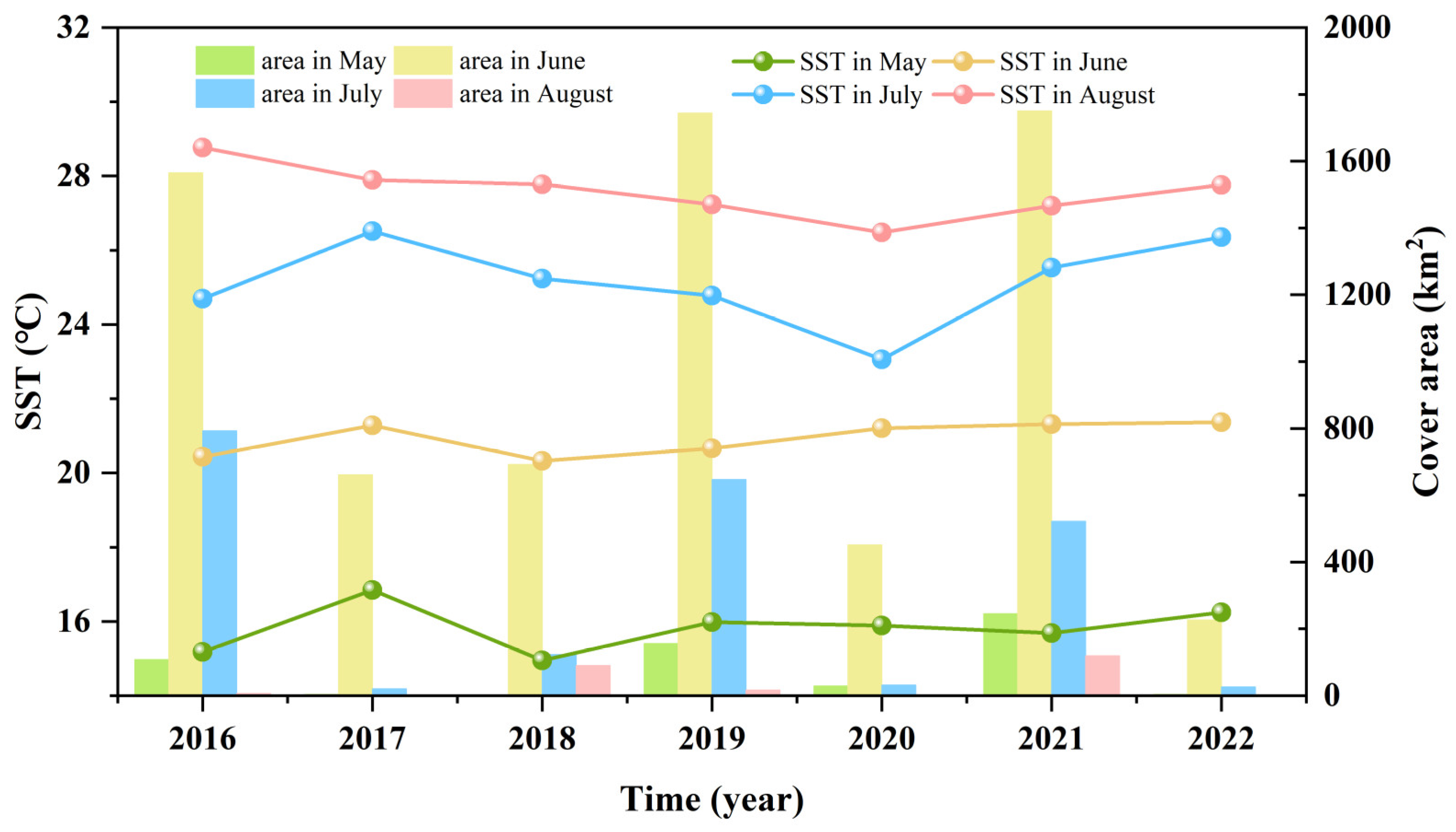
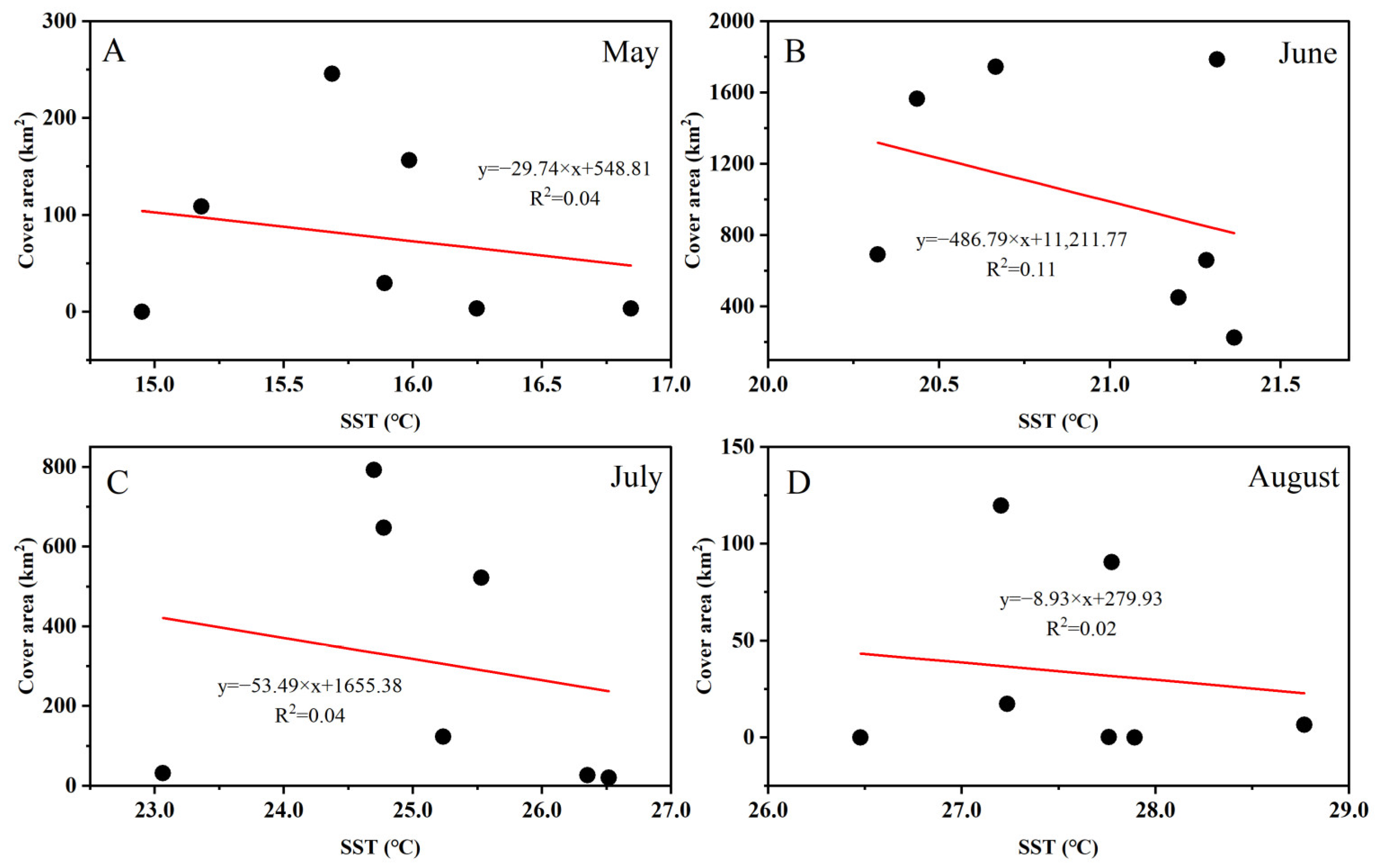
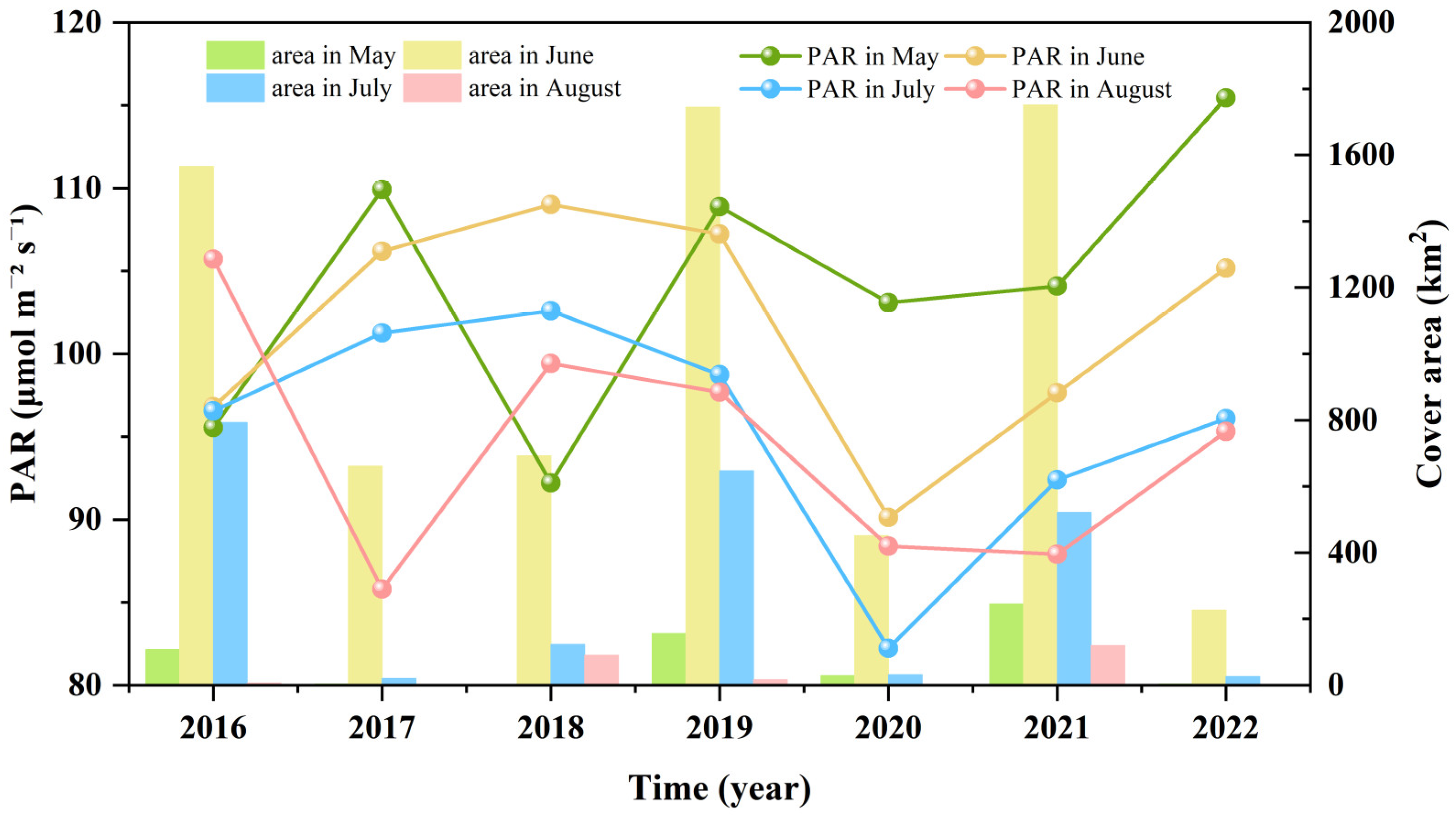
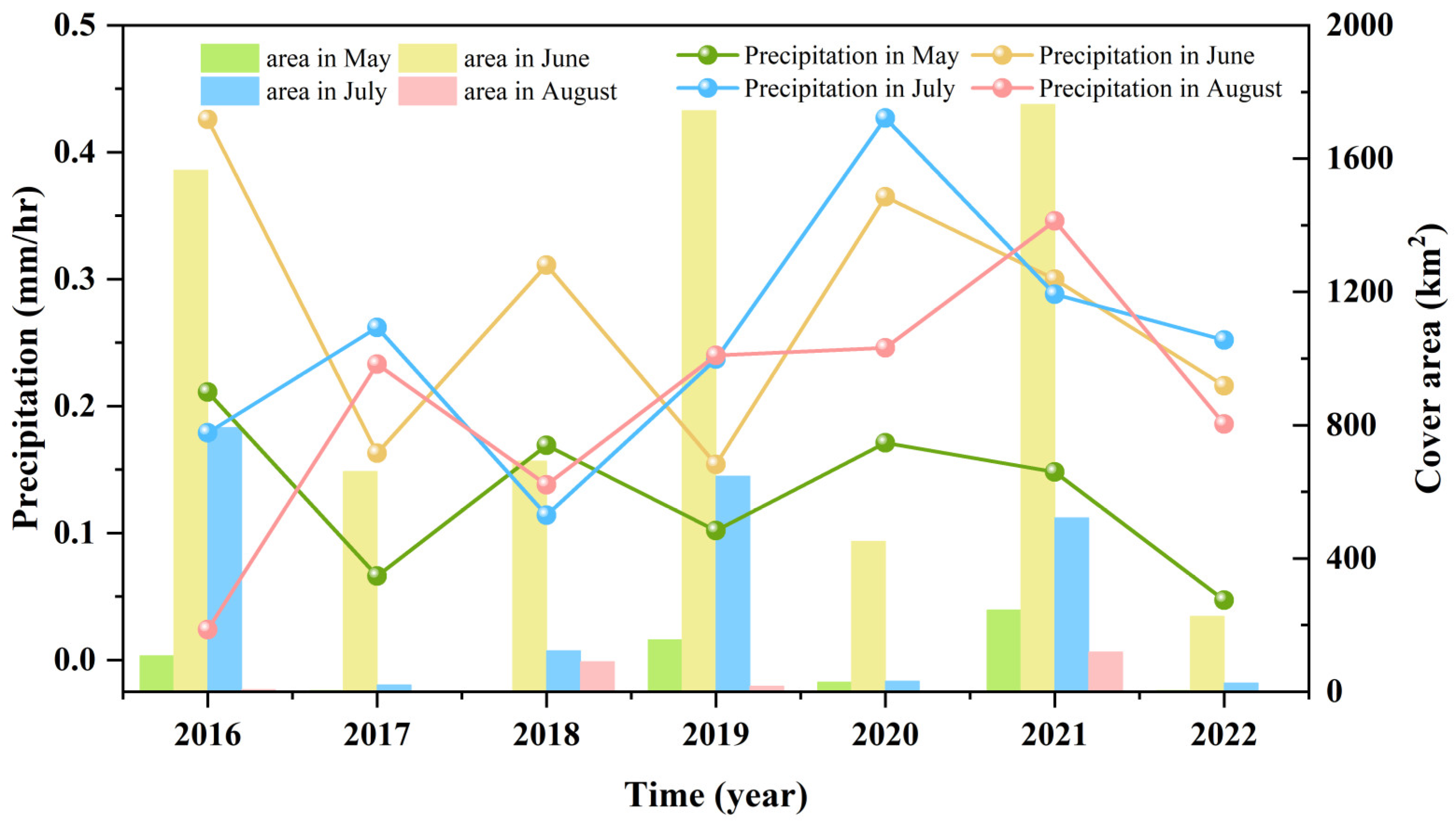
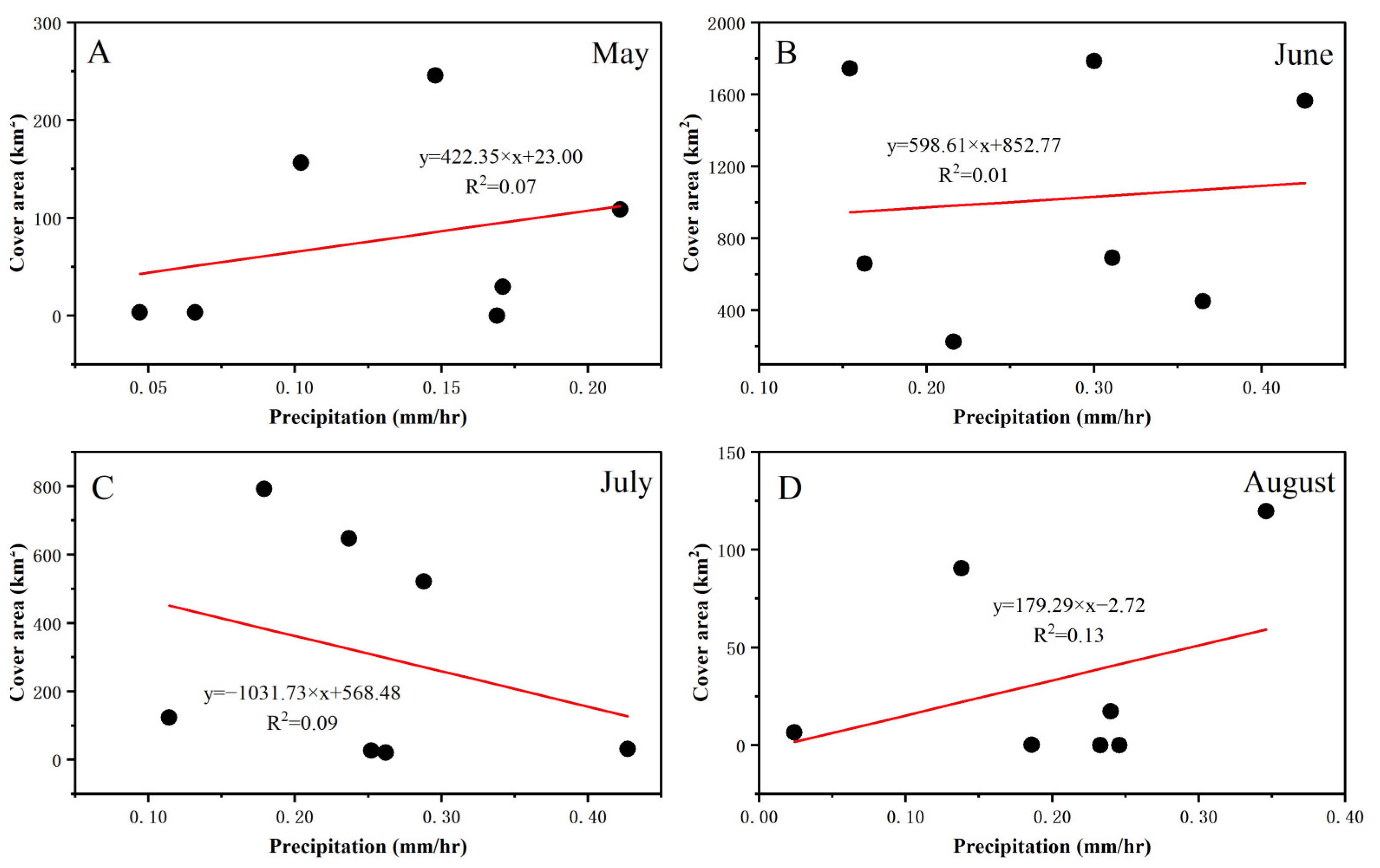
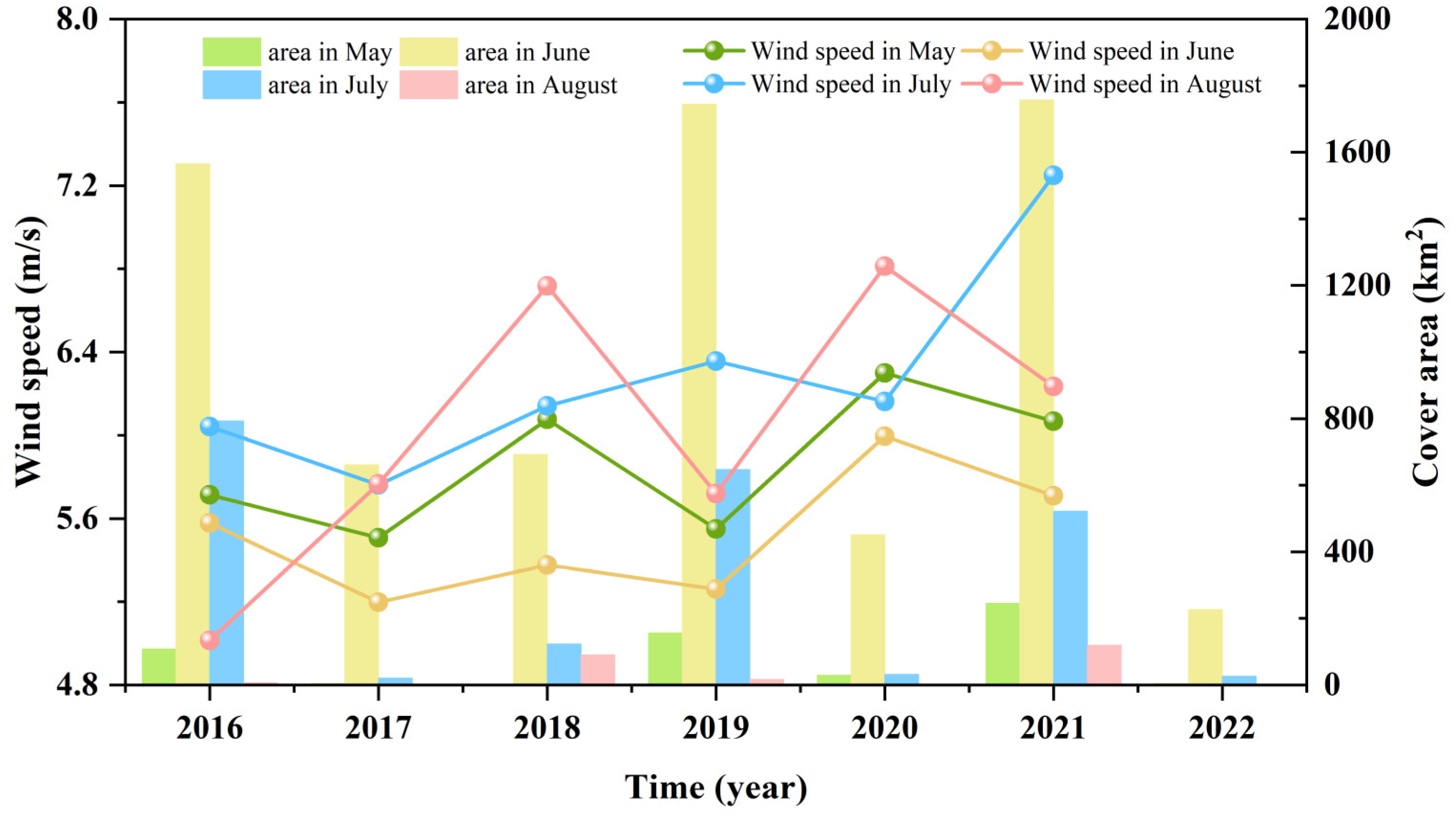
3.3. Fluctuations in Environmental Factors during Green Tide Events in the SYS
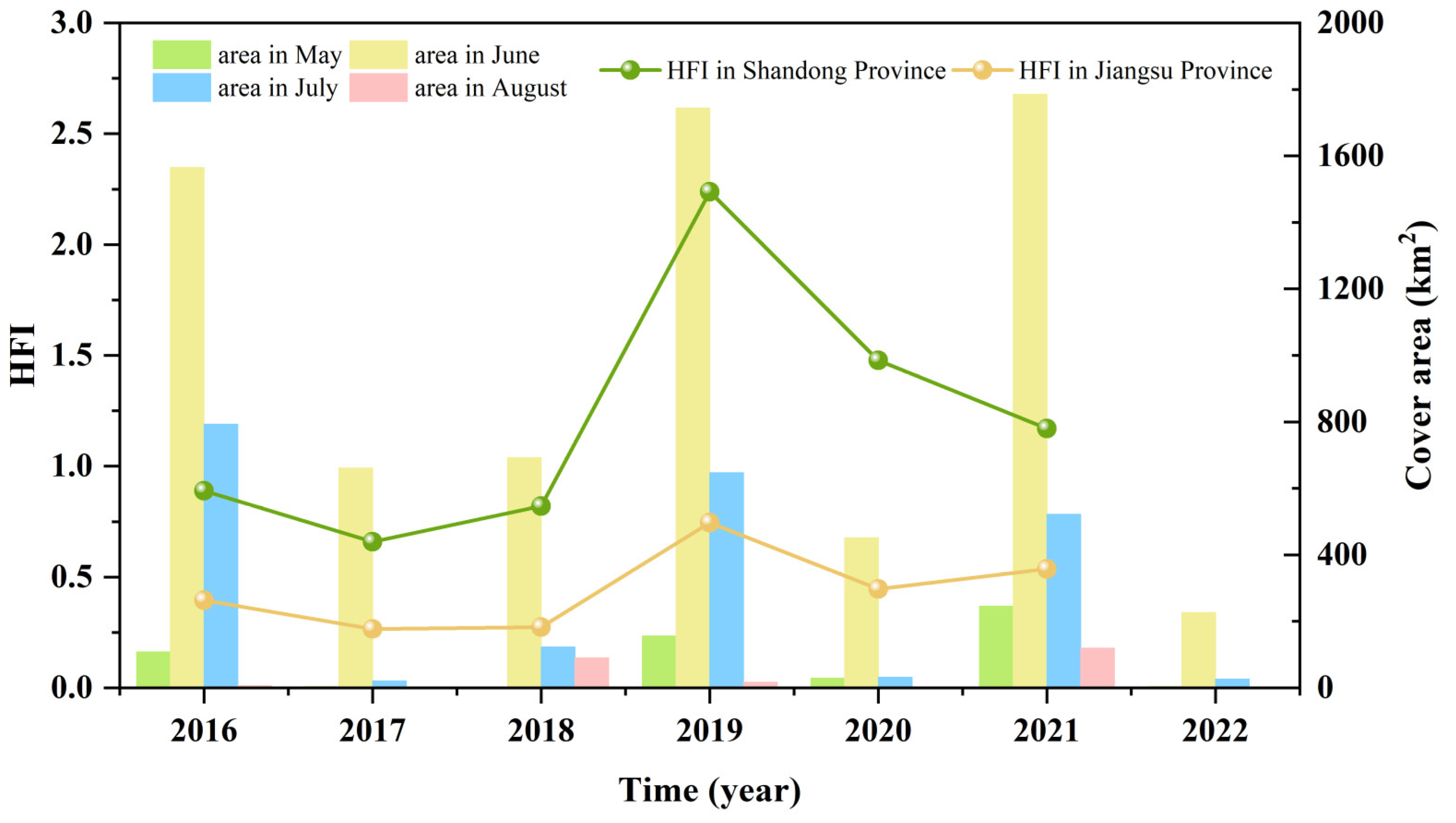
3.4. Human Footprint Index Dynamics in the SYS
4. Discussion
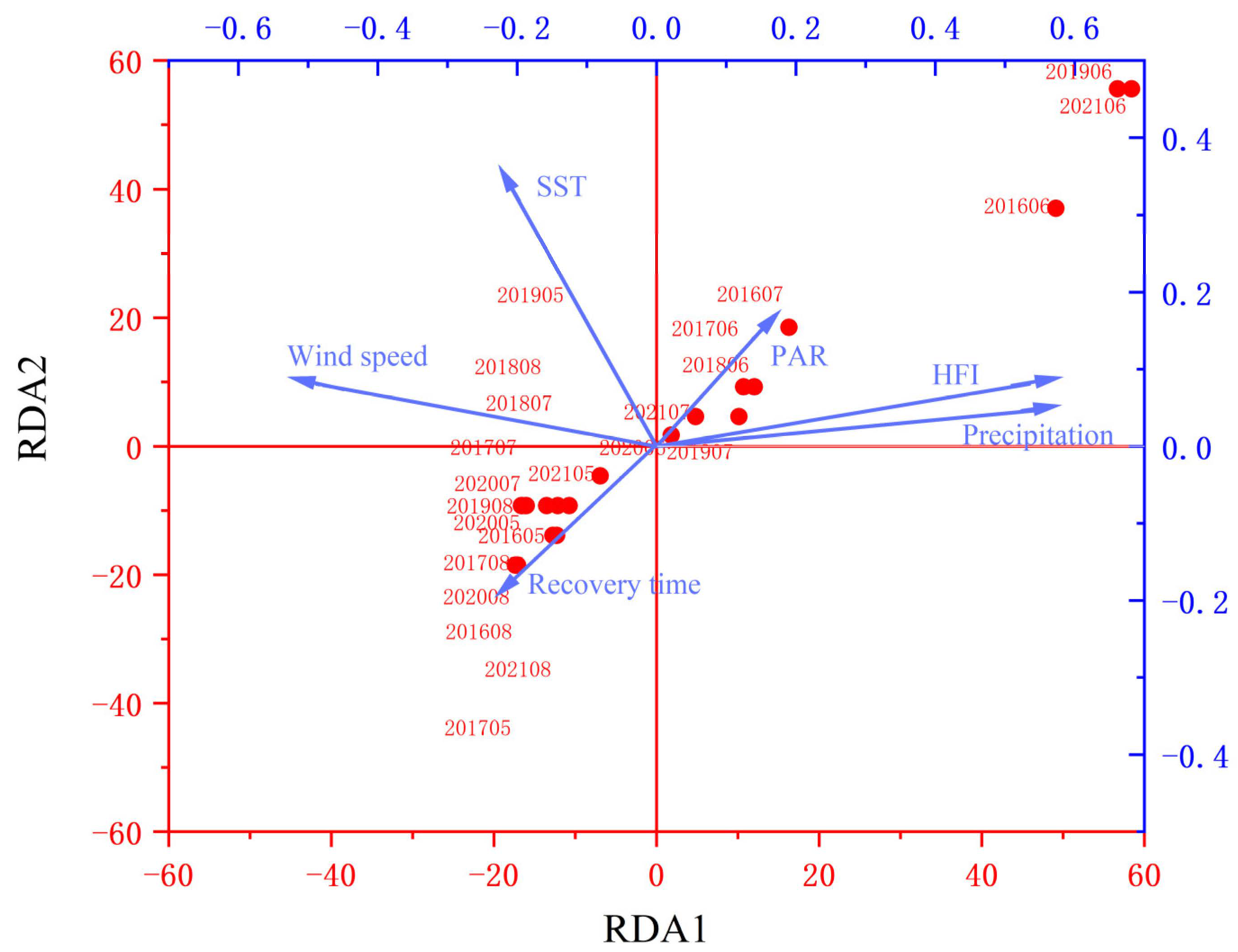
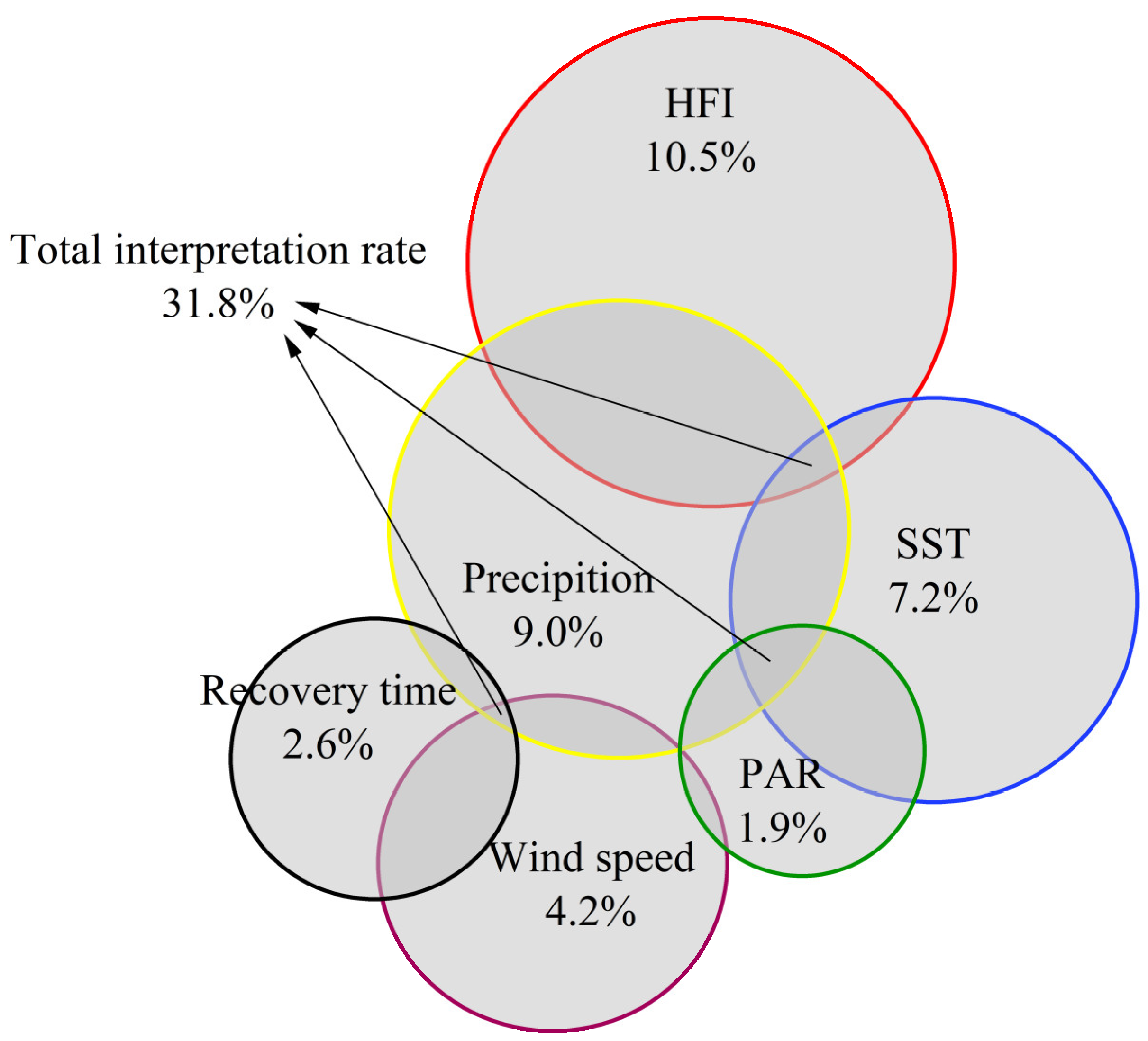
4.1. Analysis of the Contribution of Impact Factors to the Outbreak of U. prolifera in the SYS
4.2. Analysis of Factors Affecting Inter-Annual Variability in U. prolifera Scale
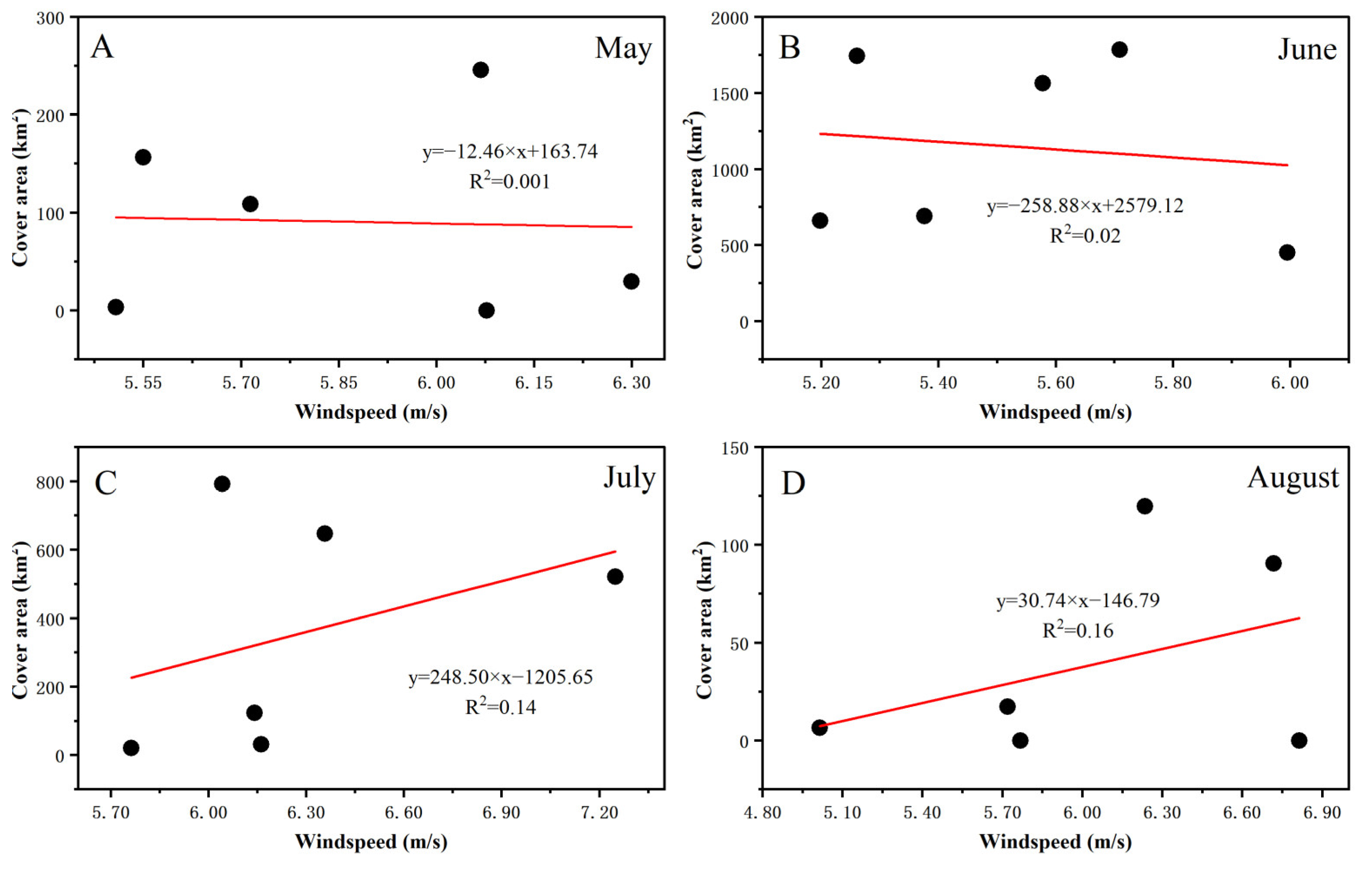
4.2.1. Analysis of Environmental Factors
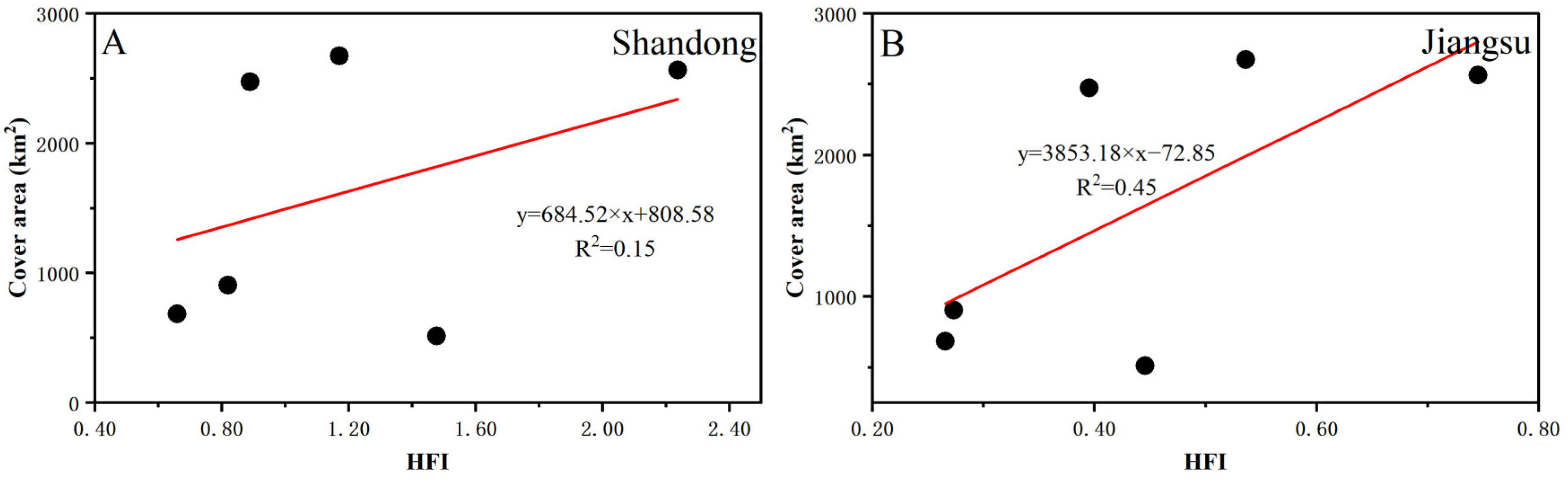
4.2.2. Human Factors Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lyons, D.A.; Arvanitidis, C.; Blight, A.J.; Chatzinikolaou, E.; Guy-Haim, T.; Kotta, J.; Orav-Kotta, H.; Queirós, A.M.; Rilov, G.; Somerfield, P.J. Macroalgal blooms alter community structure and primary productivity in marine ecosystems. Glob. Chang. Biol. 2014, 20, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, J.; Cui, Y.; Li, Y.; Lin, H.; Qin, S. Molecular phylogenetic analysis of attached Ulvaceae species and free-floating Enteromorpha from Qingdao coasts in 2007. Chin. J. Oceanol. Limnol. 2008, 26, 276–279. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Gao, S.; Huo, Y.; Cui, J.; Shen, H.; Liu, G.; He, P. Annual patterns of macroalgal blooms in the Yellow Sea during 2007–2017. PLoS ONE 2019, 14, e0210460. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, M.; Zhou, M.; Zhao, L. Spatiotemporal distribution and influencing factors of Ulva prolifera and Sargassum and their coexistence in the South Yellow Sea, China. J. Oceanol. Limnol. 2022, 40, 1070–1084. [Google Scholar] [CrossRef]
- Zhou, M.-J.; Liu, D.-Y.; Anderson, D.M.; Valiela, I. Introduction to the Special Issue on green tides in the Yellow Sea. Estuar. Coast. Shelf Sci. 2015, 163, 3–8. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, B.; Yao, Q.; Fu, M.; Sun, J.; Xu, B.; Yu, Z. Hydro-biogeochemical processes and their implications for Ulva prolifera blooms and expansion in the world’s largest green tide occurrence region (Yellow Sea, China). Sci. Total Environ. 2018, 645, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Wu, L.; Tian, L.; Cui, T.; Li, L.; Kong, F.; Gao, X.; Wu, M. Remote sensing of early-stage green tide in the Yellow Sea for floating-macroalgae collecting campaign. Mar. Pollut. Bull. 2018, 133, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Keesing, J.K.; He, P.; Wang, Z.; Shi, Y.; Wang, Y. The world’s largest macroalgal bloom in the Yellow Sea, China: Formation and implications. Estuar. Coast. Shelf Sci. 2013, 129, 2–10. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Wang, Y.; Zhang, H.; Li, F.; Zhang, Y. Distribution characteristics of green tides and its impact on environment in the Yellow Sea. Mar. Environ. Res. 2022, 181, 105756. [Google Scholar] [CrossRef]
- Fan, S.; Fu, M.; Wang, Z.; Zhang, X.; Song, W.; Li, Y.; Liu, G.; Shi, X.; Wang, X.; Zhu, M. Temporal variation of green macroalgal assemblage on Porphyra aquaculture rafts in the Subei Shoal, China. Estuar. Coast. Shelf Sci. 2015, 163, 23–28. [Google Scholar] [CrossRef]
- Liu, J.; Xia, J.; Zhuang, M.; Zhang, J.; Yu, K.; Zhao, S.; Sun, Y.; Tong, Y.; Xia, L.; Qin, Y. Controlling the source of green tides in the Yellow Sea: NaClO treatment of Ulva attached on Pyropia aquaculture rafts. Aquaculture 2021, 535, 736378. [Google Scholar] [CrossRef]
- Taylor, R.; Fletcher, R.; Raven, J. Preliminary studies on the growth of selected ‘green tide’algae in laboratory culture: Effects of irradiance, temperature, salinity and nutrients on growth rate. Bot. Mar. 2001, 44, 327–336. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.; Gao, C.; Jiang, M.; Li, R.; Wang, Z.; Li, Y.; Fan, S.; Zhang, X. Effect of temperature, salinity and irradiance on growth and photosynthesis of Ulva prolifera. Acta Oceanol. Sin. 2016, 35, 114–121. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Han, X.; Shi, X.; Rivkin, R.B.; Legendre, L. Growth responses of Ulva prolifera to inorganic and organic nutrients: Implications for macroalgal blooms in the southern Yellow Sea, China. Sci. Rep. 2016, 6, 26498. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, D.; Wang, Y.; Zhang, X.; Cao, S.; Mou, S.; Ye, N. The effect of nutrient concentrations, nutrient ratios and temperature on photosynthesis and nutrient uptake by Ulva prolifera: Implications for the explosion in green tides. J. Appl. Phycol. 2014, 26, 537–544. [Google Scholar] [CrossRef]
- Wu, H.; Gao, G.; Zhong, Z.; Li, X.; Xu, J. Physiological acclimation of the green tidal alga Ulva prolifera to a fast-changing environment. Mar. Environ. Res. 2018, 137, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, J.; Cui, T. High-precision extraction of nearshore green tides using satellite remote sensing data of the Yellow Sea, China. Int. J. Remote Sens. 2017, 38, 1626–1641. [Google Scholar] [CrossRef]
- Ma, Y.; Wong, K.; Tsou, J.Y.; Zhang, Y. Investigating spatial distribution of green-tide in the Yellow Sea in 2021 using combined optical and SAR images. J. Mar. Sci. Eng. 2022, 10, 127. [Google Scholar] [CrossRef]
- Hu, C.; Qi, L.; Hu, L.; Cui, T.; Xing, Q.; He, M.; Wang, N.; Xiao, Y.; Sun, D.; Lu, Y. Mapping Ulva prolifera green tides from space: A revisit on algorithm design and data products. Int. J. Appl. Earth Obs. Geoinf. 2023, 116, 103173. [Google Scholar] [CrossRef]
- Cuevas, E.; Uribe-Martínez, A.; Liceaga-Correa, M.d.l.Á. A satellite remote-sensing multi-index approach to discriminate pelagic Sargassum in the waters of the Yucatan Peninsula, Mexico. Int. J. Remote Sens. 2018, 39, 3608–3627. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; Dong, Z.; Zhen, Y.; Di, B.; Shi, Y.; Fearns, P.; Shi, P. Recurrence of the world’s largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Mar. Pollut. Bull. 2010, 60, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, M. Green macroalgae blooms in the Yellow Sea during the spring and summer of 2008. J. Geophys. Res. Ocean. 2009, 114, C12010. [Google Scholar] [CrossRef]
- Hu, C. A novel ocean color index to detect floating algae in the global oceans. Remote Sens. Environ. 2009, 113, 2118–2129. [Google Scholar] [CrossRef]
- Xing, Q.; Hu, C. Mapping macroalgal blooms in the Yellow Sea and East China Sea using HJ-1 and Landsat data: Application of a virtual baseline reflectance height technique. Remote Sens. Environ. 2016, 178, 113–126. [Google Scholar] [CrossRef]
- Amani, M.; Ghorbanian, A.; Ahmadi, S.A.; Kakooei, M.; Moghimi, A.; Mirmazloumi, S.M.; Moghaddam, S.H.A.; Mahdavi, S.; Ghahremanloo, M.; Parsian, S. Google earth engine cloud computing platform for remote sensing big data applications: A comprehensive review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 5326–5350. [Google Scholar] [CrossRef]
- Li, D.; Gao, Z.; Xu, F. Research on the dissipation of green tide and its influencing factors in the Yellow Sea based on Google Earth Engine. Mar. Pollut. Bull. 2021, 172, 112801. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, M.; Cui, Y.; Tian, L.; Yang, P.; Zhao, L.; Xue, M.; Liu, J. What causes the great green tide disaster in the South Yellow Sea of China in 2021? Ecol. Indic. 2022, 140, 108988. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge 1. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Tian, R.; Lin, Q.; Li, D.; Zhang, W.; Zhao, X. Atmospheric transport of nutrients during a harmful algal bloom event. Reg. Stud. Mar. Sci. 2020, 34, 101007. [Google Scholar] [CrossRef]
- Xing, Q.; Loisel, H.; Schmitt, F.; Shi, P.; Liu, D.; Keesing, J. Detection of the green tide at the Yellow Sea and tracking its wind-forced drifting by remote sensing. In Proceedings of the EGU General Assembly Conference, Vienna, Austria, 19–24 April 2009; p. 577. [Google Scholar]
- Gower, J.; Hu, C.; Borstad, G.; King, S. Ocean color satellites show extensive lines of floating Sargassum in the Gulf of Mexico. IEEE Trans. Geosci. Remote Sens. 2006, 44, 3619–3625. [Google Scholar] [CrossRef]
- Son, Y.B.; Min, J.-E.; Ryu, J.-H. Detecting massive green algae (Ulva prolifera) blooms in the Yellow Sea and East China Sea using geostationary ocean color imager (GOCI) data. Ocean Sci. J. 2012, 47, 359–375. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, M.; Wei, J.; He, Y.; Niu, L.; Li, H.; Xu, G. Adaptive threshold model in google earth engine: A case study of Ulva prolifera extraction in the south yellow sea, China. Remote Sens. 2021, 13, 3240. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, H.; Cheng, Y. Multi-sensor monitoring of Ulva prolifera blooms in the Yellow Sea using different methods. Front. Earth Sci. 2016, 10, 378–388. [Google Scholar] [CrossRef]
- Li, D.; Gao, Z.; Song, D. Analysis of environmental factors affecting the large-scale long-term sequence of green tide outbreaks in the Yellow Sea. Estuar. Coast. Shelf Sci. 2021, 260, 107504. [Google Scholar] [CrossRef]
- Sun, X.; Wu, M.; Xing, Q.; Song, X.; Zhao, D.; Han, Q.; Zhang, G. Spatio-temporal patterns of Ulva prolifera blooms and the corresponding influence on chlorophyll-a concentration in the Southern Yellow Sea, China. Sci. Total Environ. 2018, 640, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef]
- Sanderson, E.W. Personalized Measures of Consumption and Development in the Context of Biodiversity Conservation: Connecting the Ecological Footprint Calculation with the Human Footprint Map. In Biodiversity Monitoring and Conservation: Bridging the Gap between Global Commitment and Local Action; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 189–209. [Google Scholar]
- Fatemi, M.; Rezaei-Moghaddam, K.; Karami, E.; Hayati, D.; Wackernagel, M. An integrated approach of Ecological Footprint (EF) and Analytical Hierarchy Process (AHP) in human ecology: A base for planning toward sustainability. PLoS ONE 2021, 16, e0250167. [Google Scholar] [CrossRef]
- Vargas, L.G. An overview of the analytic hierarchy process and its applications. Eur. J. Oper. Res. 1990, 48, 2–8. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Principal component analysis and redundancy analysis. In Analysing Ecological Data; Springer: Berlin/Heidelberg, Germany, 2007; pp. 193–224. [Google Scholar]
- Chen, H.W.; Lin, H.C.; Chuang, Y.H.; Sun, C.T.; Chen, W.Y.; Kao, C.Y. Effects of environmental factors on benthic species in a coastal wetland by redundancy analysis. Ocean Coast. Manag. 2019, 169, 37–49. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, Y.; Li, Z.; Li, P.; Xu, G. Influence of land use and land cover patterns on seasonal water quality at multi-spatial scales. Catena 2017, 151, 182–190. [Google Scholar] [CrossRef]
- Fang, C.; Song, K.; Paerl, H.W.; Jacinthe, P.A.; Wen, Z.; Liu, G.; Tao, H.; Xu, X.; Kutser, T.; Wang, Z. Global divergent trends of algal blooms detected by satellite during 1982–2018. Glob. Chang. Biol. 2022, 28, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P. Studying beta diversity: Ecological variation partitioning by multiple regression and canonical analysis. J. Plant Ecol. 2008, 1, 3–8. [Google Scholar] [CrossRef]
- Hao, Y.; Qu, T.; Guan, C.; Zhao, X.; Hou, C.; Tang, X.; Wang, Y. Competitive advantages of Ulva prolifera from Pyropia aquaculture rafts in Subei Shoal and its implication for the green tide in the Yellow Sea. Mar. Pollut. Bull. 2020, 157, 111353. [Google Scholar] [CrossRef] [PubMed]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Yang, D.; Yuen, K.-V.; Gu, X.; Sun, C.; Gao, L. Influences of environmental factors on the dissipation of green tides in the Yellow Sea, China. Mar. Pollut. Bull. 2023, 189, 114737. [Google Scholar] [CrossRef]
- Hu, L.; He, M. Impacts of sea surface temperature anomaly to the coverage area and early appearance time of green tide in the Yellow Sea. In Proceedings of the 2014 IEEE Geoscience and Remote Sensing Symposium, Quebec City, QC, Canada, 13–18 July 2014; pp. 4465–4468. [Google Scholar]
- Hu, C.; Li, D.; Chen, C.; Ge, J.; Muller-Karger, F.E.; Liu, J.; Yu, F.; He, M.X. On the recurrent Ulva prolifera blooms in the Yellow Sea and East China Sea. J. Geophys. Res. Ocean. 2010, 115, C05017. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhu, L.; Zhou, B.; Tang, X. Comparative studies on the ecophysiological differences of two green tide macroalgae under controlled laboratory conditions. PLoS ONE 2012, 7, e38245. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, M.; Zhang, A.; Xing, Q.; Zhou, M.; Zhao, D.; Song, X.; Yu, Z. Influence of sea surface temperature on outbreak of Ulva prolifera in the Southern Yellow Sea, China. Chin. Geogr. Sci. 2020, 30, 631–642. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Le Nohaïc, M.; Ross, C.L.; Cornwall, C.E.; Comeau, S.; Lowe, R.; McCulloch, M.T.; Schoepf, V. Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia. Sci. Rep. 2017, 7, 14999. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gao, Z.; Wang, Z. Analysis of the reasons for the outbreak of Yellow Sea green tide in 2021 based on long-term multi-source data. Mar. Environ. Res. 2022, 178, 105649. [Google Scholar] [CrossRef] [PubMed]
- Droop, M.R. 25 years of algal growth kinetics a personal view. Bot. Mar. 1983, 26, 99–112. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Zhang, G.; Zhang, J.; Wu, Y.; Ju, H. Spatiotemporal dynamics of succession and growth limitation of phytoplankton for nutrients and light in a large shallow lake. Water Res. 2021, 194, 116910. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Pardal, M.; Lillebø, A.; Flindt, M.; Marques, J. Hydrodynamics as a major factor controlling the occurrence of green macroalgal blooms in a eutrophic estuary: A case study on the influence of precipitation and river management. Estuar. Coast. Shelf Sci. 2001, 52, 165–177. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Zhang, C.; Su, R.; Shi, X.; Wang, X. Characterization of the development stages and roles of nutrients and other environmental factors in green tides in the Southern Yellow Sea, China. Harmful Algae 2020, 98, 101893. [Google Scholar] [CrossRef]
- Keesing, J.K.; Liu, D.; Fearns, P.; Garcia, R. Inter-and intra-annual patterns of Ulva prolifera green tides in the Yellow Sea during 2007–2009, their origin and relationship to the expansion of coastal seaweed aquaculture in China. Mar. Pollut. Bull. 2011, 62, 1169–1182. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhang, X. A review of the green tides in the Yellow Sea, China. Mar. Environ. Res. 2016, 119, 189–196. [Google Scholar] [CrossRef]
- Pang, S.J.; Liu, F.; Shan, T.F.; Xu, N.; Zhang, Z.H.; Gao, S.Q.; Chopin, T.; Sun, S. Tracking the algal origin of the Ulva bloom in the Yellow Sea by a combination of molecular, morphological and physiological analyses. Mar. Environ. Res. 2010, 69, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gao, Z.; Wang, Y. Research on the long-term relationship between green tide and chlorophyll-a concentration in the Yellow Sea based on Google Earth Engine. Mar. Pollut. Bull. 2022, 177, 113574. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; Dutta, V. Nutrient enrichment in lake ecosystem and its effects on algae and macrophytes. In Environmental Concerns and Sustainable Development: Volume 2: Biodiversity, Soil and Waste Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 81–126. [Google Scholar]
- Li, S.; Yu, K.; Huo, Y.; Zhang, J.; Wu, H.; Cai, C.E.; Liu, Y.; Shi, D.; He, P. Effects of nitrogen and phosphorus enrichment on growth and photosynthetic assimilation of carbon in a green tide-forming species (Ulva prolifera) in the Yellow Sea. Hydrobiologia 2016, 776, 161–171. [Google Scholar] [CrossRef]
- Ye, N.-H.; Zhang, X.-W.; Mao, Y.-Z.; Liang, C.-W.; Xu, D.; Zou, J.; Zhuang, Z.-M.; Wang, Q.-Y. ‘Green tides’ are overwhelming the coastline of our blue planet: Taking the world’s largest example. Ecol. Res. 2011, 26, 477–485. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. Pollut. Bull. 2017, 114, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ni, J.; Chen, Z.; Wu, S.; Wu, R.; He, C.; Wang, J.; Liu, Y.; Zhou, W.; Xu, J. Effects of ocean acidification and eutrophication on the growth and photosynthetic performances of a green tide alga Ulva prolifera. Front. Mar. Sci. 2023, 10, 1145048. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, M.; Zhao, J.; Luan, S.; Wang, D.; Jiang, W.; Xue, M.; Liu, J.; Cui, Y. Effects of Ulva prolifera dissipation on the offshore environment based on remote sensing images and field monitoring data. Acta Oceanol. Sin. 2023, 42, 112–120. [Google Scholar] [CrossRef]


| Data Name | Data Provider | Dataset | Data Name | Data Provider |
|---|---|---|---|---|
| Sentinel-1 | European Union/ESA/Copernicus | 3 October 2014 | 10 m/6 d | COPERNICUS/S1_GRD |
| Sentinel-2 | European Union/ESA/Copernicus | 23 June 2015 | 10 m/5 d | COPERNICUS/S2_HARMONIZED |
| SST | NOPP | 2 October 1992–8 May 2023 | 8905.6 m/1 d | HYCOM/sea_temp_salinity |
| PAR | NASA LP DAAC at the USGS EROS Center | 24 February 2002–1 July 2023 | 500 m/3 h | MODIS/061/MCD18C2 |
| Precipitation | NASA GES DISC at NASA Goddard Space Flight Center | 1 June 2000–1 September 2021 | 11,132 m/3 h | NASA/GPM_L3/IMERG_MONTHLY_V06 |
| Windspeed | NOAA | 1 January 1988–31 August 2021 | 27,830 m/1 d | NOAA/CDR/ATMOS_NEAR_SURFACE/V2 |
| Actual/Predicted | Cultivation Zone (1) | Non-Cultivation Zone (2) |
|---|---|---|
| Cultivation zone (1) | TP = 246 | FN = 54 |
| Non-cultivation zone (2) | FP = 29 | TN = 271 |
| Impact Indicators | Weights |
|---|---|
| Cropland area | 0.15 |
| Deforestation area | 0.17 |
| Fisheries catch | 0.22 |
| Energy consumption | 0.27 |
| Building land area | 0.11 |
| population | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, M.; Wu, M.; Zheng, L.; Liu, J.; Liu, L.; Zhu, S.; Liu, S.; Liu, L. Multi-Factors Synthetically Contribute to Ulva prolifera Outbreaks in the South Yellow Sea of China. Remote Sens. 2023, 15, 5151. https://doi.org/10.3390/rs15215151
Xue M, Wu M, Zheng L, Liu J, Liu L, Zhu S, Liu S, Liu L. Multi-Factors Synthetically Contribute to Ulva prolifera Outbreaks in the South Yellow Sea of China. Remote Sensing. 2023; 15(21):5151. https://doi.org/10.3390/rs15215151
Chicago/Turabian StyleXue, Mingyue, Mengquan Wu, Longxiao Zheng, Jiayan Liu, Longxing Liu, Shan Zhu, Shubin Liu, and Lijuan Liu. 2023. "Multi-Factors Synthetically Contribute to Ulva prolifera Outbreaks in the South Yellow Sea of China" Remote Sensing 15, no. 21: 5151. https://doi.org/10.3390/rs15215151
APA StyleXue, M., Wu, M., Zheng, L., Liu, J., Liu, L., Zhu, S., Liu, S., & Liu, L. (2023). Multi-Factors Synthetically Contribute to Ulva prolifera Outbreaks in the South Yellow Sea of China. Remote Sensing, 15(21), 5151. https://doi.org/10.3390/rs15215151







