Satellite Imagery-Estimated Intertidal Seaweed Biomass Using UAV as an Intermediary
Abstract
1. Introduction
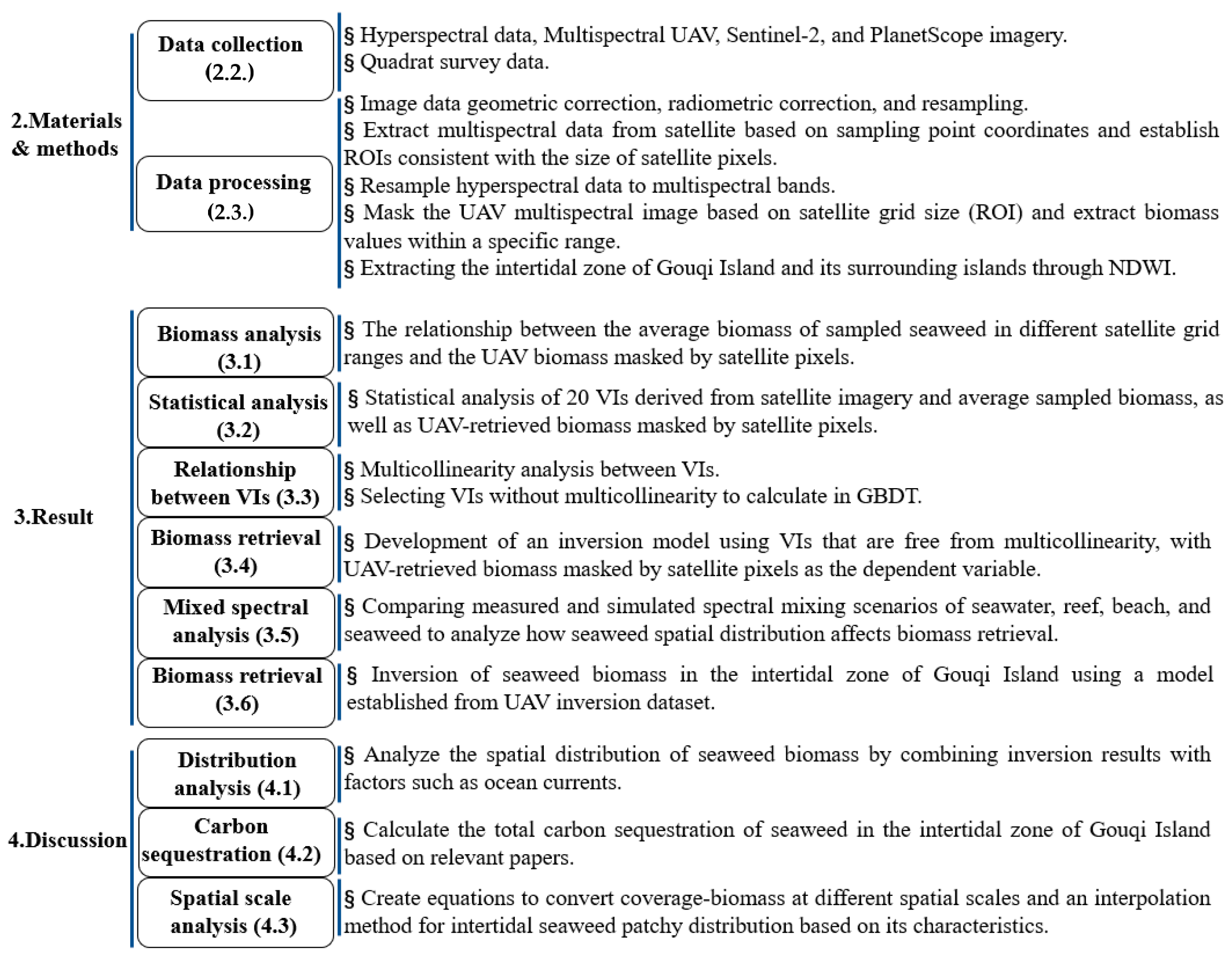
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Processing
- (i)
- Initialization
- (ii)
- Iterative training m = 1, 2,..., M trees.
- (iii)
- Obtain the final learner GBDT
3. Result
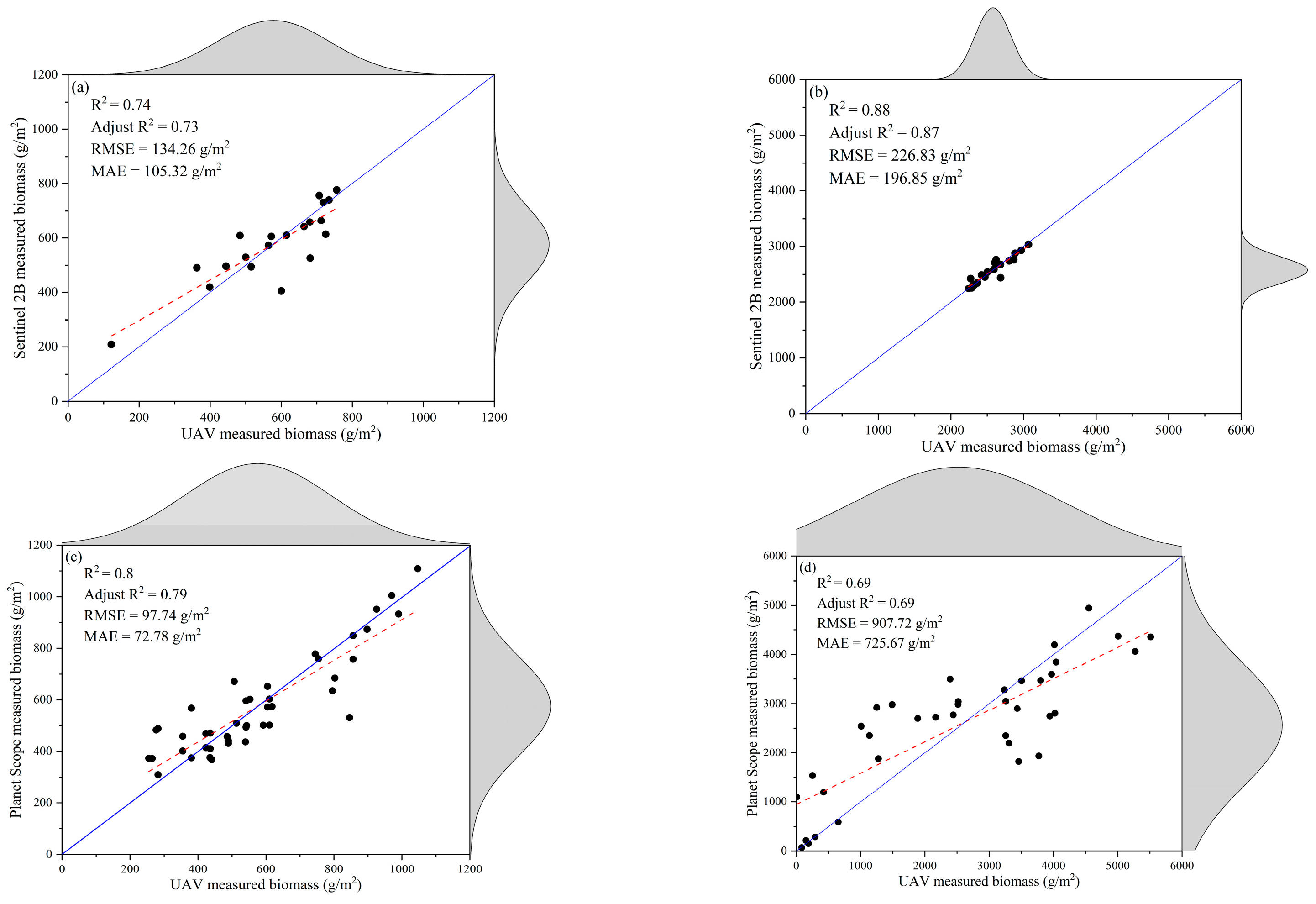
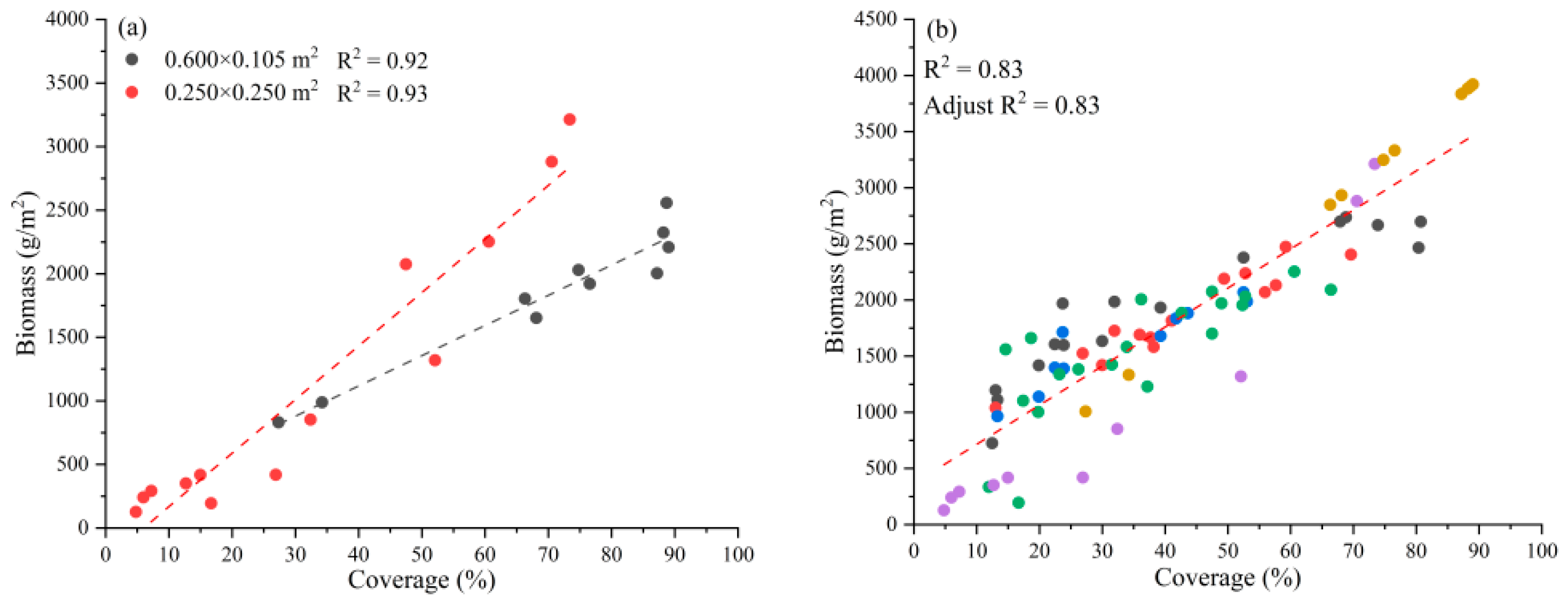
3.1. Relationship between Biomass and Satellite VIs
3.2. Relationship between Different VIs and Biomass
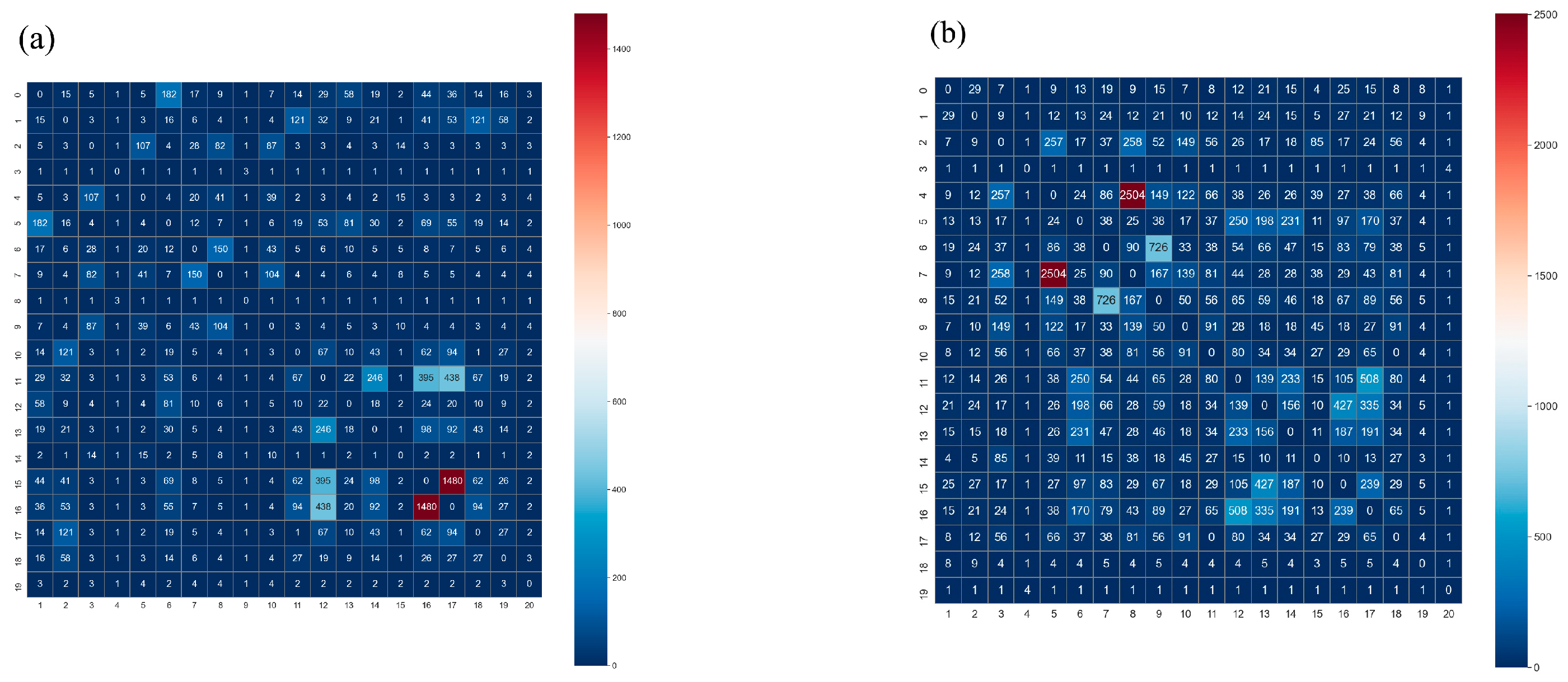
3.3. Relationship between VIs
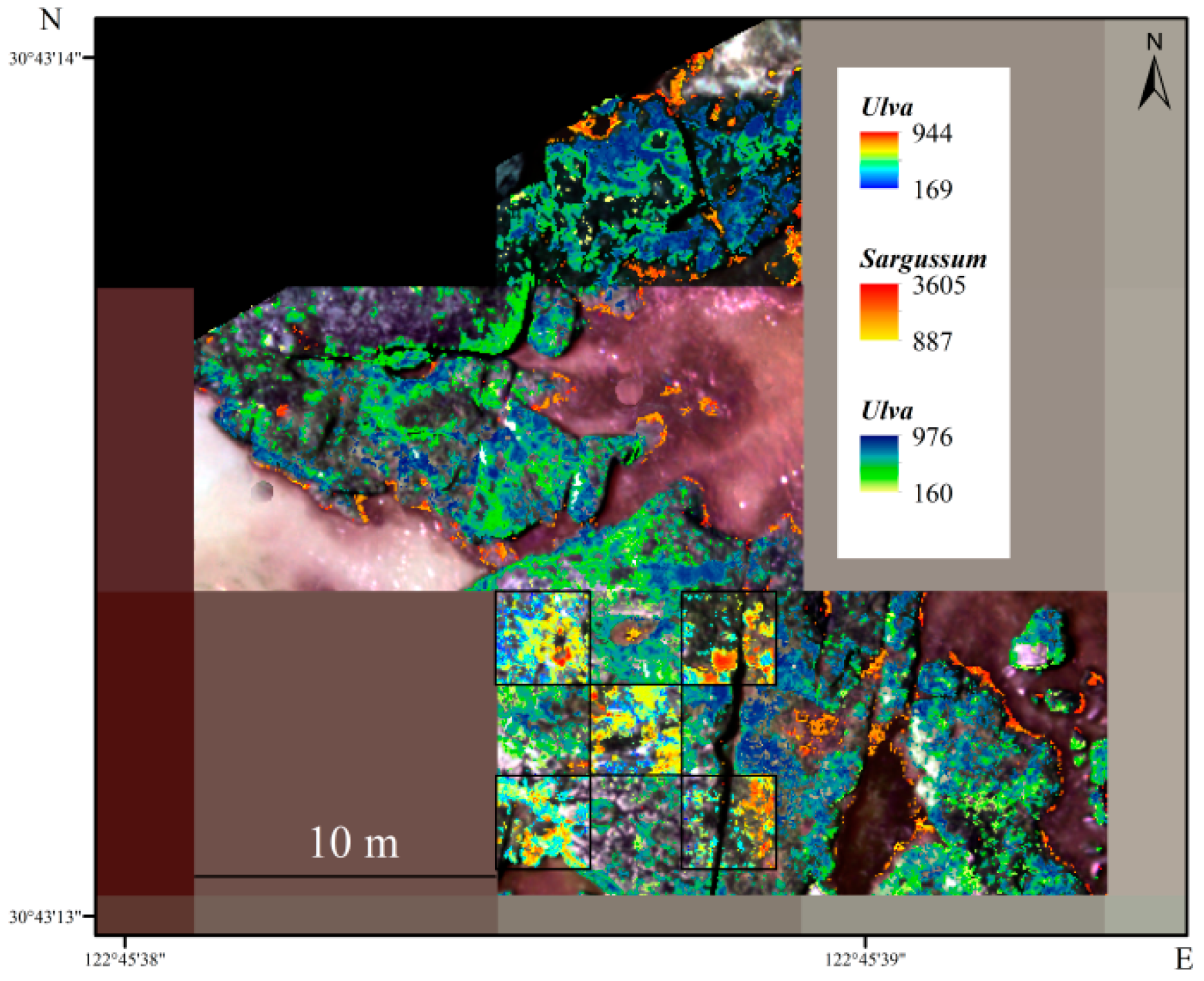
3.4. Satellite Remote Sensing Retrieval Based on UAV Biomass
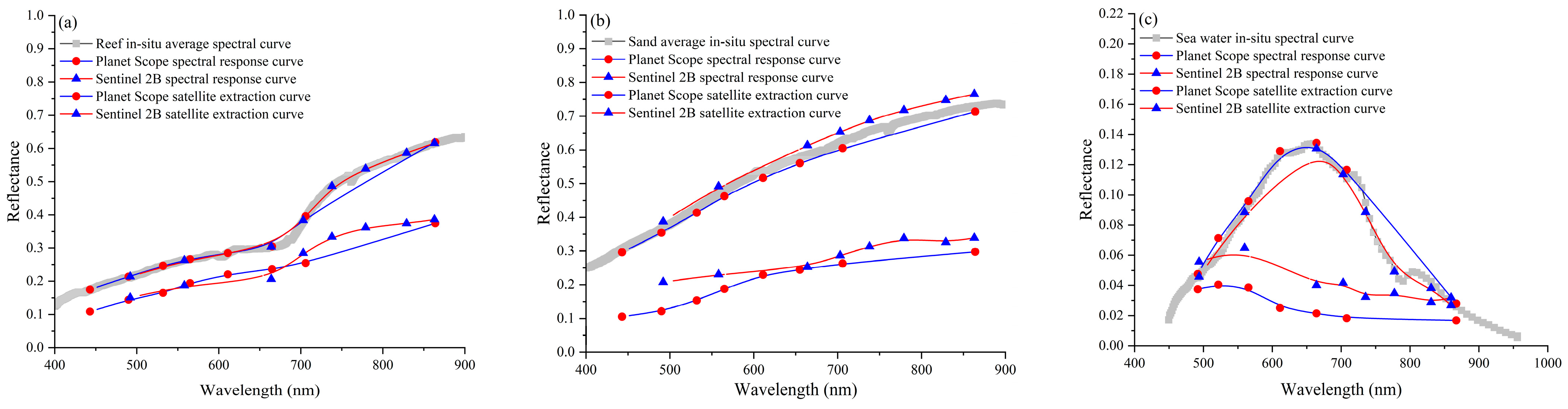
3.5. Spectral Analysis
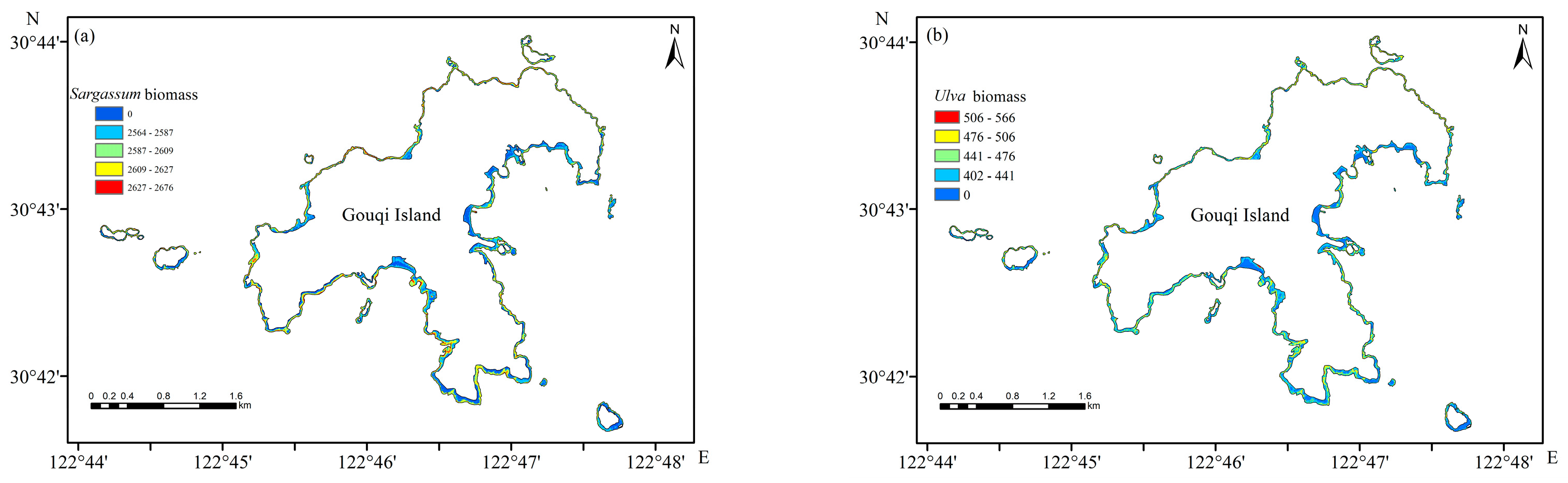
3.6. Inversion of Seaweed Biomass in the Intertidal Zone of Gouqi Island
4. Discussion
4.1. Distribution of Seaweed Biomass in Intertidal Zones
4.2. Carbon Sequestration of Seaweed in the Intertidal Zone of Gouqi Island
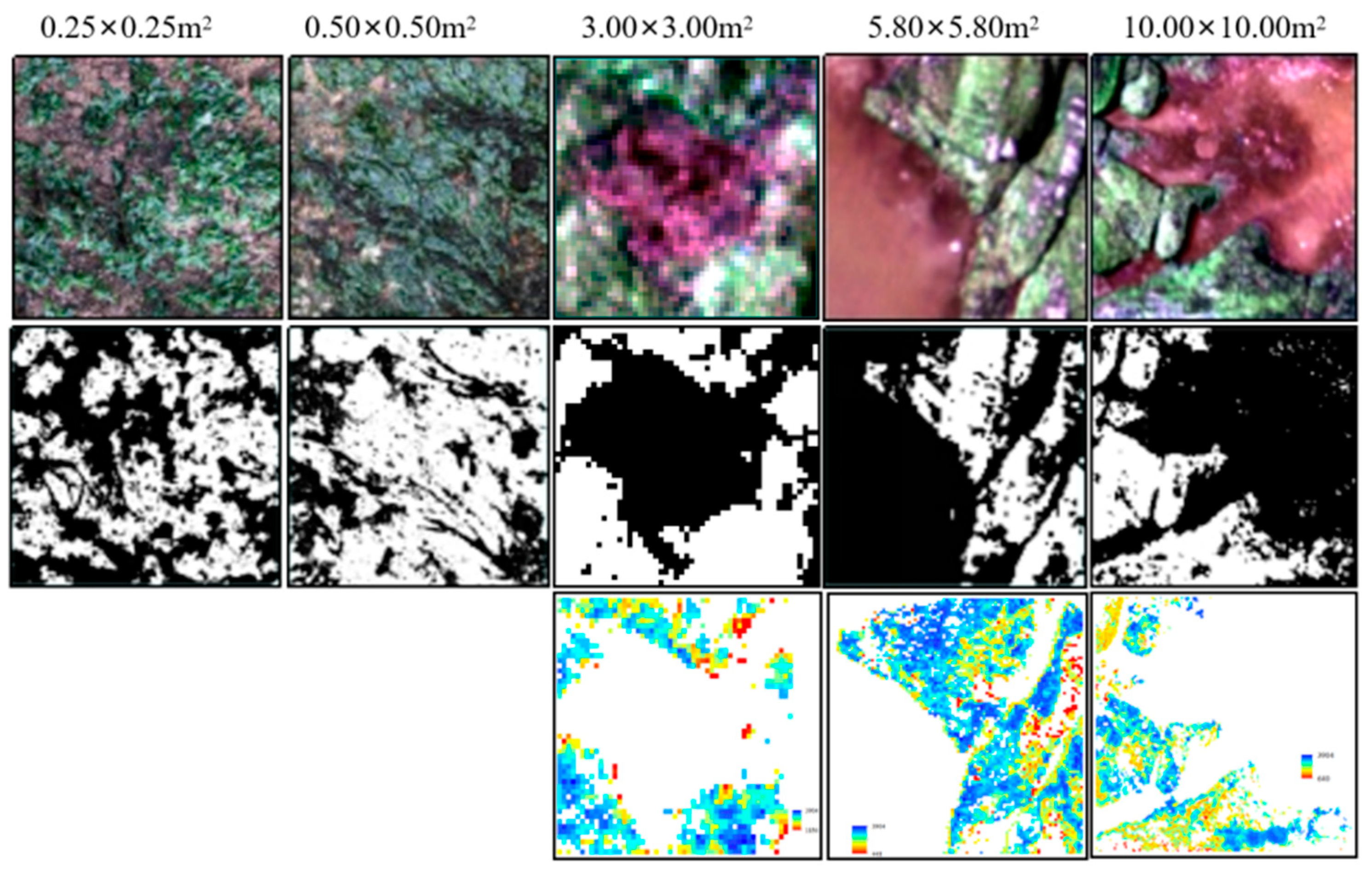
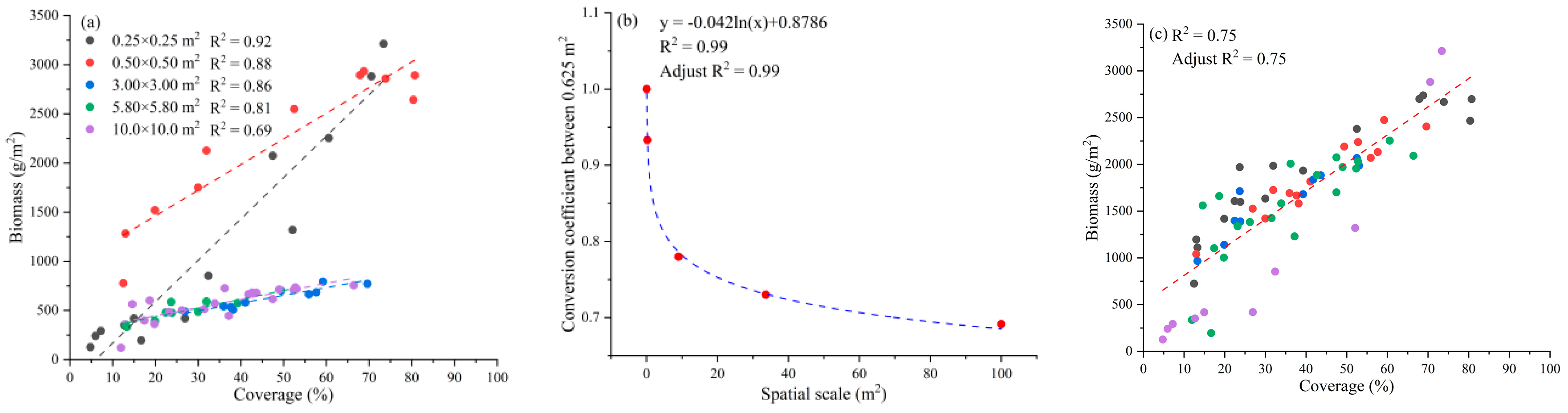
4.3. Spatial Scale Differences in Seaweed Biomass in Intertidal Zones
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Diazpulido, G.J.; Mccook, L. Macroalgae (Seaweeds). The State of the Great Barrier Reef On-Line; Australian Government: Great Barrier Reef Marine Park Authority: Townsville, Australia, 2008. [Google Scholar]
- Gellenbeck, K.W.; Chapman, D.J. Seaweed uses: The outlook for mariculture. Endeavour 1983, 7, 31–37. [Google Scholar] [CrossRef]
- Gao, K.; Mckinley, K.R. Use of macroalgae for marine biomass production and CO2 remediation: A review. J. Appl. Phycol. 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Nielsen, D.A.; Kelleway, J.J.; Atwood, T.B.; Ralph, P.J. Can we manage coastal ecosystems to sequester more blue carbon? Front. Ecol. Environ. 2017, 15, 206–213. [Google Scholar] [CrossRef]
- Kwan, V.; Fong, J.; Chin, N.; Huang, D. Temporal and spatial dynamics of tropical macroalgal contributions to blue carbon. Sci. Total Environ. 2022, 828, 154369. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Assis, J.; Filbee-dexter, K.; Burrows, M.; Gattuso, J.P.; Duarte, C.M.; Krause-Jense, D.; Moore, P.J.; Smale, D.A.; Wernberg, T. Global seaweed productivity. Sci. Adv. 2022, 8, eabn2465. [Google Scholar]
- Starko, S.; Neufeld, C.J.; Gendall, L.; Timmer, B.; Campbell, L.; Yakimishyn, J.; Druehl, L.; Baum, J.K. Microclimate predicts kelp forest extinction in the face of direct and indirect marine heatwave effects. Ecol. Appl. 2022, 32, e2673. [Google Scholar] [CrossRef]
- Menge, B.; Gravem, S.; Johnson, A.; Robinson, J.; Poirson, B. Increasing instability of a rocky intertidal meta-ecosystem. Proc. Natl. Acad. Sci. USA 2022, 119, e2114257119. [Google Scholar] [CrossRef]
- Brodie, J.; Kunzig, S.; Agate, J.; Yesson, C.; Robinson, L. The Big Seaweed Search: Evaluating a citizen science project for a difficult to identify group of organisms. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 33, 44–45. [Google Scholar] [CrossRef]
- Cappelatti, L.; Griffin, J.; Mauffrey, A. Functional diversity of habitat formers declines scale-dependently across an environmental stress gradient. Oecologia 2020, 194, 135–149. [Google Scholar] [CrossRef]
- Johnson, M. Estimating intertidal seaweed biomass at larger scales from quadrat surveys. Mar. Environ. Res. 2020, 156, 104906. [Google Scholar] [CrossRef]
- Terada, R.; Abe, M.; Abe, T.; Aoki, M.; Dazai, A.; Endo, H.; Kamiya, M.; Kawai, H.; Kurashima, A.; Motomura, T.; et al. Japan’s nationwidelong-term monitoring survey of seaweed communities known as the “Monitoring Sites 1000”: Ten-year overview and future perspectives. Phycol. Res. 2021, 69, 12–30. [Google Scholar] [CrossRef]
- Wraase, L.; Reuber, V.; Kurth, P.; Fekadu, M.; Demissew, S.; Miehe, G.; Opgenoorth, L.; Selig, U.; Woldu, Z.; Zeuss, D.; et al. Remote sensing-supported mapping of the activity of a subterranean landscape engineer across an afro-alpine ecosystem. Remote Sens. Ecol. Conserv. 2022, 9, 195–209. [Google Scholar] [CrossRef]
- Zoffoli, L.; Gernez, P.; Oiry, S.; Godet, L.; Dalloyau, S.; Davies, B.; Barillé, L. Remote sensing in seagrass ecology: Coupled dynamics between migratory herbivorous birds and intertidal meadows observed by satellite during four decades. Remote Sens. Ecol. Conserv. 2022, 9, 420–433. [Google Scholar] [CrossRef]
- Bell, T.; Siegel, D. Nutrient availability and senescence spatially structure the dynamics of a foundation species. Proc. Natl. Acad. Sci. USA 2022, 119, e2105135118. [Google Scholar] [CrossRef]
- Morgan, B.E.; Chipman, J.W.; Bolger, D.T.; Dietrich, J.T. Spatiotemporal Analysis of Vegetation Cover Change in a Large Ephemeral River: Multi-Sensor Fusion of Unmanned Aerial Vehicle (UAV) and Landsat Imagery. Remote Sens. 2021, 13, 51. [Google Scholar] [CrossRef]
- Haro, S.; Jesus, B.; Oiry, S.; Papaspyrou, S.; Lara, M.; Gonzalez, C.; Corzo, A. Microphytobenthos spatio-temporal dynamics across an intertidal gradient using Random Forest classification and Sentinel-2 imagery. Sci. Total Environ. 2021, 804, 149983. [Google Scholar] [CrossRef]
- Li, X.; Kai, W.; Shouyu, Z.; Meiping, F. Distribution and Flora of Seaweed Beds in the Coastal Waters of China. Sustainability 2021, 13, 3009. [Google Scholar] [CrossRef]
- Diruit, W.; Bris, A.; Bajjouk, T.; Richier, S.; Helias, M.; Burel, T.; Lennon, M.; Guyot, A.; Gall, E. Seaweed Habitats on the Shore: Characterization through Hyperspectral UAV Imagery and Field Sampling. Remote Sens. 2022, 14, 3124. [Google Scholar] [CrossRef]
- Chen, J.; Xunmeng, L.; Kai, W.; Shouyu, Z.; Jun, L.; Jian, Z.; Weicheng, G. Variable Optimization of Seaweed Spectral Response Characteristics and Species Identification in Gouqi Island. Sensors 2022, 22, 4656. [Google Scholar] [CrossRef]
- Candiago, S.; Remondino, F.; De Giglio, M.; Dubbini, M.; Gattelli, M. Evaluating Multispectral Images and Vegetation Indices for Precision Farming Applications from UAV Images. Remote Sens. 2015, 7, 4026–4047. [Google Scholar] [CrossRef]
- Lucas, P.O.; Mauro, S.A.; José, M.J.; Buceli, D.N.; Paula, M.R.; Akemi, S.M.; Nobuhiro, I.N.; Roberto, P.D.; Eduardo, I.N.; Roberto, P.D.; et al. A convolutional neural network approach for counting and geolocating citrus-trees in UAV multispectral imagery. ISPRS J. Photogramm. Remote Sens. 2020, 160, 97–106. [Google Scholar]
- Guo, Y.; Chen, S.; Li, X.; Maro, C.; Senthilnath, J.; Davide, C.; Yongshou, F. Machine Learning-Based Approaches for Predicting SPAD Values of Maize Using Multi-Spectral Images. Remote Sens. 2022, 14, 1337. [Google Scholar] [CrossRef]
- Qi, G.; Chang, C.; Yang, W.; Zhao, G. Soil salinity inversion incoastal cotton growing areas: A integration method of satellite-ground spectral fusion and satellite-UAV collaboration. Land Degrad. Dev. 2022, 13, 2289–2302. [Google Scholar] [CrossRef]
- Román, A.; Navarro, G.; Caballero, I.; Tovar-Sanchez, A. High-spatial resolution UAV multispectral data complementing satellite imagery to characterize a chinstrap penguin colony ecosystem on deception island (Antarctica). GIScience Remote Sens. 2022, 59, 1159–1176. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, W.; Zhang, Z.; Chen, H.; Zhao, G.; Liu, P. Fusion level of satellite and UAV image data for soil salinity inversion in the coastal area of the Yellow River Delta. Int. J. Remote Sens. 2022, 43, 7039–7063. [Google Scholar] [CrossRef]
- Ling, C.; Sun, H.; Zhang, H.; Lin, H.; Ju, H.; Liu, H. Study on Above-Ground Biomass Estimation of East Dong Ting Lake wetland Based on Worldview-2 Data. In Proceedings of the 3rd International Workshop on Earth Observation and Remote Sensing Applications, EORSA 2014—Proceedings, Changsha, China, 11–14 June 2014. [Google Scholar] [CrossRef]
- Han, L.; Liu, D.; Cheng, G.; Zhang, G.; Wang, L. Spatial distribution and genesis of salt on the saline playa at Qehan Lake, Inner Mongolia, China. CATENA 2019, 177, 22–30. [Google Scholar] [CrossRef]
- Granadeiro, J.P.; Belo, J.; Henriques, M.; Catalao, J.; Catry, T. Using Sentinel-2 Images to Estimate Topography, Tidal-Stage Lags and Exposure Periods over Large Intertidal Areas. Remote Sens. 2021, 13, 320. [Google Scholar] [CrossRef]
- Chen, J.; Xunmeng, L.; Kai, W.; Shouyu, Z.; Jun, L. Estimation of Seaweed Biomass Based on Multispectral UAV in the Intertidal Zone of Gouqi Island. Remote Sens. 2022, 14, 2143. [Google Scholar] [CrossRef]
- Ge, H.; Zheng, J.; Xu, H. Advances in Machine Learning for High Value-Added Applications of Lignocellulosic Biomass. Bioresour. Technol. 2022, 369, 128481. [Google Scholar] [CrossRef]
- Akira, O.; James, G.; Mitchel, J.G. Patchy Distribution and Diffusion. Diffus. Ecol. Probl. Mod. Perspect. 2001, 14, 268–297. [Google Scholar]
- Fales, R.J.; Smith, J.R. Long-term change in a high-intertidal rockweed (Pelvetiopsis californica) and community-level consequences. Mar. Biol. 2022, 169, 04022. [Google Scholar] [CrossRef]
- Zongling, W.; Chao, Y.; Xuelei, Z.; Yongjuan, L.; Mingzhu, F.; Jie, X. Interannual variations of Sargassum blooms in the Yellow Sea and East China Sea during 2017–2021. Harmful Algae 2023, 126, 102451. [Google Scholar]
- Melville, B.; Fisher, A.; Lucieer, A. Ultra-high spatial resolution fractional vegetation cover from unmanned aerial multispectral imagery. Int. J. Appl. Earth Obs. Geoinf. 2019, 78, 14–24. [Google Scholar] [CrossRef]
- Roca, M.; Mar, D.; Martha, R.; Caballero, I.; Zoffoli, M.; Gernez, P.; Navarro, G. Monitoring the marine invasive alien species Rugulopteryx okamurae using unmanned aerial vehicles and satellites. Front. Mar. Sci. 2022, 9, 1004012. [Google Scholar] [CrossRef]
- Lewis, P.H.; Roberts, B.P.; Moore, P.J.; Pike, S.; Scarth, A.; Medcalf, K.; Cameron, I. Combining unmanned aerial vehicles and satellite imagery to quantify areal extent of intertidal brown canopy-forming macroalgae. Remote Sens. Ecol. Conserv. 2023, 9, 540–552. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, S. Effect of Typhoon on the Distribution of Macroalgae in the Seaweed Beds of Gouqi Island, Zhejiang Province. J. Agric. Sci. Technol. 2019, 21, 159–168. [Google Scholar]
- Tang, G.; Li, Y.; Wills, P.; Hanisak, D.; Ouyang, B. Development of a macroalgal biomass sensor for an integrated multi-trophic aquaculture (IMTA) system. Conf. Big Data III Learn. Anal. Appl. 2021, 6, 1173007. [Google Scholar]
- Allen, D.M. The Relationship Between Variable Selection and Data Agumentation and a Method for Prediction. Technometrics 1999, 16, 125–127. [Google Scholar] [CrossRef]
- Stone, M. Cross-Validatory Choice and Assessment of Statistical Predictions. J. R. Stat. Soc. Ser. B 1974, 36, 111–133. [Google Scholar] [CrossRef]
- Kogan, F.; Kussul, N.; Adamenko, T.; Skakun, S.; Kravchenko, O.; Kryvobok, O.; Shelestov, A.; Kolotii, A.; Kussul, O.; Lavrenyuk, A. Winter wheat yield forecasting in Ukraine based on Earth observation, meteorologicaldata and biophysical models. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 192–203. [Google Scholar]
- Li, Y.; Guan, K.; Yu, A.; Peng, B.; Zhao, L.; Li, B.; Peng, J. Toward building a transparent statistical model for improving crop yield prediction: Modeling rainfed corn in the U.S. Field Crops Res. 2019, 234, 55–65. [Google Scholar] [CrossRef]
- Wen, Y.; Lin, J.; Yang, G. Study on hydrodynamic effect of suspended mussel aquaculture facilities and detritus transportation of epiphytic seaweed. J. Shanghai Ocean Univ. 2022, 31, 1549–1561. [Google Scholar]
- Martina, M.; Jacob, S.; Gil, R. Biomass calibration of nine dominant native and non-native Levantine seaweeds. Aquat. Bot. 2022, 178, 103496. [Google Scholar]
- Sara, B.; Wickham, C.; Darimont, C.; Reynolds, J.; Starzomske, B. Species-specific wet-dry mass calibrations for dominant Northeastern Pacific Ocean macroalgae and seagrass. Aquat. Bot. 2019, 152, 27–31. [Google Scholar]
- Li, X.; Zhao, X.; Yuan, H.; Guo, Y.; Li, J.; Zhang, S.; Chen, J.; Wang, Z.; Wang, K. Diversity and Carbon Sequestration of Seaweed in the Ma’an Archipelago, China. Diversity 2023, 15, 12. [Google Scholar] [CrossRef]
- Walter, J.; Hallett, L.; Sheppard, L.; Anderson, T.; Zhao, L.; Hobbs, R.; Suding, K.; Reuman, D. Micro-scale geography of synchrony in a serpentine plant community. J. Ecol. 2020, 2, 750–762. [Google Scholar] [CrossRef]
- Yan, G.; Li, L.; Coy, A.; Mu, X.; Chen, S.; Xie, D.; Zhang, W.; Shen, Q.; Zhou, H. Improving the estimation of fractional vegetation cover from UAV RGB imagery by colour unmixing. ISPRS J. Photogramm. Remote Sens. 2019, 158, 23–34. [Google Scholar] [CrossRef]
- Peter, K.D.; D’Oleire-Oltmanns, S.; Ries, J.B.; Marzolff, I.; Hssaine, A.A. Soil erosion in gully catchments affected by land-levelling measures in the Souss Basin, Morocco, analysed by rainfall simulation and UAV remote sensing data. Catena 2014, 113, 24–40. [Google Scholar] [CrossRef]
- Wiegert, R.G. The Selection of an Optimum Quadrat Size for Sampling the Standing Crop of Grasses and Forbs. Ecology 1962, 43, 125–129. [Google Scholar] [CrossRef]
- Lin, D.; Lai, J.; Mi, X.; Ren, H.; Ma, K. Spatial variation in community structure of a subtropical evergreen broad-leaved forest: Implications for sampling design. Chin. Sci. Bull. 2013, 58, 1181–1186. [Google Scholar] [CrossRef][Green Version]
- Yang, K.; Guan, D. Selection of gaining quadrat for harvesting the undergrowth vegetation and its biomass estimation modeling in forest. Acta Ecol. Sin. 2007, 27, 705–714. [Google Scholar]
- Su, H.; Karna, D.; Fraim, E.; Fitzgerald, M.; Dominguez, R.; Myers, J.S.; Coffland, B.; Handley, L.R.; Mace, T. Evaluation of Eelgrass Beds Mapping Using a High-Resolution Airborne Multispectral Scanner. Photogramm. Eng. Remote Sens. 2006, 9, 789–797. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Cui, T.; Gong, J.; Liu, R.; Chen, X.; Liang, X. Remote sensing estimation of the biomass of floating Ulva prolifera and analysis of the main factors driving the interannual variability of the biomass in the Yellow Sea. Mar. Pollut. Bull. 2019, 140, 330–340. [Google Scholar] [CrossRef]
- Che, S.; Du, G.; Wang, N.; He, K.; Mo, Z.; Sun, B.; Chen, Y.; Cao, Y.; Wang, J.; Mao, Y. Biomass estimation of cultivated red algae Pyropia using unmanned aerial platform based multispectral imaging. Plant Methods 2021, 17, 12. [Google Scholar] [CrossRef]
- Elvidge, C.D.; Chen, Z. Comparison of broad-band and narrow-band red and near-infrared vegetation indices. Remote Sens. Environ. 1995, 54, 38–48. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yokozawa, M.; Toritani, H.; Shibayama, M.; Ishitsuka, N.; Ohno, H. A crop phenology detection method using time-series MODIS data. Remote Sens. Environ. 2005, 96, 366–374. [Google Scholar] [CrossRef]
- Driss, H.; John, R.M.; Elizabeth, P.; Zarco-Tejada, P.; Strachan, I. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Feng, W.; Wu, Y.; He, L.; Ren, X.; Wang, Y.; Hou, G.; Wang, Y.; Liu, W.; Guo, T. An optimized non-linear vegetation index for estimating leaf area index in winter wheat. Precis. Agric. 2019, 20, 1157–1176. [Google Scholar] [CrossRef]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of Green-Red Vegetation Index for Remote Sensing of Vegetation Phenology. Remote Sens. 2010, 2, 2369–2387. [Google Scholar] [CrossRef]
- Rumora, L.; Majić, I.; Miler, M.; Medak, D. Spatial video remote sensing for urban vegetation mapping using vegetation indices. Urban Ecosyst 2021, 24, 21–33. [Google Scholar] [CrossRef]
- Wang, W.; Liu, R.; Gan, F.; Zhou, P.; Zhang, X.; Ding, L. Monitoring and Evaluating Restoration Vegetation Status in Mine Region Using Remote Sensing Data: Case Study in Inner Mongolia, China. Remote Sens. 2021, 13, 1350. [Google Scholar] [CrossRef]
- Merwe, D.; Price, K.P. Harmful Algal Bloom Characterization at Ultra-High Spatial and Temporal Resolution Using Small Unmanned Aircraft Systems. Toxins 2015, 7, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Shinya, T.; Seijiro, G.; Masayasu, M.; Akiyama, T.; Muramoto, Y.; Yoshida, K. Estimation and validation of leaf chlorophyll concentration in winter wheat at heading to anthesis stage using ground-based and aerial hyperspectral data. J. Jpn. Soc. Photogramm. Remote Sens. 2008, 47, 39–49. [Google Scholar]
- Wang, F.-M.; Huang, J.-F.; Tang, Y.-L.; Wang, X.-Z. New Vegetation Index and Its Application in Estimating Leaf Area Index of Rice. Chin. J. Rice Sci. 2007, 21, 159–166. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yang, M.; Rasheed, A.; Jin, X.; Xia, X.; Xiao, Y.; He, Z. Time-Series Multispectral Indices from Unmanned Aerial Vehicle Imagery Reveal Senescence Rate in Bread Wheat. Remote Sens. 2018, 10, 809. [Google Scholar] [CrossRef]
- Fernández, C.I.; Leblon, B.; Haddadi, A.; Wang, K.; Wang, J. Potato Late Blight Detection at the Leaf and Canopy Levels Based in the Red and Red-Edge Spectral Regions. Remote Sens. 2020, 12, 1292. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, H.B.; Xu, X.Q.; He, J.Y.; Ge, X.K.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Predicting grain yield in rice using multi-temporal vegetation indices from UAV-based multispectral and digital imagery. ISPRS J. Photogramm. Remote Sens. 2017, 130, 246–255. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, K.; Zhao, X.; Cheng, X.; Zhang, S.; Chen, J.; Li, J.; Li, X. Satellite Imagery-Estimated Intertidal Seaweed Biomass Using UAV as an Intermediary. Remote Sens. 2023, 15, 4428. https://doi.org/10.3390/rs15184428
Chen J, Wang K, Zhao X, Cheng X, Zhang S, Chen J, Li J, Li X. Satellite Imagery-Estimated Intertidal Seaweed Biomass Using UAV as an Intermediary. Remote Sensing. 2023; 15(18):4428. https://doi.org/10.3390/rs15184428
Chicago/Turabian StyleChen, Jianqu, Kai Wang, Xu Zhao, Xiaopeng Cheng, Shouyu Zhang, Jie Chen, Jun Li, and Xunmeng Li. 2023. "Satellite Imagery-Estimated Intertidal Seaweed Biomass Using UAV as an Intermediary" Remote Sensing 15, no. 18: 4428. https://doi.org/10.3390/rs15184428
APA StyleChen, J., Wang, K., Zhao, X., Cheng, X., Zhang, S., Chen, J., Li, J., & Li, X. (2023). Satellite Imagery-Estimated Intertidal Seaweed Biomass Using UAV as an Intermediary. Remote Sensing, 15(18), 4428. https://doi.org/10.3390/rs15184428









