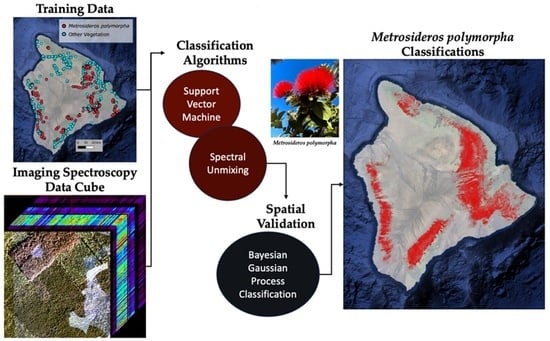
Classifying a Highly Polymorphic Tree Species across Landscapes Using Airborne Imaging Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
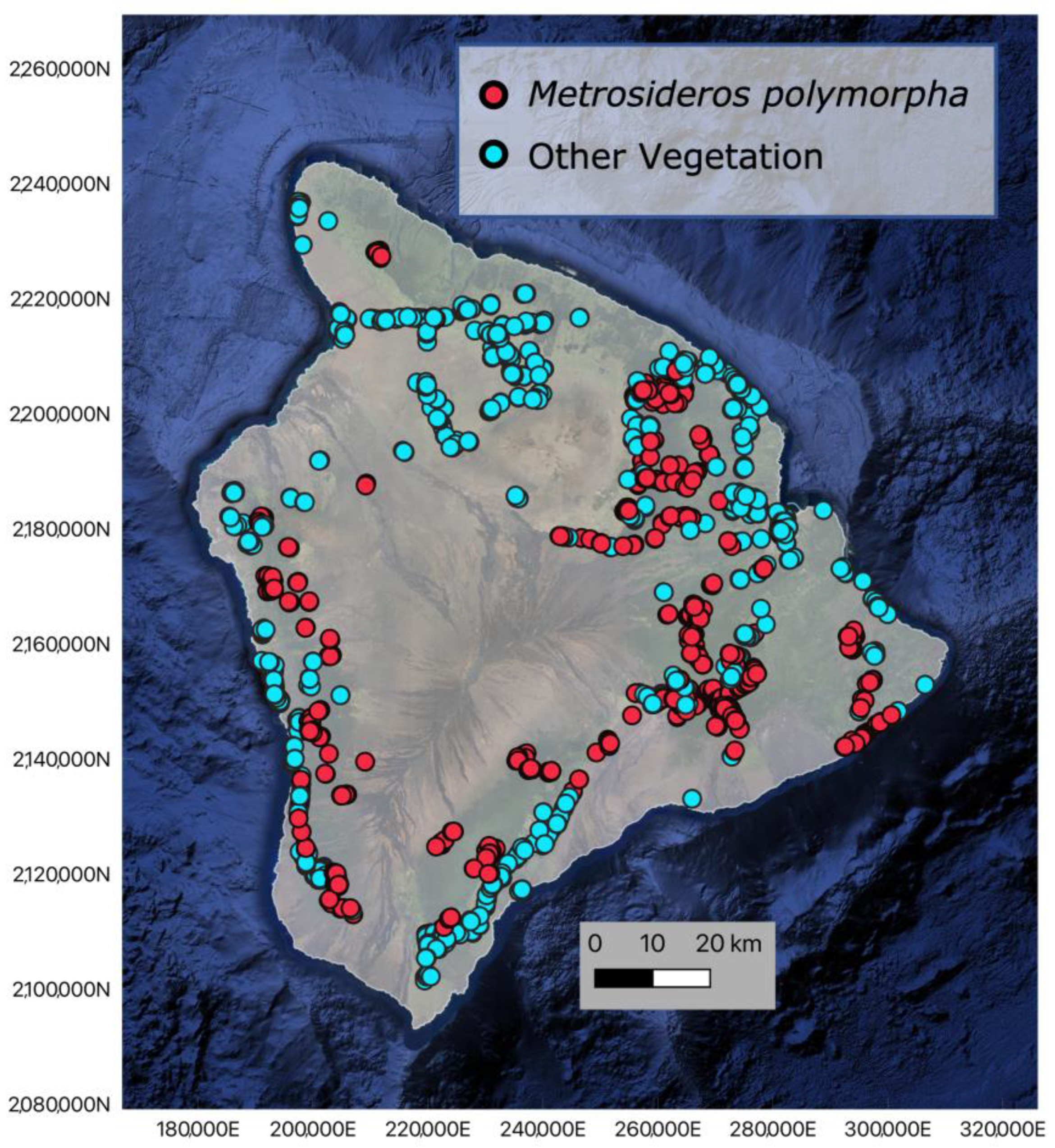
2.1. Training Data Collection
2.2. Species Classification
3. Results
3.1. Model Performance
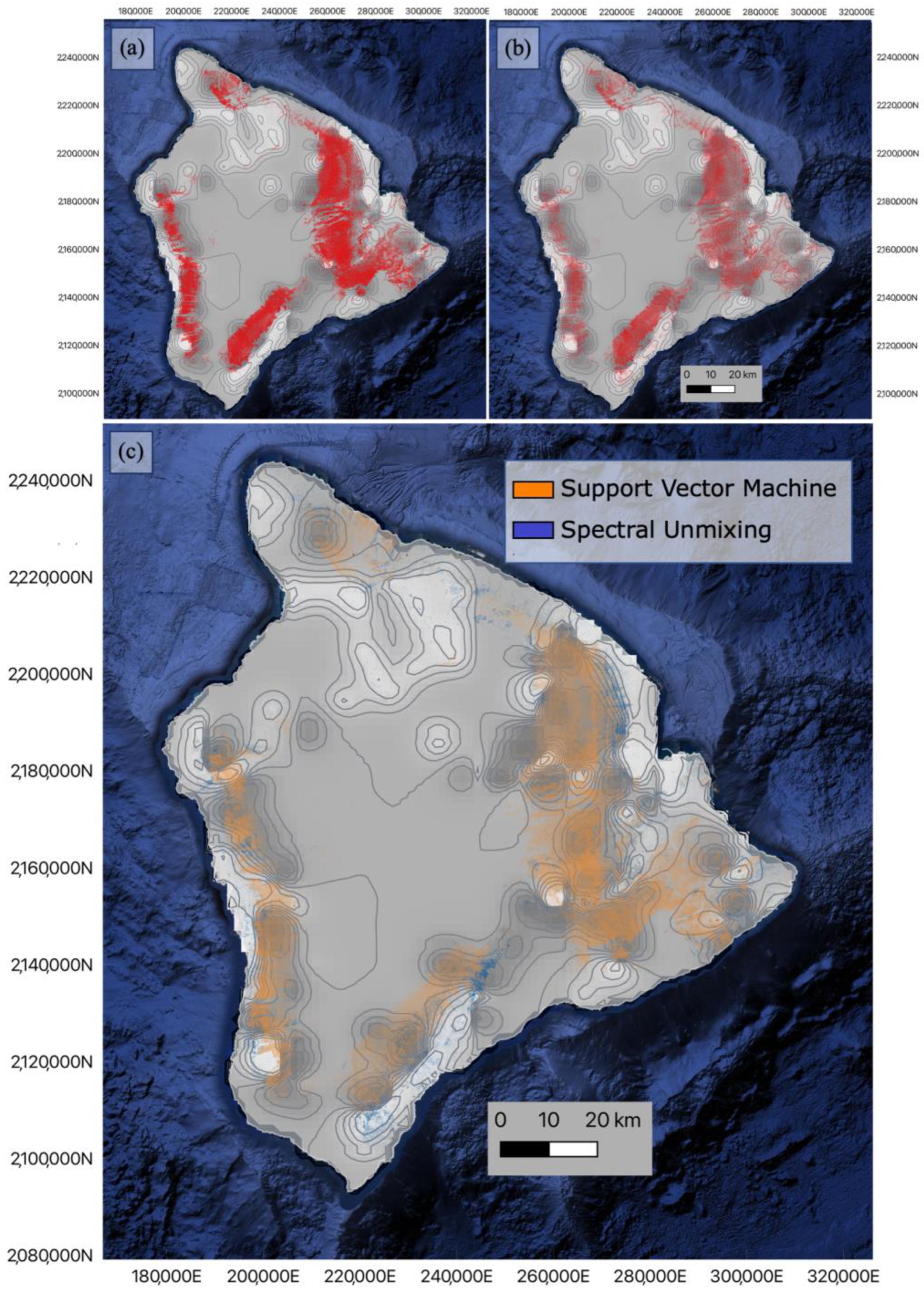
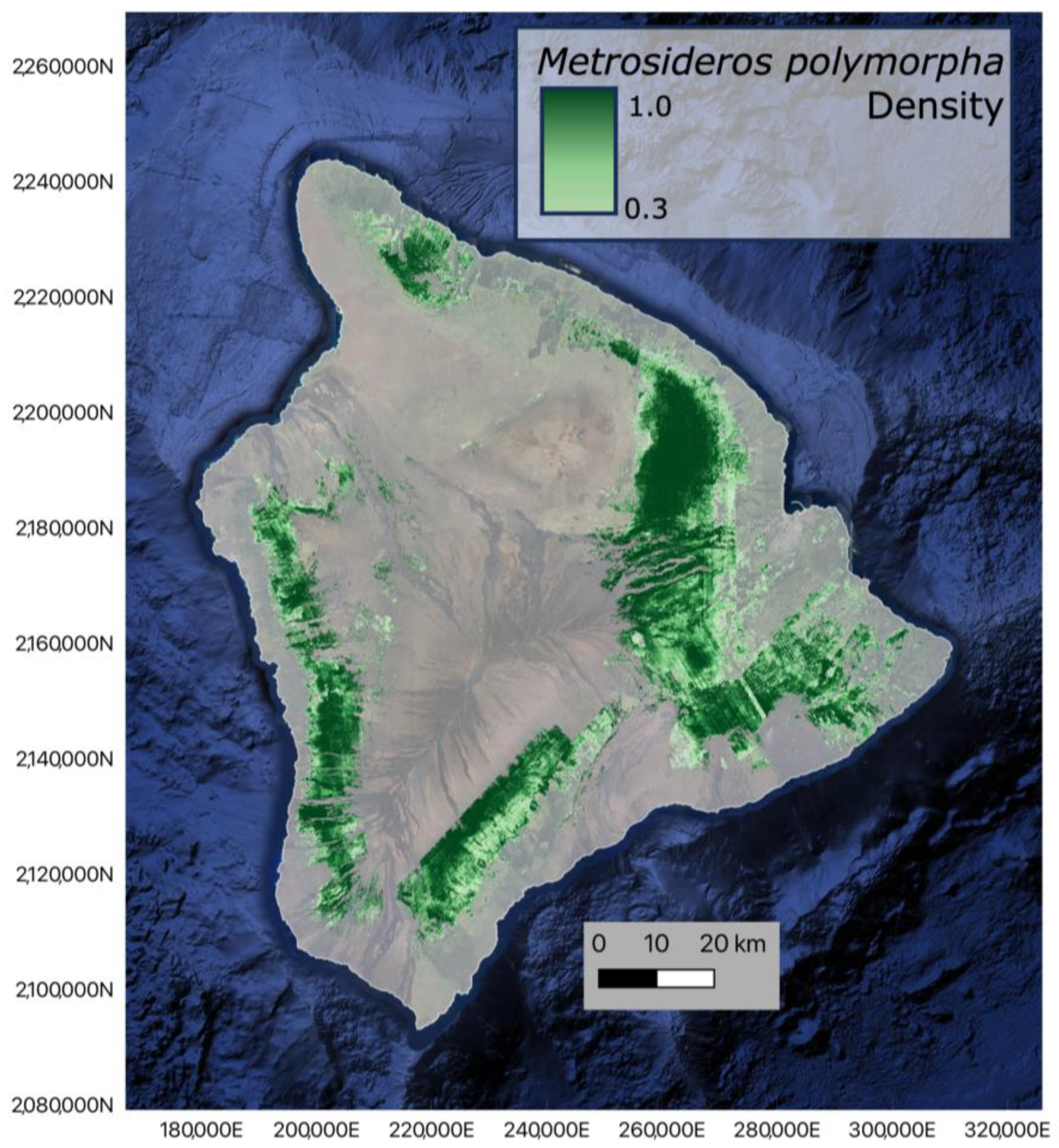
3.2. Metrosideros Polymorpha Distribution
4. Discussion
4.1. High-Resolution Model Comparison
4.2. Considerations for Future Large-Scale Modeling Efforts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fassnacht, F.E.; Latifi, H.; Stereńczak, K.; Modzelewska, A.; Lefsky, M.; Waser, L.T.; Straub, C.; Ghosh, A. Review of studies on tree species classification from remotely sensed data. Remote Sens. Environ. 2016, 186, 64–87. [Google Scholar] [CrossRef]
- Baldeck, C.A.; Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E.; Kellner, J.R.; Wright, S.J. Operational Tree Species Mapping in a Diverse Tropical Forest with Airborne Imaging Spectroscopy. PLoS ONE 2015, 10, e0118403. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.L.; Roberts, D.A.; Dennison, P.E.; Alonzo, M.; Peterson, S.H.; Beland, M. Differentiating plant species within and across diverse ecosystems with imaging spectroscopy. Remote Sens. Environ. 2015, 167, 135–151. [Google Scholar] [CrossRef]
- Laybros, A.; Schläpfer, D.; Féret, J.-B.; Descroix, L.; Bedeau, C.; Lefevre, M.-J.; Vincent, G. Across Date Species Detection Using Airborne Imaging Spectroscopy. Remote Sens. 2019, 11, 789. [Google Scholar] [CrossRef]
- Lopatin, J.; Dolos, K.; Kattenborn, T.; Fassnacht, F.E. How canopy shadow affects invasive plant species classification in high spatial resolution remote sensing. Remote Sens. Ecol. Conserv. 2019, 5, 302–317. [Google Scholar] [CrossRef]
- Roth, K.L.; Dennison, P.E.; Roberts, D.A. Comparing endmember selection techniques for accurate mapping of plant species and land cover using imaging spectrometer data. Remote Sens. Environ. 2012, 127, 139–152. [Google Scholar] [CrossRef]
- Comber, A.; Fisher, P.; Brunsdon, C.; Khmag, A. Spatial analysis of remote sensing image classification accuracy. Remote Sens. Environ. 2012, 127, 237–246. [Google Scholar] [CrossRef]
- Yu, Q.; Gong, P.; Tian, Y.; Pu, R.; Yang, J. Factors Affecting Spatial Variation of Classification Uncertainty in an Image Object-based Vegetation Mapping. Photogramm. Eng. Remote Sens. 2008, 74, 1007–1018. [Google Scholar] [CrossRef]
- Mortenson, L.A.; Hughes, R.F.; Friday, J.B.; Keith, L.M.; Barbosa, J.M.; Friday, N.J.; Liu, Z.; Sowards, T.G. Assessing spatial distribution, stand impacts and rate of Ceratocystis fimbriata induced ‘ōhi‘a (Metrosideros polymorpha) mortality in a tropical wet forest, Hawai‘i Island, USA. For. Ecol. Manag. 2016, 377, 83–92. [Google Scholar] [CrossRef]
- Loope, L.; Hughes, F.; Keith, L.; Harrington, R.; Hauff, R.; Friday, J.B.; Ewig, C.; Bennett, G.; Cannon, P.; Atkinson, C.; et al. Guidance Document for Rapid Ohia Death: Background for the 2017–2019 ROD Strategic Response Plan; University of Hawaii, College of Tropical Agriculture and Human Resources: Honolulu, HI, USA, 2016. [Google Scholar]
- Pratt, T.; Atkinson, C.; Banko, P.C.; Jacobi, J.; Woodworth, B. Conservation Biology of Hawaiian Forest Birds; Yale University Press: London, UK, 2009; Available online: https://yalebooks.yale.edu/9780300141085/conservation-biology-of-hawaiian-forest-birds (accessed on 4 August 2022).
- Chow, E.T. The Sovereign Nation of Hawai’i: Resistance in the Legacy of “Aloha ‘Oe”. SUURJ Seattle Univ. Undergrad. Res. J. 2018, 2, 15. [Google Scholar]
- Westervelt, W.D. Hawaiian Legends of Volcanoes (Mythology); Ellis Press: Los Angeles, CA, USA, 1916. [Google Scholar]
- Kagawa, A.; Sack, L.; Duarte, K.; James, S. Hawaiian native forest conserves water relative to timber plantation: Species and stand traits influence water use. Ecol. Appl. Publ. Ecol. Soc. Am. 2009, 19, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, N.R.; Asner, G.P.; Brodrick, P.G.; Martin, R.E.; Heckler, J.W.; Knapp, D.E.; Hughes, R.F. An Approach for High-Resolution Mapping of Hawaiian Metrosideros Forest Mortality Using Laser-Guided Imaging Spectroscopy. Remote Sens. 2018, 10, 502. [Google Scholar] [CrossRef]
- Wagner, W.L.; Herbst, D.R.; Sohmer, S.H. Manual of the Flowering Plants of Hawai’i; University of Hawaii Press: Honolulu, HI, USA, 1990; ISBN 978-0-8248-8577-9. Available online: https://www.degruyter.com/document/isbn/9780824885779/html?lang=en (accessed on 20 April 2023).
- Somers, B.; Asner, G.P. Invasive Species Mapping in Hawaiian Rainforests Using Multi-Temporal Hyperion Spaceborne Imaging Spectroscopy. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 351–359. [Google Scholar] [CrossRef]
- Vitousek, P.M. Nutrient Cycling and Limitation: Hawai’i as a Model System; Princeton University Press: Princeton, NJ, USA, 2004; Available online: http://www.jstor.org/stable/j.ctv39x77c (accessed on 23 September 2022).
- Cordell, S.; Goldstein, G.; Mueller-Dombois, D.; Webb, D.; Vitousek, P.M. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: The role of phenotypic plasticity. Oecologia 1998, 113, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Joel, G.; Aplet, G.; Vitousek, P.M. Leaf Morphology Along Environmental Gradients in Hawaiian Metrosideros Polymorpha. Biotropica 1994, 26, 17–22. [Google Scholar] [CrossRef]
- Martin, R.E.; Asner, G.P.; Sack, L. Genetic variation in leaf pigment, optical and photosynthetic function among diverse phenotypes of Metrosideros polymorpha grown in a common garden. Oecologia 2007, 151, 387–400. [Google Scholar] [CrossRef]
- Stacy, E.A.; Johansen, J.B.; Sakishima, T.; Price, D.K.; Pillon, Y. Incipient radiation within the dominant Hawaiian tree Metrosideros polymorpha. Heredity 2014, 113, 334–342. [Google Scholar] [CrossRef]
- Stacy, E.A.; Johansen, J.B.; Sakishima, T.; Price, D.K. Genetic analysis of an ephemeral intraspecific hybrid zone in the hypervariable tree, Metrosideros polymorpha, on Hawai‘i Island. Heredity 2016, 117, 173–183. [Google Scholar] [CrossRef]
- Seeley, M.M.; Stacy, E.A.; Martin, R.E.; Asner, G.P. Foliar functional and genetic variation in a keystone Hawaiian tree species estimated through spectroscopy. Oecologia 2023, 202, 15–28. [Google Scholar] [CrossRef]
- U.S. Geological Survey Gap. Analysis Program GAP/LANDFIRE National Terrestrial Ecosystems 2011; U.S. Geological Survey: Reston, VA, USA, 2011.
- Pascual, A.; Giardina, C.P.; Povak, N.A.; Hessburg, P.F.; Asner, G.P. Integrating ecosystem services modeling and efficiencies in decision-support models conceptualization for watershed management. Ecol. Model. 2022, 466, 109879. [Google Scholar] [CrossRef]
- Pascual, A.; Giardina, C.P.; Povak, N.A.; Hessburg, P.F.; Heider, C.; Salminen, E.; Asner, G.P. Optimizing invasive species management using mathematical programming to support stewardship of water and carbon-based ecosystem services. J. Environ. Manag. 2022, 301, 113803. [Google Scholar] [CrossRef] [PubMed]
- Povak, N.A.; Hessburg, P.F.; Giardina, C.P.; Reynolds, K.M.; Heider, C.; Salminen, E.; Salter, R.B.; MacKenzie, R.A. A watershed decision support tool for managing invasive species on Hawai‘i Island, USA. For. Ecol. Manag. 2017, 400, 300–320. [Google Scholar] [CrossRef]
- Boardman, J.W.; Green, R.O. Exploring the spectral variability of the Earth as measured by AVIRIS in 1999. In Proceedings of the Ninth JPL Airborne Earth Science Workshop; Jet Propulsion Laboratory: Pasadena, CA, USA, 2000. Available online: https://trs.jpl.nasa.gov/handle/2014/16602 (accessed on 3 January 2023).
- Green, R.O.; Boardman, J.W. Exploration of the relationship between information content and signal-to-noise ratio and spatial resolution in AVIRIS spectral data. Spectrum 2000, 7. [Google Scholar]
- Asner, G.P.; Martin, R.E. Airborne spectranomics: Mapping canopy chemical and taxonomic diversity in tropical forests. Front. Ecol. Environ. 2009, 7, 269–276. [Google Scholar] [CrossRef]
- Balzotti, C.S.; Asner, G.P.; Adkins, E.D.; Parsons, E.W. Spatial drivers of composition and connectivity across endangered tropical dry forests. J. Appl. Ecol. 2020, 57, 1593–1604. [Google Scholar] [CrossRef]
- Chakravortty, S.; Shah, E.; Chowdhury, A.S. Application of Spectral Unmixing Algorithm on Hyperspectral Data for Mangrove Species Classification. In Proceedings of the Applied Algorithms; Gupta, P., Zaroliagis, C., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 223–236. [Google Scholar]
- Pontius, J.; Hanavan, R.P.; Hallett, R.A.; Cook, B.D.; Corp, L.A. High spatial resolution spectral unmixing for mapping ash species across a complex urban environment. Remote Sens. Environ. 2017, 199, 360–369. [Google Scholar] [CrossRef]
- Shang, X.; Chisholm, L.A. Classification of Australian Native Forest Species Using Hyperspectral Remote Sensing and Machine-Learning Classification Algorithms. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2481–2489. [Google Scholar] [CrossRef]
- Somers, B.; Asner, G.P. Tree species mapping in tropical forests using multi-temporal imaging spectroscopy: Wavelength adaptive spectral mixture analysis. Int. J. Appl. Earth Obs. Geoinf. 2014, 31, 57–66. [Google Scholar] [CrossRef]
- Torabzadeh, H.; Leiterer, R.; Hueni, A.; Schaepman, M.E.; Morsdorf, F. Tree species classification in a temperate mixed forest using a combination of imaging spectroscopy and airborne laser scanning. Agric. For. Meteorol. 2019, 279, 107744. [Google Scholar] [CrossRef]
- Asner, G.P.; Knapp, D.E.; Boardman, J.; Green, R.O.; Kennedy-Bowdoin, T.; Eastwood, M.; Martin, R.E.; Anderson, C.; Field, C.B. Carnegie Airborne Observatory-2: Increasing science data dimensionality via high-fidelity multi-sensor fusion. Remote Sens. Environ. 2012, 124, 454–465. [Google Scholar] [CrossRef]
- Féret, J.-B.; Asner, G.P. Mapping tropical forest canopy diversity using high-fidelity imaging spectroscopy. Ecol. Appl. 2014, 24, 1289–1296. [Google Scholar] [CrossRef]
- Marconi, S.; Weinstein, B.G.; Zou, S.; Bohlman, S.A.; Zare, A.; Singh, A.; Stewart, D.; Harmon, I.; Steinkraus, A.; White, E.P. Continental-scale hyperspectral tree species classification in the United States National Ecological Observatory Network. Remote Sens. Environ. 2022, 282, 113264. [Google Scholar] [CrossRef]
- Miller, C.J. Performance assessment of ACORN atmospheric correction algorithm. In Proceedings of the Algorithms and Technologies for Multispectral, Hyperspectral, and Ultraspectral Imagery VIII; SPIE: Bellingham, WA, USA, 2002; Volume 4725, pp. 438–449. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/4725/0000/Performance-assessment-of-ACORN-atmospheric-correction-algorithm/10.1117/12.478777.full (accessed on 21 December 2021).
- Schaepman-Strub, G.; Schaepman, M.; Martonchik, J.; Schaaf, C. What’s in a Satellite Albedo Product? IEEE Int. Symp. Geosci. Remote Sens. 2006, 2848–2852. [Google Scholar]
- Feilhauer, H.; Asner, G.P.; Martin, R.E.; Schmidtlein, S. Brightness-normalized Partial Least Squares Regression for hyperspectral data. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1947–1957. [Google Scholar] [CrossRef]
- Colgan, M.S.; Baldeck, C.A.; Féret, J.-B.; Asner, G.P. Mapping Savanna Tree Species at Ecosystem Scales Using Support Vector Machine Classification and BRDF Correction on Airborne Hyperspectral and LiDAR Data. Remote Sens. 2012, 4, 3462–3480. [Google Scholar] [CrossRef]
- Asner, G.P.; Knapp, D.E.; Kennedy-Bowdoin, T.; Jones, M.O.; Martin, R.E.; Boardman, J.W.; Field, C.B. Carnegie Airborne Observatory: In-flight fusion of hyperspectral imaging and waveform light detection and ranging for three-dimensional studies of ecosystems. J. Appl. Remote Sens. 2007, 1, 013536. [Google Scholar] [CrossRef]
- Weingarten, E.; Martin, R.; Hughes, F.; Vaughn, N.; Schafron, E.; Asner, G.P. Early Detection of a Tree Pathogen using Airborne Remote Sensing. Ecol. Appl. 2021, 21, e2519. [Google Scholar] [CrossRef]
- Dalponte, M.; Bruzzone, L.; Gianelle, D. Tree species classification in the Southern Alps based on the fusion of very high geometrical resolution multispectral/hyperspectral images and LiDAR data. Remote Sens. Environ. 2012, 123, 258–270. [Google Scholar] [CrossRef]
- Dalponte, M.; Ørka, H.O.; Gobakken, T.; Gianelle, D.; Næsset, E. Tree Species Classification in Boreal Forests With Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2013, 51, 2632–2645. [Google Scholar] [CrossRef]
- Feret, J.-B.; Asner, G.P. Tree Species Discrimination in Tropical Forests Using Airborne Imaging Spectroscopy. IEEE Trans. Geosci. Remote Sens. 2013, 51, 73–84. [Google Scholar] [CrossRef]
- Crabbé, A.H.; Somers, B.; Roberts, D.A.; Halligan, K.; Dennison, P.; Dudley, K. MESMA QGIS Plugin. 2020. Available online: https://bitbucket.org/kul-reseco/mesma (accessed on 1 November 2022).
- Roberts, D.A.; Gardner, M.; Church, R.; Ustin, S.; Scheer, G.; Green, R.O. Mapping Chaparral in the Santa Monica Mountains Using Multiple Endmember Spectral Mixture Models. Remote Sens. Environ. 1998, 65, 267–279. [Google Scholar] [CrossRef]
- Camps-Valls, G.; Gomez-Chova, L.; Calpe-Maravilla, J.; Martin-Guerrero, J.D.; Soria-Olivas, E.; Alonso-Chorda, L.; Moreno, J. Robust support vector method for hyperspectral data classification and knowledge discovery. IEEE Trans. Geosci. Remote Sens. 2004, 42, 1530–1542. [Google Scholar] [CrossRef]
- Melgani, F.; Bruzzone, L. Classification of hyperspectral remote sensing images with support vector machines. IEEE Trans. Geosci. Remote Sens. 2004, 42, 1778–1790. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.E. Gaussian Processes in Machine Learning. In Advanced Lectures on Machine Learning; Bousquet, O., von Luxburg, U., Rätsch, G., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 3176, pp. 63–71. ISBN 978-3-540-23122-6. Available online: http://link.springer.com/10.1007/978-3-540-28650-9_4 (accessed on 19 April 2023).
- Culver, T.; Rydeen, A.; Dix, M.; Camello, M.; Gallaher, M.; Lapidus, D.; Brown, E.; Lee, C.; Luvall, J.; Stavros, N.; et al. SBG User Needs and Valuation Study; RTI Innovation Advisors: Durham, NC, USA, 2020. [Google Scholar]
- Iwasaki, A.; Tanii, J.; Kashimura, O.; Ito, Y. Prelaunch Status of Hyperspectral Imager Suite (Hisui). In Proceedings of the IGARSS 2019–2019 IEEE International Geoscience and Remote Sensing Symposium, Yokohama, Japan, 28 July–2 August 2019; pp. 5887–5890. [Google Scholar]
- Lopinto, E.; Ananasso, C. The Prisma Hyperspectral Mission. In Proceedings of the 33rd EARSeL Symposium, Towards Horizon, Matera, Italy, 3–6 June 2013; Volume 12. [Google Scholar]
- Müller, R.; Alonso, K.; Krawczyk, H.; Bachmann, M.; Cerra, D.; Krutz, D.; Dietrich, D.; Gerasch, B.; Ziel, V.; Heiden, U.; et al. Overview and Status of the DESIS Mission. In Proceedings of the 9th Whispers Conference, Amsterdam, The Netherlands, 23–26 September 2018. [Google Scholar]
- Martin, R.E.; Asner, G.P. Leaf Chemical and Optical Properties of Metrosideros polymorpha across Environmental Gradients in Hawaii. Biotropica 2009, 41, 292–301. [Google Scholar] [CrossRef]
- Seeley, M.M.; Martin, R.E.; Vaughn, N.R.; Thompson, D.R.; Dai, J.; Asner, G.P. Quantifying the Variation in Reflectance Spectra of Metrosideros polymorpha Canopies across Environmental Gradients. Remote Sens. 2023, 15, 1614. [Google Scholar] [CrossRef]
- Jensen, R.R.; Hardin, P.J.; Hardin, A.J. Classification of urban tree species using hyperspectral imagery. Geocarto Int. 2012, 27, 443–458. [Google Scholar] [CrossRef]
- Callaghan, J.; McAlpine, C.; Mitchell, D.; Thompson, J.; Bowen, M.; Rhodes, J.; Jong, C.d.; Domalewski, R.; Scott, A.; Callaghan, J.; et al. Ranking and mapping koala habitat quality for conservation planning on the basis of indirect evidence of tree-species use: A case study of Noosa Shire, south-eastern Queensland. Wildl. Res. 2011, 38, 89–102. [Google Scholar] [CrossRef]
- Fremout, T.; Thomas, E.; Gaisberger, H.; Van Meerbeek, K.; Muenchow, J.; Briers, S.; Gutierrez-Miranda, C.E.; Marcelo-Peña, J.L.; Kindt, R.; Atkinson, R.; et al. Mapping tree species vulnerability to multiple threats as a guide to restoration and conservation of tropical dry forests. Glob. Chang. Biol. 2020, 26, 3552–3568. [Google Scholar] [CrossRef]
- Jonsson, M.; Bengtsson, J.; Gamfeldt, L.; Moen, J.; Snäll, T. Levels of forest ecosystem services depend on specific mixtures of commercial tree species. Nat. Plants 2019, 5, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.A.; Martins, F.R. Rarity patterns and the conservation status of tree species in South American savannas. Flora 2021, 285, 151942. [Google Scholar] [CrossRef]
- Roth, K.L.; Roberts, D.A.; Dennison, P.E.; Peterson, S.H.; Alonzo, M. The impact of spatial resolution on the classification of plant species and functional types within imaging spectrometer data. Remote Sens. Environ. 2015, 171, 45–57. [Google Scholar] [CrossRef]
| Predicted | |||||
|---|---|---|---|---|---|
| Spectral Mixture Analysis | Support Vector Machine | ||||
| M. polymorpha | Other Vegetation | M. polymorpha | Other Vegetation | ||
| Actual | M. polymorpha | 478 | 46 | 497 | 27 |
| Other Vegetation | 61 | 1025 | 44 | 1042 | |
| High M. polymorpha Likelihood | Low M. polymorpha Likelihood | Medium M. polymorpha Likelihood | |
|---|---|---|---|
| Support Vector Machine | 47.5 | 13.9 | 38.9 |
| Spectral Mixture Analysis | 29.4 | 30.6 | 39.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seeley, M.M.; Vaughn, N.R.; Shanks, B.L.; Martin, R.E.; König, M.; Asner, G.P. Classifying a Highly Polymorphic Tree Species across Landscapes Using Airborne Imaging Spectroscopy. Remote Sens. 2023, 15, 4365. https://doi.org/10.3390/rs15184365
Seeley MM, Vaughn NR, Shanks BL, Martin RE, König M, Asner GP. Classifying a Highly Polymorphic Tree Species across Landscapes Using Airborne Imaging Spectroscopy. Remote Sensing. 2023; 15(18):4365. https://doi.org/10.3390/rs15184365
Chicago/Turabian StyleSeeley, Megan M., Nicholas R. Vaughn, Brennon L. Shanks, Roberta E. Martin, Marcel König, and Gregory P. Asner. 2023. "Classifying a Highly Polymorphic Tree Species across Landscapes Using Airborne Imaging Spectroscopy" Remote Sensing 15, no. 18: 4365. https://doi.org/10.3390/rs15184365
APA StyleSeeley, M. M., Vaughn, N. R., Shanks, B. L., Martin, R. E., König, M., & Asner, G. P. (2023). Classifying a Highly Polymorphic Tree Species across Landscapes Using Airborne Imaging Spectroscopy. Remote Sensing, 15(18), 4365. https://doi.org/10.3390/rs15184365