1. Introduction
Invasive alien species (IAS) are one of the largest threats to biodiversity around the world [
1]. It is estimated that the global costs of damage caused by IAS exceed hundreds of billions of U.S. dollars a year [
2,
3] and that IAS have negatively affected more than a hundred critically endangered native terrestrial vertebrates [
4]. The number of established IAS has exponentially increased during the last century for different biological groups [
5], and climate change is expected to further expand the distribution of some invasive species [
6]. After reaching and becoming established in a new area, IAS can impact local biodiversity through direct and indirect negative interactions with native taxa, such as predation, competition, disease spread, predator poisoning, and altering habitat characteristics [
2]. The impact of introducing an alien species can be even more dramatic for native species on islands, which may experience rapid extirpation of native fauna after the arrival and establishment of an IAS [
7,
8,
9]. Insular species often have small populations and home ranges, low genetic diversity, and lack morphological adaptations against IAS [
7]. Islands in warmer regions of the globe are hotspots for IAS and tend to have more established invasive species than mainland areas, generating a profound concern for the conservation of native species on tropical islands [
5,
8].
One of the main obstacles that wildlife managers and conservationists face in opposing the threat of invasive species is acquiring rapid, reliable, large-scale baseline information on the distribution of fauna—data which are critical in guiding effective wildlife management programs [
10]. A central challenge to this type of monitoring is how to cover a large sampling area with a limited number of researchers (an issue faced by many projects and agencies) and still complete surveys in a short period of time, which has the dual benefit of avoiding temporal bias and providing distribution data quickly. Moreover, the success of IAS control or eradication efforts is typically greater when species are detected in an early stage before they become established in a new location [
11]. Therefore, the development of early-stage detection systems is urgent in order to identify IAS before they can become a significant threat [
12,
13,
14]. The rapid evolution of remote sensing tools has made it possible to conduct large-scale monitoring in a short period of time, providing detailed baseline information of ecosystems and biodiversity [
15].
Remote sensing through satellite and airborne images has been successfully used to detect and monitor changes in forest cover, vegetation type, disturbance regimes, plant phenology, and ecosystems [
16,
17,
18,
19,
20]. However, remote sensing is still mostly overlooked for invasive fauna, though camera traps and autonomous acoustic devices have been successfully used to detect and monitor alien animal species [
15,
21,
22]. Other noninvasive methods such as eDNA have also been used recently to detect IAS [
21,
22]. The emergence of new autonomous recording units (ARUs) and platforms to store and analyze massive amounts of audio data has greatly improved the utility of passive acoustic monitoring (PAM) to monitor soniferous wildlife and its threats [
23,
24,
25,
26,
27,
28]. Taxa that regularly produce species-specific vocalizations, such as birds, anurans, bats, insects, and some mammals, are well suited for ecoacoustic surveys [
29,
30]. Although PAM has great potential for monitoring biodiversity and researching a variety of ecological issues [
31], it has not been used yet to its maximum potential for investigation of soniferous terrestrial IAS [
32,
33]. However, PAM has been used for investigating the occurrence of invasive freshwater drum in the New York State Canal System and assessing the phenology of the invasive cane toad in Australia [
34,
35].
Passive acoustic monitoring has numerous benefits for rapid assessment and early detection of sound-producing fauna, as well as large-scale and long-term monitoring, and can decrease the response time for managing soniferous IAS [
28,
31,
33,
36]. Amongst the primary benefits, PAM is a standardized noninvasive survey method that can be used simultaneously in numerous locations, allowing for the monitoring of hundreds to thousands of sites at the same time, which would otherwise be impossible if trained researchers were required to be in the field at each location [
36]. Additionally, PAM facilitates sampling during all periods of the day, covering the peak of activity of different taxa; devices can be easily deployed with little or no specialized training; and recordings can be permanently stored, providing insights on temporal patterns of biodiversity [
36,
37].
Passive acoustic monitoring generates the detection and non-detection data required for species distribution models, one of the most used tools for IAS risk assessment [
38,
39,
40,
41,
42]. Due to the substantial volume of data collected by the ARU in the field, one constraint on PAM has been the ability to examine all of the recordings collected. As a result, the development of protocols that optimize inspection of recordings together with semiautomatic or automatic techniques that speed up data analysis is necessary to extract the maximum amount of information from the acoustic data collected. In addition, to expedite biological and ecological insights from data acquired through PAM, it is important to develop user interface tools that allow availability, visibility, and management of results for the nonacademic public (e.g., citizen scientists, birdwatchers, practitioners, and wildlife managers).
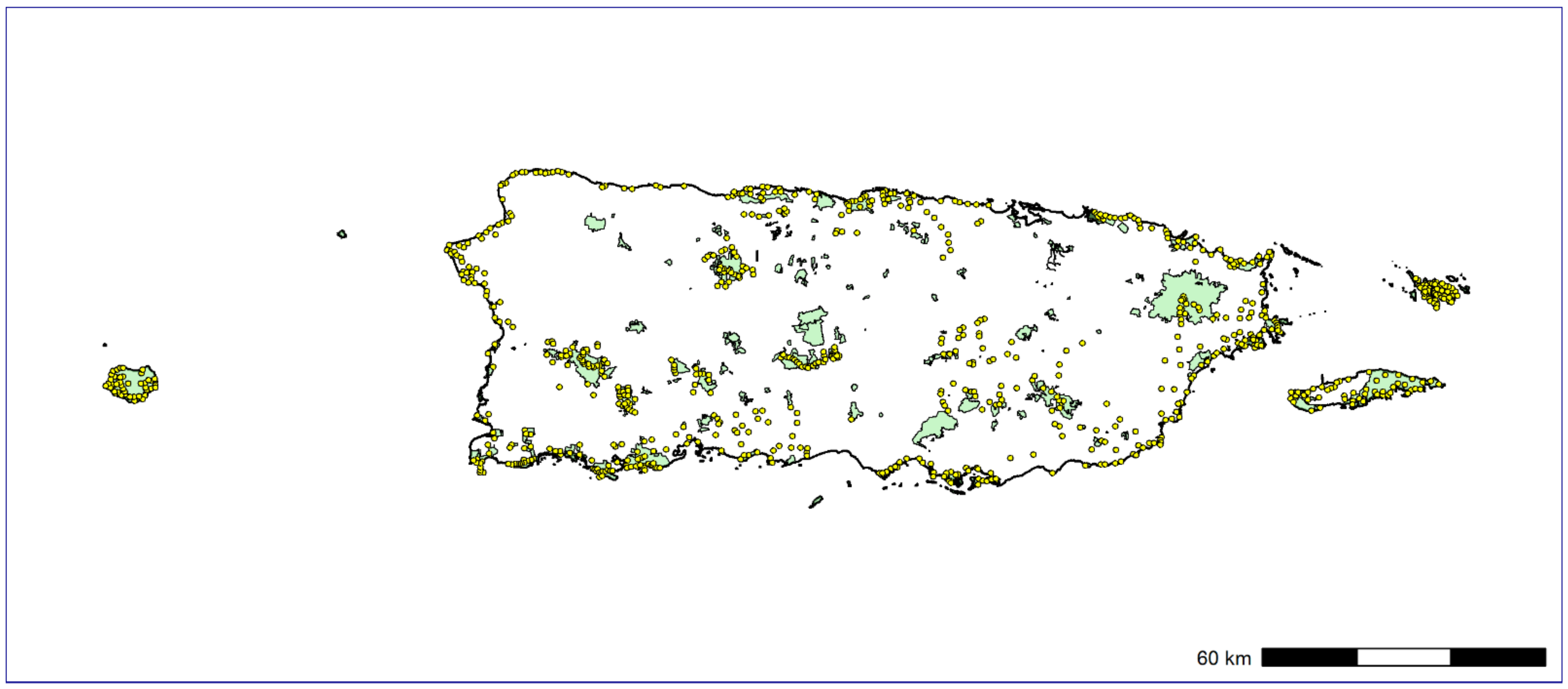
Here, we used a large-scale PAM survey across the Puerto Rican archipelago (841 sampling sites) to investigate the spatial distribution and peak of vocal activity of IAS. Our main goal was to generate baseline population data for soniferous native and IAS in Puerto Rico. Moreover, we provide a roadmap on how remote soundscape data collection can be used to rapidly provide distribution information for soniferous species through a free, user-friendly web interface designed to be accessible to diverse stakeholder groups as biologists, ecologists, wildlife managers, and citizen scientists. Our workflow offers a balance between manual inspection of recordings with semiautomatic analysis, which considerably reduces the time required to analyze the large amount of data typically generated by PAM, and providing assessment for the peak activity of species and detection/nondetection data for each day and site sampled. To assess the status of IAS populations in Puerto Rico, we used Bayesian single-species occupancy models to investigate how the spatial distribution of IAS varied through environmental gradients. The last step of our end-to-end pipeline for large-scale passive acoustic monitoring consisted in the development of a web page connecting and summarizing data from the Arbimon platform to display and share the results of the ecoacoustic analyses and close the gaps between academia and wildlife managers and decision makers. We expect that our study can provide baseline information on soniferous IAS for wildlife managers and decision makers in Puerto Rico and support future research focused on the rapid assessment or long-term monitoring of sound-producing wildlife across a broad spatial area using acoustic survey methods.
3. Results
We based our findings on 1,773,287 1-min recordings from 8.9 TB of soundscape data. Our manual inspection of recordings together with PM analysis generated an overall list of 95 species detected throughout the Puerto Rican archipelago: 74 birds, 18 frogs, and 3 mammals (
Appendix C). We found 92 species on the main island of Puerto Rico, followed by Vieques, Culebra, and Mona with 31, 25, and 19 species, respectively (
Appendix C). Overall, 16 species were considered invasive species (
Appendix C) and are potential threats to wildlife in the Puerto Rico: 10 birds (
Icterus,
Molothrus bonariensis, Gallus gallus domesticus,
Brotogeris versicolurus,
Passer domesticus,
Amazona amazonica,
Amazona viridigenalis,
Bubulcus ibis,
Myiopsitta monachus, and
Streptopelia decaocto), 3 frogs (
Lithobates catesbeianus,
Osteopilus septentrionalis, and
Rhinella marina), and 3 mammals (
Canis lupus familiaris,
Capra hircus, and
Equus ferus caballus).
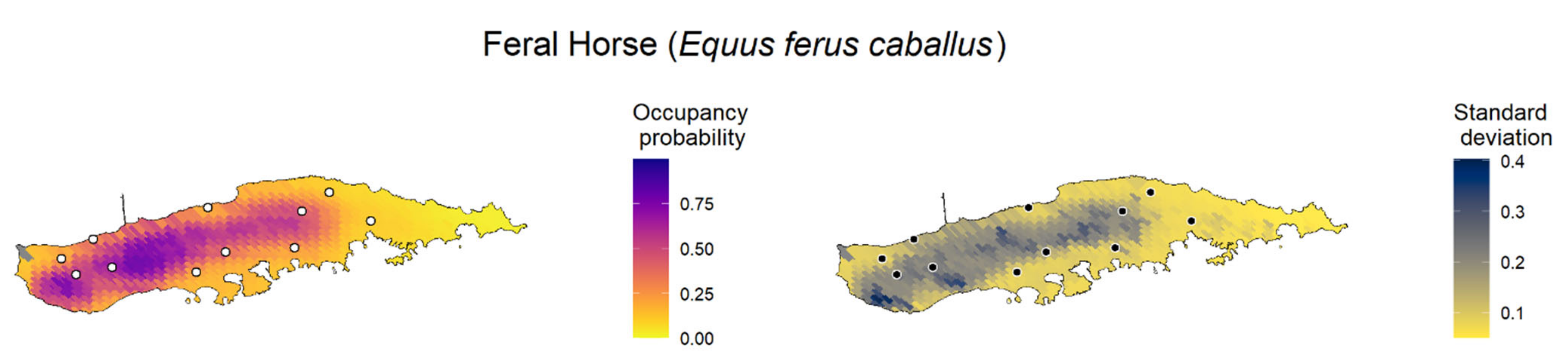
None of the invasive species were detected on Desecheo Island; therefore, this island was not used in the occupancy model. The wild goat (C. hircus) was detected at 21 sites, all of which were on Mona Island. Feral horse (E. ferus caballus) was detected at 11 sites on Vieques and two sites on the mainland; therefore, the occupancy model was performed only for Vieques. Chickens (G. gallus domesticus), Venezuelan troupial (I. icterus), shiny cowbird (M. bonariensis), and domestic dog (C. lupus familiaris) were the most widespread species, detected at 165, 131, 78, and 76 sites, respectively. Chickens (the only IAS for which the occupancy model was fitted for more than one island) were detected at 134 sites on the main island, 27 sites on Culebra, 3 sites on Vieques, and 1 site on Mona. Five bird species (A. amazonica, A. viridigenalis, B. ibis, M. monachus, and S. decaocto) had low raw detections and were detected at few sites (less than 10 sites); thus, we did not run occupancy models or a call activity pattern analysis for them. The other IAS detected were found at between 12 and 24 sites (American bullfrog (L. catesbeianus), house sparrow (P. domesticus), Cuban tree frog (O. septentrionalis), cane toad (R. marina), and white-winged parakeet (B. versicolurus)).
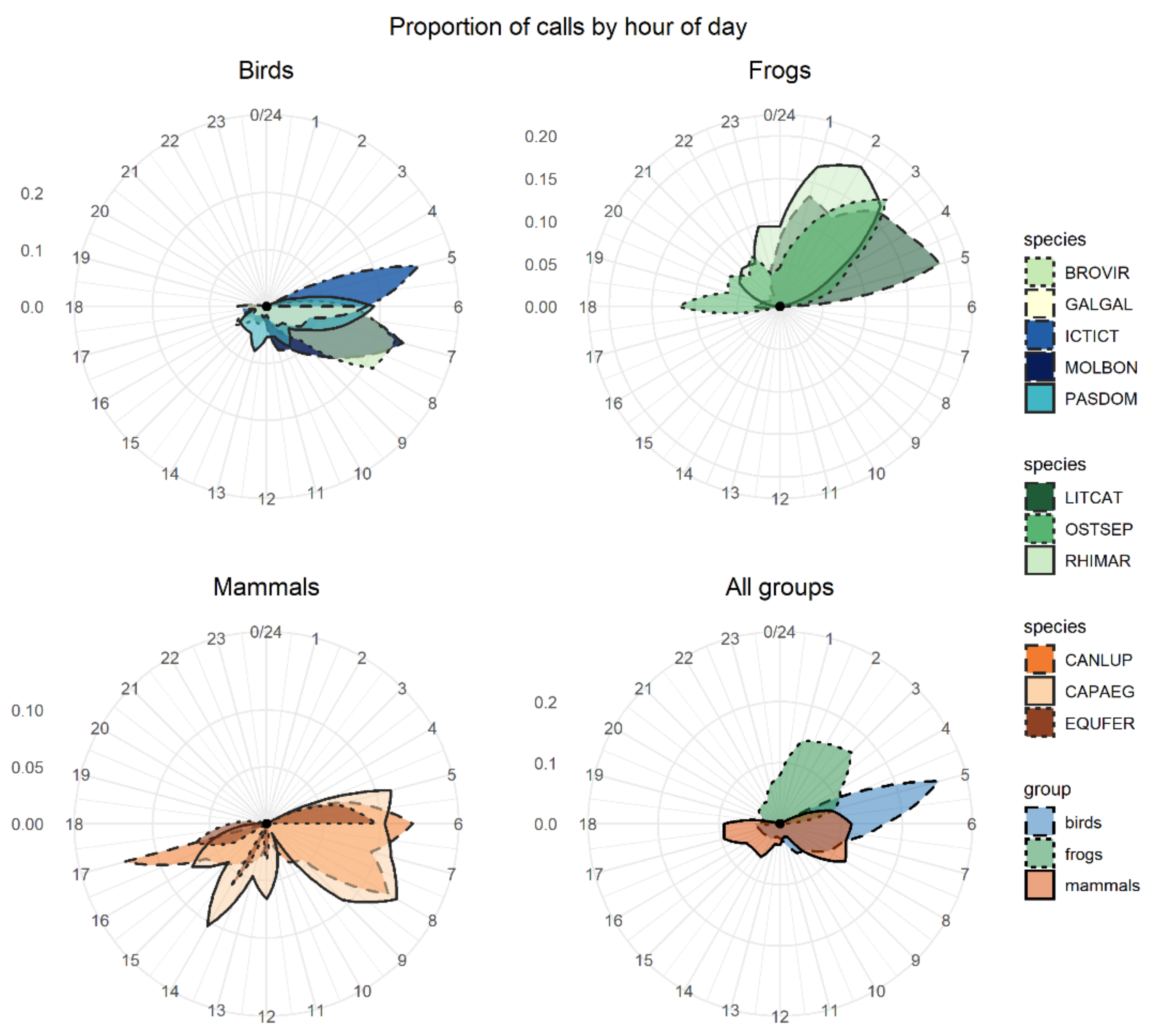
The number of calls recorded and the peak of activity varied greatly between species (
Figure 2). The group with the highest number of detections (i.e., best-scored ROI per site and day) was birds (e.g., chicken had more than 750 raw detections, Venezuelan troupial had 450 detections, and shiny cowbird had 194 detections), followed by mammals (ranging from 23 to 148 detections) and frogs (ranging from 53 to 75 detections). The bird species had a high activity peak early in the morning (between 5 a.m. and 7 a.m.). The peak activity of frog species varied between 1 a.m. and 5 a.m. depending on the species. The American bullfrog had a peak in calling activity at dawn (about 6 a.m.); the cane toad showed a peak at 2 a.m.; and the Cuban tree frog showed two peaks of activity, with the strongest at 3 a.m. and a second less-intense peak at dusk. Mammals did not show a clear calling activity peak.
We fit occupancy models for 11 IAS (all 3 frog and mammal IAS and 5 species of birds). In general, the occupancy models presented good convergence and fit well for the species when checked through the inspection of traceplots (
Appendix D) and using R-hat statistic values <1.1 (
Appendix E). However, the “top-ranked” spatial occupancy model of the Cuban tree frog did not show good convergence and efficiency diagnostics for Markov chains (
ψ(fa2 + fa3 + RSR-1000);
(elevation + date + date
2)) even after running the model with 200,000 interactions; thus, we used the second-best model for the species to show the parameter estimates (
Table 2;
Appendix E). In general, the probability of detecting IAS throughout Puerto Rico was medium to low and varied widely, ranging from 0.002 to 0.63 (
Appendix E); most species were below 0.3. The best model that explained the detection probability of all species (except for feral horse, white-winged parakeet, and the two domesticated species) included the three predictor variables: elevation, Julian date linear, and Julian date quadratic (
Appendix E). In general, the Julian date positively influenced a species’ mean detection probability. The relationship between elevation and the detection probability varied depending on the species.
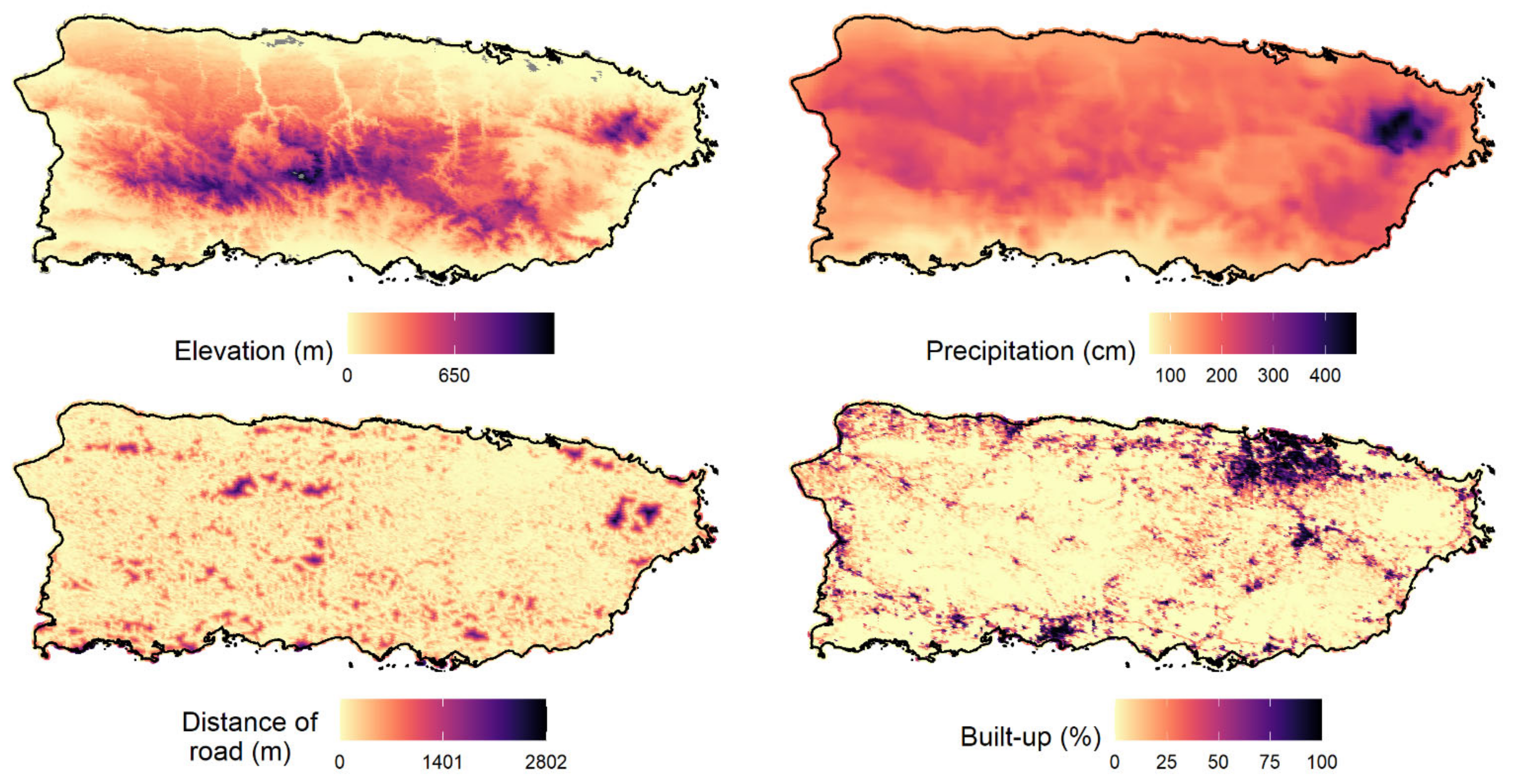
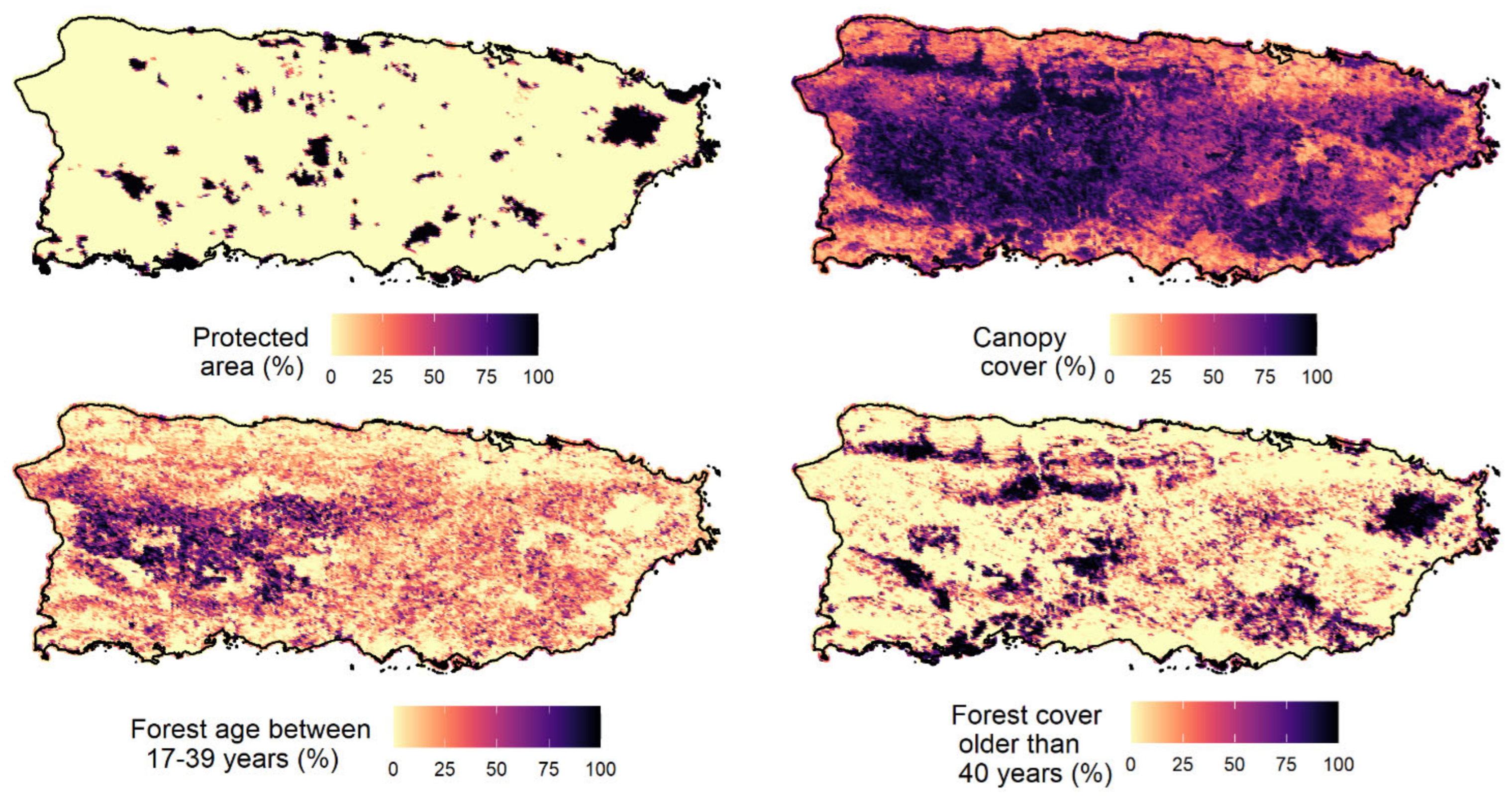
Occupancy probability ranged from 0.002 (American bullfrog) to 0.67 (wild goat on Mona). The three amphibian IAS showed a very low occupancy probability (lower than 0.08). The results showed that elevation and precipitation were the variables that best explained the occupancy probability of IAS in Puerto Rico and appeared in the top-model of six species, followed by proportion of forest cover aged between 32–54 years (fa2) and forest cover older than 54 years (fa3;
Table 2). The best model selected for four of the species (shiny cowbird, cane toad, wild goat, and house sparrow) was the null model containing only the intercept.
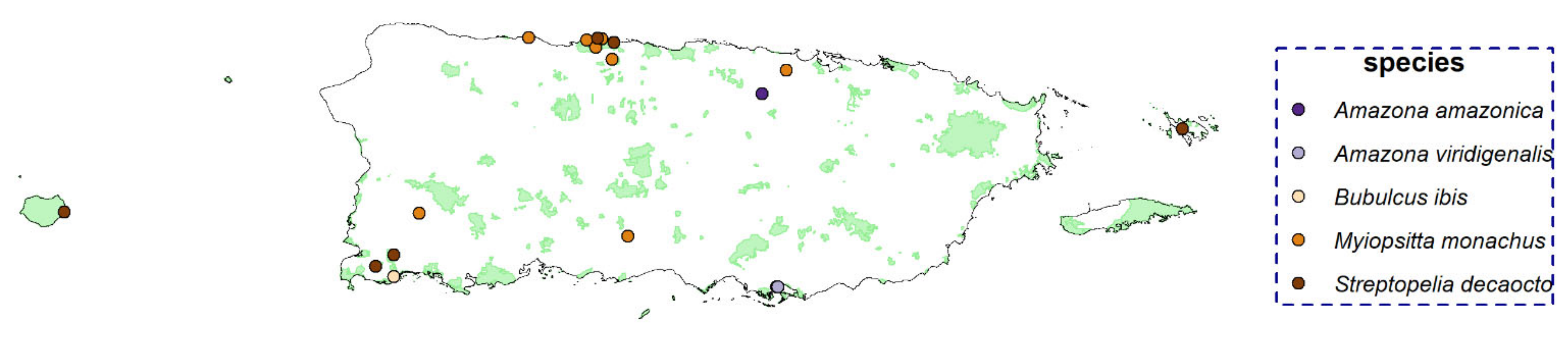
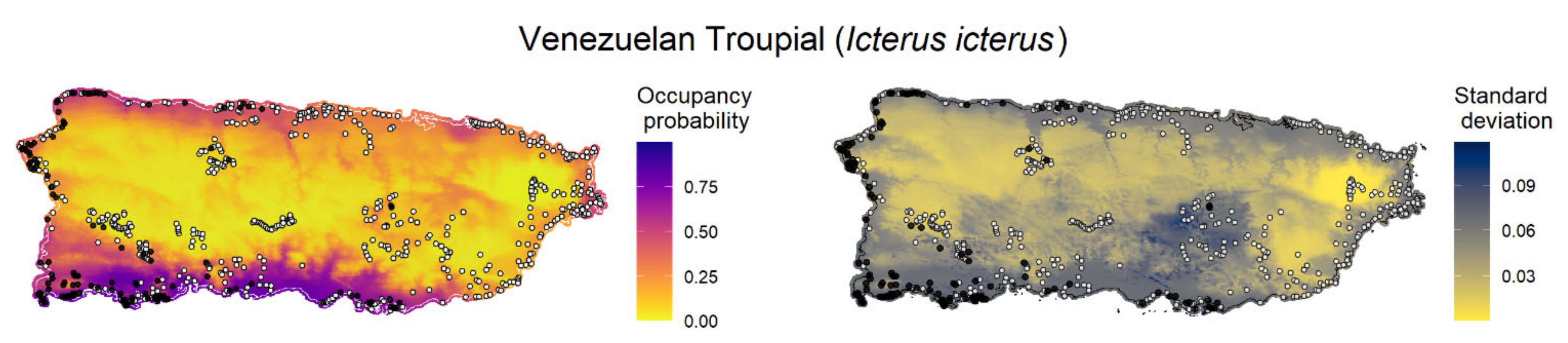
We created maps of model-predicted species occupancy probability across Puerto Rico and outlying islands for the 11 invasive species with occupancy models (
Figure 3;
Appendix F). Here, we exemplify the predicted occupancy of the Venezuelan troupial (see
Appendix F for the occupancy prediction maps for the other species). We chose the Venezuelan troupial as an example because it was the species that showed the top-ranked model with the highest difference between the other a priori candidate models, which suggested more confidence in the top-ranked model (higher ΔELPD between the top-ranked and second-best models). We limited the forecast map to the islands where the occupancy model was fitted for each species to diminish spurious predictions because the gradients between islands were different. The point-estimates occupancy probability map for the Venezuelan troupial in Puerto Rico in 2021 under the top-ranked occupancy model suggested a high probability of the species occurring in regions close to the coast, mainly in the south and southwest of the main island (
Figure 3).
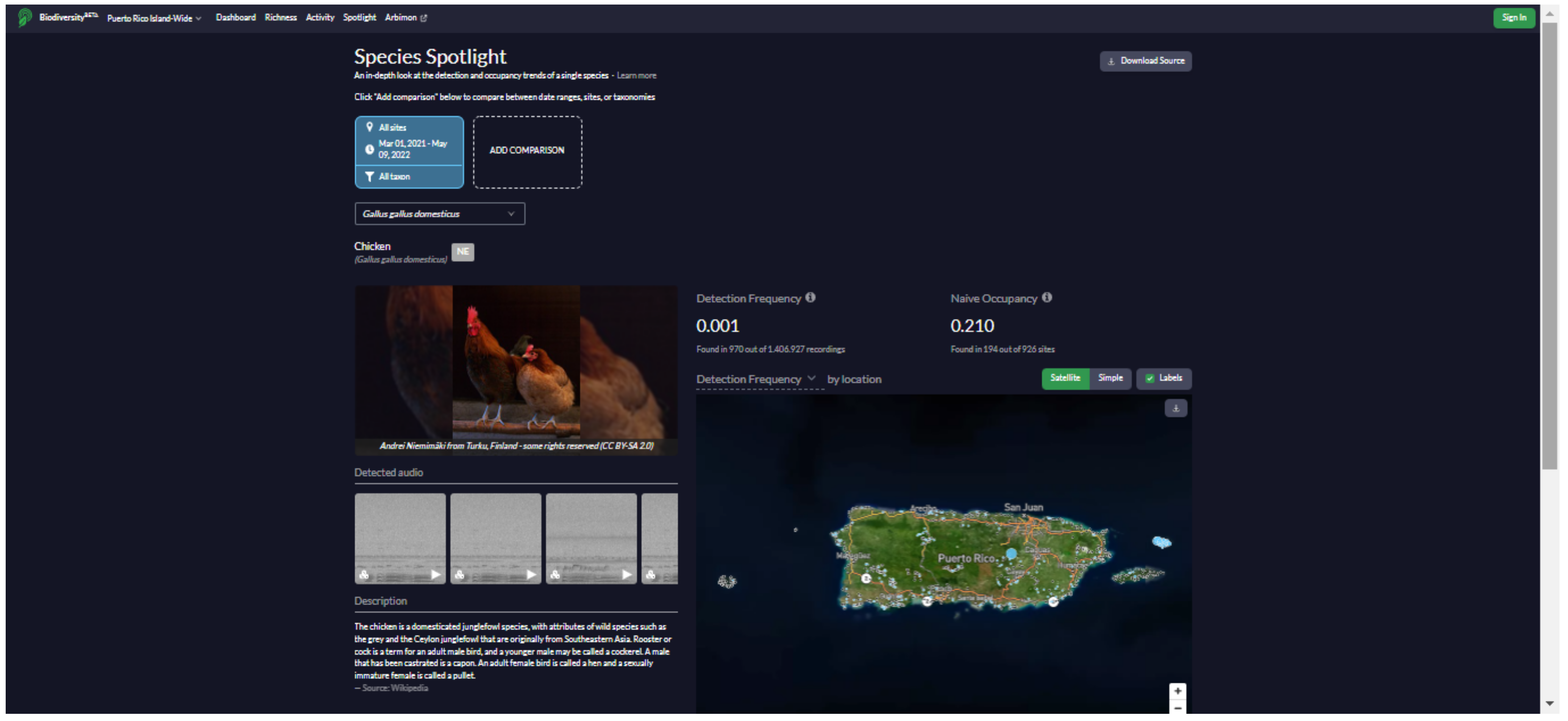
We developed a website (
https://bio.rfcx.org/puerto-rico-island-wide (accessed on 14 June 2022)) with the purpose of data sharing and summarizing the results of the study for the use of government agencies (e.g., Departamento de Recursos Naturales y Ambientales (DRNA), U.S. Fish and Wildlife Service) and the general public (e.g., educators and birdwatchers). The website contains information on the vocalizations, distribution, and ecology of the species detected at the 841 sampling sites in Puerto Rico during the project, including a searchable database with detection data, presence/absence maps, and occupancy maps for all birds, mammals, and anurans recorded (
Appendix G). All maps, plots, and data used to generate the figures can be freely downloaded. The data and figures on the website are continuously updated according to new acoustic data as they become available.
4. Discussion
The acquisition of population baseline data for native and invasive species is a fundamental step in monitoring and managing wildlife in dynamic land cover and climate change scenarios. However, detecting and monitoring animal species at large spatiotemporal scales, especially in the tropics, remains a significant challenge. In this study, we presented an end-to-end acoustic monitoring pipeline that was able to detect several soniferous native, endemic, threatened, and invasive species; access their current population status; and summarize and display results in a user-friendly platform.
Converging with our expectations, the occupancy probabilities of IAS were generally lower in environments with less human disturbance. For example, the occupancy probabilities of some IAS were negatively associated with elevation (five species) and forest older than 54 years (four species). These findings suggested that IAS may be favored by human activities because species occupancies were higher in new forest areas that were expected to be more disturbed and human activities are more common and intense at low elevations in Puerto Rico. Our findings were in agreement with an overall correlation between anthropogenically disturbed habitats and invasive species of plants, invertebrates, fishes, birds, frogs, and mammals [
45,
66,
67,
69]. Our results revealed that elevation and precipitation were the most important variables for explaining the distribution of most soniferous IAS (the best model for 6 of 11 IAS contained elevation and precipitation as explanatory variables), followed by proportion of forest cover aged between 32–54 years (fa2) and forest cover older than 54 years (fa3).
Free-range pets and domesticated species can also have a negative impact on native species, especially in island ecosystems [
68,
70]. Although the chicken, dog, wild goat, and feral horse are domesticated species, we decided to include them in the acoustic analysis because they can impact native wildlife directly (e.g., predation) and indirectly (e.g., spreading diseases and impacting on vegetation) [
71]; moreover, some feral populations exist in the archipelago. Some studies have shown that domesticated species (such as dogs) can occur widely within protected areas and may represent a threat to native species [
72,
73]. In contrast, our occupancy models suggested a lower probability of dogs and chickens occupying the protected areas on the main island of Puerto Rico (
Appendix E and
Appendix F), indicating that protected areas can offer some level of protection against domesticated species.
Our knowledge about the main drivers of IAS distribution is still limited, which was reflected in the model-selection process. The null model was the top-ranked model of four species, indicating that the explanatory variables utilized in the models were not good predictors of spatial variation of these species. The anuran IAS showed a low probability of occupancy (<0.07) in the landscape of the main island of Puerto Rico, which may have reflected the low natural availability of aquatic environments in the landscape (i.e., natural low occupancy probability of species) or may have been a result of our sampling design, which was not focused on lentic systems. The three frog IAS had call activity associated with ponds; a new sampling process could easily be designed to include more lentic systems, which would increase the chance of finding frogs with an aquatic life stage.
Estimating the probability of detection and occupancy of IAS can facilitate more efficient management actions because the estimations of parameters related to species occurrence on the landscape will be unbiased and provide an uncertainty measure [
74,
75]. In our study, most IAS showed a higher probability of detection at the end of the sampling period (late May and early June 2021), suggesting that most species were more vocal and thus more easily detected by PAM at the end of the early high rainfall season. These findings were congruent with breeding activity peaks recorded for terrestrial birds in Puerto Rico [
52]. This positive relationship was restricted to the temporal range of the study (March–June) because a year-round sampling design can have more variability and alter the relationship between the Julian day and detection probability.
Despite the large volume of data analyzed through PM, five birds were detected at only a few sites (<10), and we did not run occupancy models for them due to the small number of detections (see
Appendix H for detection locations). This low detection rate could correspond to a “real” low occurrence/density of the species in the study area or might have resulted from the species vocalizing sparingly. Even with a low number of detections, knowing where these species were found can present a valuable opportunity to prioritize surveying of locations closely similar in geographical and environmental space where the species were detected. Although working with few detection points (or even one detection point) is challenging and has several limitations, it can be useful in directing resources and field survey efforts to find undetected populations of a “rare” species in a region [
76] and monitor possible early-stage expansions.
The detection/nondetection of species is a baseline outcome of audio data analysis that can be used in various ecological analyses that make use of this type of data [
28,
36,
38]. For example, the diel and annual activities of species are fundamental aspects of their life history, and the knowledge of it can be used along with artificial advertisement calls to trap and manage IAS [
35,
77,
78]. We found that diel call activity of the species groups greatly varied, suggesting that monitoring and management programs should focus on specific periods of the day to increase their chance to detect and capture the IAS.
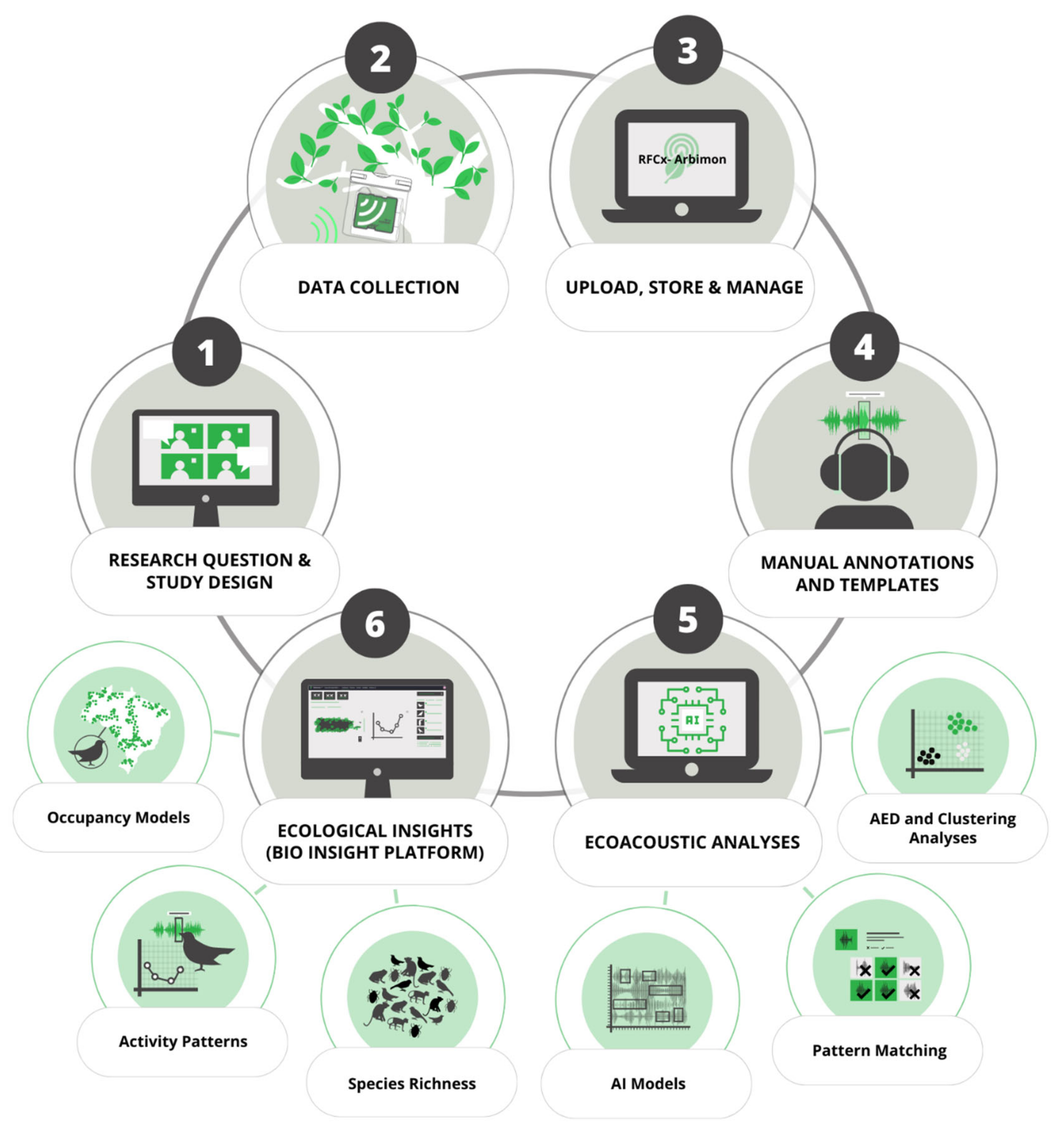
Our end-to-end pipeline provided an effective method for detecting several native, endemic, threatened, and IAS (16 species) in Puerto Rico, including birds, frogs, and mammals (95 species were detected overall). The manual validation of best ROI per site per day of PM analysis detected around 2000 call events of IAS. We took less than two days to validate the PM of the 16 IAS following our workflow. The fast analysis of this vast data set (1,773,287 1-min recordings) was only possible through a user-friendly web-based platform with annotation functionality and the creation of user-defined playlists that allowed us to combine manual annotations with PM analysis and validations by experts (see
Figure 1). While users can take advantage of a variety of available software platforms or develop their own code to analyze PAM data, multifunctional tools that have user-friendly interfaces are necessary to speed up and increase usage of PAM by a wider audience with varying skill sets [
79]. Free user-friendly interfaces may be an effective way to implement early-stage detection and assist in the long-term monitoring of IAS populations by conservationists, wildlife managers, and decision makers. Cloud-based platforms such as those used in this project also can facilitate the inclusion of citizen scientists and other experts to improve and speed up the validation of data from the target groups.
Detection and monitoring of IAS is of increasing importance for informing conservation management. Tools using an intuitive GUI can improve data exploration and narrow communication and knowledge gaps between the scientific community and other groups [
80]. Here, we introduced a webpage as part of the last step of the Arbimon ecosystem, filling a critical need for a practical and intuitive way to summarize, display, and share ecological results from acoustic-monitoring processes so that the data generated can be easily used and shared by and with environmental agencies. This web-based tool was designed to display biodiversity indicators such as number of detected species, activity patterns, and species occupancy over maps and plots that can support species management.
Previous studies have shown the benefit of combining acoustic monitoring with occupancy modeling to understand native species distribution [
38,
51,
81,
82,
83]; in accordance with these findings, our study reinforced that this approach can be useful to understand the distribution of soniferous IAS as well. Additionally, our approach generated detection/nondetection data from species of greatest conservation need beyond IAS that can be used in more complex models (e.g., two-species or multispecies models) to assess relationships and interactions between IAS and SGCN, which can help researchers to understand the potential effects of IAS on native species detection and occupancy probabilities [
84,
85,
86]. PAM has been shown to be very useful for investigating IAS, and there remain a number of avenues for expansion. Other studies have demonstrated the usefulness of PAM as a consistent tool to examine sounds in nature, involving a range of topics [
27] such as monitoring native wildlife species [
49,
82,
87], disturbance from human noise [
88,
89], agricultural pests [
90], ecosystem functions [
91], disease-transmitting mosquitoes [
92], gunshots [
93,
94], and illegal timber harvests [
95]. Although automated techniques are emerging to deal with the massive amount of acoustic data that are intrinsic to this monitoring tool, many of these methods still require manual examination of sounds for training and validating models [
47,
96,
97,
98]. Therefore, manually processing a large amount of recorded data continues to be one of the biggest challenges of PAM, and forthcoming studies may benefit from the pipeline we introduced. Furthermore, the usefulness of passive acoustic monitoring in combating invasive alien species should be boosted if used in actions organized and interconnected globally alongside other emerging tools such as environmental DNA, GIS analysis, camera traps, and citizen science [
29,
33,
99]. We also emphasize the need to develop a long-term real-time alert system using artificial intelligence models to keep a vigilant eye on the early detection of alien invasive species, which can be easily driven through passive acoustic monitoring.