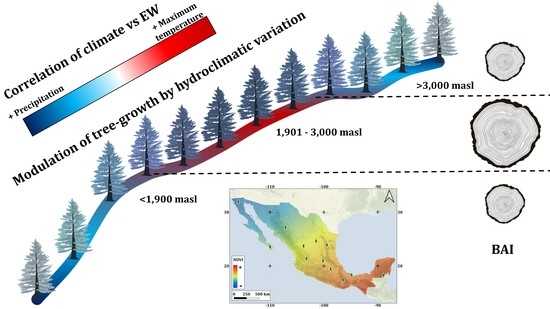
What Are Contemporary Mexican Conifers Telling Us? A Perspective Offered from Tree Rings Linked to Climate and the NDVI along a Spatial Gradient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site, Study Species and Sample Collection
2.2. Dendrochronological Processing
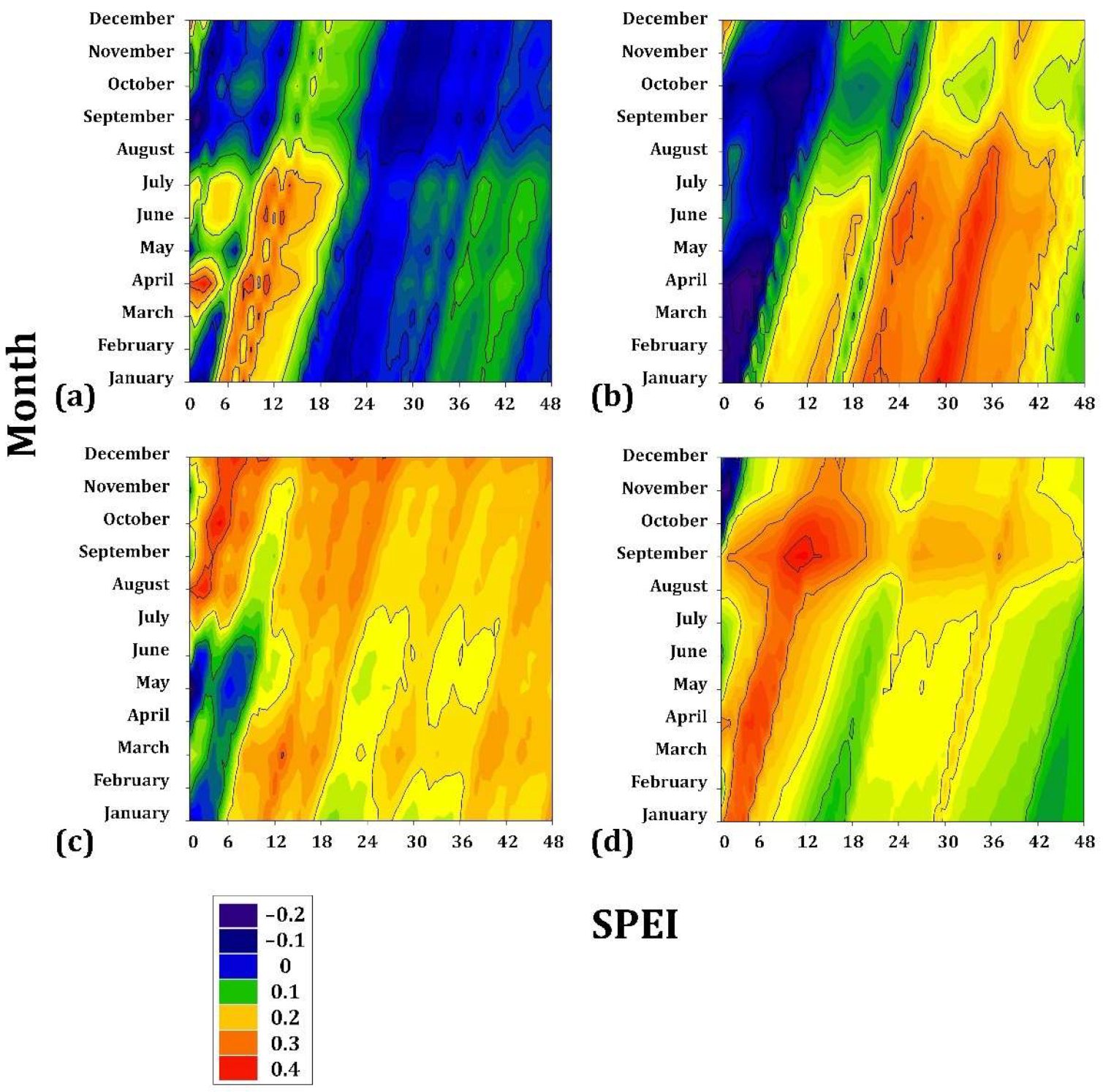
2.3. Climate-Drought-Growth Relationships
2.4. Effect of Elevation on Basal Area Increment
2.5. Effect of Latitude on NDVI
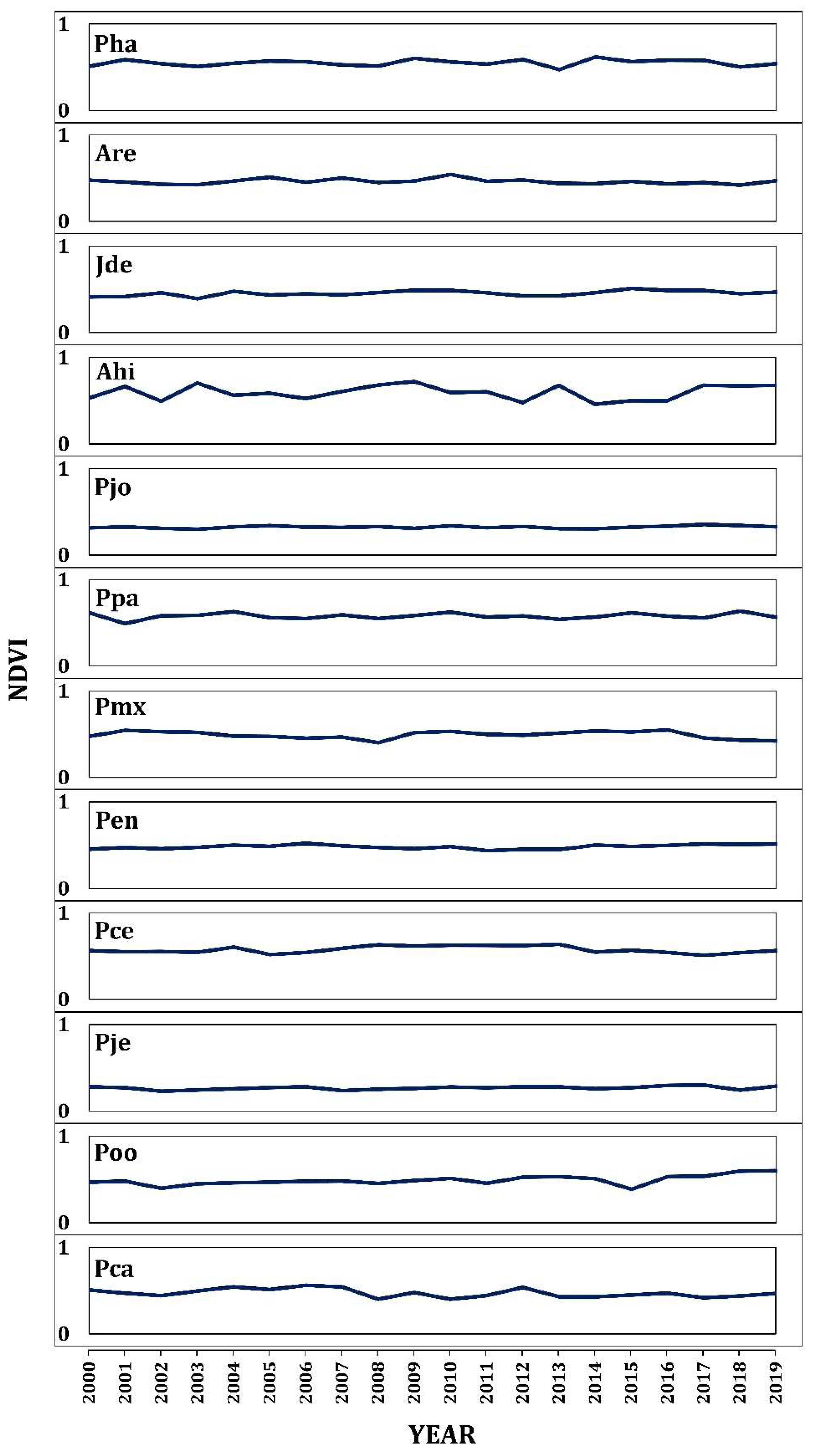
3. Results
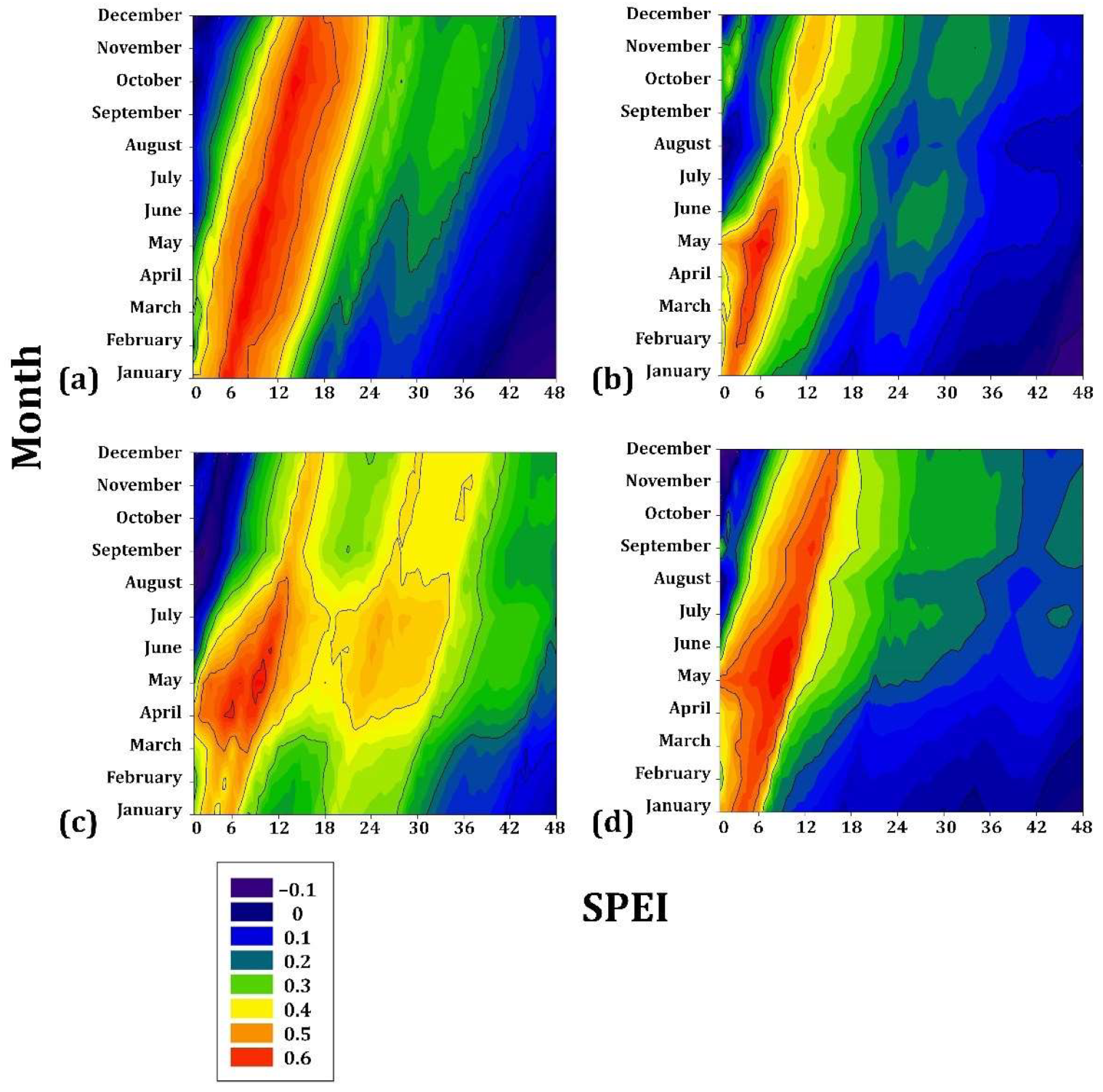
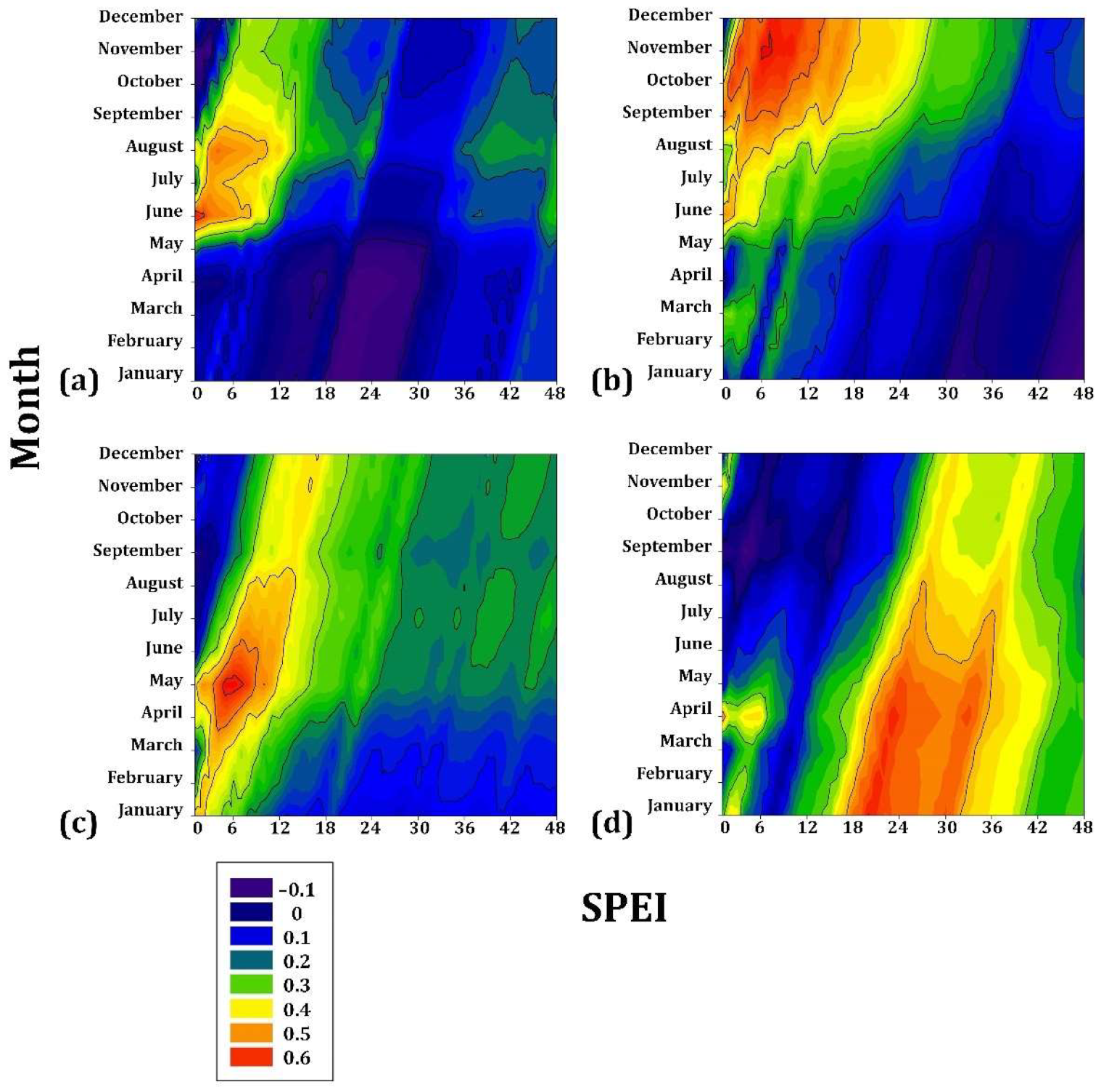
3.1. Climate–Drought–Growth Relationships
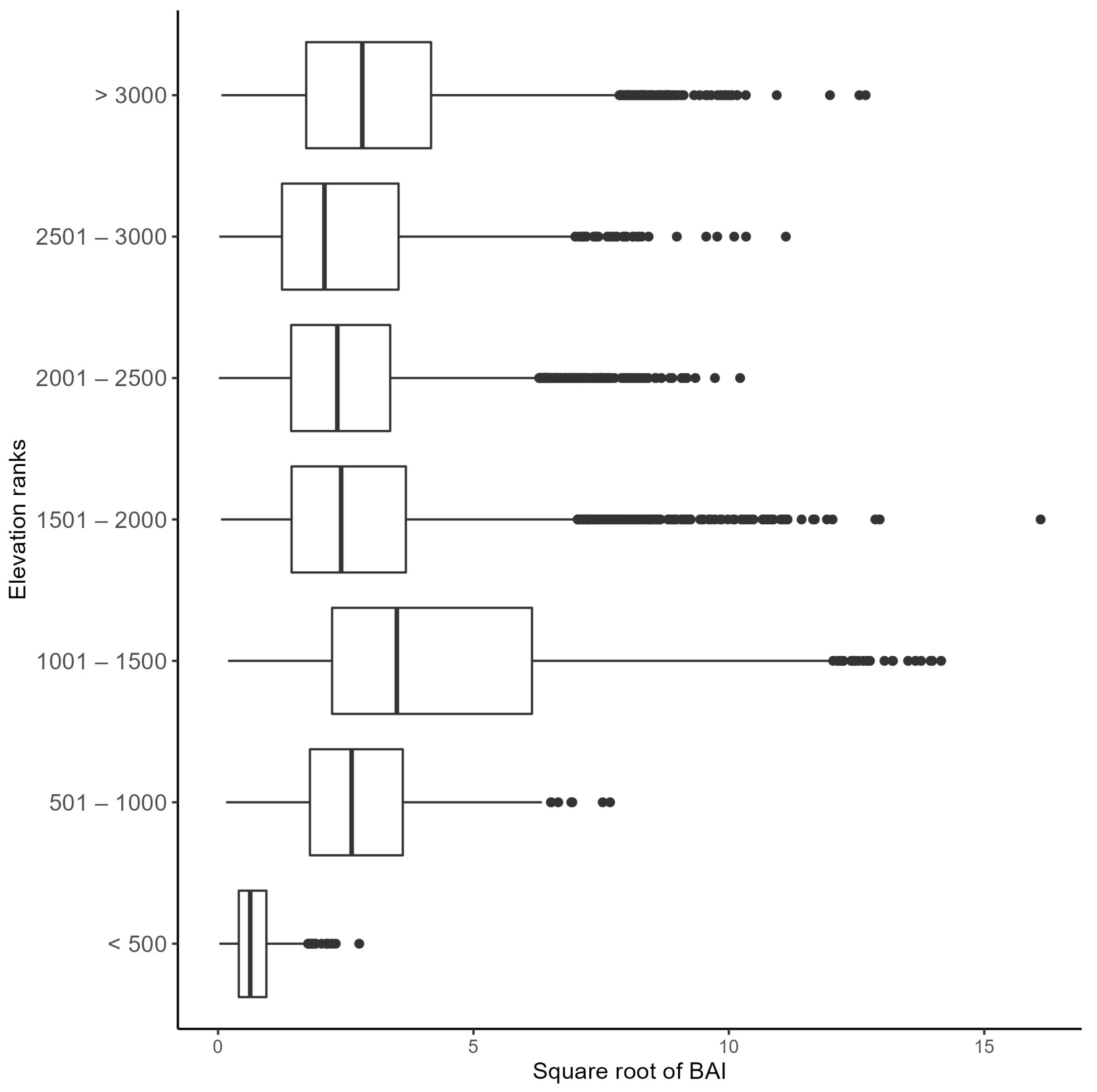
3.2. Effect of Elevation on Basal Area Increment
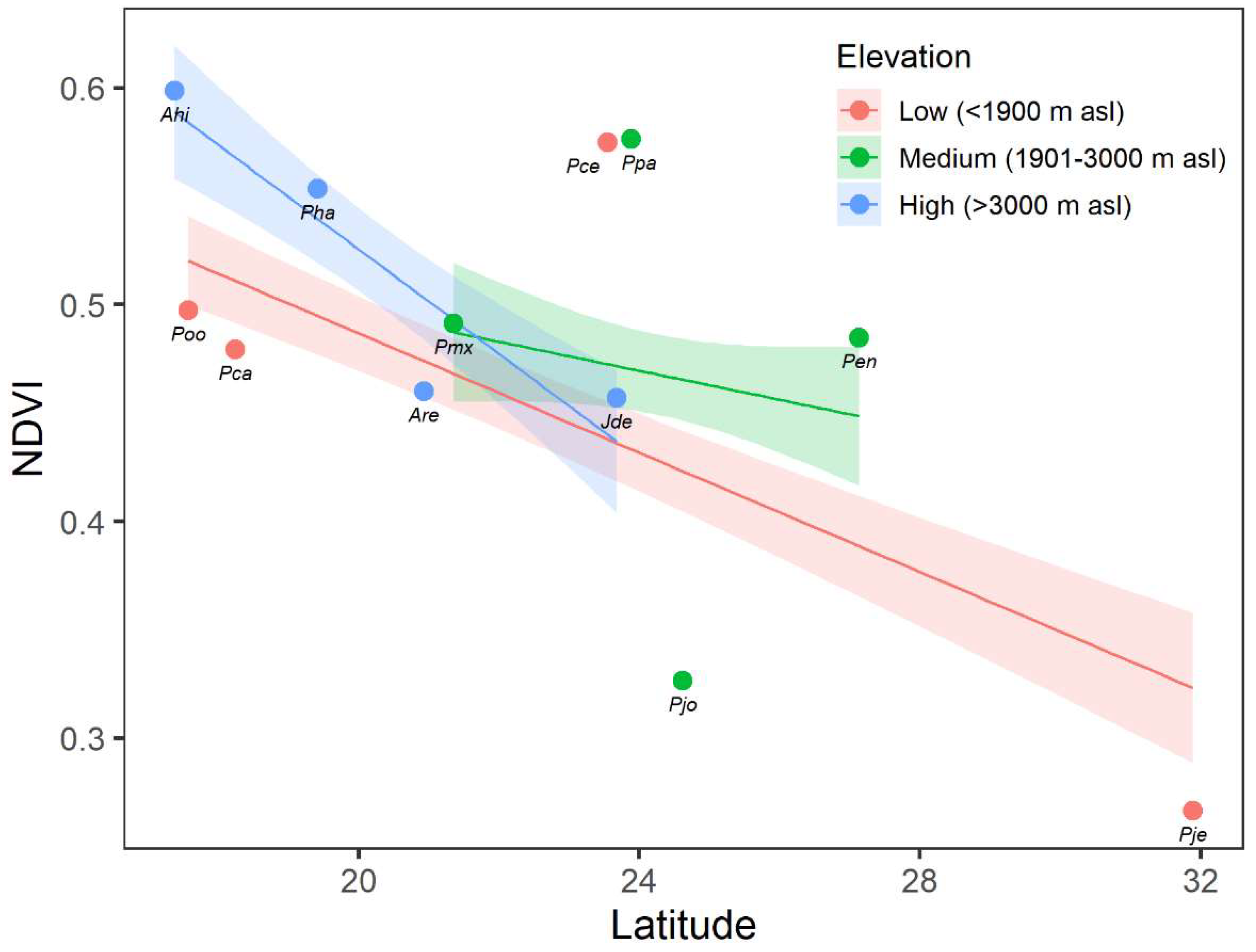
3.3. Effect of Latitude on NDVI
4. Discussion
4.1. Climate–Growth Relationships
4.2. Effect of Elevation and Latitude on Forest Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; González-Olabarria, J.R.; Lyver, P.O.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest Biodiversity, Ecosystem Functioning and the Provision of Ecosystem Services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Gauthier, S. Young and Old Forest in the Boreal: Critical Stages of Ecosystem Dynamics and Management under Global Change. For. Ecosyst. 2018, 5, 26. [Google Scholar] [CrossRef]
- Torres-Rojo, J.M.; Moreno-Sánchez, R.; Mendoza-Briseño, M.A. Sustainable Forest Management in Mexico. Curr. For. Rep. 2016, 2, 93–105. [Google Scholar] [CrossRef]
- Grantham, H.S.; Duncan, A.; Evans, T.D.; Jones, K.R.; Beyer, H.L.; Schuster, R.; Walston, J.; Ray, J.C.; Robinson, J.G.; Callow, M.; et al. Anthropogenic Modification of Forests Means Only 40% of Remaining Forests Have High Ecosystem Integrity. Nat. Commun. 2020, 11, 5978. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Angelstam, P.; Frelich, L.; Jõgiste, K.; Koivula, M.; Kubota, Y.; Lafleur, B.; Macdonald, E. Natural Disturbance-Based Forest Management: Moving beyond Retention and Continuous-Cover Forestry. Front. For. Glob. Change 2021, 4, 629020. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Báez, S.; Malizia, A.; Carilla, J.; Blundo, C.; Aguilar, M.; Aguirre, N.; Aquirre, Z.; Álvarez, E.; Cuesta, F.; Duque, Á.; et al. Large-Scale Patterns of Turnover and Basal Area Change in Andean Forests. PLoS ONE 2015, 10, e0126594. [Google Scholar] [CrossRef]
- Aquilué, N.; Filotas, É.; Craven, D.; Fortin, M.-J.; Brotons, L.; Messier, C. Evaluating Forest Resilience to Global Threats Using Functional Response Traits and Network Properties. Ecol. Appl. 2020, 30, e02095. [Google Scholar] [CrossRef]
- Mina, M.; Messier, C.; Duveneck, M.; Fortin, M.-J.; Aquilué, N. Network Analysis Can Guide Resilience-Based Management in Forest Landscapes under Global Change. Ecol. Appl. 2021, 31, e2221. [Google Scholar] [CrossRef]
- Babst, F.; Alexander, R.; Szejner, P.; Bouriaud, O.; Klesse, S.; Roden, J.; Ciais, P.; Poulter, B.; Frank, D.; Moore, D.J.P. A Tree-Ring Perspective on the Terrestrial Carbon Cycle. Oecologia 2014, 176, 307–322. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Wang, X.; Liang, P.; An, H.; Sun, H.; Han, W.; Li, Q. Tree-Ring Widths Are Good Proxies of Annual Variation in Forest Productivity in Temperate Forests. Sci. Rep. 2017, 7, 1945. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, F.; Wang, H.; Qiu, B.; Wu, M.; He, W.; Ju, W.; Zhang, Y.; Chen, J.M.; Zhou, Y. Constraining Global Terrestrial Gross Primary Productivity in a Global Carbon Assimilation System with OCO-2 Chlorophyll Fluorescence Data. Agric. For. Meteorol. 2021, 304, 108424. [Google Scholar] [CrossRef]
- Ruiz-Benito, P.; Madrigal-González, J.; Ratcliffe, S.; Coomes, D.A.; Kändler, G.; Lehtonen, A.; Wirth, C.; Zavala, M.A. Stand Structure and Recent Climate Change Constrain Stand Basal Area Change in European Forests: A Comparison Across Boreal, Temperate, and Mediterranean Biomes. Ecosystems 2014, 17, 1439–1454. [Google Scholar] [CrossRef]
- Ryan, M.G.; Binkley, D.; Fownes, J.H. Age-Related Decline in Forest Productivity: Pattern and Process. Adv. Ecol. Res. 1997, 27, 213–262. [Google Scholar]
- He, L.; Chen, J.M.; Pan, Y.; Birdsey, R.; Kattge, J. Relationships between Net Primary Productivity and Forest Stand Age in US Forests. Glob. Biogeochem. Cycles 2012, 26, 1–19. [Google Scholar] [CrossRef]
- Pompa-García, M.; Hadad, M.A. Sensitivity of Pines in Mexico to Temperature Varies with Age. Atmósfera 2016, 29, 209–219. [Google Scholar] [CrossRef]
- Metsaranta, J.M.; Bhatti, J.S. Evaluation of Whole Tree Growth Increment Derived from Tree-Ring Series for Use in Assessments of Changes in Forest Productivity across Various Spatial Scales. Forests 2016, 7, 303. [Google Scholar] [CrossRef]
- Teets, A.; Fraver, S.; Hollinger, D.Y.; Weiskittel, A.R.; Seymour, R.S.; Richardson, A.D. Linking Annual Tree Growth with Eddy-Flux Measures of Net Ecosystem Productivity across Twenty Years of Observation in a Mixed Conifer Forest. Agric. For. Meteorol. 2018, 249, 479–487. [Google Scholar] [CrossRef]
- Xu, K.; Wang, X.; Liang, P.; Wu, Y.; An, H.; Sun, H.; Wu, P.; Wu, X.; Li, Q.; Guo, X.; et al. A New Tree-Ring Sampling Method to Estimate Forest Productivity and Its Temporal Variation Accurately in Natural Forests. For. Ecol. Manag. 2019, 433, 217–227. [Google Scholar] [CrossRef]
- Mérian, P.; Lebourgeois, F. Size-Mediated Climate–Growth Relationships in Temperate Forests: A Multi-Species Analysis. For. Ecol. Manag. 2011, 261, 1382–1391. [Google Scholar] [CrossRef]
- Trouillier, M.; van der Maaten-Theunissen, M.; Scharnweber, T.; Würth, D.; Burger, A.; Schnittler, M.; Wilmking, M. Size Matters—A Comparison of Three Methods to Assess Age- and Size-Dependent Climate Sensitivity of Trees. Trees 2019, 33, 183–192. [Google Scholar] [CrossRef]
- Lockwood, B.R.; Maxwell, J.T.; Robeson, S.M.; Au, T.F. Assessing Bias in Diameter at Breast Height Estimated from Tree Rings and Its Effects on Basal Area Increment and Biomass. Dendrochronologia 2021, 67, 125844. [Google Scholar] [CrossRef]
- Babst, F.; Esper, J.; Parlow, E. Landsat TM/ETM+ and Tree-Ring Based Assessment of Spatiotemporal Patterns of the Autumnal Moth (Epirrita Autumnata) in Northernmost Fennoscandia. Remote Sens. Environ. 2010, 114, 637–646. [Google Scholar] [CrossRef]
- Erasmi, S.; Klinge, M.; Dulamsuren, C.; Schneider, F.; Hauck, M. Modelling the Productivity of Siberian Larch Forests from Landsat NDVI Time Series in Fragmented Forest Stands of the Mongolian Forest-Steppe. Environ. Monit. Assess. 2021, 193, 200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lyu, L.; Liu, W.; Liang, H.; Huang, J.; Zhang, Q.-B. Topographic Patterns of Forest Decline as Detected from Tree Rings and NDVI. CATENA 2021, 198, 105011. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.; Yang, X.; Fan, W. Spatiotemporal Variations of Forest Vegetation Phenology and Its Response to Climate Change in Northeast China. Remote Sens. 2022, 14, 2909. [Google Scholar] [CrossRef]
- Brehaut, L.; Danby, R.K. Inconsistent Relationships between Annual Tree Ring-Widths and Satellite-Measured NDVI in a Mountainous Subarctic Environment. Ecol. Indic. 2018, 91, 698–711. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-Scale Geospatial Analysis for Everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Roces-Díaz, J.V.; Vayreda, J.; De Cáceres, M.; García-Valdés, R.; Banqué-Casanovas, M.; Morán-Ordóñez, A.; Brotons, L.; de-Miguel, S.; Martínez-Vilalta, J. Temporal Changes in Mediterranean Forest Ecosystem Services Are Driven by Stand Development, Rather than by Climate-Related Disturbances. For. Ecol. Manag. 2021, 480, 118623. [Google Scholar] [CrossRef]
- Correa-Díaz, A.; Silva, L.C.R.; Horwath, W.R.; Gómez-Guerrero, A.; Vargas-Hernández, J.; Villanueva-Díaz, J.; Velázquez-Martínez, A.; Suárez-Espinoza, J. Linking Remote Sensing and Dendrochronology to Quantify Climate-Induced Shifts in High-Elevation Forests Over Space and Time. J. Geophys. Res. Biogeosci. 2019, 124, 166–183. [Google Scholar] [CrossRef]
- Vivar-Vivar, E.D.; Pompa-García, M.; Rodríguez-Trejo, D.A.; Leyva-Ovalle, A.; Wehenkel, C.; Carrillo-Parra, A.; Moreno-Anguiano, O. Drought responsiveness in two Mexican conifer species forming young stands at high elevations. For. Syst. 2021, 30, e012. [Google Scholar] [CrossRef]
- Acosta-Hernández, A.C.; Pompa-García, M.; Camarero, J.J. An Updated Review of Dendrochronological Investigations in Mexico, a Megadiverse Country with a High Potential for Tree-Ring Sciences. Forests 2017, 8, 160. [Google Scholar] [CrossRef]
- Villanueva Díaz, J.; Cerano Paredes, J.; Fulé, P.Z.; Cortés Montaño, C.; Vázquez Selem, L.; Yocom, L.L.; Ruiz-Corral, J.A. Cuatro Siglos de Variabilidad Hidroclimática En El Noroeste de Chihuahua, México, Reconstruida Con Anillos de Árboles. Investig. Geogr. 2015, 1, 141–153. [Google Scholar] [CrossRef]
- Herrera-Soto, G.; González-Cásares, M.; Pompa-García, M.; Camarero, J.J.; Solís-Moreno, R. Growth of Pinus Cembroides Zucc. in Response to Hydroclimatic Variability in Four Sites Forming the Species Latitudinal and Longitudinal Distribution Limits. Forests 2018, 9, 440. [Google Scholar] [CrossRef]
- Gernandt, D.S.; Pérez-de la Rosa, J.A. Biodiversidad de Pinophyta (Coníferas) En México. Rev. Mex. Biodivers. 2014, 85, 126–133. [Google Scholar] [CrossRef]
- Malhi, Y.; Silman, M.; Salinas, N.; Bush, M.; Meir, P.; Saatchi, S. Introduction: Elevation gradients in the tropics: Laboratories for ecosystem ecology and global change research. Glob. Change Biol. 2010, 16, 3171–3175. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Taylor, P.; Alvarez-Clare, S.; Bustamante, M.M.; Chuyong, G.; Dobrowski, S.Z.; Grierson, P.; Harms, K.E.; Houlton, B.Z.; et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: A pan-tropical analysis. Ecol. Lett. 2011, 14, 939–947. [Google Scholar] [CrossRef]
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers: An Analysis of Their Distribution, Biogeography, Diversity and Conservation Status; Brill: Leiden, The Netherlands, 2013; ISBN 978-90-04-21181-0. [Google Scholar]
- Holmes, R.L. Computer assisted quality control. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Bunn, A.G. A Dendrochronology Program Library in R (DplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the Average Value of Correlated Time-Series, with Applications in Dendroclimatology and Hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Trouet, V.; Oldenborgh, G.J. Van KNMI Climate Explorer: A Web-Based Research Tool for High-Resolution Paleoclimatology. Tree Ring Res. 2013, 69, 3–13. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS Monthly High-Resolution Gridded Multivariate Climate Dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beguería, S.; Vicente-Serrano, S.M.; Reig, F.; Latorre, B. Standardized Precipitation Evapotranspiration Index (SPEI) Revisited: Parameter Fitting, Evapotranspiration Models, Tools, Datasets and Drought Monitoring. Int. J. Climatol. 2014, 34, 3001–3023. [Google Scholar] [CrossRef]
- Pompa-García, M.; Sánchez-Salguero, R.; Camarero, J.J. Observed and projected impacts of climate on radial growth of three endangered conifers in northern Mexico indicate high vulnerability of drought-sensitive species from mesic habitats. Dendrochronologia 2017, 45, 145–155. [Google Scholar] [CrossRef]
- Dinno, A.; Dinno, M.A. Package ‘dunn. test’. CRAN Repos 2017, 10, 1–7. [Google Scholar]
- Pohlert, T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended 2021. Available online: https://www.quantargo.com/help/r/latest/packages/PMCMRplus/1.9.0 (accessed on 16 December 2021).
- Sader, S.A.; Winne, J.C. RGB-NDVI colour composites for visualizing forest change dynamics. Int. J. Remote Sens. 1992, 13, 3055–3067. [Google Scholar] [CrossRef]
- Allan, R.P.; Hawkins, E.; Bellouin, N.; Collins, B. IPCC, 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 3–32. [Google Scholar]
- González-Cásares, M.; Pompa-García, M.; Camarero, J.J. Differences in Climate–Growth Relationship Indicate Diverse Drought Tolerances among Five Pine Species Coexisting in Northwestern Mexico. Trees 2017, 31, 531–544. [Google Scholar] [CrossRef]
- Pompa-García, M.; González-Cásares, M.; Acosta-Hernández, A.C.; Camarero, J.J.; Rodríguez-Catón, M. Drought Influence over Radial Growth of Mexican Conifers Inhabiting Mesic and Xeric Sites. Forests 2017, 8, 175. [Google Scholar] [CrossRef]
- Tumajer, J.; Scharnweber, T.; Smiljanic, M.; Wilmking, M. Limitation by Vapour Pressure Deficit Shapes Different Intra-Annual Growth Patterns of Diffuse- and Ring-Porous Temperate Broadleaves. New Phytol. 2022, 233, 2429–2441. [Google Scholar] [CrossRef]
- Pompa-García, M.; González-Cásares, M.; Gazol, A.; Camarero, J.J. Run to the Hills: Forest Growth Responsiveness to Drought Increased at Higher Elevation during the Late 20th Century. Sci. Total Environ. 2021, 772, 145286. [Google Scholar] [CrossRef]
- Hughes, M.K.; Kelly, M.P.; Pilcher, J.R.; Lamarche, V.C. Climate from Tree Rings. In Climate from Tree Rings; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Adams, H.D.; Kolb, T.E. Tree Growth Response to Drought and Temperature in a Mountain Landscape in Northern Arizona, USA. J. Biogeogr. 2005, 32, 1629–1640. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Davi, N.; Jacoby, G.; Wilson, R.; Wiles, G. Dendroclimatic Studies: Tree Growth and Climate Change in Northern Forests; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 1118848713. [Google Scholar]
- Murray-Tortarolo, G.N. Seven Decades of Climate Change across Mexico. Atmósfera 2021, 34, 217–226. [Google Scholar] [CrossRef]
- Huang, J.; Tarif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial Growth Response of Four Dominant Boreal Tree Species to Climate along a Latitudinal Gradient in the Eastern Canadian Boreal Forest. Glob. Change Biol. 2010, 16, 711–731. [Google Scholar] [CrossRef]
- Commission for Environmental Cooperation. Ecological Regions of North America. Toward a Common Perspective; Commission for Environmental Cooperation: Montreal, QC, Canada, 1997. [Google Scholar]
- Urrutia-Jalabert, R.; Barichivich, J.; Rozas, V.; Lara, A.; Rojas, Y.; Bahamondez, C.; Rojas-Badilla, M.; Gipoulou-Zuñiga, T.; Cuq, E. Climate Response and Drought Resilience of Nothofagus Obliqua Secondary Forests across a Latitudinal Gradient in South-Central Chile. For. Ecol. Manag. 2021, 485, 118962. [Google Scholar] [CrossRef]
- Núñez-García, A.; Gómez-Guerrero, A.; Terrazas-Salgado, T.M.; Vargas-Hernández, J.J.; Villanueva-Díaz, J. Analysis of Basal Area Increment of Pinus hartwegii Lindl. at Different Elevations and Aspects on Jocotitlan Mountain, State of Mexico. Rev. Chapingo Ser. Ciencias For. Y Del Ambient 2020, 27, 77–88. [Google Scholar] [CrossRef]
- Coomes, D.A.; Allen, R.B. Effects of Size, Competition and Altitude on Tree Growth. J. Ecol. 2007, 95, 1084–1097. [Google Scholar] [CrossRef]
- Malizia, A.; Blundo, C.; Carilla, J.; Osinaga Acosta, O.; Cuesta, F.; Duque, A.; Aguirre, N.; Aguirre, Z.; Ataroff, M.; Baez, S.; et al. Elevation and Latitude Drives Structure and Tree Species Composition in Andean Forests: Results from a Large-Scale Plot Network. PLoS ONE 2020, 15, e0231553. [Google Scholar] [CrossRef]
- González-Cásares, M.; Camarero, J.J.; Colangelo, M.; Rita, A.; Pompa-García, M. High Responsiveness of Wood Anatomy to Water Availability and Drought near the Equatorial Rear Edge of Douglas-Fir. Can. J. For. Res. 2019, 49, 1114–1123. [Google Scholar] [CrossRef]
- Coulthard, B.L.; Touchan, R.; Anchukaitis, K.J.; Meko, D.M.; Sivrikaya, F. Tree Growth and Vegetation Activity at the Ecosystem-Scale in the Eastern Mediterranean. Environ. Res. Lett. 2017, 12, 084008. [Google Scholar] [CrossRef]
- Llyod, A.H.; Bunn, A.G.; Berner, L. A Latitudinal Gradient in Tree Growth Response to Climate Warming in the Siberian Taiga. Glob. Change Biol. 2011, 17, 1935–1945. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Andreu-Hayles, L.; D’Arrigo, R.; Anchukaitis, K.J.; Tucker, C.J.; Pinzón, J.E.; Goetz, S.J. A Large-Scale Coherent Signal of Canopy Status in Maximum Latewood Density of Tree Rings at Arctic Treeline in North America. Glob. Planet. Change 2013, 100, 109–118. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Camarero, J.J.; Olano, J.M.; Martín-Hernández, N.; Peña-Gallardo, M.; Tomás-Burguera, M.; Gazol, A.; Azorin-Molina, C.; Bhuyan, U.; El Kenawy, A. Diverse Relationships between Forest Growth and the Normalized Difference Vegetation Index at a Global Scale. Remote Sens. Environ. 2016, 187, 14–29. [Google Scholar] [CrossRef]
- Dash, J.P.; Pearse, G.D.; Watt, M.S. UAV Multispectral Imagery Can Complement Satellite Data for Monitoring Forest Health. Remote Sens. 2018, 10, 1216. [Google Scholar] [CrossRef] [Green Version]
- Che, J.; Jönsson, P.; Tamura, M.; Gu, Z.; Matsushita, B.; Eklundh, L. A simple method for reconstructing a high-quality NDVI time-series data set based on the Savitzky-Golay filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Chen, J.M.; Deng, F.; Chen, M. Locally adjusted cubic-spline capping for reconstructing seasonal trajectories of a satellite-derived surface parameter. IEEE Trans. Geosci. Remote Sens. 2006, 44, 2230–2238. [Google Scholar] [CrossRef]
- Chu, D.; Shen, H.; Guan, X.; Chen, J.M.; Li, X.; Li, J.; Zhang, L. Long time-series NDVI reconstruction in cloud-prone regions via spatio-temporal tensor completion. Remote Sens. Environ. 2021, 264, 112632. [Google Scholar] [CrossRef]
- Vivar-Vivar, E.D.; Pompa-García, M.; Martínez-Rivas, J.A.; Mora-Tembre, L.A. UAV-Based Characterization of Tree-Attributes and Multispectral Indices in an Uneven-Aged Mixed Conifer-Broadleaf Forest. Remote Sens. 2022, 14, 2775. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate, 1st ed.; Academic Press Inc.: London, UK, 1976. [Google Scholar]
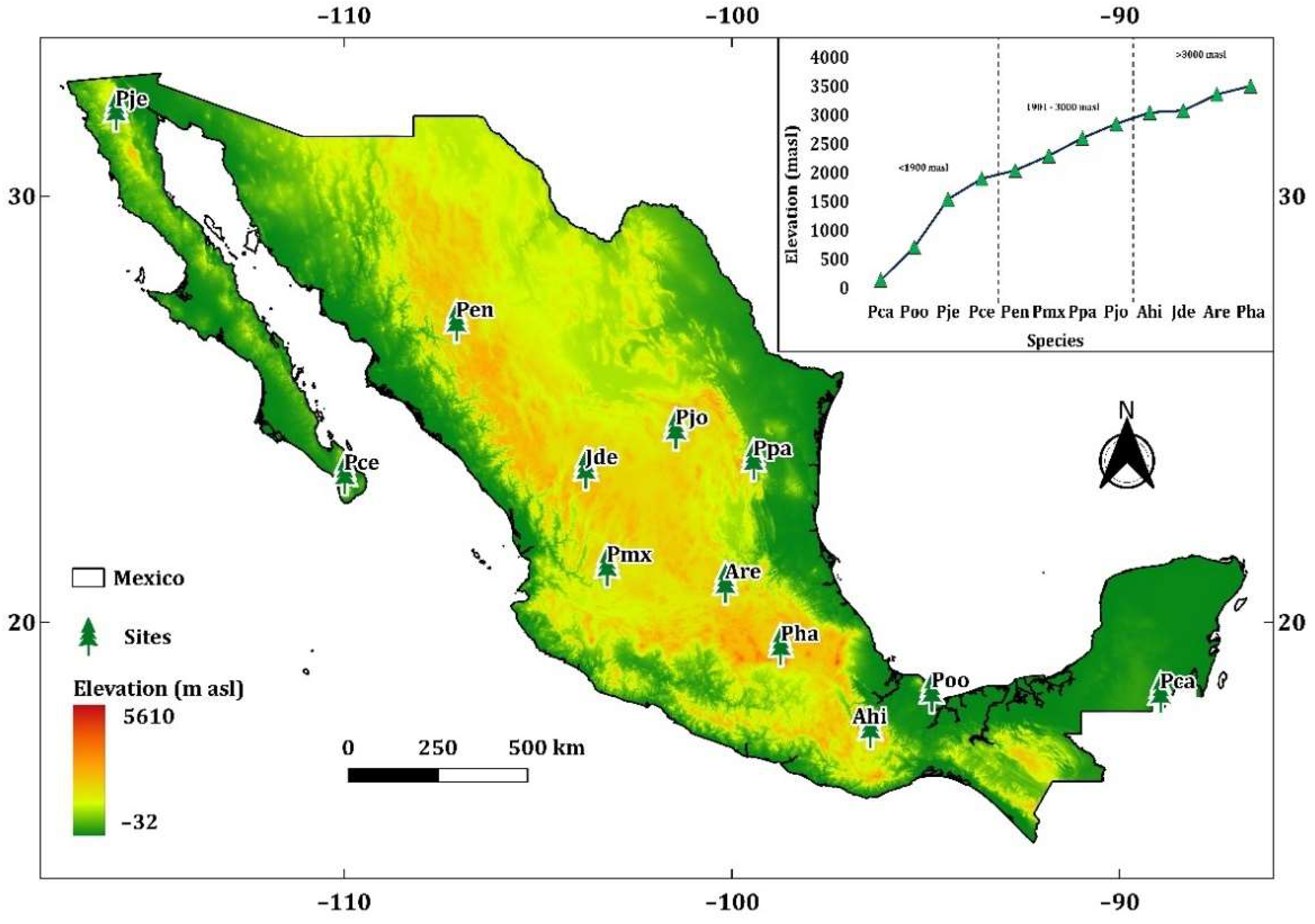


| Tree Species (Code) | Elevation (m asl) | Ecoregion | Well-Replicated Period | TRW (mm) | EW (mm) | Average Series Length | AC | MS | Rbt | Mean Annual Temperature (°C) | Total Annual Precipitation (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pca | 138 | Tropical Wet Forests | 1982–2020 | 0.57 ± 0.05 | 0.31 ± 0.03 | 54 ± 5 | 0.31 ± 0.03 | 0.43 ± 0.01 | 0.4 | 25.9 | 1168.50 |
| Poo | 713 | Tropical Wet Forests | 1986–2020 | 3.16 ± 0.20 | 2.93 ± 0.33 | 28 ± 0.5 | 0.47 ± 0.04 | 0.40 ± 0.01 | 0.26 | 23.7 | 2100.00 |
| Pje | 1540 | Mediterranean California | 1975–2019 | 2.73 ± 0.16 | 2.49 ± 0.25 | 56 ± 3 | 0.51 ± 0.02 | 0.49 ± 0.01 | 0.56 | 17.3 | 274.2 |
| Pce | 1897 | Tropical Dry Forests | 1970–2019 | 1.28 ± 0.07 | 1.16 ± 0.08 | 66 ± 5 | 0.36 ± 0.03 | 0.43 ± 0.01 | 0.32 | 22 | 420.9 |
| Pen | 2041 | Temperate Sierras | 1951–2019 | 2.13 ± 0.08 | 1.76 ± 0.13 | 60 ± 2 | 0.39 ± 0.03 | 0.45 ± 0.01 | 0.66 | 24.2 | 743.7 |
| Pmx | 2296 | Temperate Sierras | 1976–2020 | 2.41 ± 0.14 | 2.28 ± 0.19 | 53 ± 4 | 0.34 ± 0.04 | 0.48 ± 0.02 | 0.5 | 20.6 | 721.6 |
| Ppa | 2602 | Temperate Sierras | 1952–2020 | 2.46 ± 0.09 | 2.11 ± 0.16 | 67 ± 2 | 0.68 ± 0.02 | 0.36 ± 0.01 | 0.49 | 16.6 | 875.1 |
| Pjo | 2847 | North American Deserts | 1976–2020 | 0.97 ± 0.06 | 0.87 ± 0.08 | 59 ± 3 | 0.70 ± 0.03 | 0.33 ± 0.01 | 0.38 | 16.8 | 434.7 |
| Ahi | 3044 | Temperate Sierras | 1956–2020 | 2.84 ± 0.14 | 2.67 ± 0.19 | 67 ± 2 | 0.66 ± 0.04 | 0.34 ± 0.01 | 0.39 | 17.2 | 988.1 |
| Jde | 3078 | Southern Semi-arid Highlands | 1986–2020 | 2.16 ± 0.19 | 2.05 ± 0.26 | 35 ± 2 | 0.39 ± 0.04 | 0.59 ± 0.02 | 0.54 | 15.1 | 459.1 |
| Are | 3358 | Southern Semi-arid Highlands | 1960–2019 | 1.63 ± 0.13 | 1.44 ± 014 | 57 ± 5 | 0.35 ± 0.04 | 0.46 ± 0.01 | 0.46 | 15.4 | 616.5 |
| Pha | 3504 | Temperate Sierras | 1975–2019 | 1.64 ± 0.11 | 1.39 ± 0.09 | 65 ± 5 | 0.53 ± 0.04 | 0.35 ± 0.02 | 0.26 | 16.1 | 648.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompa-García, M.; Vivar-Vivar, E.D.; Sigala-Rodríguez, J.A.; Padilla-Martínez, J.R. What Are Contemporary Mexican Conifers Telling Us? A Perspective Offered from Tree Rings Linked to Climate and the NDVI along a Spatial Gradient. Remote Sens. 2022, 14, 4506. https://doi.org/10.3390/rs14184506
Pompa-García M, Vivar-Vivar ED, Sigala-Rodríguez JA, Padilla-Martínez JR. What Are Contemporary Mexican Conifers Telling Us? A Perspective Offered from Tree Rings Linked to Climate and the NDVI along a Spatial Gradient. Remote Sensing. 2022; 14(18):4506. https://doi.org/10.3390/rs14184506
Chicago/Turabian StylePompa-García, Marín, Eduardo D. Vivar-Vivar, José A. Sigala-Rodríguez, and Jaime R. Padilla-Martínez. 2022. "What Are Contemporary Mexican Conifers Telling Us? A Perspective Offered from Tree Rings Linked to Climate and the NDVI along a Spatial Gradient" Remote Sensing 14, no. 18: 4506. https://doi.org/10.3390/rs14184506
APA StylePompa-García, M., Vivar-Vivar, E. D., Sigala-Rodríguez, J. A., & Padilla-Martínez, J. R. (2022). What Are Contemporary Mexican Conifers Telling Us? A Perspective Offered from Tree Rings Linked to Climate and the NDVI along a Spatial Gradient. Remote Sensing, 14(18), 4506. https://doi.org/10.3390/rs14184506