One Shell of a Problem: Cumulative Threat Analysis of Male Sea Turtles Indicates High Anthropogenic Threat for Migratory Individuals and Gulf of Mexico Residents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area/Species Collection
2.2. Collection and Calculation of Threats/State-Space Modelling
2.3. Fishing Data
2.4. Shipping Data
2.5. Drilling Platform Data
2.6. Light Pollution Data
2.7. MPA, EEZ, and Proximity to Coast Data
2.8. Statistical Analyses
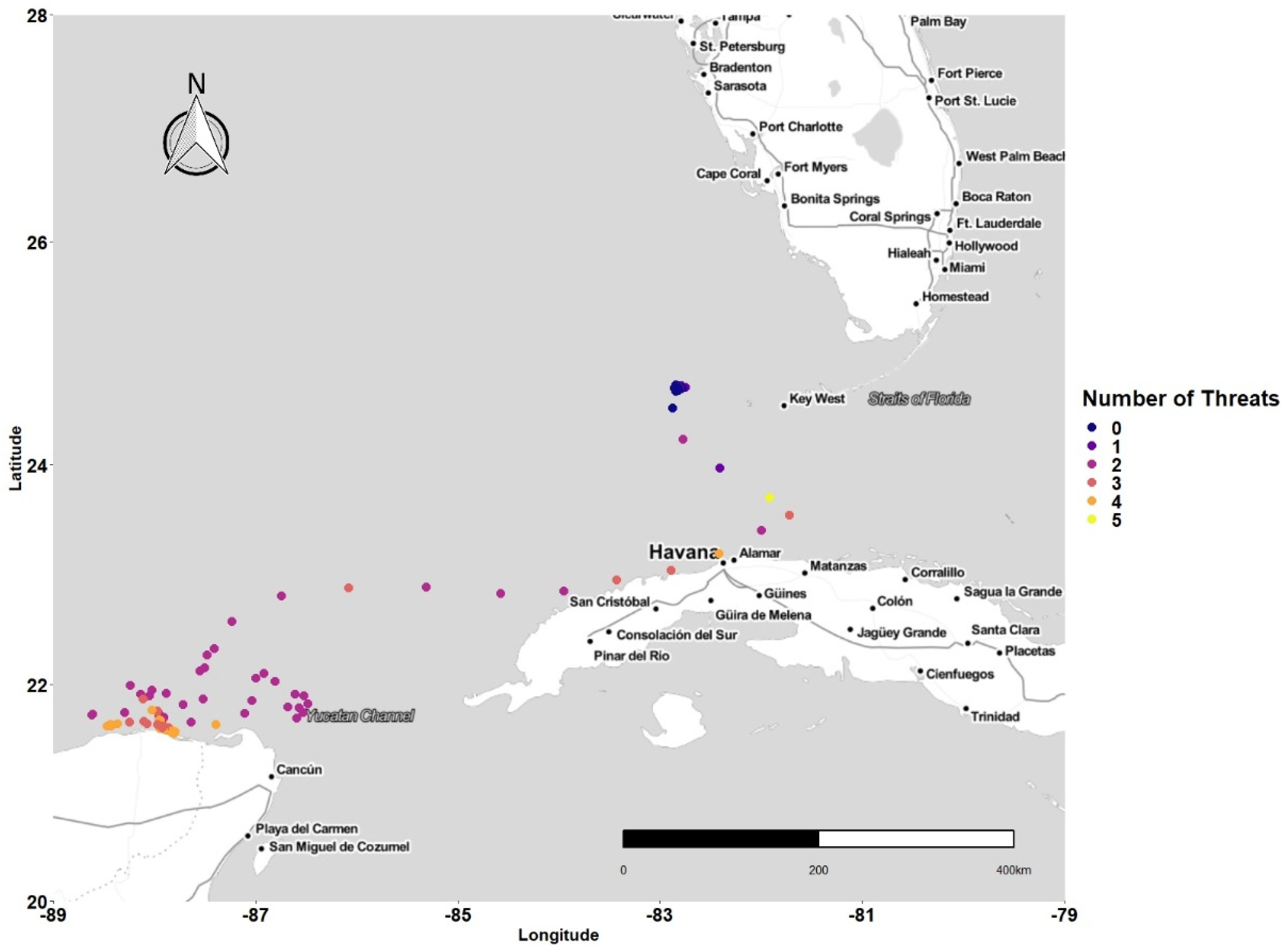
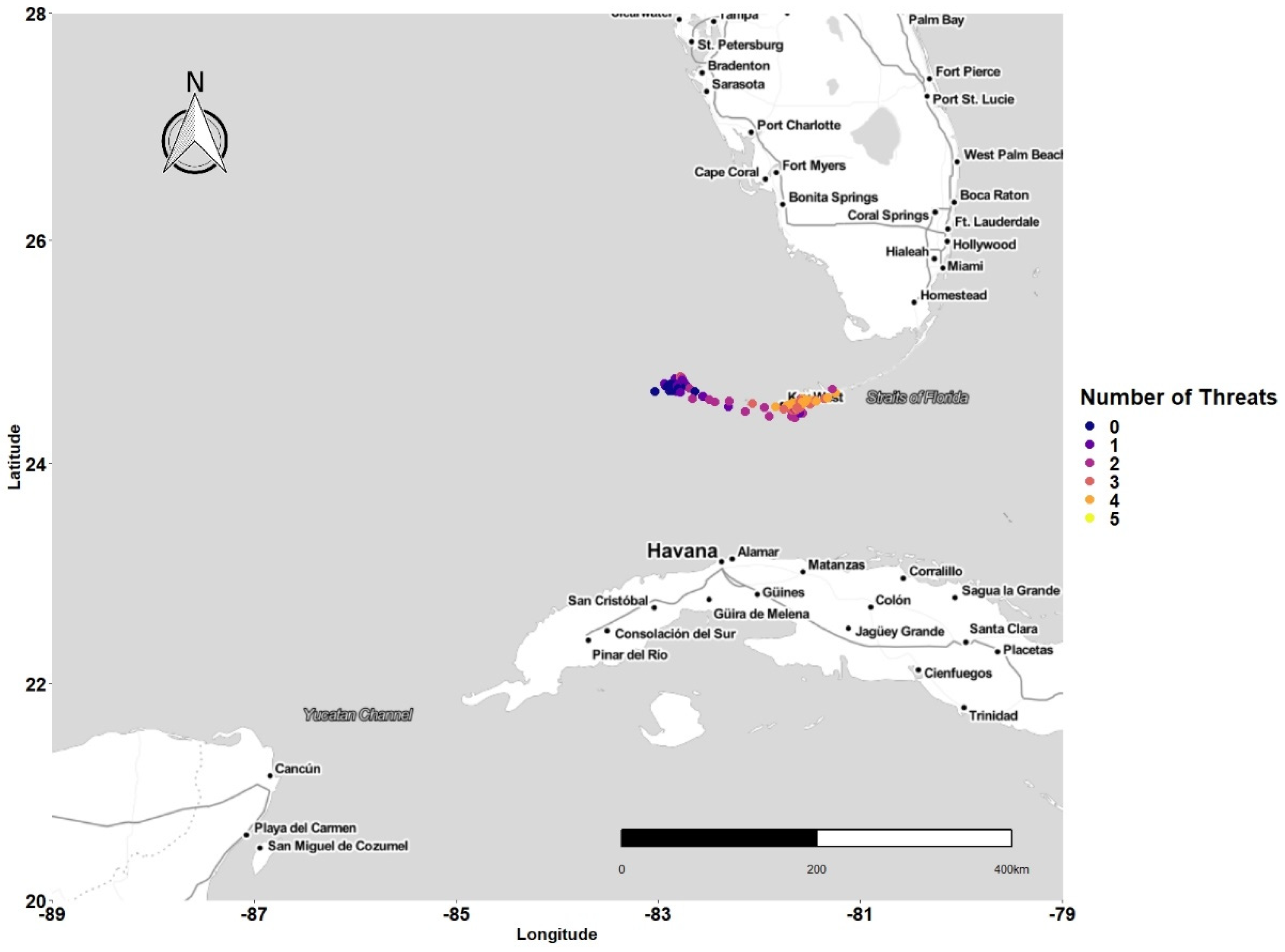
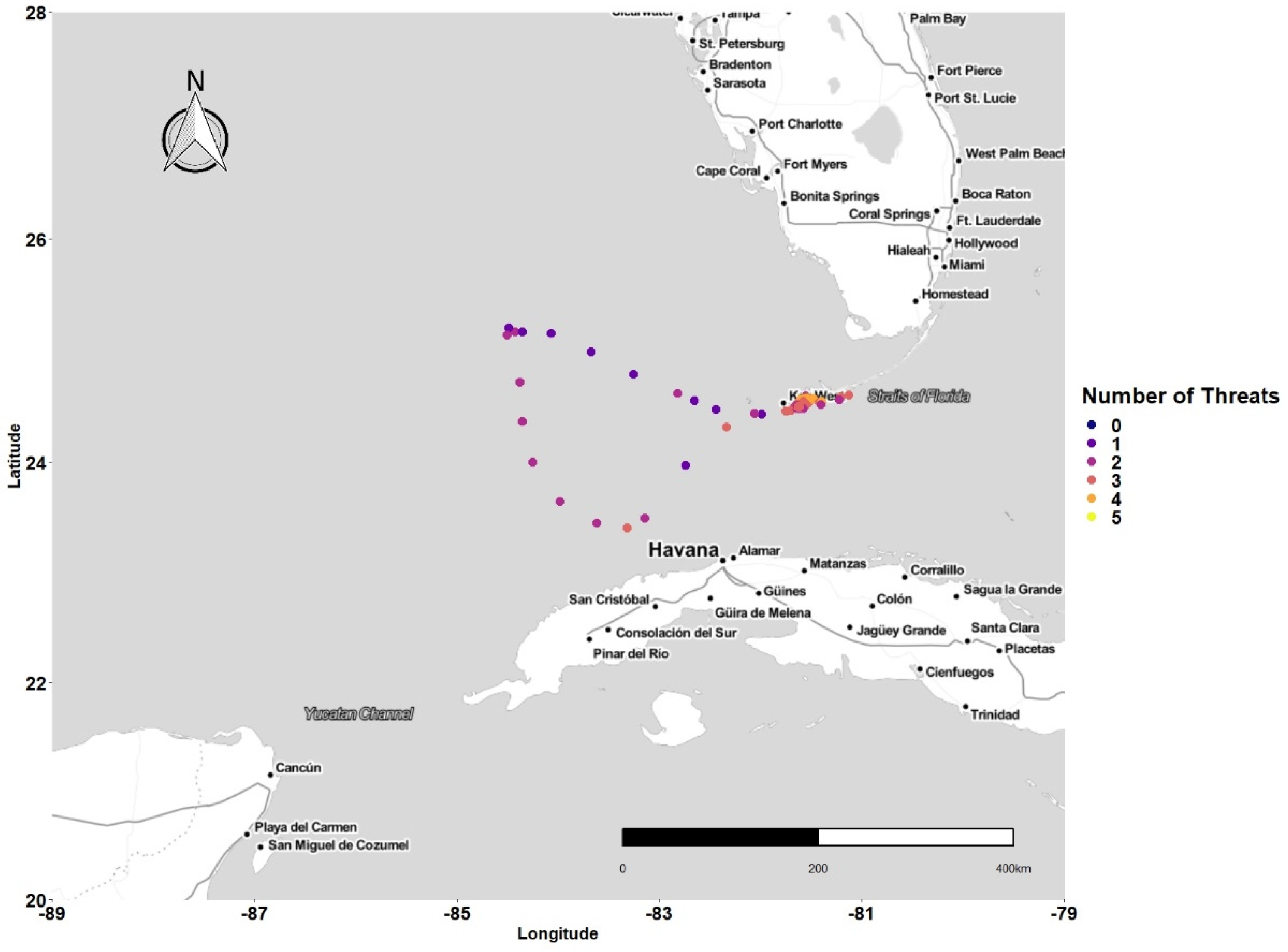
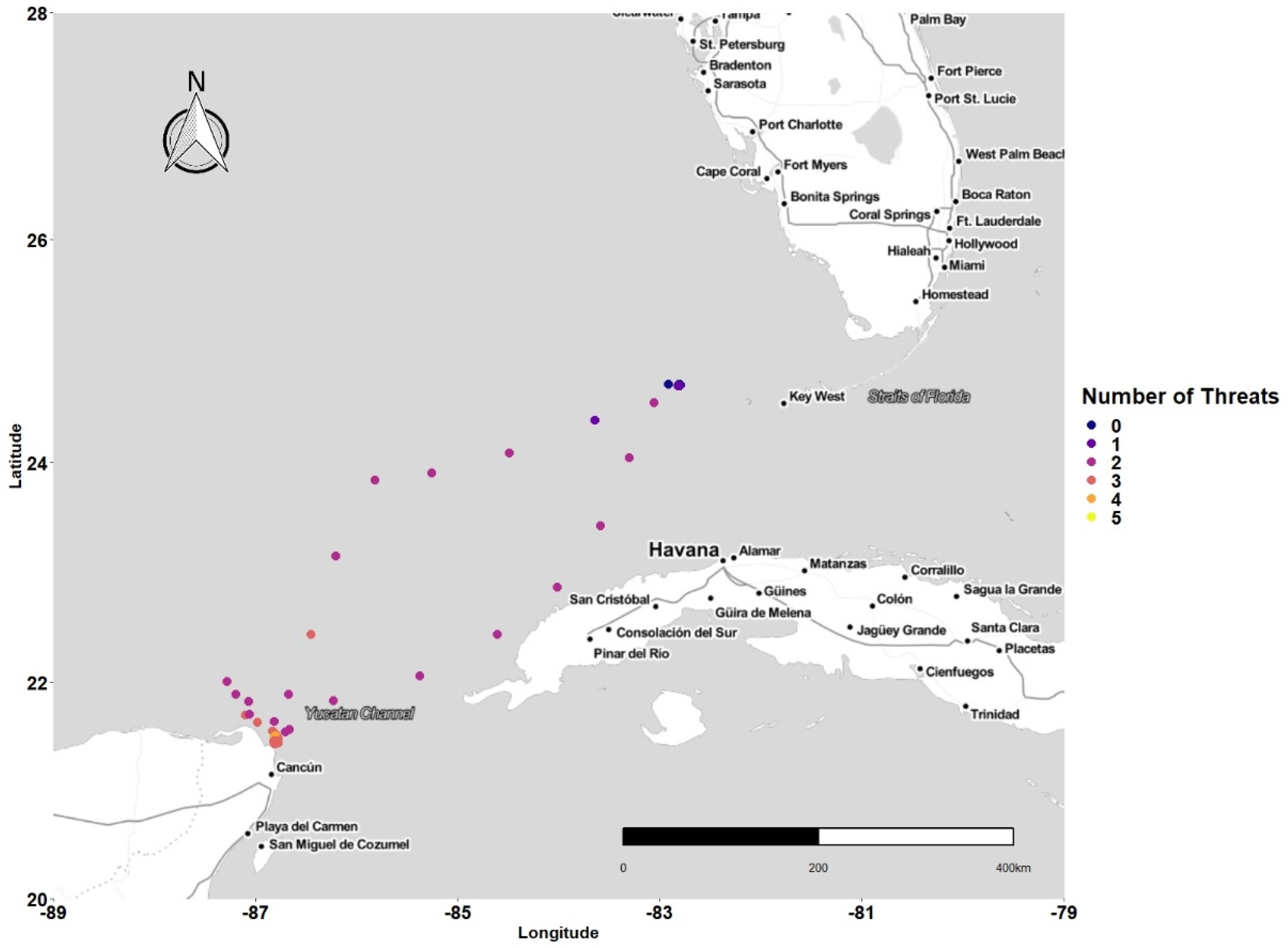
3. Results
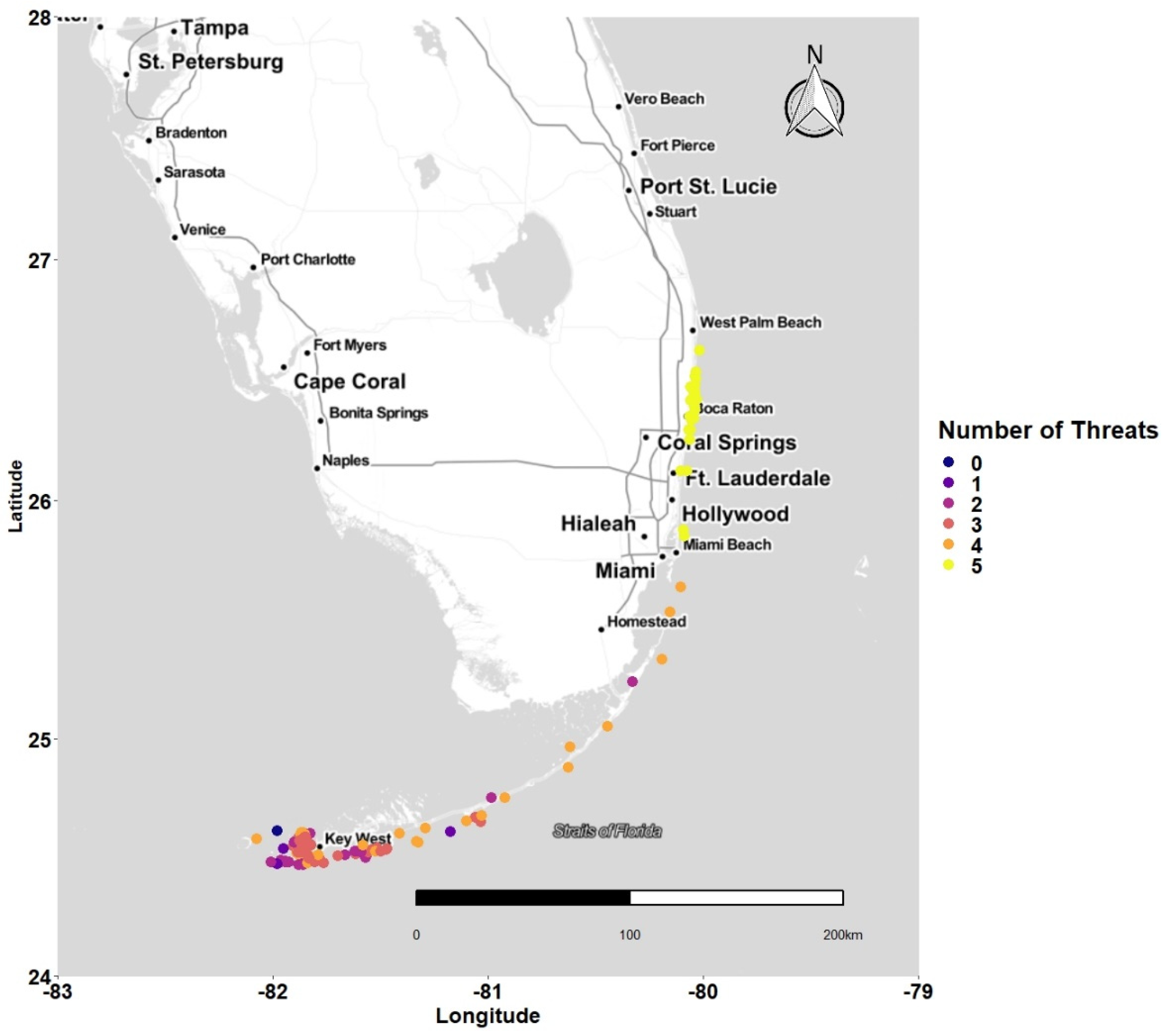
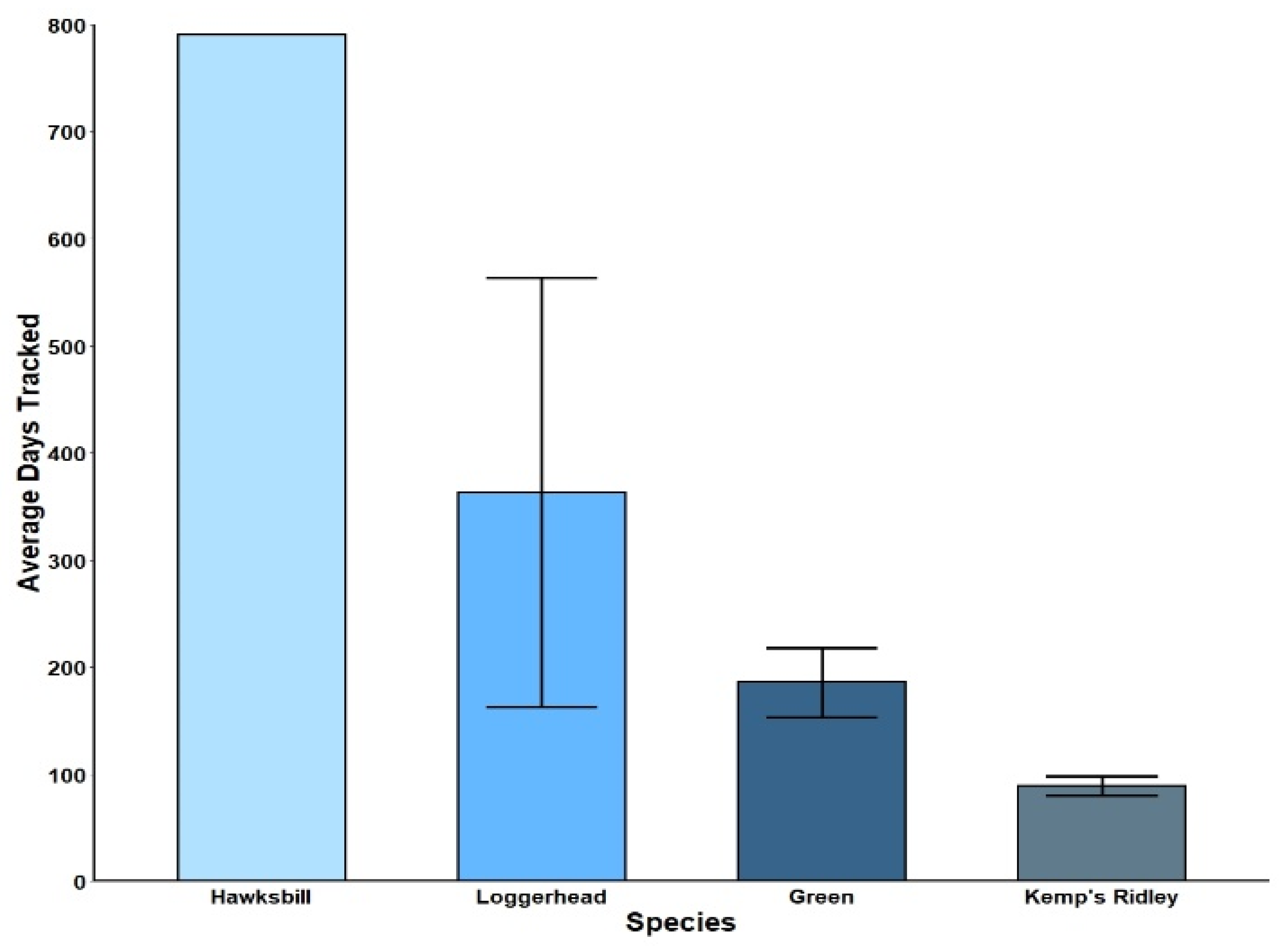
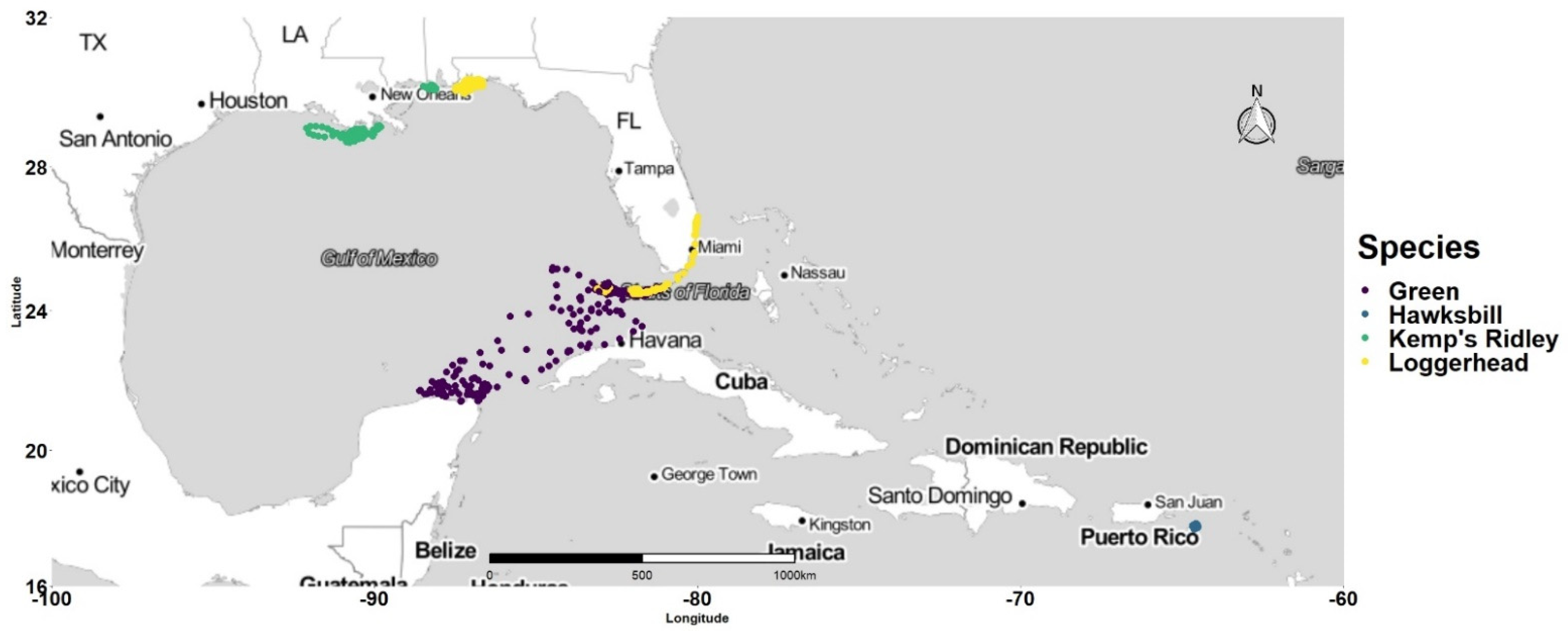
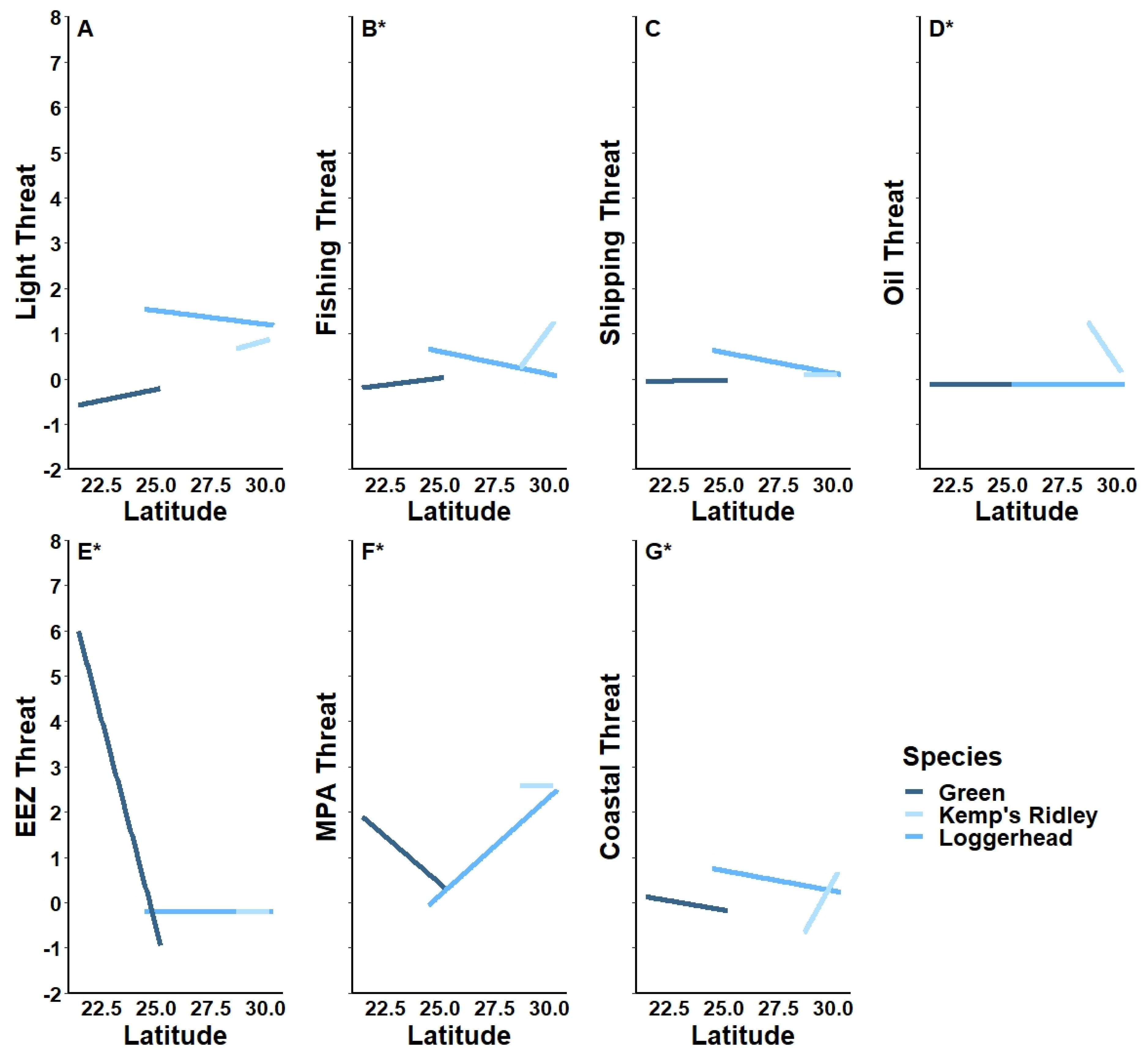
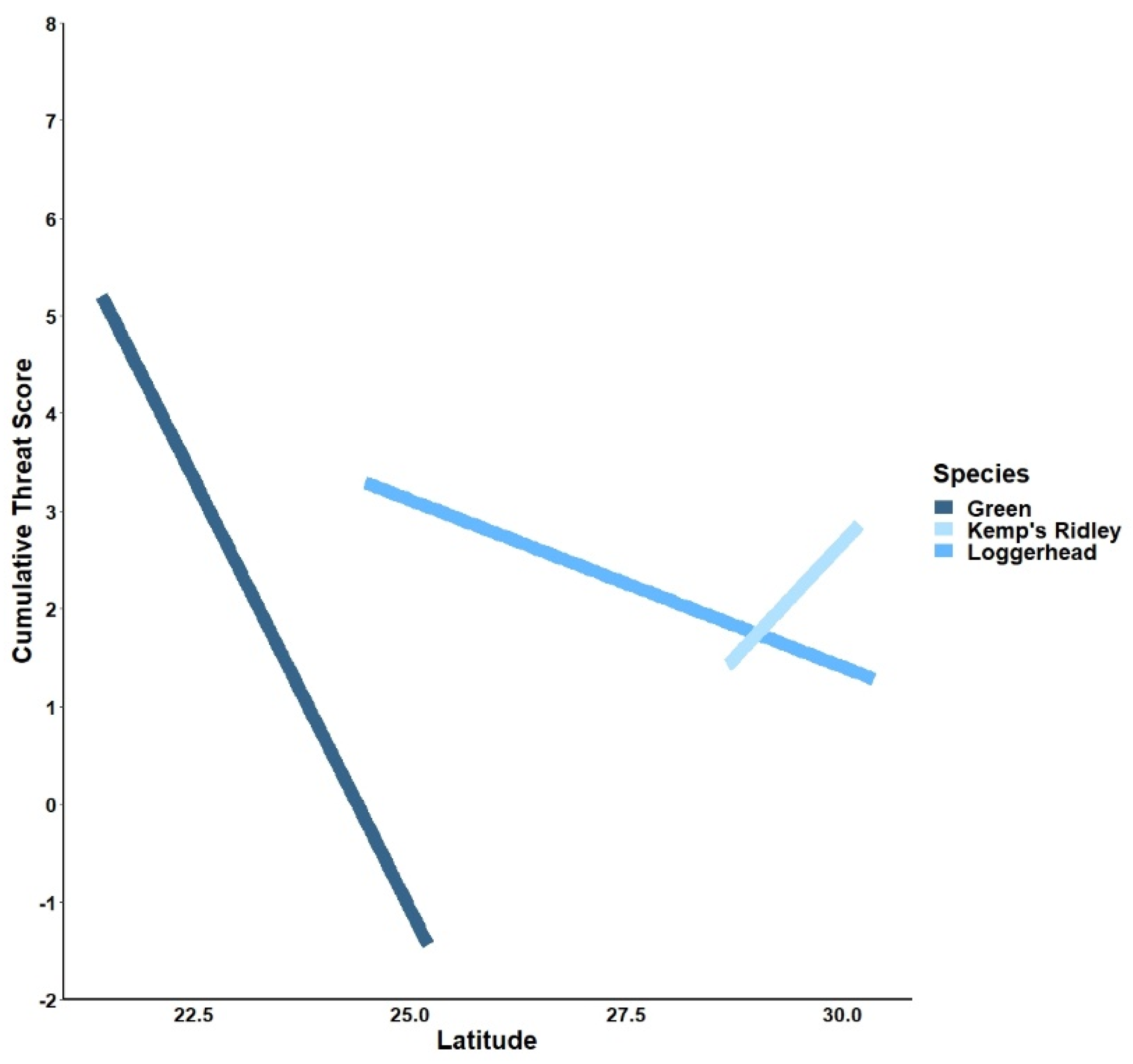
3.1. Latitudinal Gradient
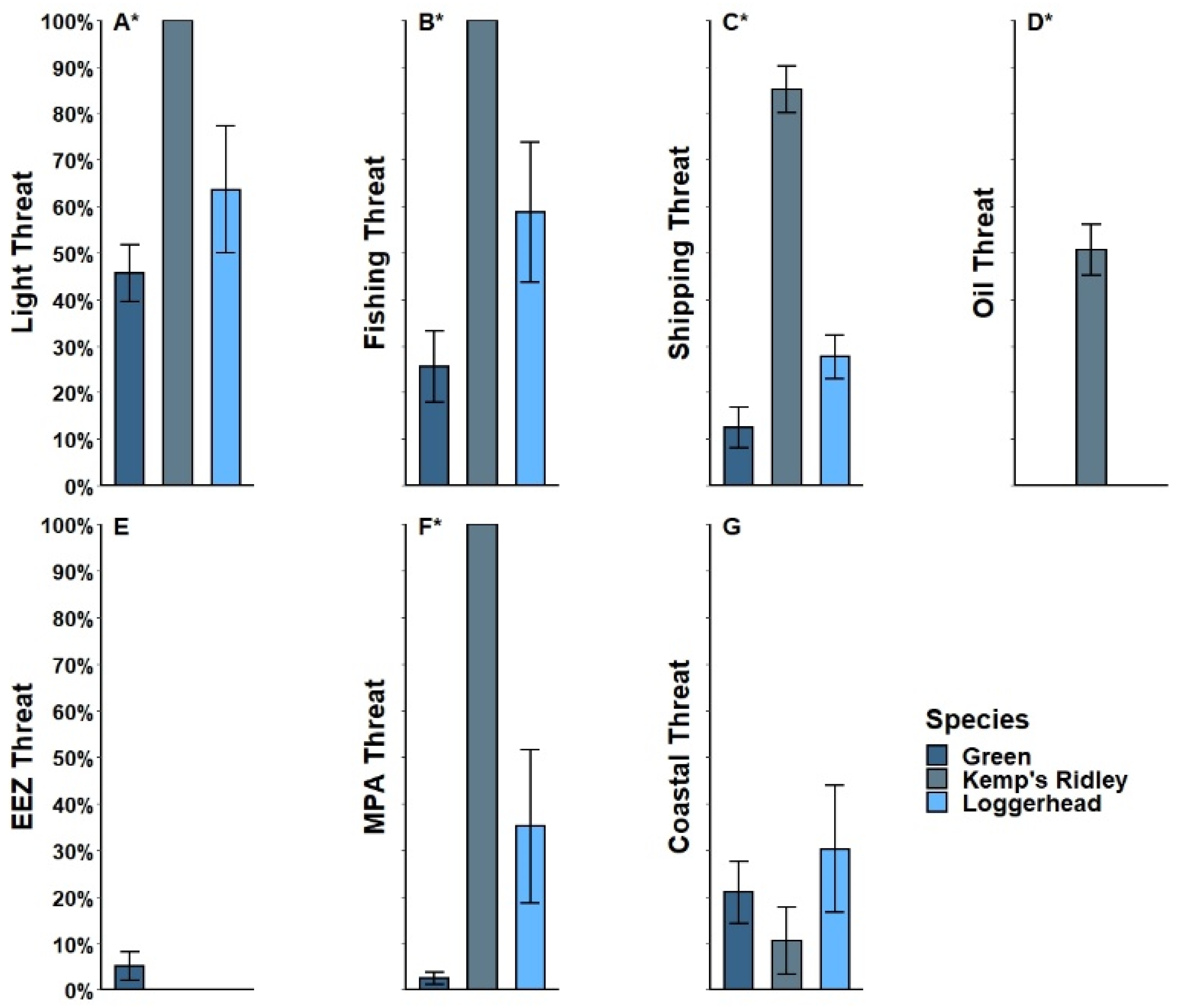
3.2. Threat Exposure
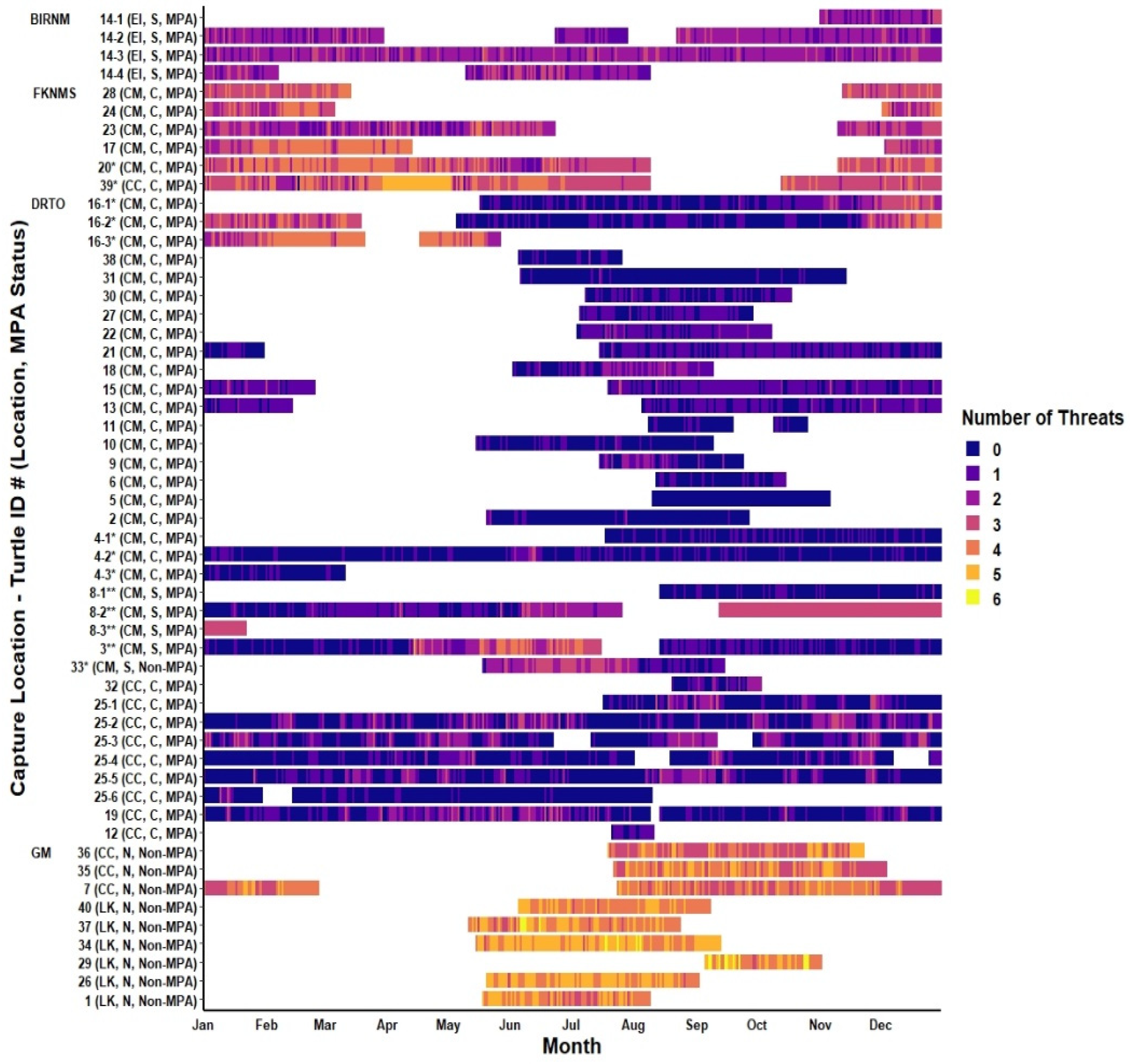
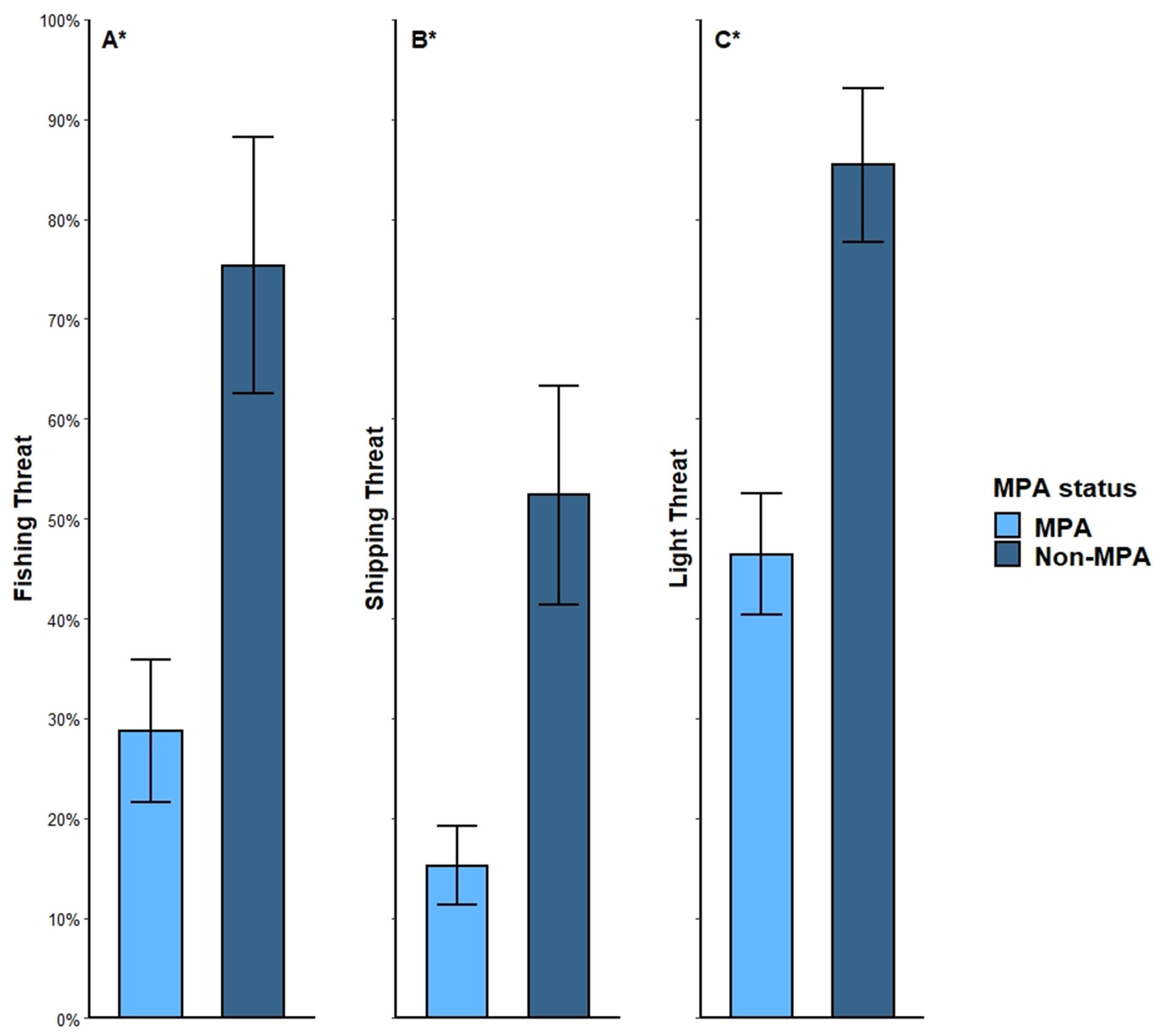
3.3. Threats and MPAs
3.4. Concentration of Points and Threat
4. Discussion
4.1. Latitudinal Gradient
4.2. Threat Exposure
4.3. Threats and MPAs
4.4. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Halpern, B.S.; Frazier, M.; Potapenko, J.; Casey, K.S.; Koenig, K.; Longo, C.; Lowndes, J.S.; Rockwood, R.C.; Selig, E.R.; Selkoe, K.A.; et al. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat. Commun. 2015, 6, 7615. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, V.J.D.; Turschwell, M.P.; Giffin, A.L.; Halpern, B.S.; Connolly, R.; Griffiths, L.; Frazer, M.; Brown, C.J. Linking threat maps with management to guide conservation investment. Biol. Conserv. 2020, 245, 108527. [Google Scholar] [CrossRef]
- Linardich, C.; Ralph, G.M.; Robertson, D.R.; Harwell, H.; Polidoro, B.A.; Lindeman, K.C.; Carpenter, K.E. Extinction risk and conservation of marine bony shorefishes of the Greater Caribbean and Gulf of Mexico. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 85–101. [Google Scholar] [CrossRef]
- Strongin, K.; Polidoro, B.; Linardich, C.; Ralph, G.; Saul, S.; Carpenter, K. Translating globally threatened marine species information into regional guidance for the Gulf of Mexico. Glob. Ecol. Conserv. 2020, 23, e01010. [Google Scholar] [CrossRef]
- O’Hara, C.C.; Frazier, M.; Halpern, B.S. At-risk marine biodiversity faces extensive, expanding, and intensifying human impacts. Science 2021, 372, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.L.; Reed, S.E.; Merenlender, A.M.; Crooks, K.R. Effects of recreation on animals revealed as widespread through a global systematic review. PLoS ONE 2016, 11, e0167259. [Google Scholar] [CrossRef]
- Schofield, G.; Scott, R.; Dimadi, A.; Fossette, S.; Katselidis, K.A.; Koutsoubas, D.; Lilley, M.K.S.; Pantis, J.D.; Karagouni, A.D.; Hays, G.C. Evidence-based marine protected area planning for a highly mobile endangered marine vertebrate. Biol. Conserv. 2013, 161, 101–109. [Google Scholar] [CrossRef]
- Schofield, G.; Dickson, L.C.D.; Westover, L.; Dujon, A.M.; Katselidis, K.A. COVID-19 disruption reveals mass-tourism pressure on nearshore sea turtle distributions and access to optimal breeding habitat. Evol. Appl. 2021, 14, 2516–2526. [Google Scholar] [CrossRef]
- Wilson, M.W.; Ridlon, A.D.; Gaynor, K.M.; Gaines, S.D.; Stier, A.C.; Halpern, B.S. Ecological impacts of human-induced animal behaviour change. Ecol. Lett. 2020, 23, 1522–1536. [Google Scholar] [CrossRef]
- Yasué, M. The effects of human presence, flock size and prey density on shorebird foraging rates. J. Ethol. 2005, 23, 199–204. [Google Scholar] [CrossRef]
- Miller, P.J.O.; Johnson, M.P.; Madsen, P.T.; Biassoni, N.; Quero, M.; Tyack, P.L. Using at-sea experiments to study the effects of airguns on the foraging behavior of sperm whales in the Gulf of Mexico. Deep Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 1168–1181. [Google Scholar] [CrossRef]
- Williams, R.; Erbe, C.; Ashe, E.; Beerman, A.; Smith, J. Severity of killer whale behavioral responses to ship noise: A dose-response study. Mar. Pollut. Bull. 2014, 79, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Erbe, C.; Ashe, E.; Clark, C.W. Quiet(er) marine protected areas. Mar. Pollut. Bull. 2015, 100, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, J.A.; Richardson, K.A.; Krueger, B.H. Impacts of the “Swim with the Turtles” attractions on endangered green turtles (Chelonia Mydas) in barbados, Barbados Sea Turtle Project Technical Report; Barbados Sea Turtle Projecty: Bridgetown, Barbados, 2007. [Google Scholar]
- Papafitsoros, K.; Panagopoulou, A.; Schofield, G. Social media reveals consistently disproportionate tourism pressure on a threatened marine vertebrate. Anim. Conserv. 2021, 24, 568–579. [Google Scholar] [CrossRef]
- Smulders, F.O.; O’Shea, O.R.; Christianen, M.J. Animal-borne video reveals atypical behaviour in provisioned green turtles: A global perspective of a widespread tourist activity. Glob. Ecol. Conserv. 2021, 25, e01417. [Google Scholar] [CrossRef]
- Stewart, K.; Norton, T.; Mohammed, H.; Browne, D.; Clements, K.; Thomas, K.; Yaw, T.; Horrocks, J. Effects of “swim with the turtles” tourist attractions on green sea turtle (Chelonia mydas) health in Barbados, West Indies. J. Wildl. Dis. 2016, 52, S104–S117. [Google Scholar] [CrossRef]
- Domènech, F.; Aznar, F.J.; Raga, J.A.; Tomás, J. Two decades of monitoring in marine debris ingestion in loggerhead sea turtle, Caretta caretta, from the western Mediterranean. Environ. Pollut. 2019, 244, 367–378. [Google Scholar] [CrossRef]
- Moore, M.R.; Vetter, W.; Gaus, C.; Shaw, G.R.; Müller, J.F. Trace organic compounds in the marine environment. Mar. Pollut. Bull. 2002, 45, 62–68. [Google Scholar] [CrossRef]
- Ylitalo, G.M.; Collier, T.K.; Anulacion, B.F.; Juaire, K.; Boyer, R.H.; da Silva, D.A.M.; Keene, J.L.; Stacy, B.A. Determining oil and dispersant exposure in sea turtles from the northern Gulf of Mexico resulting from the Deepwater Horizon oil spill. Endanger. Species Res. 2017, 33, 9–24. [Google Scholar] [CrossRef]
- Zacharias, M.A.; Gregr, E.J. Sensitivity and vulnerability in marine environments: An approach to identifying vulnerable marine areas. Conserv. Biol. 2005, 19, 86–97. [Google Scholar] [CrossRef]
- Worm, B. Averting a global fisheries disaster. Proc. Natl. Acad. Sci. USA 2016, 113, 4895–4897. [Google Scholar] [CrossRef]
- Pauly, D.; Zeller, D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 2016, 7, 10244. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, Y.; Watson, R.A.; Blanchard, J.L.; Fulton, E.A. Evolution of global marine fishing fleets and the response of fished resources. Proc. Natl. Acad. Sci. USA 2019, 116, 12238–12243. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.A.; Alverson, D.L.; Metuzals, K.I. By-catch: Problems and solutions. Mar. Pollut. Bull. 2000, 41, 204–219. [Google Scholar] [CrossRef]
- Soykan, C.U.; Moore, J.E.; Zydelis, R.; Crowder, L.B.; Safina, C.; Lewison, R.L. Why study bycatch? An introduction to the Theme Section on fisheries bycatch. Endanger. Species Res. 2008, 5, 91–102. [Google Scholar] [CrossRef]
- Wallace, B.P.; Lewison, R.L.; Mcdonald, S.L.; Mcdonald, R.K.; Kot, C.Y.; Kelez, S.; Bjorkland, R.K.; Finkbeiner, E.M.; Helmbrecht, S.; Crowder, L.B. Global patterns of marine turtle bycatch. Conserv. Lett. 2010, 3, 131–142. [Google Scholar] [CrossRef]
- Zeller, D.; Pauly, D. Good news, bad news: Global fisheries discards are declining, but so are total catches. Fish Fish. 2005, 6, 156–159. [Google Scholar] [CrossRef]
- Graham, R.T.; Witt, M.J.; Castellanos, D.W.; Remolina, F.; Maxwell, S.; Godley, B.J.; Hawkes, L.A. Satellite tracking of manta rays highlights challenges to their conservation. PLoS ONE 2012, 7, e36834. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Baum, J.K.; Clarke, S.; Compagno, L.J.; Cortés, E.; Domingo, A.; Fordham, S.; Fowler, S.; Francis, M.P.; Gibson, C.; et al. You can swim but you can’t hide: The global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 459–482. [Google Scholar] [CrossRef]
- Murray, K.T.; Hatch, J.M.; DiGiovanni, R.A.; Josephson, E. Tracking young-of-the-year gray seals Halichoerus grypus to estimate fishery encounter risk. Mar. Ecol. Prog. Ser. 2021, 671, 235–245. [Google Scholar] [CrossRef]
- Read, A.J.; Drinker, P.; Northridge, S. Bycatch of marine mammals in U.S. and global fisheries. Conserv. Biol. 2006, 20, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Redfern, J.V.; Becker, E.A.; Moore, T.J. Effects of variability in ship traffic and whale distributions on the risk of ships striking whales. Front. Mar. Sci. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Rojas-Bracho, L.; Gulland, F.M.D.; Smith, C.R.; Taylor, B.; Wells, R.S.; Thomas, P.O.; Bauer, B.; Heide-Jørgensen, M.P.; Teilmann, J.; Dietz, R.; et al. A field effort to capture critically endangered vaquitas Phocoena sinus for protection from entanglement in illegal gillnets. Endanger. Species Res. 2019, 38, 11–27. [Google Scholar] [CrossRef]
- Iverson, A.R.; Benscoter, A.M.; Fujisaki, I.; Lamont, M.M.; Hart, K.M. Migration corridors and threats in the Gulf of Mexico and Florida Straits for loggerhead sea turtles. Front. Mar. Sci. 2020, 7, 208. [Google Scholar] [CrossRef]
- Hart, K.M.; Iverson, A.R.; Fujisaki, I.; Lamont, M.M.; Bucklin, D.; Shaver, D.J. Marine threats overlap key foraging habitat for two imperiled sea turtle species in the Gulf of Mexico. Front. Mar. Sci. 2018, 5, 336. [Google Scholar] [CrossRef]
- Lewison, R.L.; Crowder, L.B.; Read, A.J.; Freeman, S.A. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 2004, 19, 598–604. [Google Scholar] [CrossRef]
- Harrison, A.L.; Costa, D.P.; Winship, A.J.; Benson, S.R.; Bograd, S.J.; Antolos, M.; Carlisle, A.B.; Dewar, H.; Dutton, P.H.; Jorgensen, S.J.; et al. The political biogeography of migratory marine predators. Nat. Ecol. Evol. 2018, 2, 1571–1578. [Google Scholar] [CrossRef]
- Hart, K.M.; Zawada, D.G.; Fujisaki, I.; Lidz, B.H. Habitat use of breeding green turtles Chelonia mydas tagged in Dry Tortugas National Park: Making use of local and regional MPAs. Biol. Conserv. 2013, 161, 142–154. [Google Scholar] [CrossRef]
- Hart, K.M.; Iverson, A.R.; Fujisaki, I.; Lamont, M.M.; Bucklin, D.; Shaver, D.J. Sympatry or syntopy? Investigating drivers of distribution and co-occurrence for two imperiled sea turtle species in Gulf of Mexico neritic waters. Ecol. Evol. 2018, 8, 12656–12669. [Google Scholar] [CrossRef]
- López-Mendilaharsu, M.; Giffoni, B.; Monteiro, D.; Prosdocimi, L.; Vélez-Rubio, G.M.; Fallabrino, A.; Estrades, A.; dos Santos, A.S.; Lara, P.H.; Pires, T.; et al. Multiple-threats analysis for loggerhead sea turtles in the southwest Atlantic Ocean. Endanger. Species Res. 2020, 41, 183–196. [Google Scholar] [CrossRef]
- Pilcher, N.J.; Antonopoulou, M.A.; Rodriguez-Zarate, C.J.; Mateos-Molina, D.; Das, H.S.; Bugla, I.; Al Ghais, S.M. Movements of green turtles from foraging areas of the United Arab Emirates: Regional habitat connectivity and use of marine protected areas. Mar. Biol. 2021, 168, 10. [Google Scholar] [CrossRef]
- Requena, S.; Oppel, S.; Bond, A.L.; Hall, J.; Cleeland, J.; Crawford, R.J.M.; Davies, D.; Dilley, B.J.; Glass, T.; Makhado, A.; et al. Marine hotspots of activity inform protection of a threatened community of pelagic species in a large oceanic jurisdiction. Anim. Conserv. 2020, 23, 585–596. [Google Scholar] [CrossRef]
- Shaver, D.J.; Schroeder, B.A.; Byles, R.A.; Burchfield, P.M.; Peña, J.; Márquez, R.; Martinez, H.J. Movements and home ranges of adult male Kemp’s ridley sea turtles (Lepidochelys kempii) in the Gulf of Mexico investigated by satellite telemetry. Chelonian Conserv. Biol. 2005, 4, 817–827. [Google Scholar]
- Notarbartolo di Sciara, G. Guidelines for the Establishment and Management of Marine Protected Areas for Cetaceans; United Nations Environment Programme: Almeria, Spain, 2007. [Google Scholar]
- Roberge, J.M.; Angelstam, P. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Vandeperre, F.; Higgins, R.M.; Sánchez-Meca, J.; Maynou, F.; Goñi, R.; Martín-Sosa, P.; Pérez-Ruzafa, A.; Afonso, P.; Bertocci, I.; Crec’hriou, R.; et al. Effects of no-take area size and age of marine protected areas on fisheries yields: A meta-analytical approach. Fish Fish. 2011, 12, 412–426. [Google Scholar] [CrossRef]
- Weigel, J.Y.; Mannle, K.O.; Bennett, N.J.; Carter, E.; Westlund, L.; Burgener, V.; Hoffman, Z.; Simão Da Silva, A.; Kane, E.A.; Sanders, J.; et al. Marine protected areas and fisheries: Bridging the divide. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 199–215. [Google Scholar] [CrossRef]
- Cuevas, E.; Abreu-Grobois, F.A.; Guzmán-Hernández, V.; Liceaga-Correa, M.A.; van Dam, R.P. Post-nesting migratory movements of hawksbill turtles Eretmochelys imbricata in waters adjacent to the Yucatan Peninsula, Mexico. Endanger. Species Res. 2008, 10, 123–133. [Google Scholar] [CrossRef]
- Casale, P.; Abitsi, G.; Aboro, M.P.; Agamboue, P.D.; Agbode, L.; Allela, N.L.; Angueko, D.; Bibang Bi Nguema, J.N.; Boussamba, F.; Cardiec, F.; et al. A first estimate of sea turtle bycatch in the industrial trawling fishery of Gabon. Biodivers. Conserv. 2017, 26, 2421–2433. [Google Scholar] [CrossRef]
- Maxwell, S.M.; Breed, G.A.; Nickel, B.A.; Makanga-Bahouna, J.; Pemo-Makaya, E.; Parnell, R.J.; Formia, A.; Ngouessono, S.; Godley, B.J.; Costa, D.P.; et al. Using satellite tracking to optimize protection of long-lived marine species: Olive ridley sea turtle conservation in central africa. PLoS ONE 2011, 6, e19905. [Google Scholar] [CrossRef]
- Hitipeuw, C.; Dutton, P.H.; Benson, S.; Thebu, J.; Bakarbessy, J. Population status and internesting movement of leatherback turtles, Dermochelys coriacea, nesting on the northwest coast of Papua, Indonesia. Chelonian Conserv. Biol. 2007, 6, 28–36. [Google Scholar] [CrossRef]
- Hays, G.C.; Bailey, H.; Bograd, S.J.; Bowen, W.D.; Campagna, C.; Carmichael, R.H.; Casale, P.; Chiaradia, A.; Costa, D.P.; Cuevas, E.; et al. Translating marine animal tracking data into conservation policy and management. Trends Ecol. Evol. 2019, 34, 459–473. [Google Scholar] [CrossRef] [PubMed]
- ConocoPhillips Australia Barossa Area Development: Off-Shore Project Proposal; ConocoPhillips: Darwin, Australia, 2018.
- Herren, R.M.; Bagley, D.A.; Bresette, M.J.; Holloway-Adkins, K.G.; Clark, D.; Witherington, B.E. Sea turtle abundance and demographic measurements in a marine protected area in the Florida Keys, USA. Herpetol. Conserv. Biol. 2018, 13, 224–239. [Google Scholar]
- Brock, R.J.; Culhane, B.F. The no-take research natural area of Dry Tortugas National Park(Florida): Wishful thinking or responsible planning? Am. Fish. Soc. Symp. 2004, 42, 67–74. [Google Scholar]
- Florida Keys National Marine Sanctuary: NOAA. 2021. Available online: https://floridakeys.noaa.gov/welcome.html (accessed on 10 October 2021).
- Suman, D.O. The Florida Keys National Marine Sanctuary: A case study of an innovative federal-state partnership in marine resource management. Coast. Manag. 1997, 25, 293–324. [Google Scholar] [CrossRef]
- Calich, H.; Estevanez, M.; Hammerschlag, N. Overlap between highly suitable habitats and longline gear management areas reveals vulnerable and protected regions for highly migratory sharks. Mar. Ecol. Prog. Ser. 2018, 602, 183–195. [Google Scholar] [CrossRef]
- Hart, K.M.; Zawada, D.G.; Sartain, A.R.; Fujisaki, I. Breeding loggerhead marine turtles Caretta caretta in Dry Tortugas National Park, USA, show high fidelity to diverse habitats near nesting beaches. Oryx 2016, 50, 283–288. [Google Scholar] [CrossRef]
- Keller, B.D.; Delaney, J.; Causey, B. Monitoring changes in the fully protected zones of the Florida Keys National Marine Sanctuary. Proc. Gulf Caribb. Fish. Inst. 2003, 54, 694–701. [Google Scholar]
- Abreu-Grobois, A.; Plotkin, P. Olive Ridley. Lepidochelys Olivacea: The IUCN Red List of Threatened Species. 2008. Available online: https://www.iucnredlist.org/species/11534/3292503 (accessed on 1 September 2021).
- Casale, P.; Tucker, A.D. Loggerhead Turtle, Caretta Caretta: The IUCN Red List of Threatened Species. 2017. Available online: https://www.iucnredlist.org/species/3897/119333622 (accessed on 1 September 2021).
- Mortimer, J.A.; Donnelly, M. Hawksbill Turtle, Eretmochelys Imbricata: The IUCN Red List of Threatened Species. 2008. Available online: https://www.iucnredlist.org/species/8005/12881238 (accessed on 1 September 2021).
- Wallace, B.P.; Tiwari, M.; Girondot, M. Leatherback: Dermochelys Coriacea: The IUCN Red List of Threatened Species. 2013. Available online: https://www.iucnredlist.org/species/6494/43526147 (accessed on 1 September 2021).
- Seminoff, J.A. Green Turtle, Chelonia Mydas: The IUCN Red List of Threatened Species. 2004. Available online: https://www.iucnredlist.org/species/4615/11037468 (accessed on 1 September 2021).
- Wibbels, T.; Bevan, E. Kemp’s Ridley, Lepidochelys Kempii: The IUCN Red List of Threatened Species. 2013. Available online: https://www.iucnredlist.org/species/11533/155057916 (accessed on 1 September 2021).
- Red List Standards & Petitions Subcommittee. Flatback, Natator Depressus: The IUCN Red List of Threatened Species. 1996. Available online: https://www.iucnredlist.org/species/14363/4435952 (accessed on 1 September 2021).
- Fossette, S.; Corbel, H.; Gaspar, P.; Le Maho, Y.; Georges, J. An alternative technique for the long-term satellite tracking of leatherback turtles. Endanger. Species Res. 2008, 4, 33–41. [Google Scholar] [CrossRef]
- Saito, T.; Kurita, M.; Okamoto, H.; Uchida, I.; Parker, D.; Balazs, G. Tracking male loggerhead turtle migrations around southwestern Japan using satellite telemetry. Chelonian Conserv. Biol. 2015, 14, 82–87. [Google Scholar] [CrossRef]
- Schofield, G.; Hobson, V.J.; Fossette, S.; Lilley, M.K.S.; Katselidis, K.A.; Hays, G.C. Fidelity to foraging sites, consistency of migration routes and habitat modulation of home range by sea turtles. Divers. Distrib. 2010, 16, 840–853. [Google Scholar] [CrossRef]
- Broderick, A.C.; Coyne, M.S.; Fuller, W.J.; Glen, F.; Godley, B.J. Fidelity and over-wintering of sea turtles. Proc. R. Soc. B Biol. Sci. 2007, 274, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.; Silva, A.C.C.D.; Castilhos, J.C.; Weber, M.I.; Soares, L.S.; Vicente, L. Strong site fidelity and longer internesting interval for solitary nesting olive ridley sea turtles in Brazil. Mar. Biol. 2012, 159, 1011–1019. [Google Scholar] [CrossRef]
- Panagopoulou, A.; Meletis, Z.A.; Margaritoulis, D.; Spotila, J.R. Caught in the same net? Small-scale fishermen’s perceptions of fisheries interactions with sea turtles and other protected species. Front. Mar. Sci. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Hojnowski, C.E.; Carter, N.H.; Brashares, J.S. The influence of human disturbance on wildlife nocturnality. Science 2018, 360, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Frid, A.; Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 16. [Google Scholar] [CrossRef]
- Whitehead, H.; Smith, T.D.; Rendell, L. Adaptation of sperm whales to open-boat whalers: Rapid social learning on a large scale? Biol. Lett. 2021, 17, 1–10. [Google Scholar] [CrossRef]
- Tyack, P.L. Implications for marine mammals of large-scale changes in the marine acoustic environment. J. Mammal. 2008, 89, 549–558. [Google Scholar] [CrossRef]
- Heithaus, M.R.; Frid, A.; Wirsing, A.J.; Dill, L.M.; Fourqurean, J.W.; Burkholder, D.; Thomson, J.; Bejder, L. State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J. Anim. Ecol. 2007, 76, 837–844. [Google Scholar] [CrossRef]
- Gaos, A.R.; Lewison, R.L.; Yañez, I.L.; Wallace, B.P.; Liles, M.J.; Nichols, W.J.; Baquero, A.; Hasbún, C.R.; Vasquez, M.; Urteaga, J.; et al. Shifting the life-history paradigm: Discovery of novel habitat use by hawksbill turtles. Biol. Lett. 2012, 8, 54–56. [Google Scholar] [CrossRef][Green Version]
- McDonald, T.L.; Schroeder, B.A.; Stacy, B.A.; Wallace, B.P.; Starcevich, L.A.; Gorham, J.; Tumlin, M.C.; Cacela, D.; Rissing, M.; McLamb, D.B.; et al. Density and exposure of surface-pelagic juvenile sea turtles to Deepwater Horizon oil. Endanger. Species Res. 2017, 33, 69–82. [Google Scholar] [CrossRef]
- Shaver, D.J.; Gredzens, C.; Walker, J.S.; Godard-Codding, C.A.J.; Yacabucci, J.E.; Frey, A.; Dutton, P.H.; Schmitt, C.J. Embryo deformities and nesting trends in Kemp’s ridley sea turtles Lepidochelys kempii before and after the Deepwater Horizon oil spill. Endanger. Species Res. 2021, 44, 277–289. [Google Scholar] [CrossRef]
- Wildermann, N.E.; Sasso, C.R.; Stokes, L.W.; Snodgrass, D.; Fuentes, M.M.P.B. Habitat use and behavior of multiple species of marine turtles at a foraging area in the Northeastern Gulf of Mexico. Front. Mar. Sci. 2019, 6, 155. [Google Scholar] [CrossRef]
- Lamont, M.M.; Iverson, A.R. Shared habitat use by juveniles of three sea turtle species. Mar. Ecol. Prog. Ser. 2018, 606, 187–200. [Google Scholar] [CrossRef]
- Godley, B.J.; Blumenthal, J.M.; Broderick, A.C.; Coyne, M.S.; Godfrey, M.H.; Hawkes, L.A.; Witt, M.J. Satellite tracking of sea turtles: Where have we been and where do we go next? Endanger. Species Res. 2008, 4, 3–22. [Google Scholar] [CrossRef]
- Hart, K.; Hyrenbach, K. Satellite telemetry of marine megavertebrates: The coming of age of an experimental science. Endanger. Species Res. 2009, 10, 9–20. [Google Scholar] [CrossRef]
- Hazen, E.; Maxwell, S.; Bailey, H.; Bograd, S.; Hamann, M.; Gaspar, P.; Godley, B.; Shillinger, G. Ontogeny in marine tagging and tracking science: Technologies and data gaps. Mar. Ecol. Prog. Ser. 2012, 457, 221–240. [Google Scholar] [CrossRef]
- Hamann, M.; Godfrey, M.H.; Seminoff, J.A.; Arthur, K.; Barata, P.C.R.; Bjorndal, K.A.; Bolten, A.B.; Broderick, A.C.; Campbell, L.M.; Carreras, C.; et al. Global research priorities for sea turtles: Informing management and conservation in the 21st century. Endanger. Species Res. 2010, 11, 245–269. [Google Scholar] [CrossRef]
- Schofield, G.; Klaassen, M.; Papafitsoros, K.; Lilley, M.K.; Katselidis, K.A.; Hays, G.C. Long-term photo-id and satellite tracking reveal sex-biased survival linked to movements in an endangered species. Ecology 2020, 101, e03027. [Google Scholar] [CrossRef]
- Arendt, M.D.; Segars, A.L.; Byrd, J.I.; Boynton, J.; Schwenter, J.A.; Whitaker, J.D.; Parker, L. Migration, distribution, and diving behavior of adult male loggerhead sea turtles (Caretta caretta) following dispersal from a major breeding aggregation in the Western North Atlantic. Mar. Biol. 2012, 159, 113–125. [Google Scholar] [CrossRef]
- Foley, A.; Schroeder, B.; Hardy, R.; MacPherson, S.; Nicholas, M.; Coyne, M. Postnesting migratory behavior of loggerhead sea turtles Caretta caretta from three Florida rookeries. Endanger. Species Res. 2013, 21, 129–142. [Google Scholar] [CrossRef][Green Version]
- Brei, M.; Pérez-Barahona, A.; Strobl, E. Environmental pollution and biodiversity: Light pollution and sea turtles in the Caribbean. J. Environ. Econ. Manag. 2016, 77, 95–116. [Google Scholar] [CrossRef]
- Price, J.T.; Drye, B.; Domangue, R.J.; Paladino, F.V. Exploring the role of artificial lighting in loggerhead turtle (Caretta caretta) nest-site selection and hatchling disorientation. Herpetol. Conserv. Biol. 2018, 13, 415–422. [Google Scholar]
- Hamed, A.; Madani, K.; Von Holle, B.; Wright, J.; Milon, J.W.; Bossick, M. How much are Floridians willing to pay for protecting sea turtles from sea level rise? Environ. Manag. 2016, 57, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.P.; von Holle, B.; Caffrey, M.A.; Weishampel, J.F. Quantifying the impacts of future sea level rise on nesting sea turtles in the southeastern United States. Ecol. Appl. 2020, 30, e02100. [Google Scholar] [CrossRef] [PubMed]
- Biddiscombe, S.J.; Smith, E.A.; Hawkes, L.A. A global analysis of anthropogenic development of marine turtle nesting beaches. Remote Sens. 2020, 12, 1492. [Google Scholar] [CrossRef]
- Bjorndal, K.A.; Parsons, J.; Mustin, W.; Bolten, A.B. Variation in age and size at sexual maturity in Kemp’s ridley sea turtles. Endanger. Species Res. 2014, 25, 57–67. [Google Scholar] [CrossRef]
- Richardson, J.I.; Bell, R.; Richardson, T.H. Population ecology and demographic implications drawn from an 11-year study of nesting hawksbill, Eretmochelys imbricata, at Jumby Bay, Long Island, Antigua, West Indies. Chelonian Conserv. Biol. 1999, 3, 244–250. [Google Scholar]
- Van Houtan, K.S.; Hargrove, S.K.; Balazs, G.H. Modeling sea turtle maturity age from partial life history records. Pac. Sci. 2014, 68, 465–477. [Google Scholar] [CrossRef]
- Hart, K.M.; Guzy, J.C.; Smith, B.J. Drivers of realized satellite tracking duration in marine turtles. Mov. Ecol. 2021, 9, 1. [Google Scholar] [CrossRef]
- Hart, K.M.; Guzy, J.C.; Cherkiss, M.S.; Smith, B.J. Tracking Durations for Marine Turtles Satellite-Tagged in Gulf of Mexico and Caribbean Sites, 2008-2019: U.S. Geological Survey Data Release. 2020. Available online: https://www.sciencebase.gov/catalog/item/5f9041f482cec5ff998af2e7 (accessed on 18 December 2020).
- Jonsen, I. Joint Estimation over multiple individuals improves behavioural state inference from animal movement data. Sci. Rep. 2016, 6, 20625. [Google Scholar] [CrossRef]
- Jonsen, I.D.; Flemming, J.M.; Myers, R.A. Robust state–space modeling of animal movement data. Ecology 2005, 86, 2874–2880. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 October 2020).
- Roberts, K.E.; Smith, B.J.; Burkholder, D.; Hart, K.M. Evaluating the use of marine protected areas by endangered species: A Habitat Selection Approach. Ecol. Solut. Evid. 2021, 2, e12035. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Lieske, D.J.; Tranquilla, L.M.F.; Ronconi, R.A.; Abbott, S. “Seas of risk”: Assessing the threats to colonial-nesting seabirds in Eastern Canada. Mar. Policy 2020, 115, 103863. [Google Scholar] [CrossRef]
- Lloret, J.; Biton-Porsmoguer, S.; Carreño, A.; Di Franco, A.; Sahyoun, R.; Melià, P.; Claudet, J.; Sève, C.; Ligas, A.; Belharet, M.; et al. Recreational and small-scale fisheries may pose a threat to vulnerable species in coastal and offshore waters of the western Mediterranean. ICES J. Mar. Sci. 2020, 77, 2255–2264. [Google Scholar] [CrossRef]
- Nel, R.; Punt, A.E.; Hughes, G.R. Are coastal protected areas always effective in achieving population recovery for nesting sea turtles? PLoS ONE 2013, 8, e63525. [Google Scholar] [CrossRef] [PubMed]
- Global Fishing Watch. 2021. Available online: www.globalfishingwatch.org (accessed on 1 October 2020).
- Marine Cadastre: An Ocean of Information. 2022. Available online: https://marinecadastre.gov (accessed on 29 September 2020).
- Esri Inc. ArcGIS Pro; Esri Inc.: Redlands, CA, USA, 2020; Available online: https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview (accessed on 1 October 2020).
- Lujala, P.; Jan, K.R.; Nadja, T. Fighting over oil: Introducing a new dataset. Confl. Manag. Peace Sci. 2007, 24, 239–256. [Google Scholar] [CrossRef]
- Suomi NPP Visible Infrared Imagine Radiometer Suite (VIIRS): NOAA Star Calibration Center. 2021. Available online: https://ncc.nesdis.noaa.gov/VIIRS/ (accessed on 2 September 2021).
- IUCN: Protected Areas. 2022. Available online: https://www.iucn.org/theme/protected-areas/our-work/world-database-protected-areas (accessed on 1 February 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 September 2021).
- Vito, M.; Muggeo, R. Segmented: An R Package to Fit Regression Models with Broken-Line Relationships. R News 2008, 8/1, 20–25. Available online: https://cran.r-project.org/doc/Rnews/ (accessed on 1 September 2021).
- Ashford, M.N.; Watling, J.I.; Hart, K.M. Male Sea Turtle Telemetry (2009–2020) Reveals High Overlap with Anthropogenic Threats in the Gulf of Mexico and Caribbean. U.S. Geological Survey Data Release. 2022. Available online: https://www.sciencebase.gov/catalog/item/62a8921ed34ec53d2770ec16 (accessed on 31 May 2022).
- Hart, K.M.; Lamont, M.M.; Sartain, A.R.; Fujisaki, I. Migration, foraging, and residency patterns for Northern Gulf loggerheads: Implications of local threats and international movements. PLoS ONE 2014, 9, e103453. [Google Scholar] [CrossRef]
- Allan, J.R.; Watson, J.E.M.; Di Marco, M.; O’Bryan, C.J.; Possingham, H.P.; Atkinson, S.C.; Venter, O. Hotspots of human impact on threatened terrestrial vertebrates. PLoS Biol. 2019, 17, 1–18. [Google Scholar] [CrossRef]
- Halpern, B.S.; Kappel, C.V.; Selkoe, K.A.; Micheli, F.; Ebert, C.M.; Kontgis, C.; Crain, C.M.; Martone, R.G.; Shearer, C.; Teck, S.J. Mapping cumulative human impacts to California Current marine ecosystems. Conserv. Lett. 2009, 2, 138–148. [Google Scholar] [CrossRef]
- Finkbeiner, E.M.; Wallace, B.P.; Moore, J.E.; Lewison, R.L.; Crowder, L.B.; Read, A.J. Cumulative estimates of sea turtle bycatch and mortality in USA fisheries between 1990 and 2007. Biol. Conserv. 2011, 144, 2719–2727. [Google Scholar] [CrossRef]
- Heaton, A.J.; Pulis, E.E.; Pitchford, J.L.; Hatchett, W.L.; Carron, A.M.; Solangi, M. Prevalence and transience of ingested fishing hooks in Kemp’s ridley sea turtles. Chelonian Conserv. Biol. 2016, 15, 257–264. [Google Scholar] [CrossRef][Green Version]
- Reich, K.; López-Castro, M.; Shaver, D.; Iseton, C.; Hart, K.; Hooper, M.; Schmitt, C. δ13C and δ15N in the endangered Kemp’s ridley sea turtle Lepidochelys kempii after the Deepwater Horizon oil spill. Endanger. Species Res. 2017, 33, 281–289. [Google Scholar] [CrossRef]
- Takeshita, R.; Bursian, S.J.; Colegrove, K.M.; Collier, T.K.; Deak, K.; Dean, K.M.; De Guise, S.; DiPinto, L.M.; Elferink, C.J.; Esbaugh, A.J.; et al. A review of the toxicology of oil in vertebrates: What we have learned following the Deepwater Horizon oil spill. J. Toxicol. Environ. Health Part B 2021, 24, 355–394. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.P.; Stacy, B.A.; Rissing, M.; Cacela, D.; Garrison, L.P.; Graettinger, G.D.; Holmes, J.V.; McDonald, T.; McLamb, D.; Schroeder, B. Estimating sea turtle exposures to Deepwater Horizon oil. Endanger. Species Res. 2017, 33, 51–67. [Google Scholar] [CrossRef]
- Caillouet, C.W.; Raborn, S.W.; Shaver, D.J.; Putman, N.F.; Gallaway, B.J.; Mansfield, K.L. Did declining carrying capacity for the Kemp’s ridley sea turtle population within the Gulf of Mexico contribute to the nesting setback in 2010–2017? Chelonian Conserv. Biol. 2018, 17, 123–133. [Google Scholar] [CrossRef]
- Bevan, E.; Wibbels, T.; Najera, B.M.; Martinez, M.A.; Martinez, L.A.; Reyes, D.J.; Hernandez, M.H.; Gamez, D.G.; Pena, L.J.; Burchfield, P.M. In situ nest and hatchling survival at Rancho Nuevo, the primary nesting beach of the Kemp’s ridley sea turtle, Lepidochelys kempii. Herpetol. Conserv. Biol. 2014, 9, 563–577. [Google Scholar]
- Kocmoud, A.R.; Wang, H.H.; Grant, W.E.; Gallaway, B.J. Population dynamics of the endangered Kemp’s ridley sea turtle following the 2010 oil spill in the Gulf of Mexico: Simulation of potential cause-effect relationships. Ecol. Modell. 2019, 392, 159–178. [Google Scholar] [CrossRef]
- Gerber, L.R.; Heppell, S.S. The use of demographic sensitivity analysis in marine species conservation planning. Biol. Conserv. 2004, 120, 121–128. [Google Scholar] [CrossRef]
- Revuelta, O.; Hawkes, L.; León, Y.M.; Godley, B.J.; Raga, J.A.; Tomás, J. Evaluating the importance of Marine Protected Areas for the conservation of hawksbill turtles Eretmochelys imbricata nesting in the Dominican Republic. Endanger. Species Res. 2015, 27, 169–180. [Google Scholar] [CrossRef]
- Zupan, M.; Bulleri, F.; Evans, J.; Fraschetti, S.; Guidetti, P.; Garcia-Rubies, A.; Sostres, M.; Asnaghi, V.; Caro, A.; Deudero, S.; et al. How good is your marine protected area at curbing threats? Biol. Conserv. 2018, 221, 237–245. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; Herman, P.M.J.; Bouma, T.J.; Lamers, L.P.M.; Van Katwijk, M.M.; Van Der Heide, T.; Mumby, P.J.; Silliman, B.R.; Engelhard, S.L.; Van De Kerk, M.; et al. Habitat collapse due to overgrazing threatens turtle conservation in marine protected areas. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132890. [Google Scholar] [CrossRef] [PubMed]
- Zupan, M.; Fragkopoulou, E.; Claudet, J.; Erzini, K.; Horta e Costa, B.; Gonçalves, E.J. Marine partially protected areas: Drivers of ecological effectiveness. Front. Ecol. Environ. 2018, 16, 381–387. [Google Scholar] [CrossRef]
- Guidetti, P.; Milazzo, M.; Bussotti, S.; Molinari, A.; Murenu, M.; Pais, A.; Spanò, N.; Balzano, R.; Agardy, T.; Boero, F.; et al. Italian marine reserve effectiveness: Does enforcement matter? Biol. Conserv. 2008, 141, 699–709. [Google Scholar] [CrossRef]
- Pilcher, N.J.; Rodriguez-Zarate, C.J.; Antonopoulou, M.A.; Mateos-Molina, D.; Das, H.S.; Bugla, I.A. Combining laparoscopy and satellite tracking: Successful round-trip tracking of female green turtles from feeding areas to nesting grounds and back. Glob. Ecol. Conserv. 2020, 23, e01169. [Google Scholar] [CrossRef]
- Cuevas, E.; Putman, N.F.; Uribe-Martínez, A.; López-Castro, M.C.; Guzmán-Hernández, V.; Gallegos-Fernández, S.A.; Liceaga-Correa, M.D.L.Á.; Trujillo-Córdova, J.A.; González-Díaz-Mirón, R.D.J.; Negrete-Phillipe, A.; et al. First spatial distribution analysis of male sea turtles in the southern Gulf of Mexico. Front. Mar. Sci. 2020, 7, 561846. [Google Scholar] [CrossRef]
- Chen, M.; Chen, T. Spatial and seasonal distribution of microplastics on sandy beaches along the coast of the Hengchun Peninsula, Taiwan. Mar. Pollut. Bull. 2020, 151, 110861. [Google Scholar] [CrossRef]
- Garrod, B.; Wilson, J.C. Nature on the Edge? Marine ecotourism in peripheral coastal areas. J. Sustain. Tour. 2004, 12, 95–120. [Google Scholar] [CrossRef]
- Key West Offshore World Championship. 2022. Available online: https://raceworldoffshore.com/key-west/ (accessed on 22 April 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashford, M.; Watling, J.I.; Hart, K. One Shell of a Problem: Cumulative Threat Analysis of Male Sea Turtles Indicates High Anthropogenic Threat for Migratory Individuals and Gulf of Mexico Residents. Remote Sens. 2022, 14, 3887. https://doi.org/10.3390/rs14163887
Ashford M, Watling JI, Hart K. One Shell of a Problem: Cumulative Threat Analysis of Male Sea Turtles Indicates High Anthropogenic Threat for Migratory Individuals and Gulf of Mexico Residents. Remote Sensing. 2022; 14(16):3887. https://doi.org/10.3390/rs14163887
Chicago/Turabian StyleAshford, Micah, James I. Watling, and Kristen Hart. 2022. "One Shell of a Problem: Cumulative Threat Analysis of Male Sea Turtles Indicates High Anthropogenic Threat for Migratory Individuals and Gulf of Mexico Residents" Remote Sensing 14, no. 16: 3887. https://doi.org/10.3390/rs14163887
APA StyleAshford, M., Watling, J. I., & Hart, K. (2022). One Shell of a Problem: Cumulative Threat Analysis of Male Sea Turtles Indicates High Anthropogenic Threat for Migratory Individuals and Gulf of Mexico Residents. Remote Sensing, 14(16), 3887. https://doi.org/10.3390/rs14163887




