Genus-Level Mapping of Invasive Floating Aquatic Vegetation Using Sentinel-2 Satellite Remote Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Acquisition and Pre-Processing of Sentinel-2 Imagery
2.3. Imaging Spectroscopy Acquisitions and Classification Process
2.4. Sentinel-2 Image Classification
2.4.1. Training and Validation Data
2.4.2. Sentinel-2 Random Forest Model Selection
3. Results
3.1. Model Accuracies
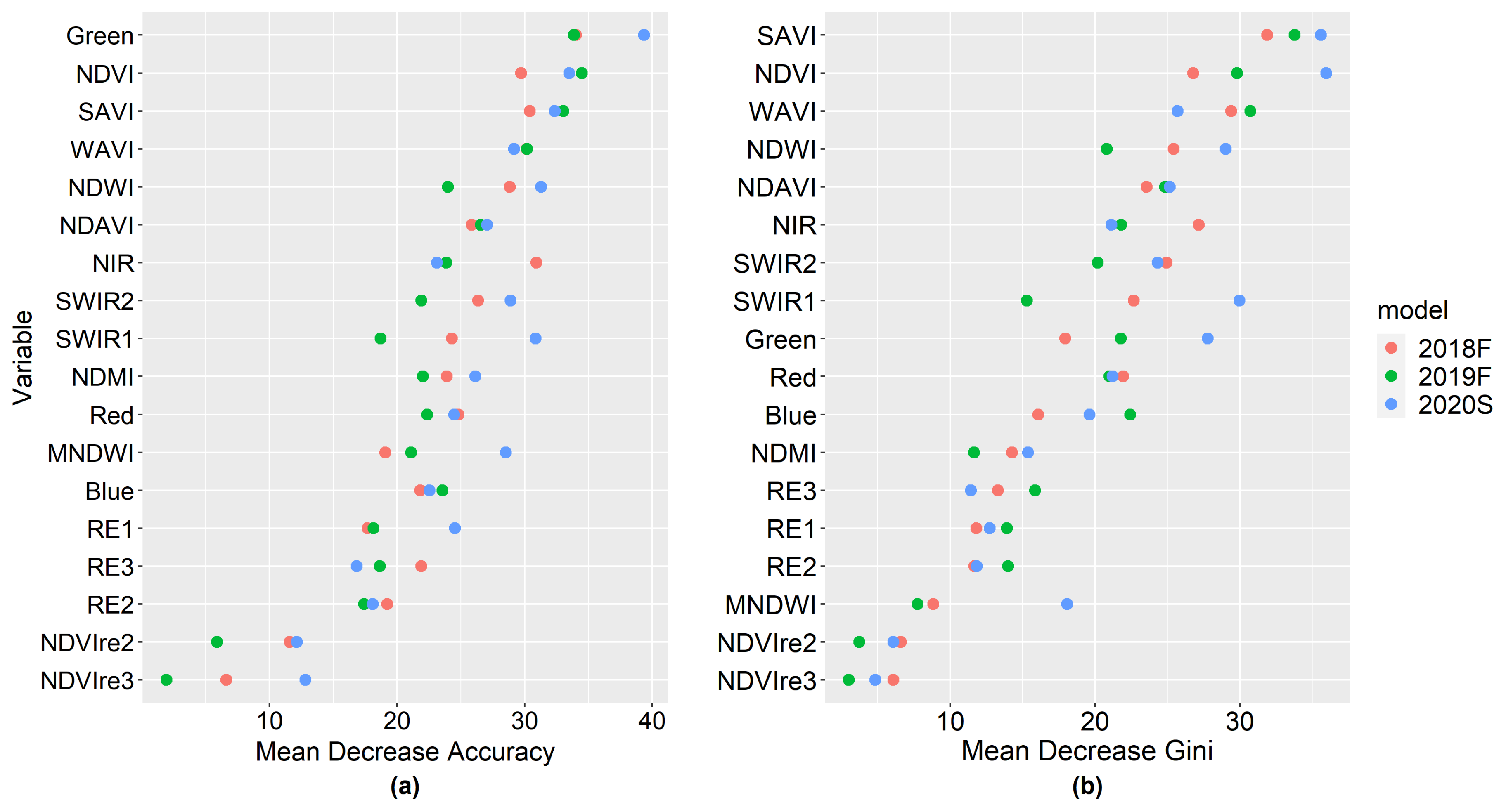
3.2. Sentinel-2 Forest Variable Importance
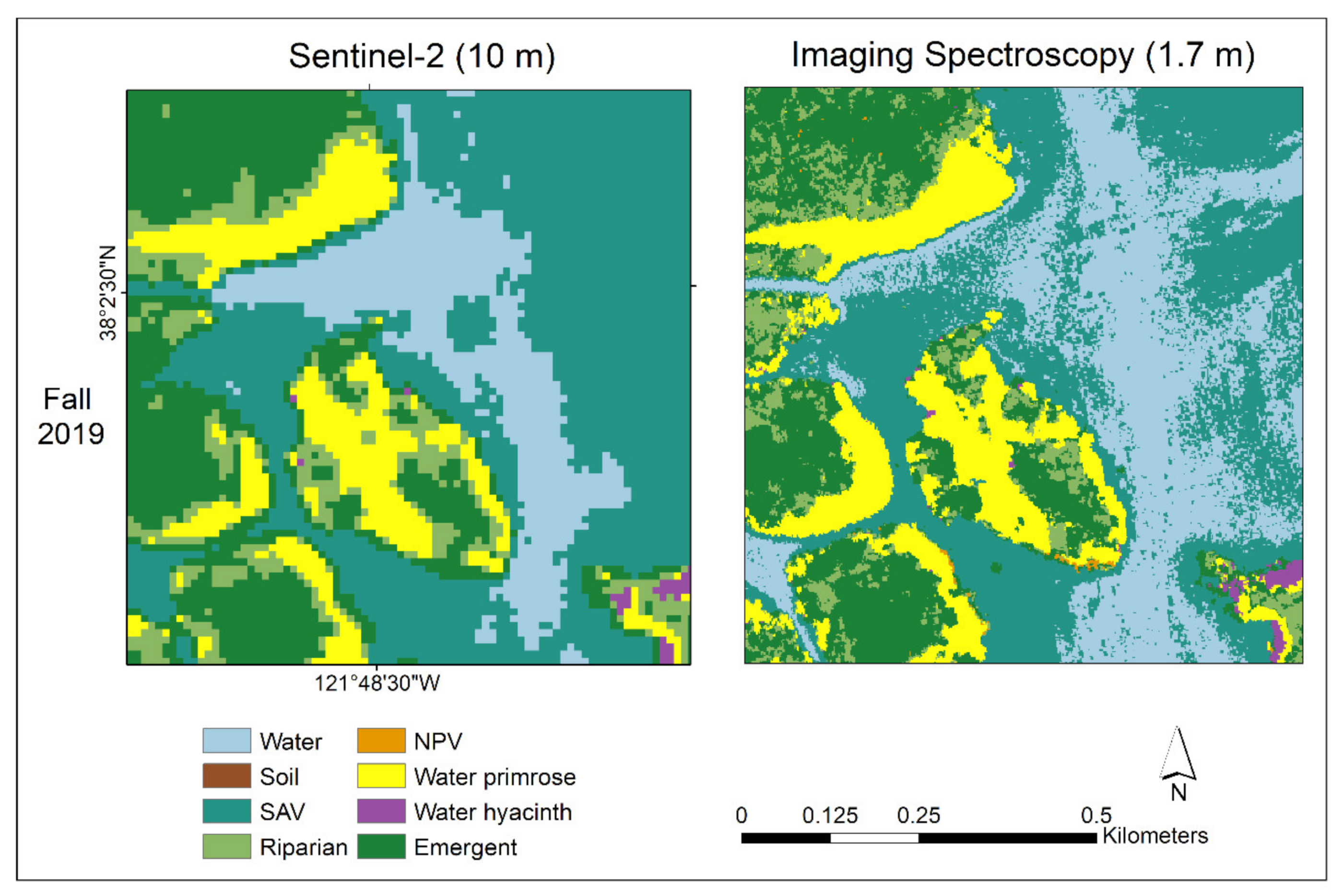
3.3. Sentinel-2 and AIS Genus-Level Map Comparison
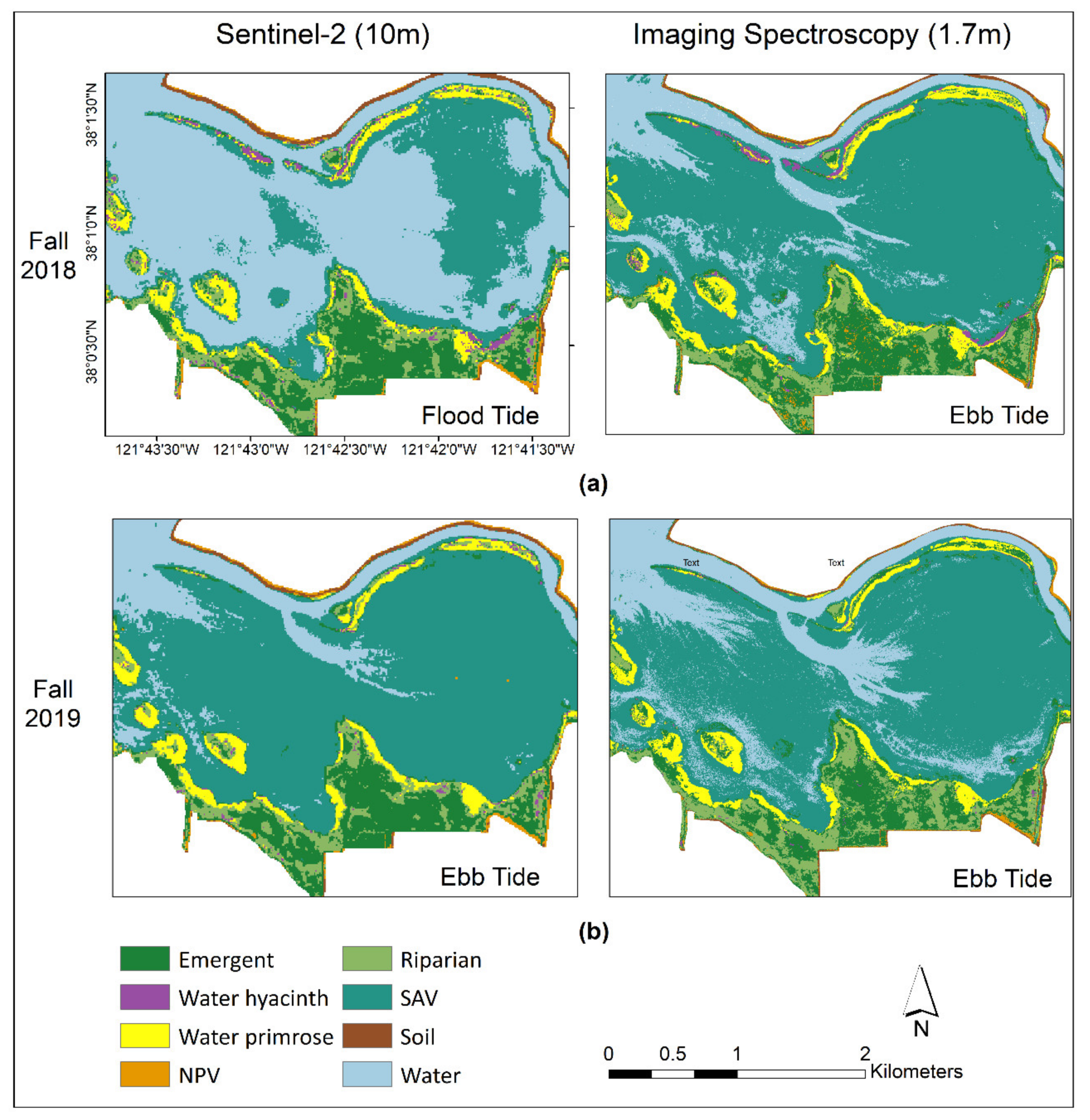
3.3.1. Visual Comparison in Two Sites
3.3.2. Percent Coverage Comparison
4. Discussion
4.1. Model Accuracies
4.2. Percent Coverage of FAV Classes
4.3. Impact of Environmental Conditions: Tidal Stage
4.4. Sentinel-2 Characteristics That Enable Differentiation between FAV Classes and the Neighboring Vegetation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Date | Water Hyacinth | Water Primrose | Emergent | Riparian | SAV | Water | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S2 | AIS | S2 | AIS | S2 | AIS | S2 | AIS | S2 | AIS | S2 | AIS | ||
| 2018F | Ward Cut | 1.2 | 1.4 | 5.0 | 5.3 | 14.7 | 17.6 | 10.3 | 7.5 | 19.5 | 18.8 | 39.6 | 41.7 |
| Rhode Island | 5.0 | 4.6 | 32.3 | 38.8 | 4.1 | 5.4 | 14.7 | 4.3 | 19.1 | 17.2 | 22.2 | 27.4 | |
| Big Break | 1.5 | 1.3 | 5.6 | 6.4 | 11.0 | 13.2 | 7.7 | 5.9 | 19.1 | 50.6 | 51.8 | 19.7 | |
| Liberty | 0.3 | 0.3 | 1.9 | 2.4 | 21.9 | 23.2 | 7.2 | 4.6 | 24.2 | 26.4 | 42.1 | 39.4 | |
| Delta | 0.9 | 0.7 | 1.6 | 2.2 | 11.8 | 14.5 | 7.5 | 5.5 | 13.6 | 19.4 | 49.9 | 50.4 | |
| 2019F | Ward Cut | 1.4 | 1.6 | 6.0 | 6.2 | 14.2 | 11.9 | 10.8 | 13.4 | 13.6 | 14.0 | 45.0 | 45.1 |
| Rhode Island | 5.8 | 4.0 | 30.0 | 31.9 | 6.0 | 8.2 | 17.4 | 12.0 | 15.6 | 14.8 | 22.4 | 24.5 | |
| Big Break | 0.7 | 0.4 | 6.6 | 6.6 | 12.0 | 11.9 | 6.8 | 7.9 | 53.4 | 46.7 | 17.3 | 23.5 | |
| Liberty | 0.4 | 0.2 | 1.6 | 2.3 | 25.5 | 19.2 | 5.5 | 12.3 | 33.2 | 26.2 | 32.4 | 36.9 | |
| Delta | 0.6 | 0.7 | 1.7 | 2.0 | 15.7 | 11.6 | 6.1 | 10.7 | 15.5 | 17.9 | 47.2 | 47.1 | |
| 2020S | Ward Cut | 2.0 | 1.6 | 5.0 | 6.9 | 13.3 | 10.8 | 13.2 | 12 | 14.9 | 17.6 | 41.8 | 42.8 |
| Rhode Island | 6.0 | 4.9 | 30.7 | 34.4 | 6.0 | 4.9 | 30.7 | 34.4 | 6.0 | 4.9 | 30.7 | 34.4 | |
| Big Break | 1.4 | 1.1 | 5.9 | 5.3 | 12.2 | 12.7 | 7.6 | 8.0 | 42.6 | 50.6 | 27.1 | 17.8 | |
| Liberty | 0.5 | 0.5 | 1.9 | 0.8 | 25.3 | 19.6 | 5.9 | 9.0 | 38.1 | 30.3 | 26.1 | 34.4 | |
| Delta | 1.4 | 1.0 | 1.5 | 1.9 | 15.4 | 10.1 | 6.9 | 10.2 | 13.1 | 13.5 | 47.5 | 53.8 | |
References
- Gordon, D.R. Effects of invasive, non-indigenous plant species on ecosystem processes: Lessons from florida. Conserv. Biol. 975 Ecol. Appl. 1998, 8, 975–989. [Google Scholar] [CrossRef]
- Scheffer, M.; Szabo, S.; Gragnani, A.; Van Nes, E.H.; Rinaldi, S.; Kautsky, N.; Norberg, J.; Roijackers, R.M.M.; Franken, R.J.M. Floating Plant Dominance as a Stable State. Proc. Natl. Acad. Sci. USA 2003, 100, 4040–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dukes, J.S.; Mooney, H.A. Disruption of Ecosystem Processes in Western North America by Invasive Species. Rev. Chil. Hist. Nat. 2004, 77, 411–437. [Google Scholar] [CrossRef]
- O’Farrell, I.; De Tezanos Pinto, P.; Rodríguez, P.L.; Chaparro, G.; Pizarro, H.N. Experimental Evidence of the Dynamic Effect of Free-Floating Plants on Phytoplankton Ecology. Freshw. Biol. 2009, 54, 363–375. [Google Scholar] [CrossRef]
- Ongore, C.O.; Aura, C.M.; Ogari, Z.; Njiru, J.M.; Nyamweya, C.S. Spatial-Temporal Dynamics of Water Hyacinth, Eichhornia crassipes (Mart.) and Other Macrophytes and Their Impact on Fisheries in Lake Victoria, Kenya. J. Great Lakes Res. 2018, 44, 1273–1280. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Thouvenot, L.; Haury, J.; Thiebaut, G. A Success Story: Water Primroses, Aquatic Plant Pests. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 790–803. [Google Scholar] [CrossRef]
- Thamaga, K.H.; Dube, T. Remote Sensing of Invasive Water Hyacinth (Eichhornia crassipes): A Review on Applications and Challenges. Remote Sens. Appl. Soc. Environ. 2018, 10, 36–46. [Google Scholar] [CrossRef]
- Adam, E.; Mutanga, O.; Rugege, D. Multispectral and Hyperspectral Remote Sensing for Identification and Mapping of Wetland Vegetation: A Review. Wetl. Ecol. Manag. 2010, 18, 281–296. [Google Scholar] [CrossRef]
- Gallant, A.L. The Challenges of Remote Monitoring of Wetlands. Remote Sens. 2015, 7, 10938–10950. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Li, J.; Sheng, C.; Xu, J.; Wu, L. A Review of Wetland Remote Sensing. Sensors 2017, 17, 777. [Google Scholar] [CrossRef] [Green Version]
- Everitt, J.H.; Yang, C.; Summy, K.R.; Owens, C.S.; Glomski, L.M.; Smart, R.M. Using in Situ Hyperspectral Reflectance Data to Distinguish Nine Aquatic Plant Species. Geocarto Int. 2011, 26, 459–473. [Google Scholar] [CrossRef]
- Hestir, E.L.; Khanna, S.; Andrew, M.E.; Santos, M.J.; Viers, J.H.; Greenberg, J.A.; Rajapakse, S.S.; Ustin, S.L. Identification of Invasive Vegetation Using Hyperspectral Remote Sensing in the California Delta Ecosystem. Remote Sens. Environ. 2008, 112, 4034–4047. [Google Scholar] [CrossRef]
- Khanna, S.; Santos, M.J.; Ustin, S.L.; Haverkamp, P.J. An Integrated Approach to a Biophysiologically Based Classification of Floating Aquatic Macrophytes. Int. J. Remote Sens. 2011, 32, 1067–1094. [Google Scholar] [CrossRef]
- Santos, M.J.; Hestir, E.L.; Khanna, S.; Ustin, S.L. Image Spectroscopy and Stable Isotopes Elucidate Functional Dissimilarity between Native and Nonnative Plant Species in the Aquatic Environment. New Phytol. 2012, 193, 683–695. [Google Scholar] [CrossRef]
- Schmidt, K.S.; Skidmore, A.K. Spectral Discrimination of Vegetation Types in a Coastal Wetland. Remote Sens. Environ. 2003, 85, 92–108. [Google Scholar] [CrossRef]
- Bolch, E.A.; Hestir, E.L.; Khanna, S. Performance and Feasibility of Drone-Mounted Imaging Spectroscopy for Invasive Aquatic Vegetation Detection. Remote Sens. 2021, 13, 582. [Google Scholar] [CrossRef]
- Khanna, S.; Santos, M.J.; Hestir, E.L.; Ustin, S.L. Plant Community Dynamics Relative to the Changing Distribution of a Highly Invasive Species, Eichhornia crassipes: A Remote Sensing Perspective. Biol. Invasions 2012, 14, 717–733. [Google Scholar] [CrossRef]
- Kleinschroth, F.; Winton, R.S.; Calamita, E.; Niggemann, F.; Botter, M.; Wehrli, B.; Ghazoul, J. Living with Floating Vegetation Invasions. Ambio 2021, 50, 125–137. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Dong, D.; Wang, C.; Yan, J.; He, Q.; Zeng, J.; Wei, Z. Combing Sentinel-1 and Sentinel-2 Image Time Series for Invasive Spartina Alterniflora Mapping on Google Earth Engine: A Case Study in Zhangjiang Estuary. J. Appl. Remote Sens. 2020, 14, 1–18. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, C.; Zhang, L.; Bai, J.; Zhou, D. Assessing Spatiotemporal Characteristics of Native and Invasive Species with Multi-Temporal Remote Sensing Images in the Yellow River Delta, China. L. Degrad. Dev. 2021, 32, 1338–1352. [Google Scholar] [CrossRef]
- Dersseh, M.G.; Tilahun, S.A.; Worqlul, A.W.; Moges, M.A.; Abebe, W.B.; Mhiret, D.A.; Melesse, A.M. Spatial and Temporal Dynamics of Water Hyacinth and Its Linkage with Lake-Level Fluctuation: Lake Tana, a Sub-Humid Region of the Ethiopian Highlands. Water 2020, 12, 1435. [Google Scholar] [CrossRef]
- Singh, G.; Reynolds, C.; Byrne, M.; Rosman, B. A Remote Sensing Method to Monitor Water, Aquatic Vegetation, and Invasive Water Hyacinth at National Extents. Remote Sens. 2020, 12, 4021. [Google Scholar] [CrossRef]
- Thamaga, K.H.; Dube, T. Testing Two Methods for Mapping Water Hyacinth (Eichhornia crassipes) in the Greater Letaba River System, South Africa: Discrimination and Mapping Potential of the Polar-Orbiting Sentinel-2 MSI and Landsat 8 OLI Sensors. Int. J. Remote Sens. 2018, 39, 8041–8059. [Google Scholar] [CrossRef]
- Khanna, S.; Ustin, S.L.; Hestir, E.L.; Santos, M.J.; Andrew, M.; Lay, M.; Tuil, J.; Bellvert, J.; Greenberg, J.; Shapiro, K.D.; et al. The Sacramento-San Joaquin Delta Genus and Community Level Classification Maps Derived. Airborne Spectroscopy Data. 2022. Available online: https://search-dev.test.dataone.org/view/doi%3A10.5063%2FF1K9360F (accessed on 3 May 2022).
- Jetter, K.M.; Nes, K.; Tahoe, L. The Cost to Manage Invasive Aquatic Weeds in the California Bay-Delta. ARE Updat. 2018, 21, 9–11. [Google Scholar]
- Rejmánková, E. Ecology of Creeping Macrophytes with Special Reference to Ludwigia peploides (H.B.K.) Raven. Aquat. Bot. 1992, 43, 283–299. [Google Scholar] [CrossRef]
- Penfound, W.T.; Earle, T.T. The Biology of the Water Hyacinth. Ecol. Monogr. 1948, 18, 447–472. [Google Scholar] [CrossRef]
- Grewell, B.J.; Gillard, M.B.; Futrell, C.J.; Castillo, J.M. Seedling Emergence from Seed Banks in Ludwigia hexapetala-Invaded Wetlands: Implications for Restoration. Plants 2019, 8, 451. [Google Scholar] [CrossRef] [Green Version]
- Malik, A. Environmental Challenge Vis a Vis Opportunity: The Case of Water Hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef]
- Ta, J.; Anderson, L.W.J.; Christman, M.A.; Khanna, S.; Kratville, D.; Madsen, J.D.; Moran, P.J.; Viers, J.H.; Ta, J.; Anderson, L.W.J.; et al. Invasive Aquatic Vegetation Management in the Sacramento-San Joaquin River Delta: Status and Recommendations. San Fr. Estuary Watershed Sci. 2017, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.N.; Carlton, J.T. Accelerating Invasion Rate in a Highly Invaded Estuary. Science 1998, 279, 555–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DBW. Aquatic Invasive Plant Control Program 2019 Annual Monitoring Report; California Department of Boating and Waterways: Sacramento, CA, USA, 2019. [Google Scholar]
- Khanna, S.; Acuna, S.; Contreras, D.; Griffiths, W.K.; Lesmeister, S.; Reyes, R.C.; Schreier, B.; Wu, B.J. Invasive Aquatic Vegetation Impacts on Delta Operations, Monitoring, and Ecosystem and Human Health. IEP Newsl. 2019, 36, 8–18. [Google Scholar]
- Hestir, E.L.; Greenberg, J.A.; Ustin, S.L. Classification Trees for Aquatic Vegetation Community Prediction from Imaging Spectroscopy. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 1572–1584. [Google Scholar] [CrossRef]
- Ustin, S.L.; Khanna, S.; Lay, M. Remote Sensing of the Sacramento-San Joaquin Delta to Enhance Mapping for Invasive and Native Aquatic Plant Species; University of California, Davis. Report to The Delta Stewardship Council; University of California Davis: Davis, CA, USA, 2021. [Google Scholar]
- Ranghetti, L.; Boschetti, M.; Nutini, F.; Busetto, L. “Sen2r”: An R Toolbox for Automatically Downloading and Preprocessing Sentinel-2 Satellite Data. Comput. Geosci. 2020, 139, 104473. [Google Scholar] [CrossRef]
- Khanna, S.; Santos, M.J.; Boyer, J.D.; Shapiro, K.D.; Bellvert, J.; Ustin, S.L. Water Primrose Invasion Changes Successional Pathways in an Estuarine Ecosystem. Ecosphere 2018, 9, e02418. [Google Scholar] [CrossRef]
- Rosenfield, G.H.; Fitzpatrick-Lins, K. A Coefficient of Agreement as a Measure of Thematic Classification Accuracy. Photogramm. Eng. Remote Sens. 1986, 52, 223–227. [Google Scholar]
- Story, M.; Congalton, R.G. Accuracy Assessment: A User’s Perspective. Photogramm. Eng. Remote Sens. 1986, 52, 397–399. [Google Scholar]
- Berhane, T.M.; Lane, C.R.; Wu, Q.; Autrey, B.C.; Anenkhonov, O.A.; Chepinoga, V.V.; Liu, H. Decision-Tree, Rule-Based, and Random Forest Classification of High-Resolution Multispectral Imagery for Wetland Mapping and Inventory. Remote Sens. 2018, 10, 580. [Google Scholar] [CrossRef] [Green Version]
- Magidi, J.; Nhamo, L.; Mpandeli, S.; Mabhaudhi, T. Application of the Random Forest Classifier to Map Irrigated Areas Using Google Earth Engine. Remote Sens. 2021, 13, 876. [Google Scholar] [CrossRef]
- Mahdianpari, M.; Salehi, B.; Mohammadimanesh, F.; Motagh, M. Random Forest Wetland Classification Using ALOS-2 L-Band, RADARSAT-2 C-Band, and TerraSAR-X Imagery. ISPRS J. Photogramm. Remote Sens. 2017, 130, 13–31. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2, 18–22. [Google Scholar]
- Kuhn, M. Building Predictive Models in R Using the Caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Villa, P.; Laini, A.; Bresciani, M.; Bolpagni, R. A Remote Sensing Approach to Monitor the Conservation Status of Lacustrine Phragmites australis Beds. Wetl. Ecol. Manag. 2013, 21, 399–416. [Google Scholar] [CrossRef]
- Villa, P.; Mousivand, A.; Bresciani, M. Aquatic Vegetation Indices Assessment through Radiative Transfer Modeling and Linear Mixture Simulation. Int. J. Appl. Earth Obs. Geoinf. 2014, 30, 113–127. [Google Scholar] [CrossRef]
- Huete, A.R. A Soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus Hippocastanum L. and Acer Platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Fernández-Manso, A.; Fernández-Manso, O.; Quintano, C. SENTINEL-2A Red-Edge Spectral Indices Suitability for Discriminating Burn Severity. Int. J. Appl. Earth Obs. Geoinf. 2016, 50, 170–175. [Google Scholar] [CrossRef]
- McFeeters, S.K. The Use of the Normalized Difference Water Index (NDWI) in the Delineation of Open Water Features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A Normalized Difference Water Index for Remote Sensing of Vegetation Liquid Water from Space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Xu, H. Modification of Normalised Difference Water Index (NDWI) to Enhance Open Water Features in Remotely Sensed Imagery. Int. J. Remote Sens. 2006, 27, 3025–3033. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Han, H.; Guo, X.; Yu, H. Variable Selection Using Mean Decrease Accuracy and Mean Decrease Gini Based on Random Forest. In Proceedings of the 2016 7th IEEE International Conference on Software Engineering and Service Science (ICSESS), Beijing, China, 26–28 August 2016; pp. 219–224. [Google Scholar] [CrossRef]
- Dogliotti, A.I.; Gossn, J.I.; Vanhellemont, Q.; Ruddick, K.G. Detecting and Quantifying a Massive Invasion of Floating Aquatic Plants in the Río de La Plata Turbid Waters Using High Spatial Resolution Ocean Color Imagery. Remote Sens. 2018, 10, 1140. [Google Scholar] [CrossRef] [Green Version]
- Thamaga, K.H.; Dube, T. Understanding Seasonal Dynamics of Invasive Water Hyacinth (Eichhornia crassipes) in the Greater Letaba River System Using Sentinel-2 Satellite Data. GISci. Remote Sens. 2019, 56, 1355–1377. [Google Scholar] [CrossRef]
- Villa, P.; Bresciani, M.; Bolpagni, R.; Pinardi, M.; Giardino, C. A Rule-Based Approach for Mapping Macrophyte Communities Using Multi-Temporal Aquatic Vegetation Indices. Remote Sens. Environ. 2015, 171, 218–233. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Dronova, I.; Gong, P.; Yang, W.; Li, Y.; Liu, Q. A New Time Series Vegetation-Water Index of Phenological-Hydrological Trait across Species and Functional Types for Poyang Lake Wetland Ecosystem. Remote Sens. Environ. 2012, 125, 49–63. [Google Scholar] [CrossRef]
- Frazier, A.E. Landscape Heterogeneity and Scale Considerations for Super-Resolution Mapping. Int. J. Remote Sens. 2015, 36, 2395–2408. [Google Scholar] [CrossRef]
- Knight, J.F.; Lunetta, R.S. An Experimental Assessment of Minimum Mapping Unit Size. IEEE Trans. Geosci. Remote Sens. 2003, 41, 2132–2134. [Google Scholar] [CrossRef]
- Roy, D.P.; Li, J.; Zhang, H.K.; Yan, L. Best Practices for the Reprojection and Resampling of Sentinel-2 Multi Spectral Instrument Level 1C Data. Remote Sens. Lett. 2016, 7, 1023–1032. [Google Scholar] [CrossRef]
- Zheng, H.; Du, P.; Chen, J.; Xia, J.; Li, E.; Xu, Z.; Li, X.; Yokoya, N. Performance Evaluation of Downscaling Sentinel-2 Imagery for Land Use and Land Cover Classification by Spectral-Spatial Features. Remote Sens. 2017, 9, 1274. [Google Scholar] [CrossRef] [Green Version]
- Morrison, B.D.; Heath, K.; Greenberg, J.A. Spatial Scale Affects Novel and Disappeared Climate Change Projections in Alaska. Ecol. Evol. 2019, 9, 12026–12044. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, V.C.; Khanna, S.; Ustin, S.L. Impact of Pixel Size on Mapping Surface Water in Subsolar Imagery. Remote Sens. Environ. 2007, 109, 1–9. [Google Scholar] [CrossRef]
- Tian, Y.; Jia, M.; Wang, Z.; Mao, D.; Du, B.; Wang, C. Monitoring Invasion Process of Spartina Alterniflora by Seasonal Sentinel-2 Imagery and an Object-Based Random Forest Classification. Remote Sens. 2020, 12, 1383. [Google Scholar] [CrossRef]
- Amani, M.; Salehi, B.; Mahdavi, S.; Brisco, B. Spectral Analysis of Wetlands Using Multi-Source Optical Satellite Imagery. ISPRS J. Photogramm. Remote Sens. 2018, 144, 119–136. [Google Scholar] [CrossRef]
- Hestir, E.L.; Brando, V.E.; Bresciani, M.; Giardino, C.; Matta, E.; Villa, P.; Dekker, A.G. Measuring Freshwater Aquatic Ecosystems: The Need for a Hyperspectral Global Mapping Satellite Mission. Remote Sens. Environ. 2015, 167, 181–195. [Google Scholar] [CrossRef] [Green Version]
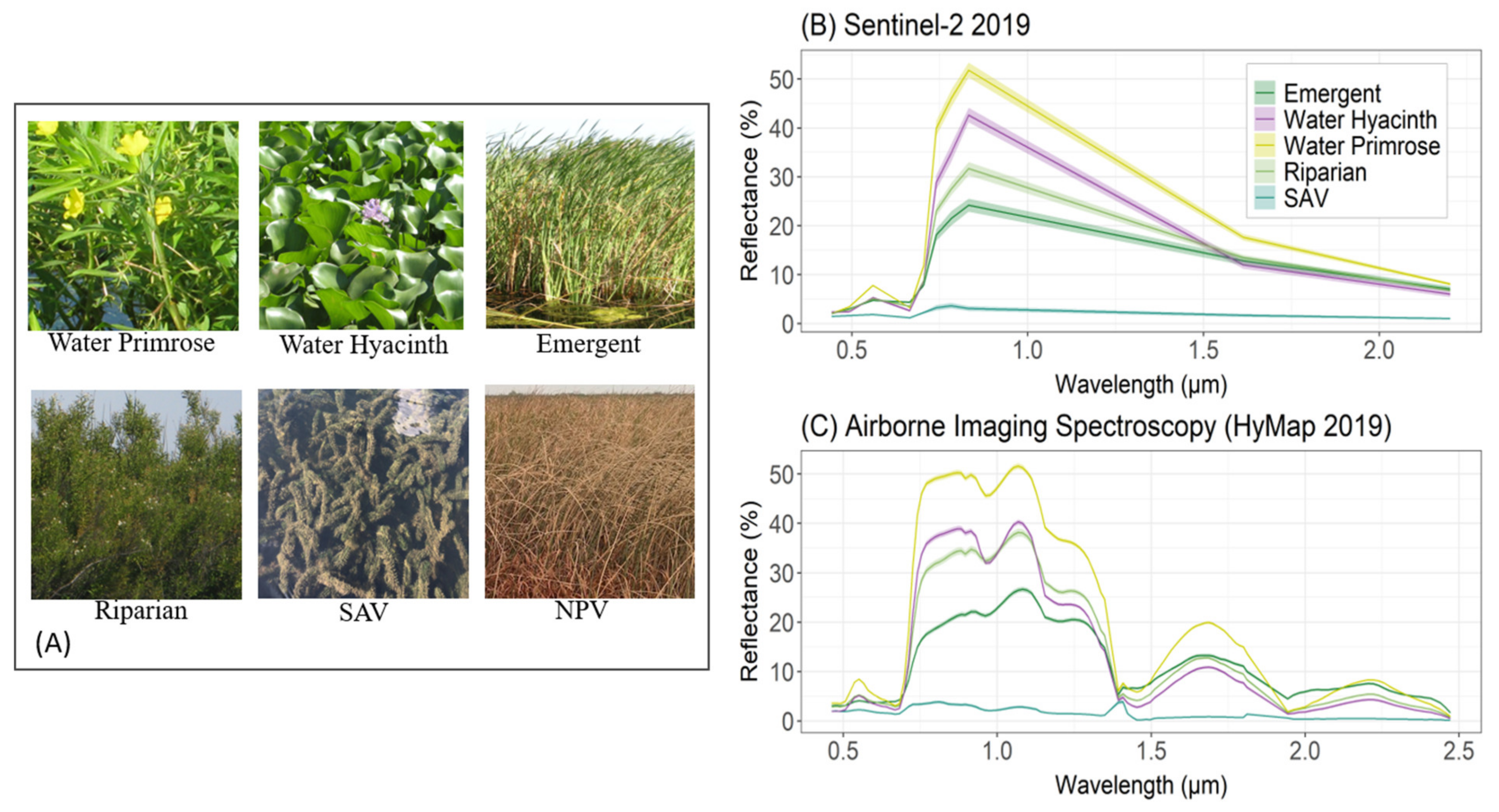
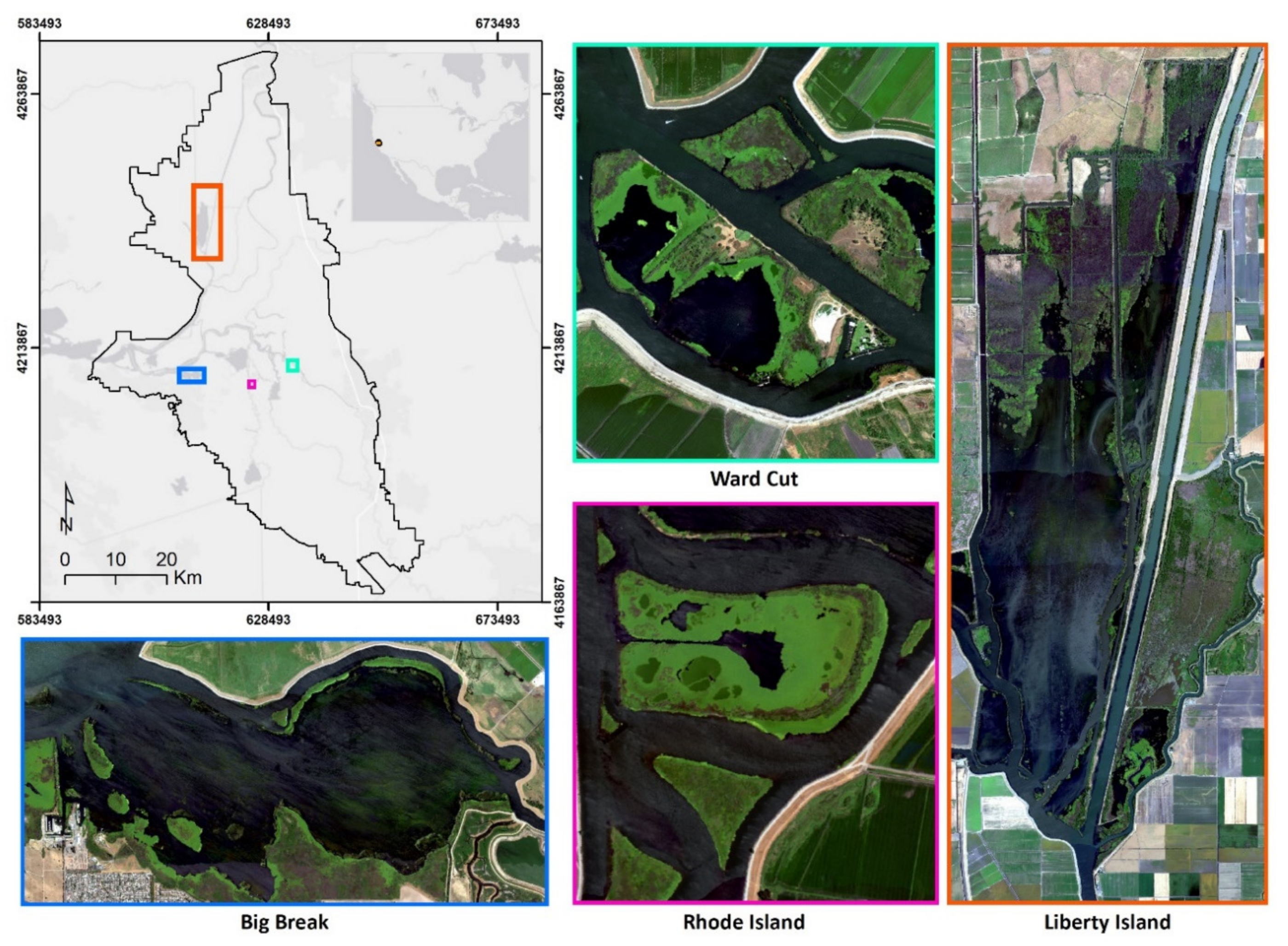


| Map Class | Description |
|---|---|
| Water Hyacinth | Pontederia crassipes |
| Water Primrose | Ludwigia spp. |
| Emergent Vegetation (EAV) | Cattail (Typha spp.) Common reed (Phragmites australis) Tule (Schoenoplectus spp.) |
| Riparian | For example: Willow species (Salix spp.), Oak species (Quercus spp.), and Cottonwood (Populus spp.) |
| Submerged Aquatic Vegetation (SAV) | Algae mats Brazilian waterweed (Egeria densa) Coontail (Ceratophyllum demersum) Curly leaf pondweed (Pomatogedon crispus) Fanwort (Cabomba caroliniana) Sago pondweed (Stuckenia pectinata) Watermilfoil (Myriophyllum spicatum) Waterweed (Elodea canadensis) |
| Non-Photosynthetic Vegetation (NPV) | Senescent or dead vegetation |
| Soil | Soil |
| Water | Water |
| Match up Date | Imaging Spectrometer Acquisition Date | Closest Sentinel-2 Image Date | Imaging Spectrometer | Sentinel-2 Sensor | Tidal Range AIS 1 (m) | Tidal Range S2 (m) |
|---|---|---|---|---|---|---|
| Fall 2018 | 6–9 October 2018 | 7 October 2018 | HyMap | S2B | 0.01–0.25 | 0.49–0.64 |
| Fall 2019 | 23–27 September 2019 | 2 October 2019 | HyMap | S2B | 0.01–1.02 | 0.28–0.39 |
| Summer 2020 | 15–18 July 2020 | 18 July 2020 | Fenix 1K | S2B | 0.01–0.37 | 0.17–0.30 |
| Sentinel-2 Bands | Wavelengths (μm) | Spatial Resolution (m) |
|---|---|---|
| Band 1—Coastal Aerosol | 0.430–0.457 | 60 |
| Band 2—Blue | 0.440–0.538 | 10 |
| Band 3—Green | 0.537–0.582 | 10 |
| Band 4—Red | 0.646–0.684 | 10 |
| Band 5—Vegetation Red Edge 1 | 0.694–0.713 | 20 |
| Band 6—Vegetation Red Edge 2 | 0.731–0.749 | 20 |
| Band 7—Vegetation Red Edge 3 | 0.769–0.797 | 20 |
| Band 8—NIR | 0.785–0.900 | 10 |
| Band 8A—Narrow NIR * | 0.849–0.881 | 20 |
| Band 9—Water Vapor * | 0.932–0.958 | 60 |
| Band 10—Cirrus * | 1.337–1.412 | 60 |
| Band 11—SWIR 1 | 1.539–1.682 | 20 |
| Band 12—SWIR 2 | 2.078–2.320 | 20 |
| Index | Formula | Source |
|---|---|---|
| NDVI | [48] | |
| NDAVI | [49] | |
| WAVI | * Here, L = 0.5 | [50] |
| SAVI | * Here, L = 0.5 | [51] |
| NDVIRe2 | [52] | |
| NDVIRe3 | [53] | |
| NDWI | [54] | |
| NDMI | [55] | |
| MNDWI | [56] |
| Class | Type of Accuracy | 2018F | 2019F | 2020S | |||
|---|---|---|---|---|---|---|---|
| S2 | AIS | S2 | AIS | S2 | AIS | ||
| OA (%) | 87 | 91 | 89 | 90 | 90 | 90 | |
| Kappa | 0.85 | 0.9 | 0.88 | 0.89 | 0.89 | 0.89 | |
| Water Hyacinth | PA (%) | 79 | 86 | 78 | 94 | 80 | 88 |
| UA (%) | 94 | 89 | 85 | 89 | 93 | 92 | |
| Water Primrose | PA (%) | 91 | 94 | 94 | 95 | 96 | 91 |
| UA (%) | 83 | 92 | 92 | 95 | 80 | 89 | |
| Emergent | PA (%) | 82 | 83 a|73 b | 81 | 83 a|84 b | 86 | 85 a|61 b |
| UA (%) | 77 | 87 a|83 b | 79 | 87 a|88 b | 86 | 81 a|76 b | |
| Riparian | PA (%) | 74 | 97 | 78 | 90 | 78 | 92 |
| UA (%) | 80 | 94 | 75 | 90 | 85 | 84 | |
| SAV | PA (%) | 86 | 91 | 90 | 79 | 96 | 91 |
| UA (%) | 84 | 83 | 92 | 82 | 91 | 97 | |
| Water | PA (%) | 88 | 91 | 92 | 84 | 90 | 99 |
| UA (%) | 90 | 92 | 90 | 83 | 96 | 91 | |
| Date | Water Hyacinth | Water Primrose | EAV | Riparian | SAV | Water | |
|---|---|---|---|---|---|---|---|
| 2018F | Ward Cut | −0.2 | −0.3 | −2.9 | 2.8 | 0.7 | −2.1 |
| Rhode Island | 0.4 | −6.5 | −1.3 | 10.4 | 1.9 | −5.2 | |
| Big Break | 0.2 | −0.8 | −2.2 | 1.8 | −31.5 | 32.1 | |
| Liberty | 0 | −0.5 | −1.3 | 2.6 | −2.2 | 2.7 | |
| Delta | 0.2 | −0.6 | −2.7 | 2 | −5.8 | −0.5 | |
| 2019F | Ward Cut | −0.2 | −0.2 | 2.3 | −2.6 | −0.4 | −0.1 |
| Rhode Island | 1.8 | −1.9 | −2.2 | 5.4 | 0.8 | −2.1 | |
| Big Break | 0.3 | 0 | 0.1 | −1.1 | 6.7 | −6.2 | |
| Liberty | 0.2 | −0.7 | 6.3 | −6.8 | 7 | −4.5 | |
| Delta | −0.1 | −0.3 | 4.1 | −4.6 | −2.4 | 0.1 | |
| 2020S | Ward Cut | 0.4 | −1.9 * | 2.5 * | 1.2 * | −2.7 | −1 |
| Rhode Island | 1.1 | −3.7 * | 1.1 * | 7.7 * | 7.4 | −12.7 | |
| Big Break | 0.3 | 0.6 * | −0.5 * | −0.4 * | −8 | 9.3 | |
| Liberty | 0 | 1.1 * | 5.7 * | −3.1 * | 7.8 | −8.3 | |
| Delta | 0.4 | −0.4 * | 5.3 * | −3.3 * | −0.4 | −6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ade, C.; Khanna, S.; Lay, M.; Ustin, S.L.; Hestir, E.L. Genus-Level Mapping of Invasive Floating Aquatic Vegetation Using Sentinel-2 Satellite Remote Sensing. Remote Sens. 2022, 14, 3013. https://doi.org/10.3390/rs14133013
Ade C, Khanna S, Lay M, Ustin SL, Hestir EL. Genus-Level Mapping of Invasive Floating Aquatic Vegetation Using Sentinel-2 Satellite Remote Sensing. Remote Sensing. 2022; 14(13):3013. https://doi.org/10.3390/rs14133013
Chicago/Turabian StyleAde, Christiana, Shruti Khanna, Mui Lay, Susan L. Ustin, and Erin L. Hestir. 2022. "Genus-Level Mapping of Invasive Floating Aquatic Vegetation Using Sentinel-2 Satellite Remote Sensing" Remote Sensing 14, no. 13: 3013. https://doi.org/10.3390/rs14133013
APA StyleAde, C., Khanna, S., Lay, M., Ustin, S. L., & Hestir, E. L. (2022). Genus-Level Mapping of Invasive Floating Aquatic Vegetation Using Sentinel-2 Satellite Remote Sensing. Remote Sensing, 14(13), 3013. https://doi.org/10.3390/rs14133013







