Maize Yield Estimation in Intercropped Smallholder Fields Using Satellite Data in Southern Malawi
Abstract
:1. Introduction
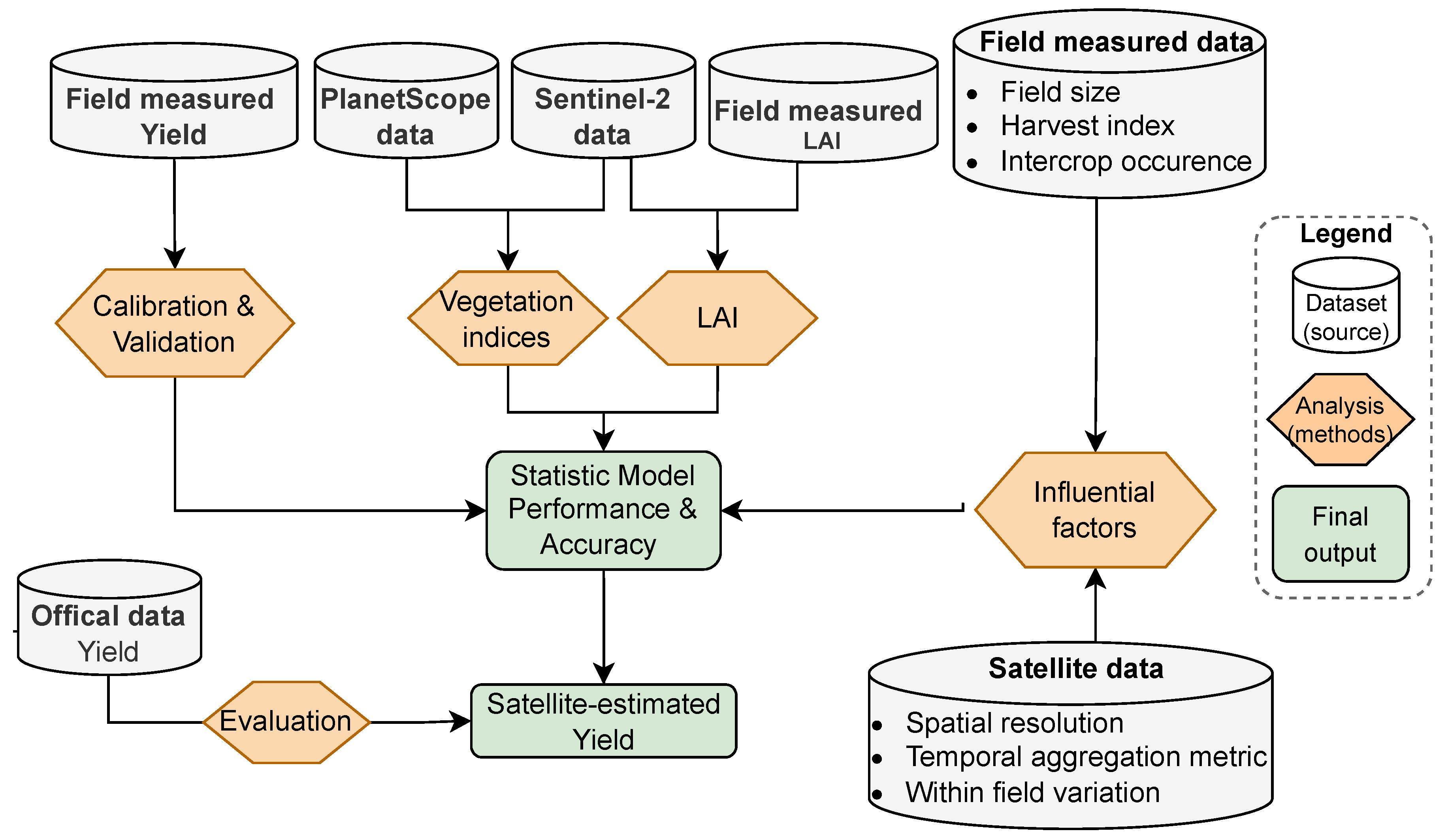
2. Data and Methods
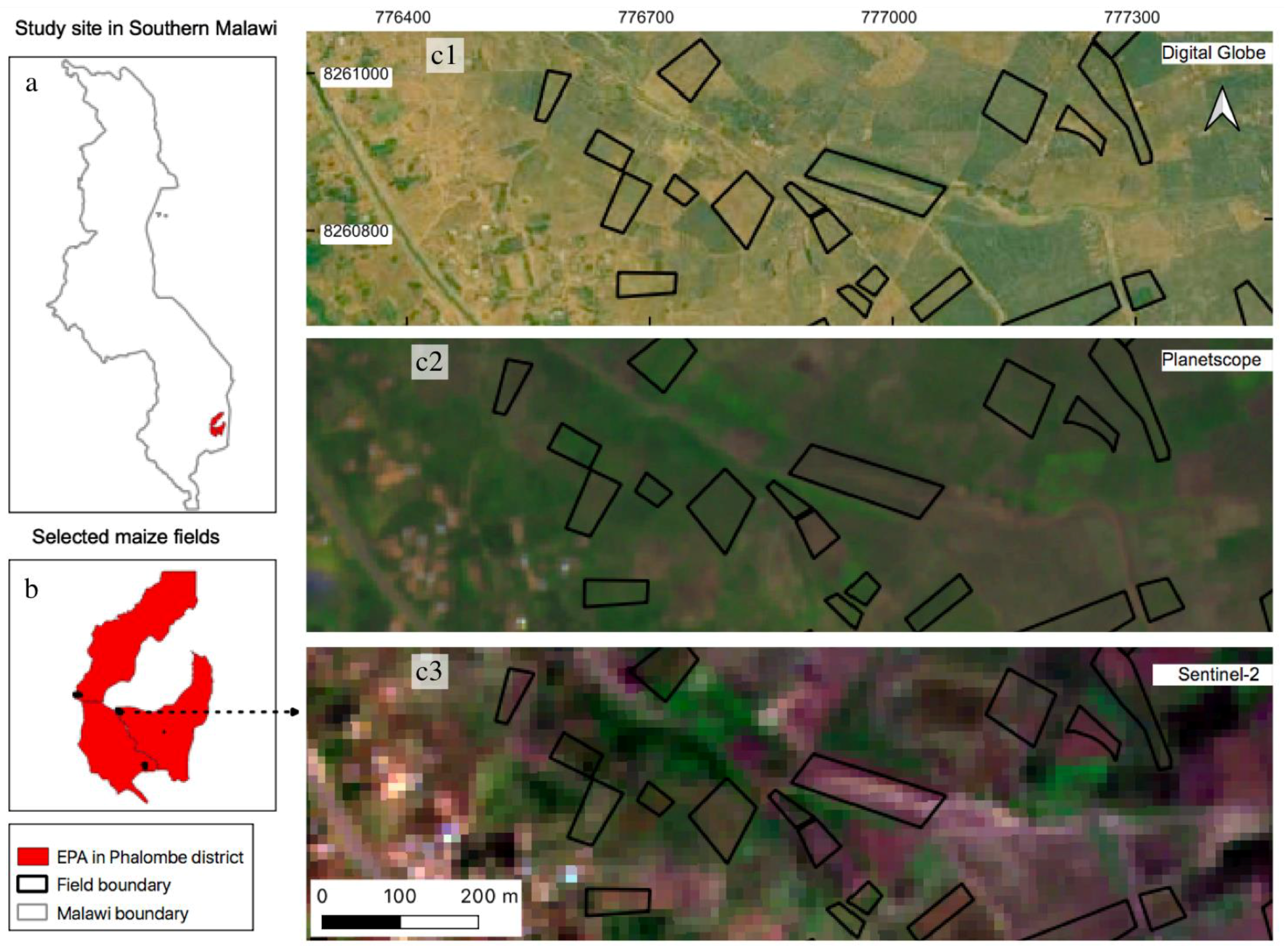
2.1. Study Area Description
2.2. Field Data Collection
2.2.1. LAI Measurements
2.2.2. Biomass and Yield Measurements
2.3. Official Yield Data and Maize Mask Dataset
2.4. PlanetScope and Sentinel-2 Data
2.4.1. Vegetation Indices (VIs)
2.4.2. LAI Retrieval
2.5. Model Development and Performance Evaluation
2.5.1. Linear Regression Model and Its Accuracy Assessment
2.5.2. Evaluating Factors Influencing Model Performance
3. Results
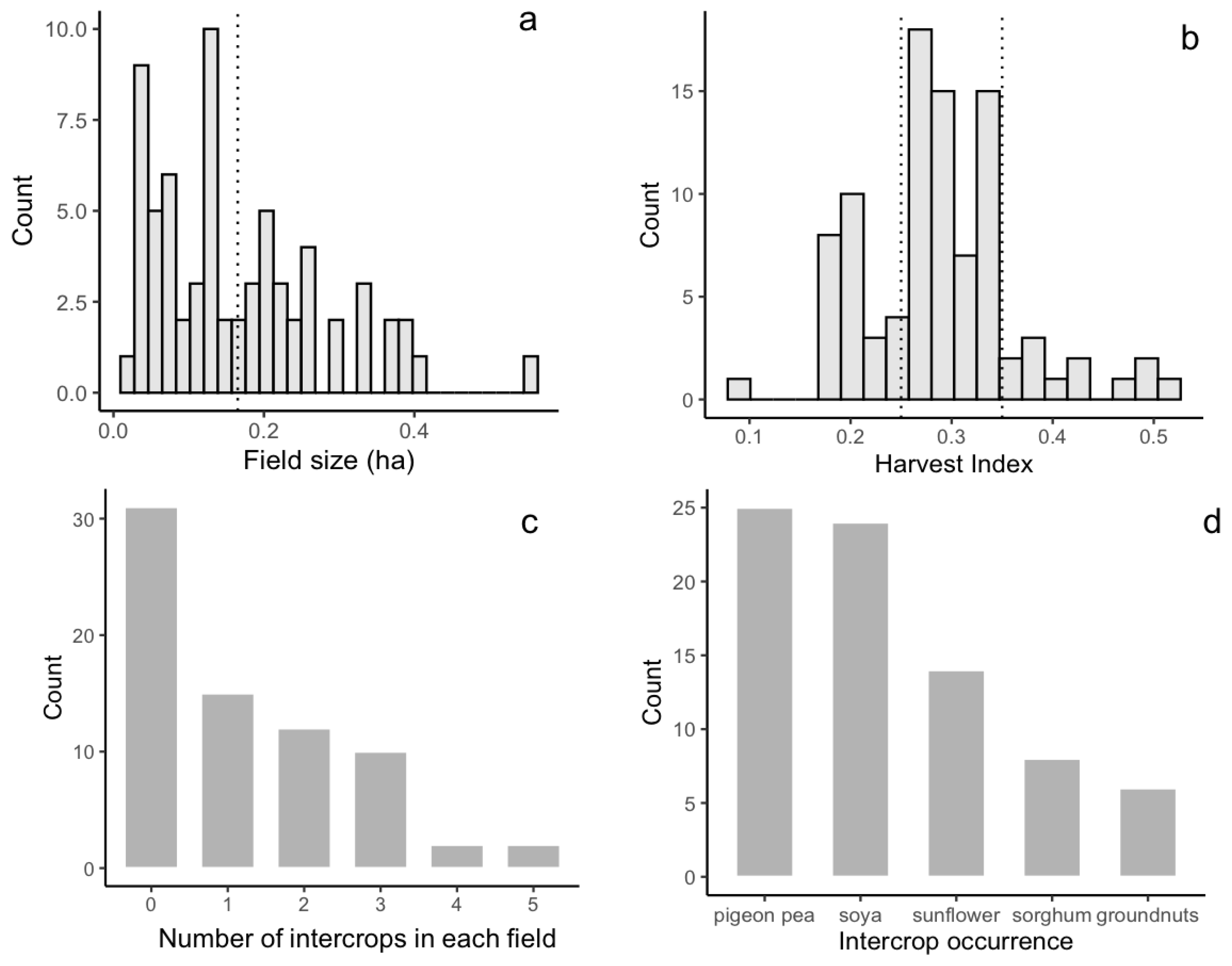
3.1. Field Characteristics and Intercropping Practices
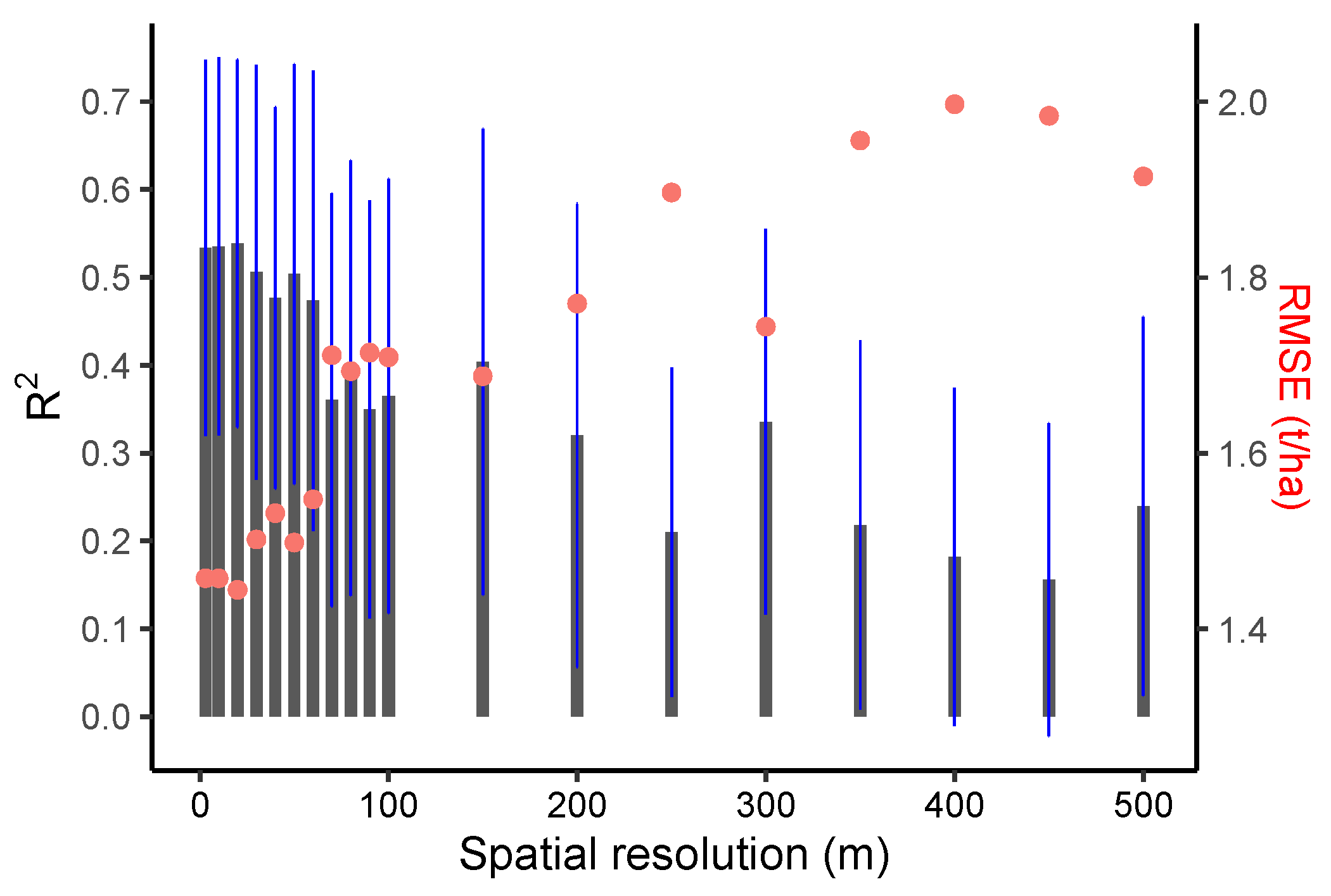
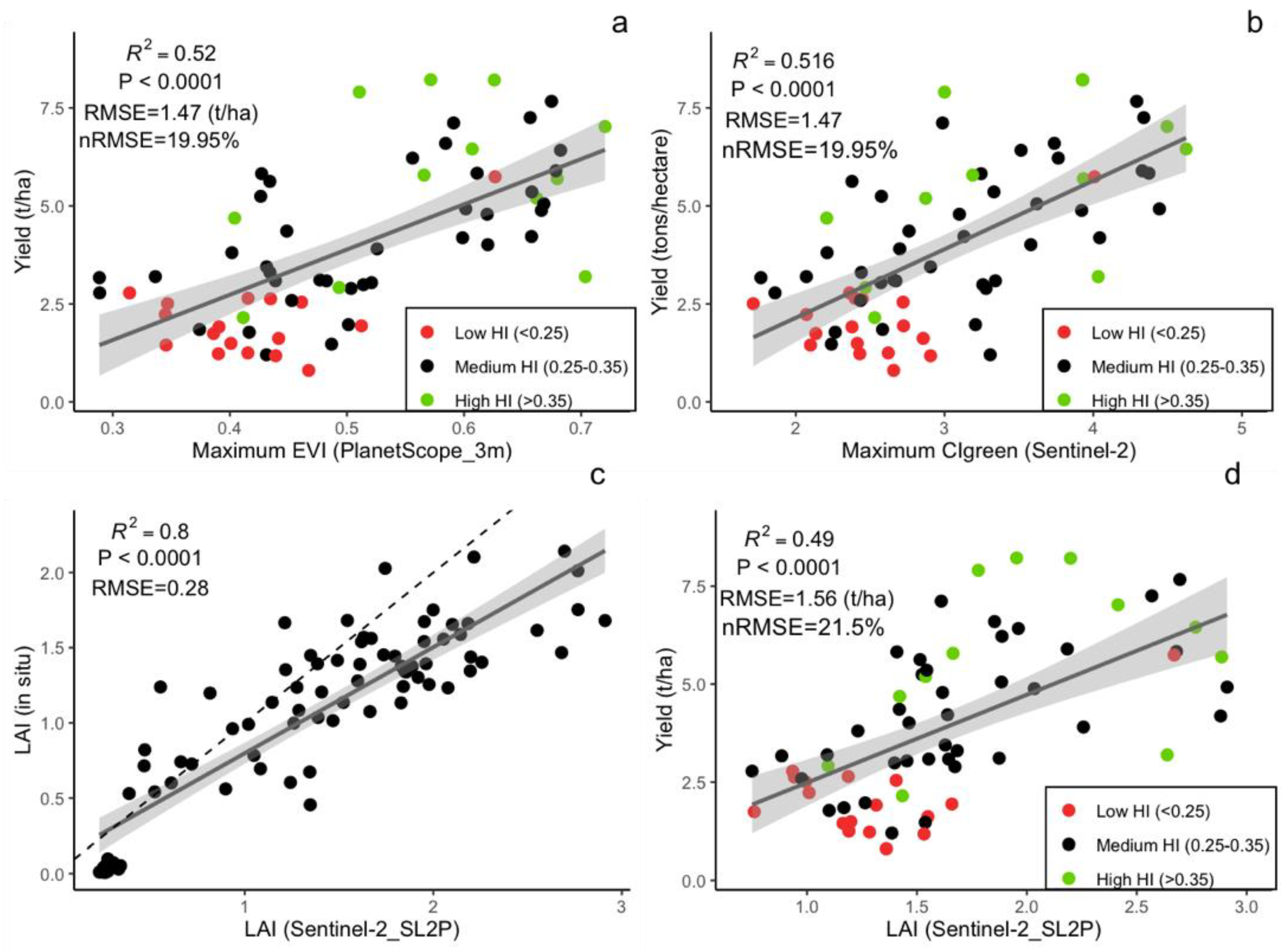
3.2. Crop Yield Estimation Using VIs and LAI with Different Spatial Resolution
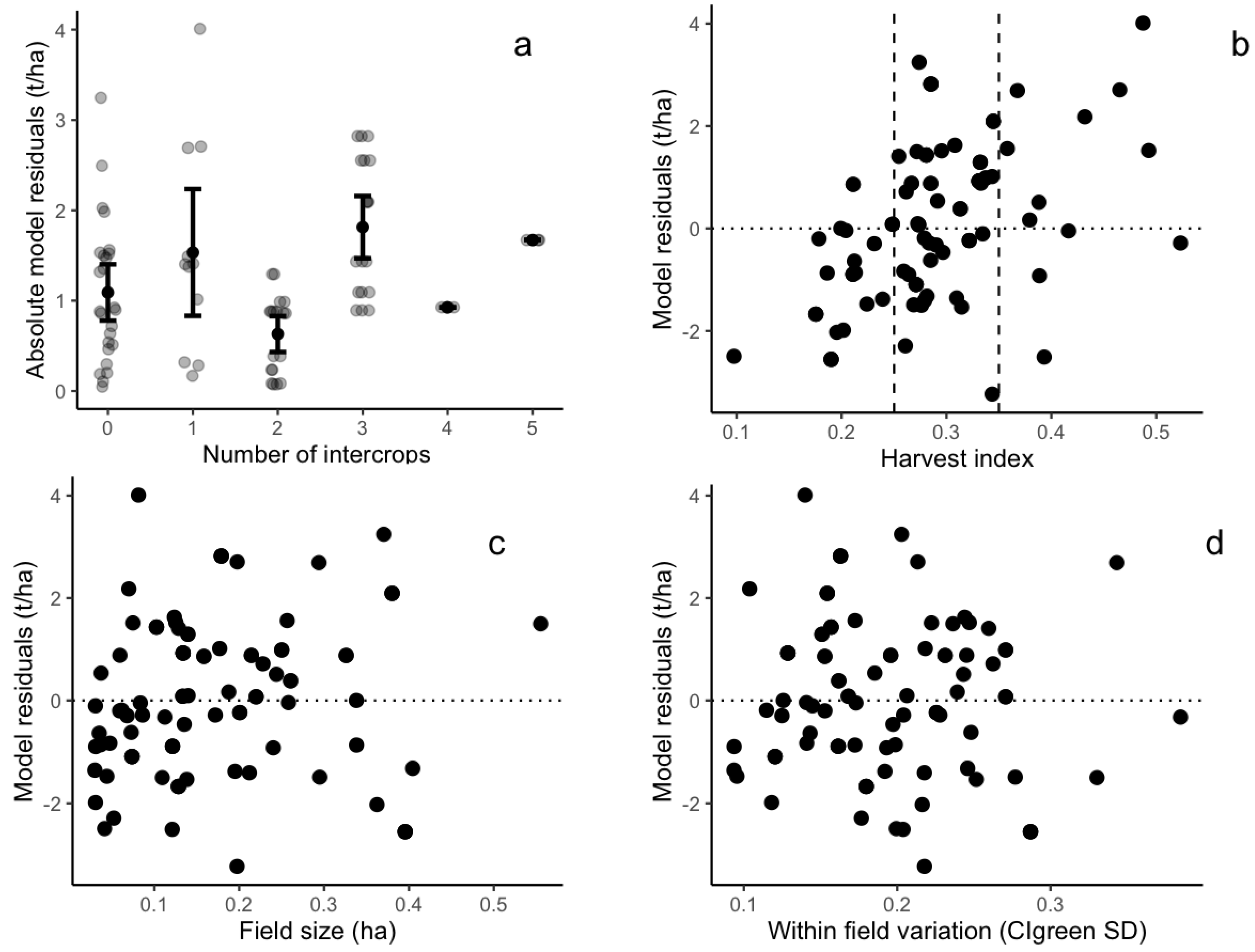
3.3. Factors Influencing Model Accuracy
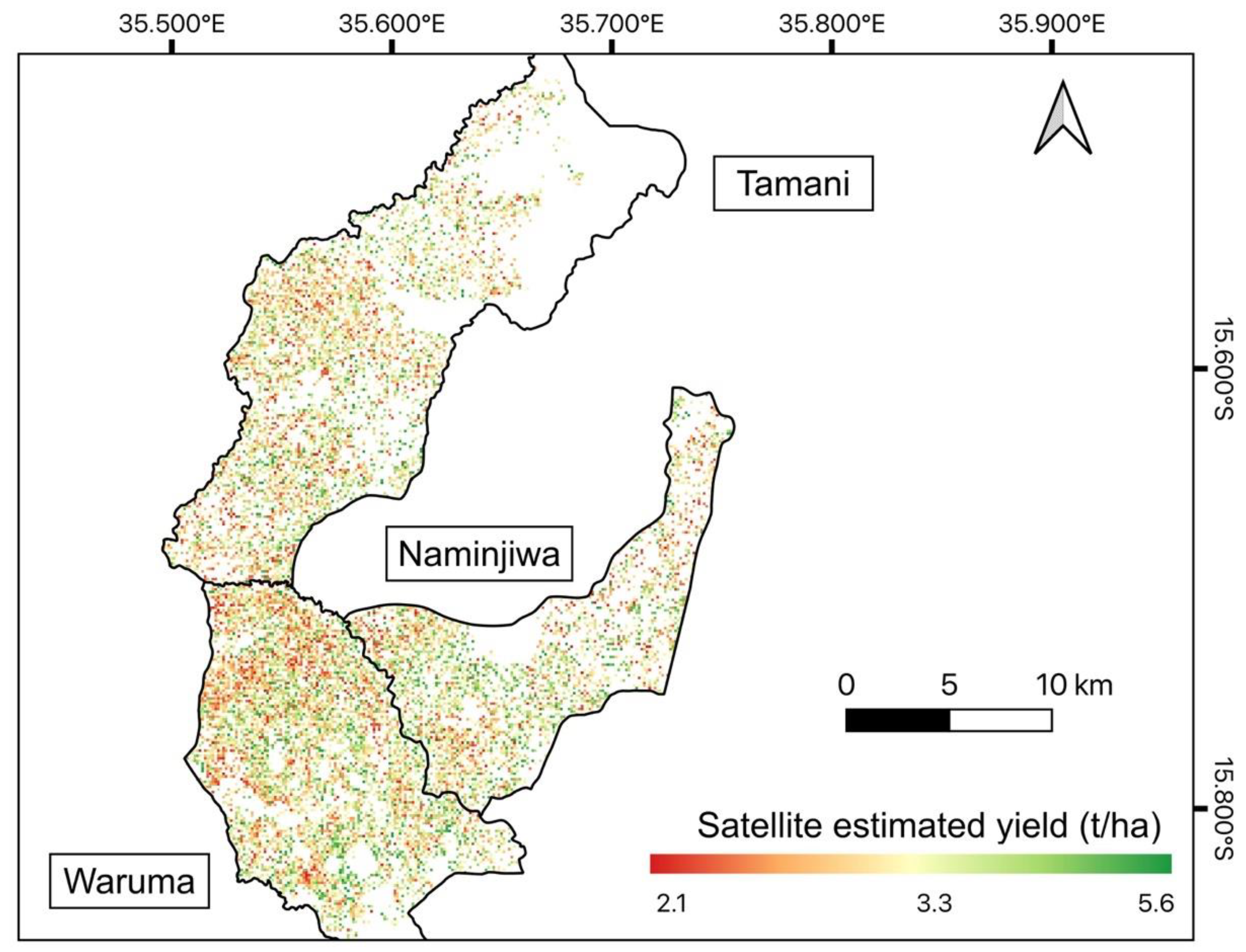
3.4. Validation of the Models over a Larger Region Using Official Statistics
4. Discussion
4.1. Performance of Optical EO Data in Estimating Yield in Intercropping Fields
4.2. Impact of EO Spatial Resolution on Yield Estimation Accuracy
4.3. Future Research Paths for Understanding Intercropping Practice and Better Estimating Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, R.A.; Connor, D.J. Issues for cropping and agricultural science in the next 20 years. Field Crops Res. 2018, 222, 121–142. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hochard, J.P. Land degradation and poverty. Nat. Sustain. 2018, 1, 623–631. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Agutu, N.O.; Awange, J.L.; Zerihun, A.; Ndehedehe, C.E.; Kuhn, M.; Fukuda, Y. Assessing multi-satellite remote sensing, reanalysis, and land surface models’ products in characterizing agricultural drought in East Africa. Remote Sens. Environ. 2017, 194, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Huang, J.; Gómez-Dans, J.L.; Huang, H.; Ma, H.; Wu, Q.; Lewis, P.E.; Liang, S.; Chen, Z.; Xue, J.-H.; Wu, Y.; et al. Assimilation of remote sensing into crop growth models: Current status and perspectives. Agric. For. Meteorol. 2019, 276-277, 107609. [Google Scholar] [CrossRef]
- Burke, M.; Lobell, D.B. Satellite-based assessment of yield variation and its determinants in smallholder African systems. Proc. Natl. Acad. Sci. USA 2017, 114, 2189–2194. [Google Scholar] [CrossRef] [Green Version]
- Burke, M.; Driscoll, A.; Lobell, D.B.; Ermon, S. Using satellite imagery to understand and promote sustainable development. Science 2021, 371, eabe8628. [Google Scholar] [CrossRef]
- Jain, M.; Balwinder, S.; Rao, P.; Srivastava, A.K.; Poonia, S.; Blesh, J.; Azzari, G.; McDonald, A.J.; Lobell, D.B. The impact of agricultural interventions can be doubled by using satellite data. Nat. Sustain. 2019, 2, 931–934. [Google Scholar] [CrossRef]
- Lambert, M.-J.; Traoré, P.C.S.; Blaes, X.; Baret, P.; Defourny, P. Estimating smallholder crops production at village level from Sentinel-2 time series in Mali’s cotton belt. Remote Sens. Environ. 2018, 216, 647–657. [Google Scholar] [CrossRef]
- Funk, C.; Budde, M.E. Phenologically-tuned MODIS NDVI-based production anomaly estimates for Zimbabwe. Remote Sens. Environ. 2009, 113, 115–125. [Google Scholar] [CrossRef]
- Lobell, D.B. The use of satellite data for crop yield gap analysis. Field Crops Res. 2013, 143, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Qader, S.H.; Dash, J.; Atkinson, P.M. Forecasting wheat and barley crop production in arid and semi-arid regions using remotely sensed primary productivity and crop phenology: A case study in Iraq. Sci. Total Environ. 2018, 613-614, 250–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, M.; Jacob, F.; Duveiller, G. Remote sensing for agricultural applications: A meta-review. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Leroux, L.; Castets, M.; Baron, C.; Escorihuela, M.-J.; Bégué, A.; Lo Seen, D. Maize yield estimation in West Africa from crop process-induced combinations of multi-domain remote sensing indices. Eur. J. Agron. 2019, 108, 11–26. [Google Scholar] [CrossRef]
- Johnson, M.D.; Hsieh, W.W.; Cannon, A.J.; Davidson, A.; Bédard, F. Crop yield forecasting on the Canadian Prairies by remotely sensed vegetation indices and machine learning methods. Agric. For. Meteorol. 2016, 218–219, 74–84. [Google Scholar] [CrossRef]
- Bolton, D.K.; Friedl, M.A. Forecasting crop yield using remotely sensed vegetation indices and crop phenology metrics. Agric. For. Meteorol. 2013, 173, 74–84. [Google Scholar] [CrossRef]
- Jin, Z.; Azzari, G.; Burke, M.; Aston, S.; Lobell, D.B. Mapping Smallholder Yield Heterogeneity at Multiple Scales in Eastern Africa. Remote Sens. 2017, 9, 931. [Google Scholar] [CrossRef] [Green Version]
- Leroux, L.; Baron, C.; Zoungrana, B.; Traoré, S.B.; Seen, D.L.; Bégué, A. Crop Monitoring Using Vegetation and Thermal Indices for Yield Estimates: Case Study of a Rainfed Cereal in Semi-Arid West Africa. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Diouf, A.A.; Brandt, M.; Verger, A.; Jarroudi, M.E.; Djaby, B.; Fensholt, R.; Ndione, J.A.; Tychon, B. Fodder Biomass Monitoring in Sahelian Rangelands Using Phenological Metrics from FAPAR Time Series. Remote Sens. 2015, 7, 9122–9148. [Google Scholar] [CrossRef] [Green Version]
- Bégué, A.; Leroux, L.; Soumaré, M.; Faure, J.-F.; Diouf, A.A.; Augusseau, X.; Touré, L.; Tonneau, J.-P. Remote Sensing Products and Services in Support of Agricultural Public Policies in Africa: Overview and Challenges. Front. Sustain. Food Syst. 2020, 4, 58. [Google Scholar] [CrossRef]
- Inbal, B.-R.; Christina, J.; Brian, B.; Michael, H.; Felix, R.; Rogerio, B.; Mario, Z.; Mike, B.; Tamuka, M.; Chris, S.; et al. Strengthening agricultural decisions in countries at risk of food insecurity: The GEOGLAM Crop Monitor for Early Warning. Remote Sens. Environ. 2020, 237, 111553. [Google Scholar] [CrossRef]
- Fritz, S.; See, L.; Bayas, J.C.L.; Waldner, F.; Jacques, D.; Becker-Reshef, I.; Whitcraft, A.; Baruth, B.; Bonifacio, R.; Crutchfield, J.; et al. A comparison of global agricultural monitoring systems and current gaps. Agric. Syst. 2019, 168, 258–272. [Google Scholar] [CrossRef]
- Seguini, L.; Bussay, A.; Baruth, B. From extreme weather to impacts: The role of the areas of concern maps in the JRC MARS bulletin. Agric. Syst. 2019, 168, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Azzari, G.; You, C.; Di Tommaso, S.; Aston, S.; Burke, M.; Lobell, D.B. Smallholder maize area and yield mapping at national scales with Google Earth Engine. Remote Sens. Environ. 2019, 228, 115–128. [Google Scholar] [CrossRef]
- Karst, I.G.; Mank, I.; Traoré, I.; Sorgho, R.; Stückemann, K.-J.; Simboro, S.; Sié, A.; Franke, J.; Sauerborn, R. Estimating Yields of Household Fields in Rural Subsistence Farming Systems to Study Food Security in Burkina Faso. Remote Sens. 2020, 12, 1717. [Google Scholar] [CrossRef]
- Lobell, D.B.; Azzari, G.; Burke, M.; Gourlay, S.; Jin, Z.; Kilic, T.; Murray, S. Eyes in the Sky, Boots on the Ground: Assessing Satellite- and Ground-Based Approaches to Crop Yield Measurement and Analysis. Am. J. Agric. Econ. 2020, 102, 202–219. [Google Scholar] [CrossRef]
- Carletto, C.; Jolliffe, D.; Banerjee, R. From Tragedy to Renaissance: Improving Agricultural Data for Better Policies. J. Dev. Stud. 2015, 51, 133–148. [Google Scholar] [CrossRef]
- Choularton, R.J.; Krishnamurthy, P.K. How accurate is food security early warning? Evaluation of FEWS NET accuracy in Ethiopia. Food Secur. 2019, 11, 333–344. [Google Scholar] [CrossRef]
- Anghileri, D.; Bozzini, V.; Molnar, P.; Jamali, A.A.J.; Sheffield, J. Comparison of hydrological and vegetation remote sensing datasets as proxies for rainfed maize yield in Malawi. Agric. Water Manag. 2022, 262, 107375. [Google Scholar] [CrossRef]
- Lyle, G.; Bryan, B.A.; Ostendorf, B. Post-processing methods to eliminate erroneous grain yield measurements: Review and directions for future development. Precis. Agric. 2014, 15, 377–402. [Google Scholar] [CrossRef]
- Inbal, B.-R.; Brian, B.; Michael, H.; Estefania, P.; Antonio, S.; Ritvik, S.; Katie, M.; Christopher, J.; Bettina, B.; Bingfang, W.; et al. The GEOGLAM crop monitor for AMIS: Assessing crop conditions in the context of global markets. Glob. Food Secur. 2019, 23, 173–181. [Google Scholar] [CrossRef]
- Lowder, S.K.; Skoet, J.; Raney, T. The Number, Size, and Distribution of Farms, Smallholder Farms, and Family Farms Worldwide. World Dev. 2016, 87, 16–29. [Google Scholar] [CrossRef] [Green Version]
- John, I.; Snapp, S.; Nord, A.; Chimonyo, V.; Gwenambira, C.; Chikowo, R. Marginal more than mesic sites benefit from groundnut diversification of maize: Increased yield, protein, stability, and profits. Agric. Ecosyst. Environ. 2021, 320, 107585. [Google Scholar] [CrossRef]
- Ogindo, H.O.; Walker, S. Comparison of measured changes in seasonal soil water content by rainfed maize-bean intercrop and component cropping systems in a semi-arid region of southern Africa. Phys. Chem. Earth Parts A/B/C 2005, 30, 799–808. [Google Scholar] [CrossRef]
- Denning, G.; Kabambe, P.; Sanchez, P.; Malik, A.; Flor, R.; Harawa, R.; Nkhoma, P.; Zamba, C.; Banda, C.; Magombo, C.; et al. Input subsidies to improve smallholder maize productivity in Malawi: Toward an african green revolution. PLoS Biol. 2009, 7, e23. [Google Scholar] [CrossRef] [Green Version]
- Mucavele, F.G. True Contribution of Agriculture to Economic Growth and Poverty Reduction: Malawi, Mozambique and Zambia Synthesis Report; Food, Agriculture, and Natural Resources Policy Analysis Network (FANRPAN): Pretoria, South Africa, 2009. [Google Scholar]
- Mdee, A.; Manda, S.; Dedaa, A.O.; Djurfeldt, A.A. A Political Economy of Inclusive Agricultural Intensification Zambia Country Report; University of Leeds: Leeds, UK, 2019. [Google Scholar]
- Li, C.; Kandel, M.; Anghileri, D.; Oloo, F.; Kambombe, O.; Chibarabada, T.P.; Ngongondo, C.; Sheffield, J.; Dash, J. Recent changes in cropland area and productivity indicate unsustainable cropland expansion in Malawi. Environ. Res. Lett. 2021, 16, 084052. [Google Scholar] [CrossRef]
- Sato, G.J.; Joshua, M.K.; Ngongondo, C.; Chipungu, F.; Malidadi, C.; Monjerezi, M. Evaluation of Different Tillage Systems for Improved Agricultural Production in Drought-Prone Areas of Malawi. In Climate Variability and Change in Africa: Perspectives, Experiences and Sustainability; Matondo, J.I., Alemaw, B.F., Sandwidi, W.J.P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 157–167. [Google Scholar]
- Baret, F.; Camacho, F.; Fang, H.; Garrigues, S.; Gobron, N.; Lang, M.; Lacaze, R.; LeBlanc, S.; Meroni, M.; Martinez, B. Global Leaf area Index Product Validation Good Practices; Academia: Cambridge, MA, USA, 2014. [Google Scholar]
- Demarez, V.; Duthoit, S.; Baret, F.; Weiss, M.; Dedieu, G. Estimation of leaf area and clumping indexes of crops with hemispherical photographs. Agric. For. Meteorol. 2008, 148, 644–655. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.A.; Meier, C.; Morris, H.; Pastor-Guzman, J.; Bai, G.; Lerebourg, C.; Gobron, N.; Lanconelli, C.; Clerici, M.; Dash, J. Evaluation of global leaf area index and fraction of absorbed photosynthetically active radiation products over North America using Copernicus Ground Based Observations for Validation data. Remote Sens. Environ. 2020, 247, 111935. [Google Scholar] [CrossRef]
- FAO. Handbook on Crop Statistics: Improving Methods for Measuring Crop Area, Production and Yield; FAO: Rome, Italy, 2018. [Google Scholar]
- Armstrong, P.R.; McNeil, S.; Manu, N.; Bosomtwe, A.; Danso, J.K.; Osekre, E.; Opit, G. Development and Evaluation of a Low-Cost Probe-Type Instrument to Measure the Equilibrium Moisture Content of Grain. Appl. Eng. Agric. 2017, 33, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Ngoune Tandzi, L.; Mutengwa, C.S. Estimation of Maize (Zea mays L.) Yield Per Harvest Area: Appropriate Methods. Agronomy 2020, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Hütsch, B.W.; Schubert, S. Chapter Two—Harvest Index of Maize (Zea mays L.): Are There Possibilities for Improvement? In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 146, pp. 37–82. [Google Scholar]
- Azzari, G.; Jain, S.; Jeffries, G.; Kilic, T.; Murray, S. Understanding the Requirements for Surveys to Support Satellite-Based Crop Type Mapping: Evidence from Sub-Saharan Africa. Remote Sens. 2021, 13, 4749. [Google Scholar] [CrossRef]
- Magdalena, M.-K.; Bringfried, P.; Jerome, L.; Vincent, D.; Uwe, M.-W.; Ferran, G. Sen2Cor for Sentinel-2. In Proceedings of SPIE; International Society for Optics and Photonics, SPIE: Bellingham, WA, USA, 2017. [Google Scholar]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Gitelson, A.A. Remote estimation of crop and grass chlorophyll and nitrogen content using red-edge bands on Sentinel-2 and -3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.; Gitelson, A.; Peng, Y.; Viña, A.; Arkebauer, T.; Rundquist, D. Green Leaf Area Index Estimation in Maize and Soybean: Combining Vegetation Indices to Achieve Maximal Sensitivity. Agron. J. 2012, 104, 1336–1347. [Google Scholar] [CrossRef] [Green Version]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Xie, Q.; Dash, J.; Huete, A.; Jiang, A.; Yin, G.; Ding, Y.; Peng, D.; Hall, C.C.; Brown, L.; Shi, Y.; et al. Retrieval of crop biophysical parameters from Sentinel-2 remote sensing imagery. Int. J. Appl. Earth Obs. Geoinf. 2019, 80, 187–195. [Google Scholar] [CrossRef]
- Brown, L.A.; Ogutu, B.O.; Dash, J. Estimating Forest Leaf Area Index and Canopy Chlorophyll Content with Sentinel-2: An Evaluation of Two Hybrid Retrieval Algorithms. Remote Sens. 2019, 11, 1752. [Google Scholar] [CrossRef] [Green Version]
- Waldner, F.; Horan, H.; Chen, Y.; Hochman, Z. High temporal resolution of leaf area data improves empirical estimation of grain yield. Sci. Rep. 2019, 9, 15714. [Google Scholar] [CrossRef] [Green Version]
- Moody, A.; Johnson, D.M. Land-Surface Phenologies from AVHRR Using the Discrete Fourier Transform. Remote Sens. Environ. 2001, 75, 305–323. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.F.; Dash, J.; Atkinson, P.M. Characterising the Land Surface Phenology of Europe Using Decadal MERIS Data. Remote Sens. 2015, 7, 9390–9409. [Google Scholar] [CrossRef] [Green Version]
- Tucker, C.; Miller, L.; Pearson, R. Measurement of the combined effect of green biomass, chlorophyll, and leaf water on canopy spectroreflectance of the shortgrass prairie. Remote Sens. Earth Resour. 2013, 1973, 601–627. [Google Scholar]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30, 1248. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef] [Green Version]
- Dash, J.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F. S2ToolBox Level 2 Products, Version 1. Available online: http://Step.esa.int/docs/extra/ATBD_S2ToolBox_L2B_V1 (accessed on 24 March 2022).
- Brown, L.A.; Fernandes, R.; Djamai, N.; Meier, C.; Gobron, N.; Morris, H.; Canisius, F.; Bai, G.; Lerebourg, C.; Lanconelli, C.; et al. Validation of baseline and modified Sentinel-2 Level 2 Prototype Processor leaf area index retrievals over the United States. ISPRS J. Photogramm. Remote Sens. 2021, 175, 71–87. [Google Scholar] [CrossRef]
- Rudorff, B.F.T.; Batista, G.T. Spectral response of wheat and its relationship to agronomic variables in the tropical region. Remote Sens. Environ. 1990, 31, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Di Tommaso, S.; You, C.; Yacoubou Djima, I.; Burke, M.; Kilic, T. Sight for Sorghums: Comparisons of Satellite- and Ground-Based Sorghum Yield Estimates in Mali. Remote Sens. 2020, 12, 100. [Google Scholar] [CrossRef] [Green Version]
- Wahab, I.; Hall, O.; Jirström, M. Remote Sensing of Yields: Application of UAV Imagery-Derived NDVI for Estimating Maize Vigor and Yields in Complex Farming Systems in Sub-Saharan Africa. Drones 2018, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, K.; Bhutoria, A.J.; Sharma, J.K.; Sinha, A.; Pandey, P.C. UAVs technology for the development of GUI based application for precision agriculture and environmental research. Remote Sens. Appl. Soc. Environ. 2019, 16, 100258. [Google Scholar] [CrossRef]
- Peng, Y.; Gitelson, A.A. Remote estimation of gross primary productivity in soybean and maize based on total crop chlorophyll content. Remote Sens. Environ. 2012, 117, 440–448. [Google Scholar] [CrossRef]
- Zhao, Y.; Potgieter, A.B.; Zhang, M.; Wu, B.; Hammer, G.L. Predicting Wheat Yield at the Field Scale by Combining High-Resolution Sentinel-2 Satellite Imagery and Crop Modelling. Remote Sens. 2020, 12, 1024. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Guan, K.; Lobell, D.; Potgieter, A.B.; Wang, S.; Peng, J.; Xu, T.; Asseng, S.; Zhang, Y.; You, L.; et al. Integrating satellite and climate data to predict wheat yield in Australia using machine learning approaches. Agric. For. Meteorol. 2019, 274, 144–159. [Google Scholar] [CrossRef]
- Guan, K.; Wu, J.; Kimball, J.S.; Anderson, M.C.; Frolking, S.; Li, B.; Hain, C.R.; Lobell, D.B. The shared and unique values of optical, fluorescence, thermal and microwave satellite data for estimating large-scale crop yields. Remote Sens. Environ. 2017, 199, 333–349. [Google Scholar] [CrossRef] [Green Version]
- Brooker, R.W.; Bennett, A.E.; Cong, W.-F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef] [PubMed]


| Satellite Data | Dates | |||||
|---|---|---|---|---|---|---|
| November 2019 | December 2019 | January 2020 | February 2020 | March 2020 | April 2020 | |
| PlanetScope | 2, 3, 14, 29 | 2, 4, 8, 16, 27 | 10, 13, 27 | 4, 8, 10, 19, 22, 23 | 2, 9, 13, 15, 21, 23, 26 | 6, 8, 17, 25 |
| Sentinel-2 | 2, 7 | 2, 7, 22, 27 | 26 | 2, 05, 20 | 3, 6, 21, 31 | 5, 10, 15, 25, 30 |
| VI | Formulation | Sentinel-2 (10 m) | PlanetScope (3 m) | Reference |
|---|---|---|---|---|
| NDVI | (NIR − Red)/(NIR + Red) | B8, B4 | Red, NIR | [60] |
| EVI | 2.5 × (NIR − Red)/(NIR + 6 × Red + 7 × Blue − 1) | B8, B4, B2 | Blue, Red, NIR | [61] |
| MSAVI | (2 × NIR + 1 − sqrt ((2 × NIR + 1)2 − 8 × (NIR − Red)))/2 | B8, B4 | Red, NIR | [62] |
| SAVI | ((NIR − Red) / (NIR + Red + 0.5)) × (1.5) | B8, B4 | Red, NIR | [62] |
| OSAVI | (1 + 0.16) × (NIR − Red)/(NIR+ R+0.16) | B8, B4 | Red, NIR | [63] |
| TVI | 0.5 × [120 × (NIR − Green) − 200 × (Red − Green)] | B8, B4, B3 | Red, Green, NIR | [64] |
| CIgreen | (NIR/Green − 1) | B7, B3 | - | [65,66,67] |
| CIred | NIR/RE − 1 | B7, B5 | - | [65,66,67] |
| MTCI | (NIR − RE)/(RE − Red) | B7, B5, B4 | - | [68] |
| MCAR/ OSAVI | (750 − 705) − 0.2× (750 − 550) × (750/705)/OSAVI | B6, B5, B3 | - | [54] |
| TCARI/ OSAVI | 3× [(750 − 705) − 0.2× (750 − 550) × (750/705)]/OSAVI | B6, B5, B3 | - | [54] |
| Extension Planning Areas (EPAs) | Naminjiwa | Tamani | Waruma |
|---|---|---|---|
| Satellite estimated yield (t/ha) | 4.05 | 3.95 | 3.96 |
| Official estimated yield (t/ha) | 3.27 | 2.56 | 3.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chimimba, E.G.; Kambombe, O.; Brown, L.A.; Chibarabada, T.P.; Lu, Y.; Anghileri, D.; Ngongondo, C.; Sheffield, J.; Dash, J. Maize Yield Estimation in Intercropped Smallholder Fields Using Satellite Data in Southern Malawi. Remote Sens. 2022, 14, 2458. https://doi.org/10.3390/rs14102458
Li C, Chimimba EG, Kambombe O, Brown LA, Chibarabada TP, Lu Y, Anghileri D, Ngongondo C, Sheffield J, Dash J. Maize Yield Estimation in Intercropped Smallholder Fields Using Satellite Data in Southern Malawi. Remote Sensing. 2022; 14(10):2458. https://doi.org/10.3390/rs14102458
Chicago/Turabian StyleLi, Chengxiu, Ellasy Gulule Chimimba, Oscar Kambombe, Luke A. Brown, Tendai Polite Chibarabada, Yang Lu, Daniela Anghileri, Cosmo Ngongondo, Justin Sheffield, and Jadunandan Dash. 2022. "Maize Yield Estimation in Intercropped Smallholder Fields Using Satellite Data in Southern Malawi" Remote Sensing 14, no. 10: 2458. https://doi.org/10.3390/rs14102458
APA StyleLi, C., Chimimba, E. G., Kambombe, O., Brown, L. A., Chibarabada, T. P., Lu, Y., Anghileri, D., Ngongondo, C., Sheffield, J., & Dash, J. (2022). Maize Yield Estimation in Intercropped Smallholder Fields Using Satellite Data in Southern Malawi. Remote Sensing, 14(10), 2458. https://doi.org/10.3390/rs14102458








