Satellite-Based Monitoring of Primary Production in a Mediterranean Islet Post Black Rat Eradication
Abstract
1. Introduction
2. Materials and Methods
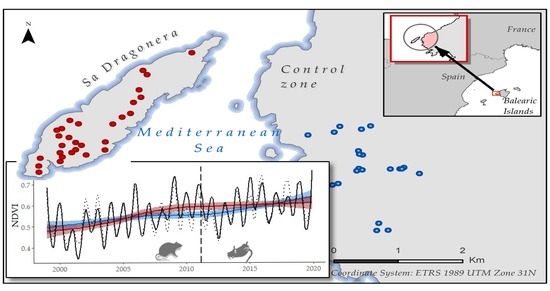
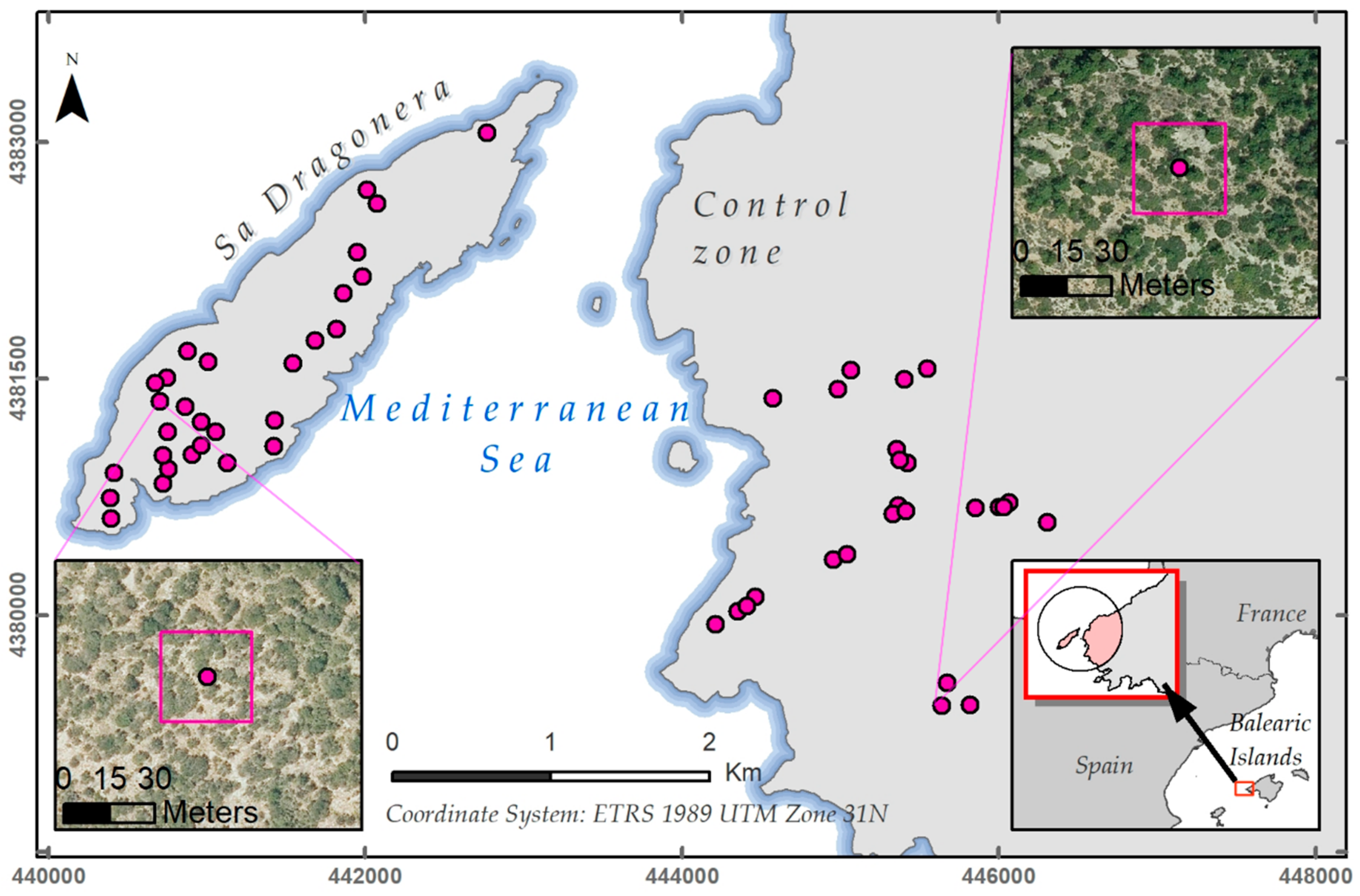
2.1. Study Area
2.2. NDVI Time Series Construction
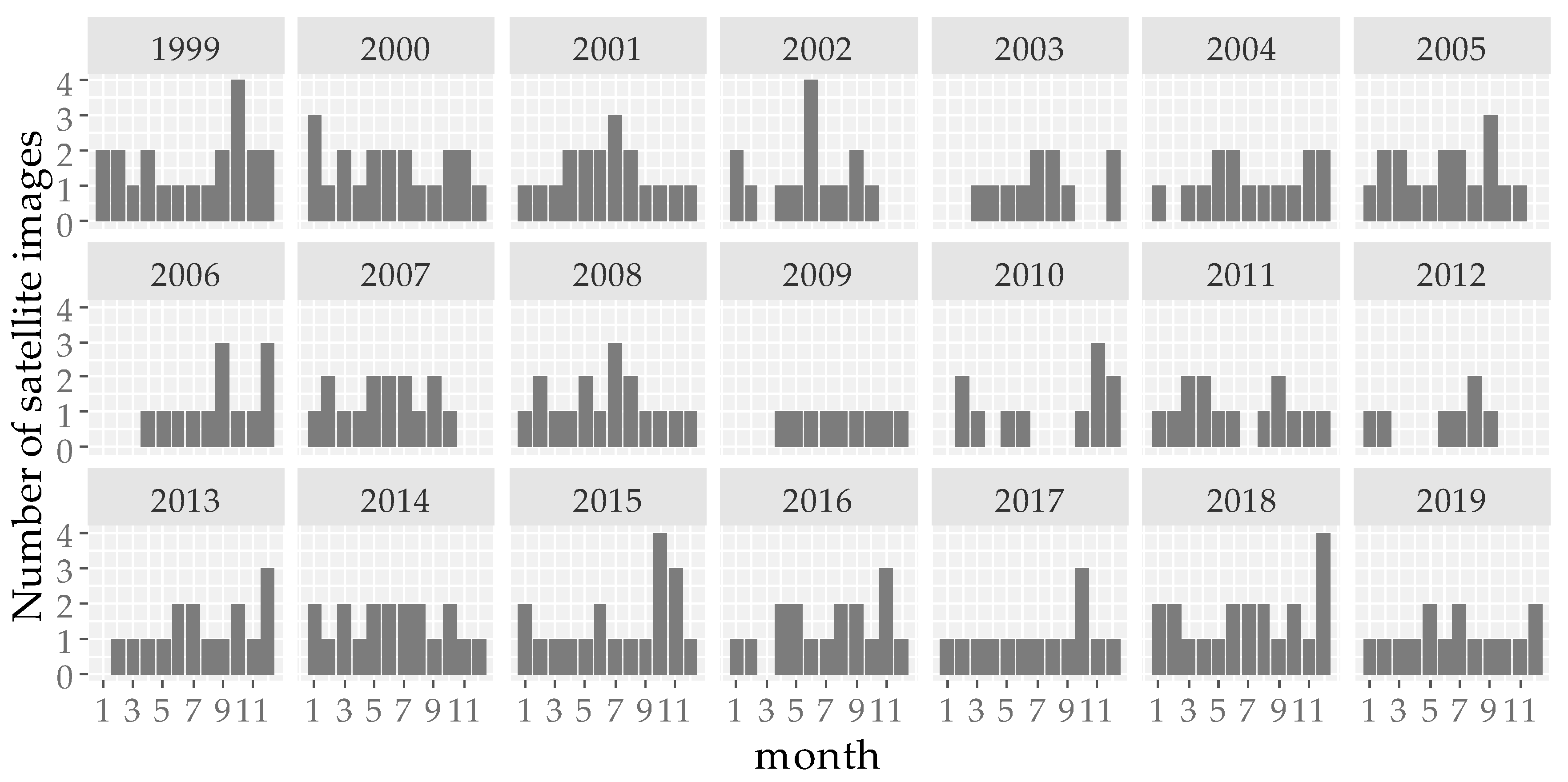
2.2.1. Satellite Images
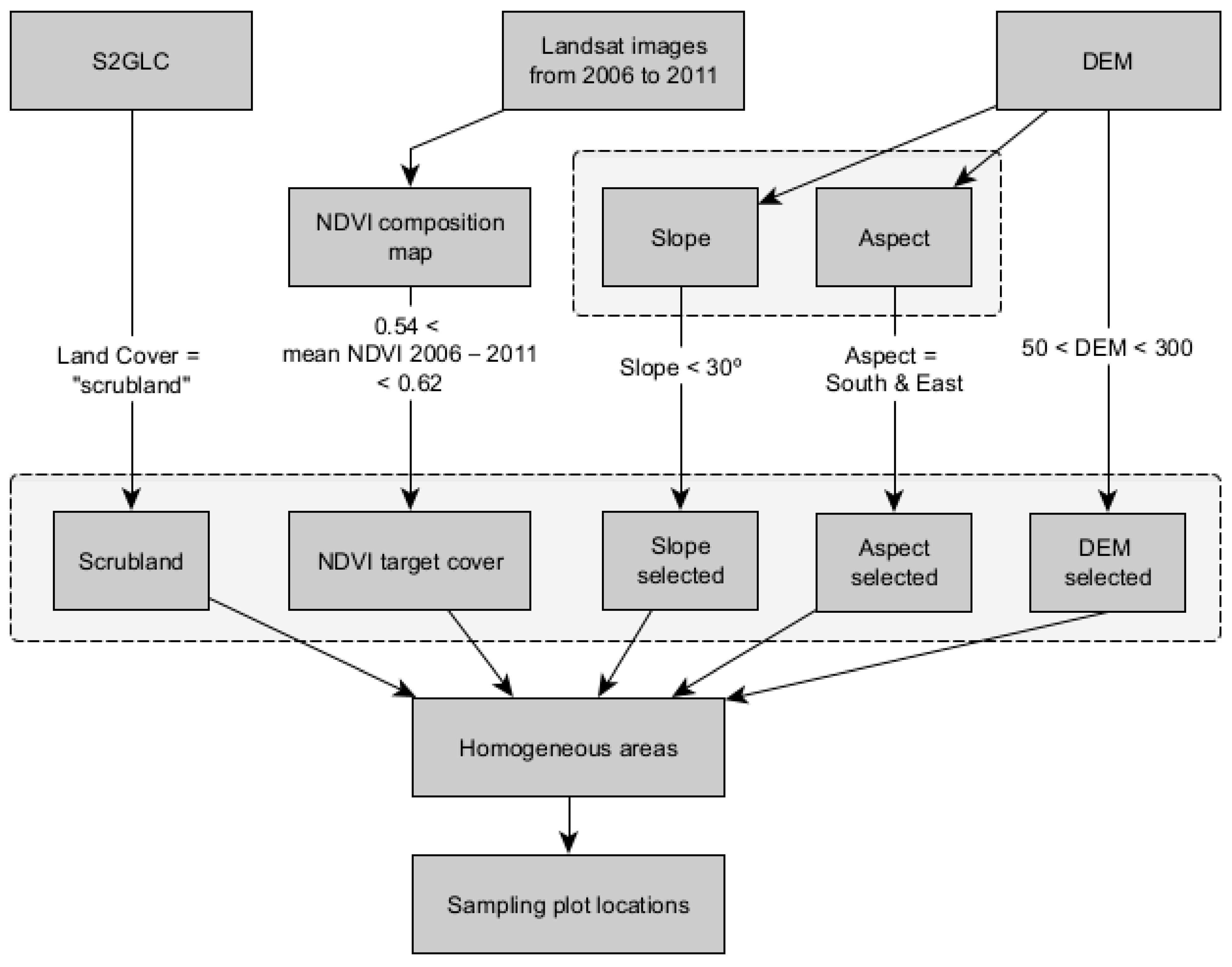
2.2.2. Plot Selection Procedure
2.2.3. Monthly Landsat NDVI Time Series Construction
2.3. Historical Time Series Analysis
3. Results
Historical Time Series Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, D.M.; Pyšek, P.; Carlton, J.T. A Compendium of Essential Concepts and Terminology in Invasion Ecology. In Fifty Years Invasion Ecology: The Legacy of Charles Elton; WILEY: Hoboken, NJ, USA, 2011; pp. 409–420. [Google Scholar]
- Dukes, J.S.; Mooney, H.A. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999, 14, 135–139. [Google Scholar] [CrossRef]
- Berger, J.J. Ecological Restoration and Nonindigenous Plant Species: A Review. Restor. Ecol. 1993, 1, 74–82. [Google Scholar] [CrossRef]
- Tsutsumida, N.; Saizen, I.; Matsuoka, M.; Ishii, R. Land cover change detection in Ulaanbaatar using the breaks for additive seasonal and trend method. Land 2013, 2, 534–549. [Google Scholar] [CrossRef]
- Vilà, M. Efectos de la introducción de especies vegetales en el funcionamiento de los ecosistemas terrestres. Montes 1999, 55, 26–30. [Google Scholar]
- Tershy, B.R.; Shen, K.W.; Newton, K.M.; Holmes, N.D.; Croll, D.A. The importance of islands for the protection of biological and linguistic diversity. Bioscience 2015, 65, 592–597. [Google Scholar] [CrossRef]
- Towns, D.R.; Atkinson, I.A.E.; Daugherty, C.H. Have the harmful effects of introduced rats on islands been exaggerated? Biol. Invasions 2006, 8, 863–891. [Google Scholar] [CrossRef]
- Howald, G.; Donlan, C.J.; Galván, J.P.; Russell, J.C.; Parkes, J.; Samaniego, A.; Wang, Y.; Veitch, D.; Genovesi, P.; Pascal, M.; et al. Invasive rodent eradication on islands. Conserv. Biol. 2007, 21, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, D.; Bertolino, S.; Mortelliti, A. Rating the rat: Global patterns and research priorities in impacts and management of rodent pests. Mamm. Rev. 2014, 44, 148–162. [Google Scholar] [CrossRef]
- Cheylan, G. Prédation du rat noir Rattus rattus sur les oiseaux de mer qui nichent dans les îles méditerranéennes. Ann. Crop 1985, 2, 27–29. [Google Scholar]
- Igual, J.M.; Forero, M.G.; Gomez, T.; Orueta, J.F.; Oro, D. Rat control and breeding performance in Cory’s shearwater (Calonectris diomedea): Effects of poisoning effort and habitat features. Anim. Conserv. 2006, 9, 59–65. [Google Scholar] [CrossRef]
- Atkinson, I.A. The spread of commensal species of Rattus to oceanic islands and their effects on island avifaunas. ICPB Tech Publ. 1985, 3, 35–81. [Google Scholar]
- Russell, J.C.; Holmes, N.D. Tropical Island conservation: Rat eradication for species recovery. Biol. Conserv. 2015, 185, 1–7. [Google Scholar] [CrossRef]
- Ruffino, L.; Russell, J.; Vidal, E. Anthropogenic subsidies mitigate environmental variability for insular rodents. Oecologia 2013, 172, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.; Rutherford, M. Home range and population density of black rats (Rattus rattus) on a seabird island: A case for a marine subsidised effect? N. Z. J. Ecol. 2016, 40, 219–228. [Google Scholar] [CrossRef]
- Oppel, S.; McClelland, G.T.W.; Lavers, J.L.; Churchyard, T.; Donaldson, A.; Duffield, N.; Havery, S.; Kelly, J.; Proud, T.; Russell, J.C.; et al. Seasonal variation in movements and survival of invasive Pacific rats on sub-tropical Henderson Island: Implications for eradication. In Proceedings of the 21st International Conference on Aquatic Invasive Species, Montreal, QC, Canada, 27–31 October 2019; pp. 200–208. [Google Scholar]
- Ruffino, L.; Bourgeois, K.; Vidal, E.; Duhem, C.; Paracuellos, M.; Escribano, F.; Sposimo, P.; Baccetti, N.; Pascal, M.; Oro, D. Invasive rats and seabirds after 2000 years of an unwanted coexistence on Mediterranean islands. Biol. Invasions 2009, 11, 1631–1651. [Google Scholar] [CrossRef]
- Martin, J.L.; Thibault, J.C.; Bretagnolle, V. Black rats, island characteristics, and colonial nesting birds in the Mediterranean: Consequences of an ancient introduction. Conserv. Biol. 2000, 14, 1452–1466. [Google Scholar] [CrossRef]
- Traveset, A.; Nogales, M.; Alcover, J.A.; Delgado, J.D.; López-Darias, M.; Godoy, D.; Igual, J.M.; Bover, P. A review on the effects of alien rodents in the Balearic (western Mediterranean sea) and Canary islands (eastern Atlantic ocean). Biol. Invasions 2009, 11, 1653–1670. [Google Scholar] [CrossRef]
- Russell, J.C.; Jones, H.P.; Armstrong, D.P.; Courchamp, F.; Kappes, P.J.; Seddon, P.J.; Oppel, S.; Rauzon, M.J.; Cowan, P.E.; Rocamora, G.; et al. Importance of lethal control of invasive predators for island conservation. Conserv. Biol. 2016, 30, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J.; Atkinson, I.A.E. Effects of kiore (Rattus exulans Peale) on recruitment of indigenous coastal trees on northern offshore islands of New Zealand. J. R. Soc. N. Z. 1999, 29, 290. [Google Scholar] [CrossRef]
- Borchert, M.I.; Jain, S.K. The effect of rodent seed predation on four species of California annual grasses. Oecologia 1978, 33, 101–113. [Google Scholar] [CrossRef]
- Shiels, A.B. Ecology and Impacts of Introduced Rodents (Rattus spp. and Mus Musculus) in the Hawaiian Islands. Ph.D. Thesis, University of Hawaii at Manoa, Honolulu, HI, USA, 2010. [Google Scholar]
- Grant-Hoffman, M.N.; Barboza, P.S. Herbivory in invasive rats: Criteria for food selection. Biol. Invasions 2010, 12, 805–825. [Google Scholar] [CrossRef]
- Auld, T.D.; Hutton, I.; Ooi, M.K.J.; Denham, A.J. Disruption of recruitment in two endemic palms on Lord Howe Island by invasive rats. Biol. Invasions 2010, 12, 3351–3361. [Google Scholar] [CrossRef]
- Campbell, D.J.; Atkinson, I.A.E. Depression of tree recruitment by the Pacific rat (Rattus exulans Peale) on New Zealand’s northern offshore islands. Biol. Conserv. 2002, 107, 19–35. [Google Scholar] [CrossRef]
- Grant-Hoffman, M.N.; Mulder, C.P.; Bellingham, P.J. Effects of invasive rats and burrowing seabirds on seeds and seedlings on New Zealand islands. Oecologia 2010, 162, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T. Rethinking the fall of Easter Island. New evidence points to and alternative explanation for a civilization’s collapse. Am. Sci. 2006, 94, 412–419. [Google Scholar] [CrossRef]
- Le Corre, M.; Danckwerts, D.K.; Ringler, D.; Bastien, M.; Orlowski, S.; Morey Rubio, C.; Pinaud, D.; Micol, T. Seabird recovery and vegetation dynamics after Norway rat eradication at Tromelin Island, western Indian Ocean. Biol. Conserv. 2015, 185, 85–94. [Google Scholar] [CrossRef]
- Weber, C. Ecological Impacts of Invasive Rat Removal on Mediterranean Sea Islands. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2014. [Google Scholar]
- Blackburn, T.M.; Cassey, P.; Duncan, R.P.; Evans, K.L.; Gaston, K.J. Avian extinction and mammalian introductions on oceanic islands. Science 2004, 305, 1955–1958. [Google Scholar] [CrossRef]
- Jones, H.P. Prognosis for ecosystem recovery following rodent eradication and seabird restoration in an island archipelago. Ecol. Appl. 2010, 20, 1204–1216. [Google Scholar] [CrossRef]
- Block, W.M.; Franklin, A.B.; Ward, J.P.; Ganey, J.L.; White, G.C. Design and implementation of monitoring studies to evaluate the success of ecological restoration on wildlife. Restor. Ecol. 2001, 9, 293–303. [Google Scholar] [CrossRef]
- Lohr, C.; Van Dongen, R.; Huntley, B.; Gibson, L.; Morris, K. Remotely monitoring change in vegetation cover on the Montebello Islands, Western Australia, in response to introduced rodent eradication. PLoS ONE 2014, 9, e114095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bastille-Rousseau, G.; Gibbs, J.P.; Campbell, K.; Yackulic, C.B.; Blake, S. Ecosystem implications of conserving endemic versus eradicating introduced large herbivores in the Galapagos Archipelago. Biol. Conserv. 2017, 209, 1–10. [Google Scholar] [CrossRef]
- Graham, M.F.; Veitch, C.R. Changes in Bird Numbers on Tiritiri Matangi Island, New Zealand, Over the Period of Rat Eradication. In Turning the Tide, The Eradication of Invasive Species; IUCN Species Survival. Commission: Gland, Switzerland, 2002; pp. 120–123. [Google Scholar]
- McClelland, P.J. Eradication of Pacific rats (Rattus exulans) from Whenua Hou Nature Reserve (Codfish Island), Putauhinu and Rarotoka Islands, New Zealand. Turning the Tide, The Eradication of Invasive Species; IUCN Species Survival. Commission: Gland, Switzerland, 2002; pp. 173–181. [Google Scholar]
- Towns, D.R.; Atkinson, I.A.E.; Daugherty, C.H. Reconstructing the Ambiguous: Can Island Ecosystems be Restored. In Ecological Restoration of New Zealand Islands; Department of Conservation: Wellington, New Zealand, 1990. [Google Scholar]
- Schweizer, D.; Jones, H.P.; Holmes, N.D. Literature Review and Meta-Analysis of Vegetation Responses to Goat and European Rabbit Eradications on Islands. Pac. Sci. 2016, 70, 55–71. [Google Scholar] [CrossRef]
- Jones, H.P.; Holmes, N.D.; Butchart, S.H.M.; Tershy, B.R.; Kappes, P.J.; Corkery, I.; Aguirre-Muñoz, A.; Armstrong, D.P.; Bonnaud, E.; Burbidge, A.A.; et al. Invasive mammal eradication on islands results in substantial conservation gains. Proc. Natl. Acad. Sci. USA 2016, 113, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Pettorelli, N.; Safi, K.; Turner, W. Satellite remote sensing, biodiversity research and conservation of the future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef]
- Sader, S.A.; Jin, S. MODIS time-series imagery for forest disturbance detection and quantification of patch size effects Article in Remote Sensing of Environment December 2005 SEE PROFILE MODIS time-series imagery for forest disturbance detection and quantification of patch size effects. Remote Sens. Environ. 2005, 99, 462–470. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Newnham, G.; Culvenor, D. Detecting trend and seasonal changes in satellite image time series. Remote Sens. Environ. 2010, 114, 106–115. [Google Scholar] [CrossRef]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef]
- Sellers, P.J. Canopy reflectance, photosynthesis and transpiration. Int. J. Remote Sens. 1985, 6, 1335–1372. [Google Scholar] [CrossRef]
- Reed, B.C.; Brown, J.F.; VanderZee, D.; Loveland, T.R.; Merchant, J.W.; Ohlen, D.O. Measuring phenological variability from satellite imagery. J. Veg. Sci. 1994, 5, 703–714. [Google Scholar] [CrossRef]
- Running, S.W. Estimating Terrestrial Primary Productivity by Combining Remote Sensing and Ecosystem Simulation. In Remote Sensing of Biosphere Functioning; Springer: New York, NY, USA, 1990; pp. 65–86. [Google Scholar]
- Myneni, R.B.; Hall, F.G.; Sellers, P.J.; Marshak, A.L. The interpretation of spectral vegetation indexes. IEEE Trans. Geosci. Remote Sens. 2019, 33, 481–486. [Google Scholar] [CrossRef]
- Potter, C.; Tan, P.N.; Steinbach, M.; Klooster, S.; Kumar, V.; Myneni, R.; Genovese, V. Major disturbance events in terrestrial ecosystems detected using global satellite data sets. Glob. Chang. Biol. 2003, 9, 1005–1021. [Google Scholar] [CrossRef]
- De Jong, R.; Verbesselt, J.; Zeileis, A.; Schaepman, M.E. Shifts in global vegetation activity trends. Remote Sens. 2013, 5, 1117–1133. [Google Scholar] [CrossRef]
- Gaitán, J.J.; Bran, D.; Oliva, G.; Ciari, G.; Nakamatsu, V.; Salomone, J.; Ferrante, D.; Buono, G.; Massara, V.; Humano, G.; et al. Evaluating the performance of multiple remote sensing indices to predict the spatial variability of ecosystem structure and functioning in Patagonian steppes. Ecol. Indic. 2013, 34, 181–191. [Google Scholar] [CrossRef]
- Helman, D.; Mussery, A.; Lensky, I.M.; Leu, S. Detecting changes in biomass productivity in a different land management regimes in drylands using satellite-derived vegetation index. Soil Use Manag. 2014, 30, 32–39. [Google Scholar] [CrossRef]
- Alcaraz-Segura, D.; Cabello, J.; Paruelo, J. Baseline characterization of major Iberian vegetation types based on the NDVI dynamics. Plant Ecol. 2008, 202, 13–29. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Gouveia, C.; Camarero, J.J.; Beguería, S.; Trigo, R.; López-Moreno, J.I.; Azorín-Molina, C.; Pasho, E.; Lorenzo-Lacruz, J.; Revuelto, J.; et al. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef]
- Verbesselt, J.; Zeileis, A.; Herold, M. Near real-time disturbance detection using satellite image time series. Remote Sens. Environ. 2012, 123, 98–108. [Google Scholar] [CrossRef]
- Geng, L.; Che, T.; Wang, X.; Wang, H. Detecting spatiotemporal changes in vegetation with the BFAST model in the Qilian Mountain region during 2000–2017. Remote Sens. 2019, 11, 103. [Google Scholar] [CrossRef]
- Gillespie, T.W.; Ostermann-Kelm, S.; Dong, C.; Willis, K.S.; Okin, G.S.; MacDonald, G.M. Monitoring changes of NDVI in protected areas of southern California. Ecol. Indic. 2018, 88, 485–494. [Google Scholar] [CrossRef]
- Fang, X.; Zhu, Q.; Ren, L.; Chen, H.; Wang, K.; Peng, C. Large-scale detection of vegetation dynamics and their potential drivers using MODIS images and BFAST: A case study in Quebec, Canada. Remote Sens. Environ. 2018, 206, 391–402. [Google Scholar] [CrossRef]
- Watts, L.M.; Laffan, S.W. Effectiveness of the BFAST algorithm for detecting vegetation response patterns in a semi-arid region. Remote Sens. Environ. 2014, 154, 234–245. [Google Scholar] [CrossRef]
- Bernardino, P.N.; De Keersmaecker, W.; Fensholt, R.; Verbesselt, J.; Somers, B.; Horion, S. Global-scale characterization of turning points in arid and semi-arid ecosystem functioning. Glob. Ecol. Biogeogr. 2020, 29, 1230–1245. [Google Scholar] [CrossRef]
- Chen, L.; Michishita, R.; Xu, B. Abrupt spatiotemporal land and water changes and their potential drivers in Poyang Lake, 2000-2012. ISPRS J. Photogramm. Remote Sens. 2014, 98, 85–93. [Google Scholar] [CrossRef]
- Spanish Meteorology Agency—AEMET Government of Spain. 2021. Available online: http://www.aemet.es/ (accessed on 25 February 2021).
- Ginés, Á.; Ginés, J.; Gómez-Pujol, L.; Onac, B.; Fornós, J. Mallorca: A Mediterranean Benchmark for Quaternary Studies; Societat d’Història Natural de les Balears: Palma, Spain, 2012. [Google Scholar]
- Vogiatzakis, I.N.; Pungetti, G.; Mannion, A.M. Mediterranean Island Landscapes: Natural and Cultural Approaches; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Volume 9. [Google Scholar]
- Focardi, S.; Tinelli, A. Herbivory in a Mediterranean forest: Browsing impact and plant compensation. Acta Oecologica 2005, 28, 239–247. [Google Scholar] [CrossRef]
- Bashan, D.; Bar-Massada, A. Regeneration dynamics of woody vegetation in a Mediterranean landscape under different disturbance-based management treatments. Appl. Veg. Sci. 2017, 20, 106–114. [Google Scholar] [CrossRef]
- Dell, B.; Hopkins, A.J.; Lamont, B.B. Resilience at the Level of the Plant Community. In Resilience in Mediterranean-Type Ecosystems; Springer Netherlands: Dordrecht, The Netherlands, 1986; Volume 16. [Google Scholar]
- Morey, M.; Ruiz-Pérez, M. The Balearic Islands. In Mediterranean Island Landscapes; Springer: Dordrecht, The Netherlands, 2008; pp. 271–296. [Google Scholar]
- Campbell, K.; Donlan, C.J. Feral Goat Eradications on Islands. Conserv. Biol. 2005, 19, 1362–1374. [Google Scholar] [CrossRef]
- Genovesi, P. Eradications of invasive alien species in Europe: A review. Biol. Invasions 2005, 7, 127–133. [Google Scholar] [CrossRef]
- Mayol, J.; Mayol, M.; Domenech, O.; Oliver, J.; McMinn, M.; Rodríguez, A. Aerial broadcast of rodenticide on the island of Sa Dragonera (Balearic Islands, Spain). A promising rodent eradication expe-rience on a Mediterranean island. Aliens Invasive Species Bull. 2012, 32, 29–32. [Google Scholar]
- Granjon, L.; Cheylan, G.; Duryadi, D.; Piraud, N.; Ganem, G. Premières données sur l’écologie et l’évolution des populations de rats noirs (Rattus rattus L. 1758) des îles Cerbicale (Corse du Sud). Trav. Sci. Parc Nat. Régional Réserves Nat. Corse 1992, 39, 97–111. [Google Scholar]
- NATURA 2000 Standard Data Form. Available online: https://natura2000.eea.europa.eu/natura2000/SDF.aspx?site=ES0000221 (accessed on 24 February 2021).
- Ministry of the Environment and Territory—Baleares Islands Government. Plan Rector de Uso y Gestión (PRUG). Plan for Use and Management of the Natural Park of sa Dragonera. Order of the Minister of the Environment of June 8, 2001, Approving the Master. (BOIB No. 73 of June 19, 2001). Available online: https://www.caib.es/sites/espaisnaturalsprotegits/ca/parc_natural_de_sa_dragonera-21716/ (accessed on 24 February 2021).
- Mcminn, M.; Sevilla, G.; Rodríguez, A.; Pons, G.X. Verificación de la presencia de roedores en el Parque Natural de sa Dragonera. Bolletí Soc. D’història Nat. Balear. 2017, 60, 171–192. [Google Scholar]
- Grivé, M.M. Acciones con Aves Marinas 2010 Phalacrocorax Aristotelis Cormorán Moñudo Acciones Aves Marinas 2009 Phalacrocorax Aristotelis Cormorán Moñudo; Skua Gabinet d’estudis ambientals S.L.P: Palma de Mallorca, Spain, 2010; pp. 1–13. [Google Scholar]
- Russell, J.C.; Beaven, B.M.; Mackay, J.W.B.; Towns, D.R.; Clout, M.N. Testing island biosecurity systems for invasive rats. Wildl. Res. 2008. [Google Scholar] [CrossRef]
- Aomar, G.; Mayol, M.; González, J.M. Notes naturalístiques del parc natural de sa Dragonera (2011-2014). Monogr. Soc. d’Historia Nat. Balear. 2015, 2015, 355–358. [Google Scholar]
- Alcover, J.A.; Alcover, J.A. The First Mallorcans: Prehistoric Colonization in the Western Mediterranean. J. World Prehistory 2008, 21, 19–84. [Google Scholar] [CrossRef]
- Seguí, B.; Payeras, L.; Ramis, D.; Martínez, A.; Delgado, J.V.; Quiroz, J. La cabra salvaje mallorquina: Origen, genética, morgología, notas ecológicas e implicaciones taxonómicas. Bolleti Soc. d’Historia Nat. Balear. 2005, 48, 121–151. [Google Scholar]
- USGS. Landsat 7 Science Data Users Handbook; USGS: Reston, VA, USA, 1998.
- Gutman, G.G. Vegetation indices from AVHRR: An update and future prospects. Remote Sens. Environ. 1991, 35, 121–136. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. Cran. 2021. R Package Version 3.5-9. Available online: https://CRAN.R-project.org/package=raster (accessed on 24 February 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Gromny, E.; Lewiński, S.; Rybicki, M.; Malinowski, R.; Krupiński, M.; Nowakowsk, A.; Jenerowicz, M. Creation of training dataset for Sentinel-2 land cover classification. In Proceedings of the Photonics Applications in Astronomy, Communications, Industry, and High-Energy Physics Experiments, Wilga, Poland, 26 May–2 June 2019; p. 106. [Google Scholar]
- North, M.A. A method for implementing a statistically significant number of data classes in the Jenks algorithm. In Proceedings of the 6th International Conference on Fuzzy Systems and Knowledge Discovery, Tianjin, China, 14–16 August 2009; pp. 35–38. [Google Scholar]
- Thomas, W. A three-dimensional model for calculating reflection functions of inhomogeneous and orographically structured natural landscapes. Remote Sens. Environ. 1997, 59, 44–63. [Google Scholar] [CrossRef]
- Verbesselt, J.; Jönsson, P.; Lhermitte, S.; Van Aardt, J.; Coppin, P. Evaluating satellite and climate data-derived indices as fire risk indicators in savanna ecosystems. IEEE Trans. Geosci. Remote Sens. 2006, 44, 1622–1632. [Google Scholar] [CrossRef]
- Chen, J.; Jönsson, P.; Tamura, M.; Gu, Z.; Matsushita, B.; Eklundh, L. A simple method for reconstructing a high-quality NDVI time-series data set based on the Savitzky-Golay filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Zeng, L.; Wardlow, B.D.; Xiang, D.; Hu, S.; Li, D. A review of vegetation phenological metrics extraction using time-series, multispectral satellite data. Remote Sens. Environ. 2020, 237. [Google Scholar] [CrossRef]
- Stanimirova, R.; Cai, Z.; Melaas, E.K.; Gray, J.M.; Eklundh, L.; Jönsson, P.; Friedl, M.A. An empirical assessment of the MODIS land cover dynamics and TIMESAT land surface phenology algorithms. Remote Sens. 2019, 11, 2201. [Google Scholar] [CrossRef]
- Kennedy, R.E.; Andréfouët, S.; Cohen, W.B.; Gómez, C.; Griffiths, P.; Hais, M.; Healey, S.P.; Helmer, E.H.; Hostert, P.; Lyons, M.B.; et al. Bringing an ecological view of change to Landsat-based remote sensing. Front. Ecol. Environ. 2014, 12, 339–346. [Google Scholar] [CrossRef]
- Funk, C.; Budde, M.E. Phenologically-tuned MODIS NDVI-based production anomaly estimates for Zimbabwe. Remote Sens. Environ. 2009, 113, 115–125. [Google Scholar] [CrossRef]
- Vrieling, A.; de Beurs, K.M.; Brown, M.E. Variability of African farming systems from phenological analysis of NDVI time series. Clim. Chang. 2011, 109, 455–477. [Google Scholar] [CrossRef]
- Conselleria medi ambient i pesca, Direcció General Recurs Hídrics. Plan Especial de Actuación en Situaciones de Alerta y Eventual Sequía; Direcció General Recurs Hídrics: Palma de Malloca, Spain, 2017. [Google Scholar]
- von Keyserlingk, J.; de Hoop, M.; Mayor, A.G.; Dekker, S.C.; Rietkerk, M.; Foerster, S. Resilience of vegetation to drought: Studying the effect of grazing in a Mediterranean rangeland using satellite time series. Remote Sens. Environ. 2021, 255, 112270. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Zeileis, A.; Culvenor, D. Phenological change detection while accounting for abrupt and gradual trends in satellite image time series. Remote Sens. Environ. 2010, 114, 2970–2980. [Google Scholar] [CrossRef]
- Alcover, J.A.; Perez-Obiol, R.; Yll, E.-I.; Bover, P. The diet of Myotragus balearicus Bate 1909 (Artiodactyla: Caprinae), an extinct bovid from the Balearic Islands: Evidence from coprolites. Biol. J. Linn. Soc. 1999, 66, 57–74. [Google Scholar] [CrossRef]
- Dobson, A.P.; Bradshaw, A.D.; Baker, A.J.M. Hopes for the future: Restoration ecology and conservation biology. Science 1997, 277, 515–522. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Ginés, À.; Ginés, J. La Cova de sa Font (o Cova des Moro) i l’origen del topònim de s’ illa de sa Dragonera: Una hipòtesi espeleològica. Endins: Publicació D’espeleologia 2010, 34, 9–18. [Google Scholar]
- Zavaleta, E.S.; Hobbs, R.J.; Mooney, H.A. Viewing invasive species removal in a whole-ecosystem context. Trends Ecol. Evol. 2001, 16, 454–459. [Google Scholar] [CrossRef]
- Strayer, D.L.; Eviner, V.T.; Jeschke, J.M.; Pace, M.L. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 2006, 21, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Blondel, J.; Aronson, J.; Bodiou, J.; Boeuf, G. The Mediterranean Region: Biological Diversity in Space and Time; Oxford University Press: Oxford, UK, 2010. [Google Scholar]

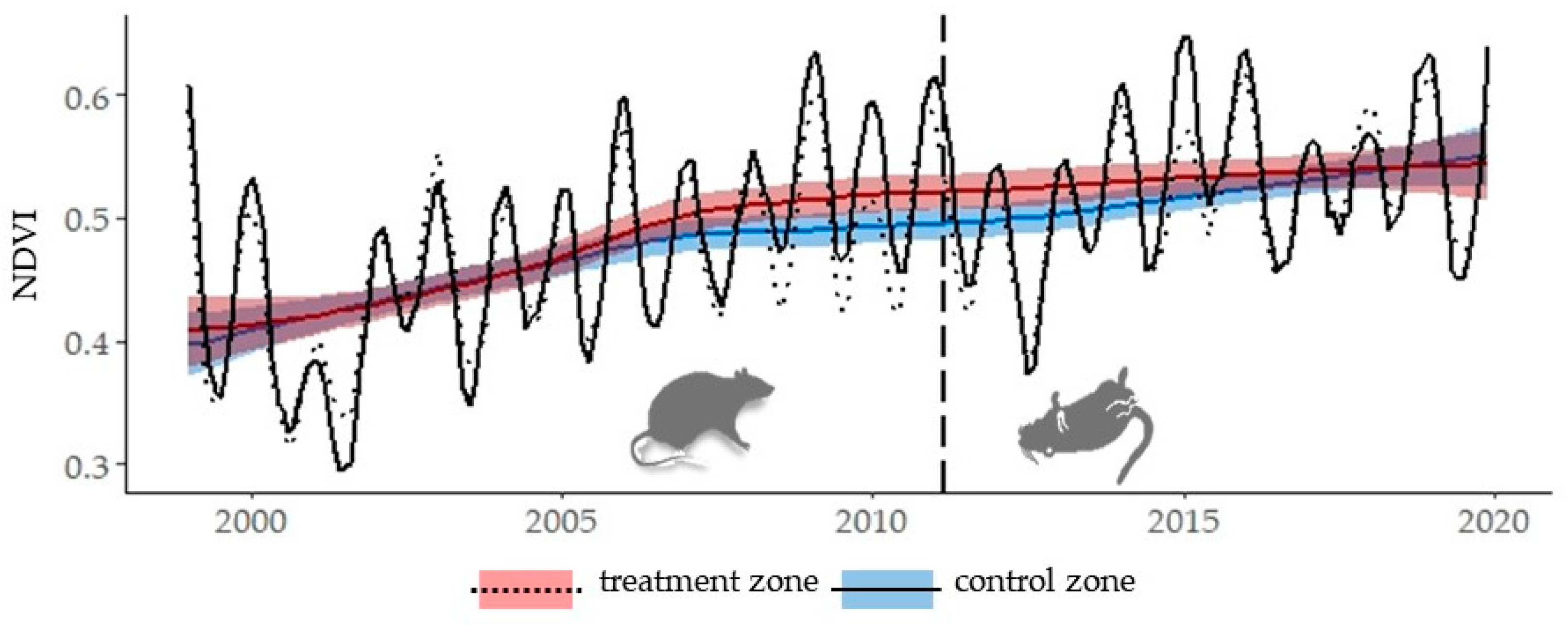
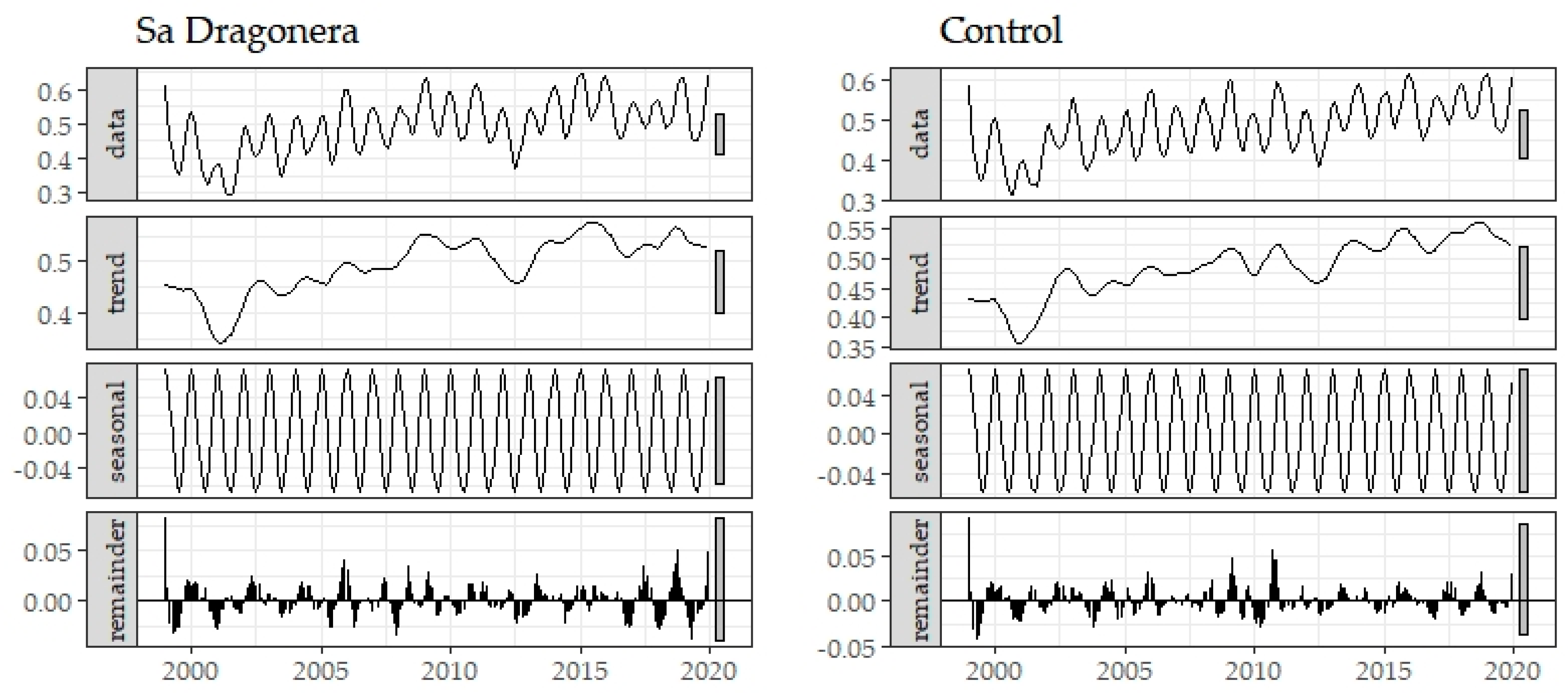
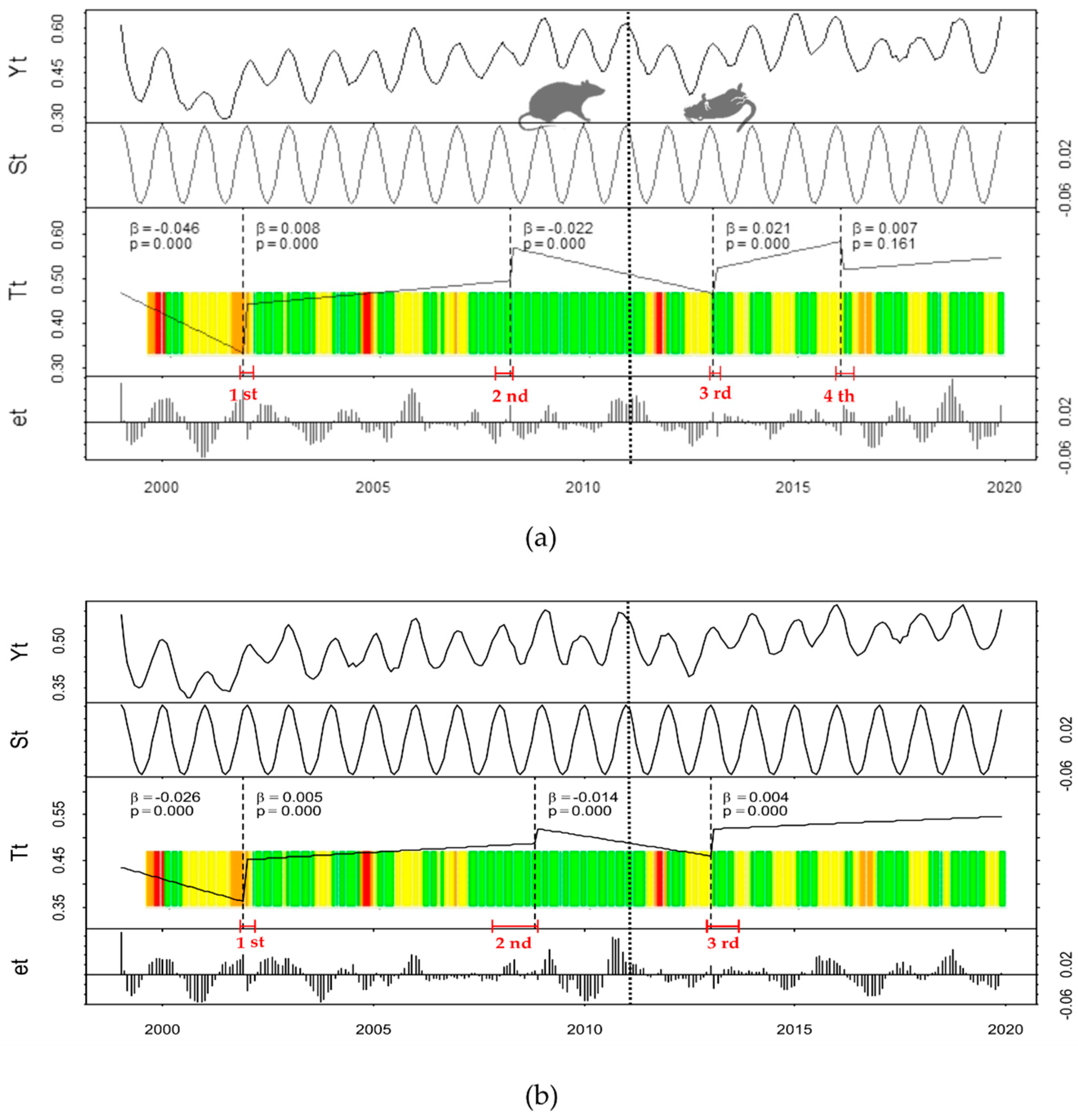
| Zone | Breakpoint | Breakpoints Dates | Change Magnitude (Absolute NDVI Units) | Linear Regression Parameters of Trend Component | |||
|---|---|---|---|---|---|---|---|
| 2.5% CI Limit | Breakpoint Timing | 97.5% CI Limit | Intercepts | Slopes | |||
| Treatment | 1st | 2001(11) | 2001(12) | 2002(03) | 0.109 | 0.442 | 0.008 |
| 2nd | 2007(12) | 2008(04) | 2008(05) | 0.074 | 0.569 | −0.022 | |
| 3rd | 2013(01) | 2013(02) | 2013(04) | 0.057 | 0.523 | 0.021 | |
| 4th | 2016(01) | 2016(02) | 2016(06) | −0.063 | 0.521 | 0.007 | |
| Control | 1st | 2001(11) | 2001(12) | 2002(03) | 0.091 | 0.453 | 0.005 |
| 2nd | 2007(11) | 2008(11) | 2008(12 | 0.031 | 0.519 | −0.014 | |
| 3rd | 2012(12) | 2013(01) | 2013(09) | 0.059 | 0.519 | 0.004 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibañez-Álvarez, M.; Farràs Santasusana, P.; Calleja, J.A.; Rouco, C.; Brolly, M.; Burnside, N.G.; Baraza, E.; Bartolomé, J.; Serrano, E. Satellite-Based Monitoring of Primary Production in a Mediterranean Islet Post Black Rat Eradication. Remote Sens. 2022, 14, 101. https://doi.org/10.3390/rs14010101
Ibañez-Álvarez M, Farràs Santasusana P, Calleja JA, Rouco C, Brolly M, Burnside NG, Baraza E, Bartolomé J, Serrano E. Satellite-Based Monitoring of Primary Production in a Mediterranean Islet Post Black Rat Eradication. Remote Sensing. 2022; 14(1):101. https://doi.org/10.3390/rs14010101
Chicago/Turabian StyleIbañez-Álvarez, Miguel, Pol Farràs Santasusana, Juan Antonio Calleja, Carlos Rouco, Matthew Brolly, Niall G. Burnside, Elena Baraza, Jordi Bartolomé, and Emmanuel Serrano. 2022. "Satellite-Based Monitoring of Primary Production in a Mediterranean Islet Post Black Rat Eradication" Remote Sensing 14, no. 1: 101. https://doi.org/10.3390/rs14010101
APA StyleIbañez-Álvarez, M., Farràs Santasusana, P., Calleja, J. A., Rouco, C., Brolly, M., Burnside, N. G., Baraza, E., Bartolomé, J., & Serrano, E. (2022). Satellite-Based Monitoring of Primary Production in a Mediterranean Islet Post Black Rat Eradication. Remote Sensing, 14(1), 101. https://doi.org/10.3390/rs14010101