Detecting Desert Locust Breeding Grounds: A Satellite-Assisted Modeling Approach
Abstract
1. Introduction
2. Data and Methods
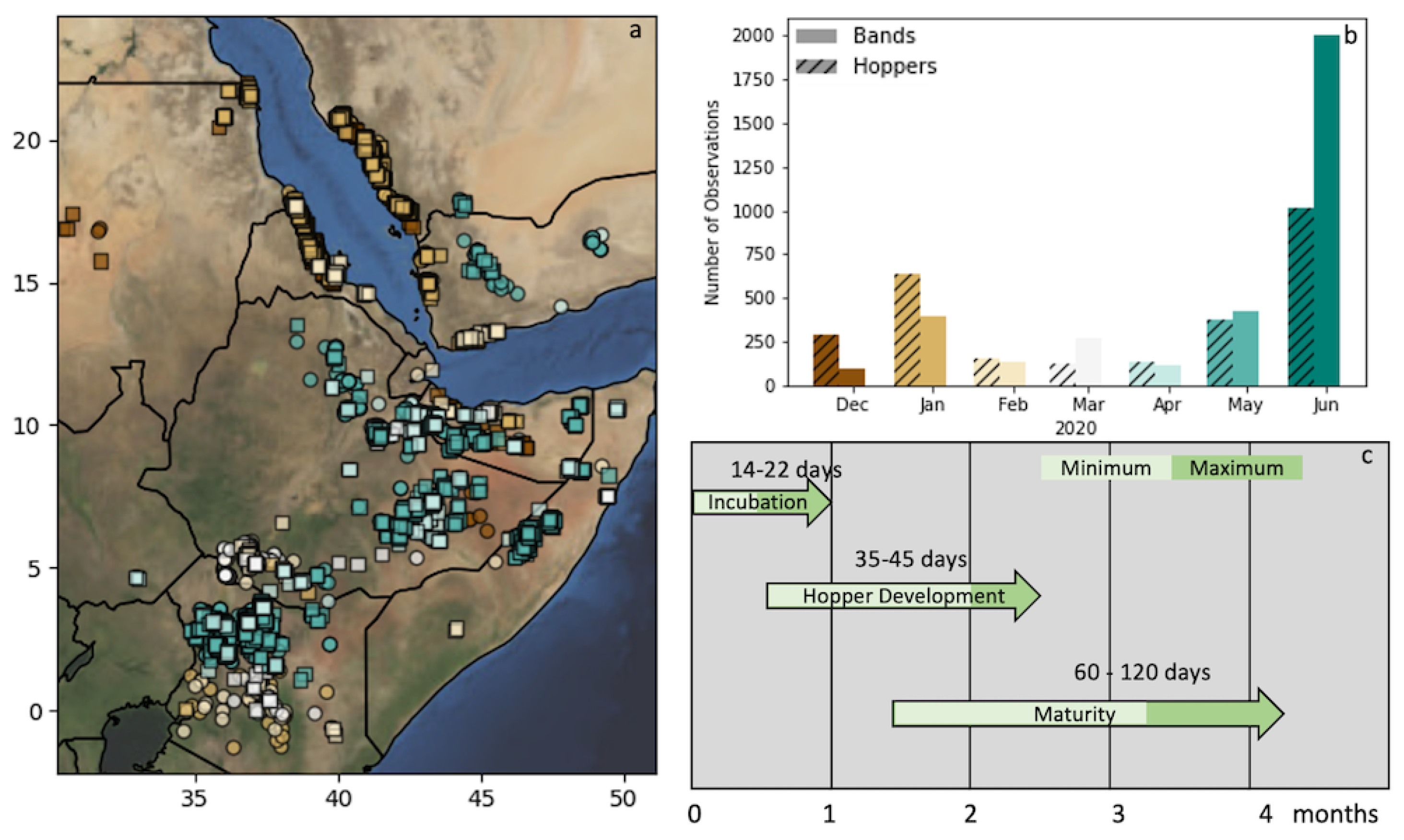
2.1. Study Area and Time Period
2.2. Locust Observations
2.3. Soil Moisture and Texture Datasets
2.3.1. Soil Moisture from LIS
2.3.2. ISRIC Soil Texture
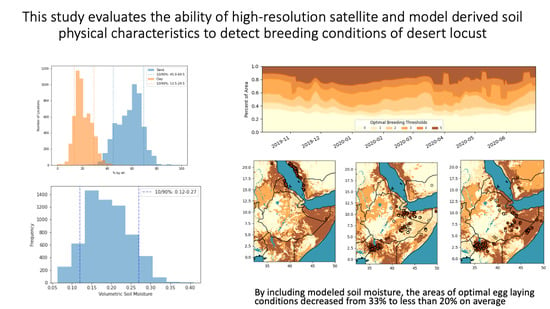
3. Results and Discussion
3.1. Soil Texture Analysis
3.2. Soil Moisture Analysis
3.3. Combined Analysis
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latchininsky, A.; Piou, C.; Franc, A.; Soti, V. Applications of remote sensing to locust management. In Land Surface Remote Sensing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 263–293. [Google Scholar]
- Van Huis, A.; Cressman, K.; Magor, J.I. Preventing desert locust plagues: optimizing management interventions. Entomol. Exp. Appl. 2007, 122, 191–214. [Google Scholar] [CrossRef]
- Mukerji, M.; Gage, S. A model for estimating hatch and mortality of grasshopper egg populations based on soil moisture and heat. Ann. Entomol. Soc. Am. 1978, 71, 183–190. [Google Scholar] [CrossRef]
- Padgham, D. Hatching rhythms in the desert locust, Schistocerca gregaria. Physiol. Entomol. 1981, 6, 191–198. [Google Scholar] [CrossRef]
- Batten, A. The senegalese grasshopper Oedaleus senegalensis Krauss. J. Appl. Ecol. 1969, 6, 27–45. [Google Scholar] [CrossRef]
- Pener, M.P.; Simpson, S.J. Locust phase polyphenism: an update. Adv. Insect Physiol. 2009, 36, 1–272. [Google Scholar]
- Peng, W.; Ma, N.L.; Zhang, D.; Zhou, Q.; Yue, X.; Khoo, S.C.; Yang, H.; Guan, R.; Chen, H.; Zhang, X.; et al. A review of historical and recent locust outbreaks: Links to global warming, food security and mitigation strategies. Environ. Res. 2020, 191, 110046. [Google Scholar] [CrossRef]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, C.; Taratiel, D.; Casanova, J. Desert locust detection using Earth observation satellite data in Mauritania. J. Arid. Environ. 2019, 164, 29–37. [Google Scholar] [CrossRef]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, J.L. Modelling desert locust presences using 32-year soil moisture data on a large-scale. Ecol. Indic. 2020, 117, 106655. [Google Scholar] [CrossRef]
- Escorihuela, M.J.; Merlin, O.; Stefan, V.; Moyano, G.; Eweys, O.A.; Zribi, M.; Kamara, S.; Benahi, A.S.; Ebbe, M.A.B.; Chihrane, J.; et al. SMOS based high resolution soil moisture estimates for desert locust preventive management. Remote. Sens. Appl. Soc. Environ. 2018, 11, 140–150. [Google Scholar]
- Piou, C.; Gay, P.E.; Benahi, A.S.; Babah Ebbe, M.A.O.; Chihrane, J.; Ghaout, S.; Cisse, S.; Diakite, F.; Lazar, M.; Cressman, K.; et al. Soil moisture from remote sensing to forecast desert locust presence. J. Appl. Ecol. 2019, 56, 966–975. [Google Scholar] [CrossRef]
- Kimathi, E.; Tonnang, H.E.; Subramanian, S.; Cressman, K.; Abdel-Rahman, E.M.; Tesfayohannes, M.; Niassy, S.; Torto, B.; Dubois, T.; Tanga, C.M.; et al. Prediction of breeding regions for the desert locust Schistocerca gregaria in East Africa. Sci. Rep. 2020, 10, 11937. [Google Scholar] [CrossRef]
- Stein, A.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.; Cohen, M.; Ngan, F. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Popov, G.B. Ecological Studies on Oviposition by Swarms of the Desert Locust (Schistocerca gregaria Forskal) in Eastern Africa; Anti-Locust Research Centre: London, UK, 1958. [Google Scholar]
- Popov, G.; Zeller, W.; Cochemé, J. Ecological Survey Report on Studies in India, Pakistan and Iran During 1963–1964; Plant Production and Protection Division, Food and Agriculture Organization: Rome, Italy, 1965. [Google Scholar]
- Symmons, P.; Cressman, K. Desert Locust Guidelines, Vol 1. Biology and Behaviour; FAO: Rome, Italy, 2001. [Google Scholar]
- Dinku, T.; Connor, S.J.; Ceccato, P. Comparison of CMORPH and TRMM-3B42 over mountainous regions of Africa and South America. In Satellite Rainfall Applications for Surface Hydrology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 193–204. [Google Scholar]
- Cressman, K. Role of remote sensing in desert locust early warning. J. Appl. Remote. Sens. 2013, 7, 075098. [Google Scholar] [CrossRef]
- Mishra, V.; Ellenburg, W.L.; Griffin, R.E.; Mecikalski, J.R.; Cruise, J.F.; Hain, C.R.; Anderson, M.C. An initial assessment of a SMAP soil moisture disaggregation scheme using TIR surface evaporation data over the continental United States. Int. J. Appl. Earth Obs. Geoinf. 2018, 68, 92–104. [Google Scholar] [CrossRef]
- Entekhabi, D.; Reichle, R.H.; Koster, R.D.; Crow, W.T. Performance metrics for soil moisture retrievals and application requirements. J. Hydrometeorol. 2010, 11, 832–840. [Google Scholar] [CrossRef]
- Escorihuela, M.J.; Chanzy, A.; Wigneron, J.P.; Kerr, Y. Effective soil moisture sampling depth of L-band radiometry: A case study. Remote. Sens. Environ. 2010, 114, 995–1001. [Google Scholar] [CrossRef]
- Shellito, P.J.; Small, E.E.; Colliander, A.; Bindlish, R.; Cosh, M.H.; Berg, A.A.; Bosch, D.D.; Caldwell, T.G.; Goodrich, D.C.; McNairn, H.; et al. SMAP soil moisture drying more rapid than observed in situ following rainfall events. Geophys. Res. Lett. 2016, 43, 8068–8075. [Google Scholar] [CrossRef]
- Rondinelli, W.J.; Hornbuckle, B.K.; Patton, J.C.; Cosh, M.H.; Walker, V.A.; Carr, B.D.; Logsdon, S.D. Different rates of soil drying after rainfall are observed by the SMOS satellite and the South Fork in situ soil moisture network. J. Hydrometeorol. 2015, 16, 889–903. [Google Scholar] [CrossRef]
- Kumar, S.V.; Peters-Lidard, C.D.; Eastman, J.L.; Tao, W.K. An integrated high-resolution hydrometeorological modeling testbed using LIS and WRF. Environ. Model. Softw. 2008, 23, 169–181. [Google Scholar] [CrossRef][Green Version]
- Case, J.L.; LaFontaine, F.J.; Bell, J.R.; Jedlovec, G.J.; Kumar, S.V.; Peters-Lidard, C.D. A real-time MODIS vegetation product for land surface and numerical weather prediction models. IEEE Trans. Geosci. Remote Sens. 2013, 52, 1772–1786. [Google Scholar] [CrossRef]
- Hengl, T.; Mendes de Jesus, J.; Heuvelink, G.B.; Ruiperez Gonzalez, M.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Camberlin, P. Climate of eastern Africa. In Oxford Research Encyclopedia of Climate Science; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- WMO/FAO. Weather and Desert Locusts, World Meteorological Organization and Food and Agriculture Organization of the United Nations; WMO: Geneva, Switzerland, 2016. [Google Scholar]
- Ariel, G.; Ayali, A. Locust collective motion and its modeling. PLoS Comput. Biol. 2015, 11, e1004522. [Google Scholar] [CrossRef] [PubMed]
- FAO. Current Upsurge (2019–2020); FAO: Rome, Italy, 2020. [Google Scholar]
- Ek, M.; Mitchell, K.; Lin, Y.; Rogers, E.; Grunmann, P.; Koren, V.; Gayno, G.; Tarpley, J. Implementation of Noah land surface model advances in the National Centers for Environmental Prediction operational mesoscale Eta model. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Case, J.L. From drought to flooding in less than a week over South Carolina. Results Phys. 2016, 6, 1183–1184. [Google Scholar] [CrossRef]
- Parrish, D.F.; Derber, J.C. The National Meteorological Center’s spectral statistical-interpolation analysis system. Mon. Weather. Rev. 1992, 120, 1747–1763. [Google Scholar] [CrossRef]
- Mesinger, F.; DiMego, G.; Kalnay, E.; Shafran, P.; Ebisuzaki, W.; Jovic, D.; Woollen, J.; Mitchell, K.; Rogers, E.; Ek, M.; et al. NCEP North American Regional Reanalysis; American Meteorological Society: Boston, MA, USA, 2004. [Google Scholar]
- Zobler, L. A World Soil File Grobal Climate Modeling; NASA Technical Memo; NASA: Washington, DC, USA, 1986; Volume 32. [Google Scholar]
- Miller, D.A.; White, R.A. A conterminous United States multilayer soil characteristics dataset for regional climate and hydrology modeling. Earth Interact. 1998, 2, 1–26. [Google Scholar] [CrossRef]
- Loveland, T.R.; Reed, B.C.; Brown, J.F.; Ohlen, D.O.; Zhu, Z.; Yang, L.; Merchant, J.W. Development of a global land cover characteristics database and IGBP DISCover from 1 km AVHRR data. Int. J. Remote Sens. 2000, 21, 1303–1330. [Google Scholar] [CrossRef]
- Chen, F.; Dudhia, J. Coupling an advanced land surface–hydrology model with the Penn State–NCAR MM5 modeling system. Part I: Model implementation and sensitivity. Mon. Weather Rev. 2001, 129, 569–585. [Google Scholar] [CrossRef]
- Vargas, M.; Jiang, Z.; Ju, J.; Csiszar, I. Real-time daily rolling weekly Green Vegetation Fraction (GVF) derived from the Visible Imaging Radiometer Suite (VIIRS) sensor onboard the SNPP satellite. In Proceedings of the Satellite Meteorology and Oceanography, Phoenix, AZ, USA, 4–8 January 2015; Volume 210. [Google Scholar]


| Breeding Threshold | ||||||
|---|---|---|---|---|---|---|
| Month | 0 | 1 | 2 | 3 | 4 | 5 |
| 10 (n = 430) | 2.0 [0.0] | 2.1 [0.0] | 5.6 [5.3] | 0.9 [0.0] | 7.1 [0.9] | 84.0 [93.7] |
| 11 (n = 1003) | 2.0 [0.1] | 0.5 [0.1] | 4.9 [1.1] | 2.9 [0.0] | 8.1 [0.5] | 83.4 [98.2] |
| 12 (n = 339) | 2.9 [1.2] | 7.3 [3.5] | 15.2 [15.0] | 9.1 [0.0] | 18.5 [6.2] | 46.9 [74.2] |
| 1 (n = 351) | 3.0 [1.2] | 4.4 [0.8] | 16.6 [14.8] | 20.2 [6.2] | 22.3 [5.1] | 33.5 [71.7] |
| 2 (n = 224) | 19.6 [0.8] | 8.2 [3.3] | 26.9 [26.8] | 11.4 [4.1] | 14.7 [8.1] | 19.2 [56.9] |
| 3 (n = 1051) | 3.6 [0.7] | 2.9 [0.6] | 33.6 [3.0] | 7.4 [2.5] | 7.1 [2.6] | 45.3 [63.5] |
| 4 (n = 2763) | 0.9 [0.2] | 2.0 [0.2] | 9.2 [4.8] | 9.9 [0.1] | 12.6 [3.5] | 65.4 [90.1] |
| Average (wt) | 2.1 [0.4] | 2.5 [0.5] | 13.2 [9.7] | 0.083 [1.1] | 11.4 [3.5] | 62.5 [85.2] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellenburg, W.L.; Mishra, V.; Roberts, J.B.; Limaye, A.S.; Case, J.L.; Blankenship, C.B.; Cressman, K. Detecting Desert Locust Breeding Grounds: A Satellite-Assisted Modeling Approach. Remote Sens. 2021, 13, 1276. https://doi.org/10.3390/rs13071276
Ellenburg WL, Mishra V, Roberts JB, Limaye AS, Case JL, Blankenship CB, Cressman K. Detecting Desert Locust Breeding Grounds: A Satellite-Assisted Modeling Approach. Remote Sensing. 2021; 13(7):1276. https://doi.org/10.3390/rs13071276
Chicago/Turabian StyleEllenburg, W. Lee, Vikalp Mishra, Jason B. Roberts, Ashutosh S. Limaye, Jonathan L. Case, Clay B. Blankenship, and Keith Cressman. 2021. "Detecting Desert Locust Breeding Grounds: A Satellite-Assisted Modeling Approach" Remote Sensing 13, no. 7: 1276. https://doi.org/10.3390/rs13071276
APA StyleEllenburg, W. L., Mishra, V., Roberts, J. B., Limaye, A. S., Case, J. L., Blankenship, C. B., & Cressman, K. (2021). Detecting Desert Locust Breeding Grounds: A Satellite-Assisted Modeling Approach. Remote Sensing, 13(7), 1276. https://doi.org/10.3390/rs13071276