Drivers of Bornean Orangutan Distribution across a Multiple-Use Tropical Landscape
Abstract
1. Introduction
- How do nest counts derived from aerial surveys compare to those derived from ground-transects?
- How is orangutan nest density abundance affected by conversion of forests to alternative land uses?
- How does the density of orangutan nests respond to variation in forest quality, strangler fig density, and elevation within a multiple-use landscape in Borneo?
2. Materials and Methods
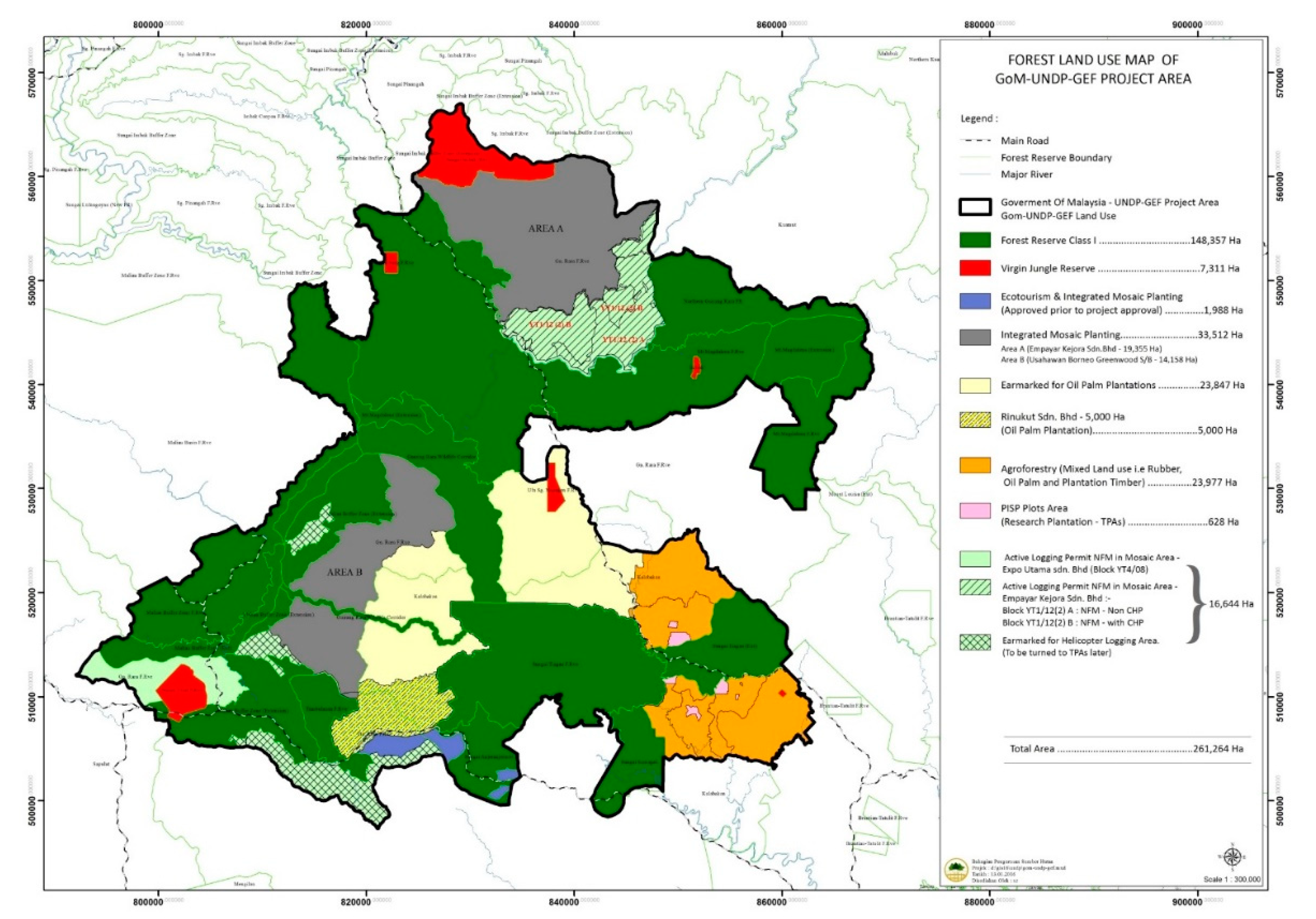
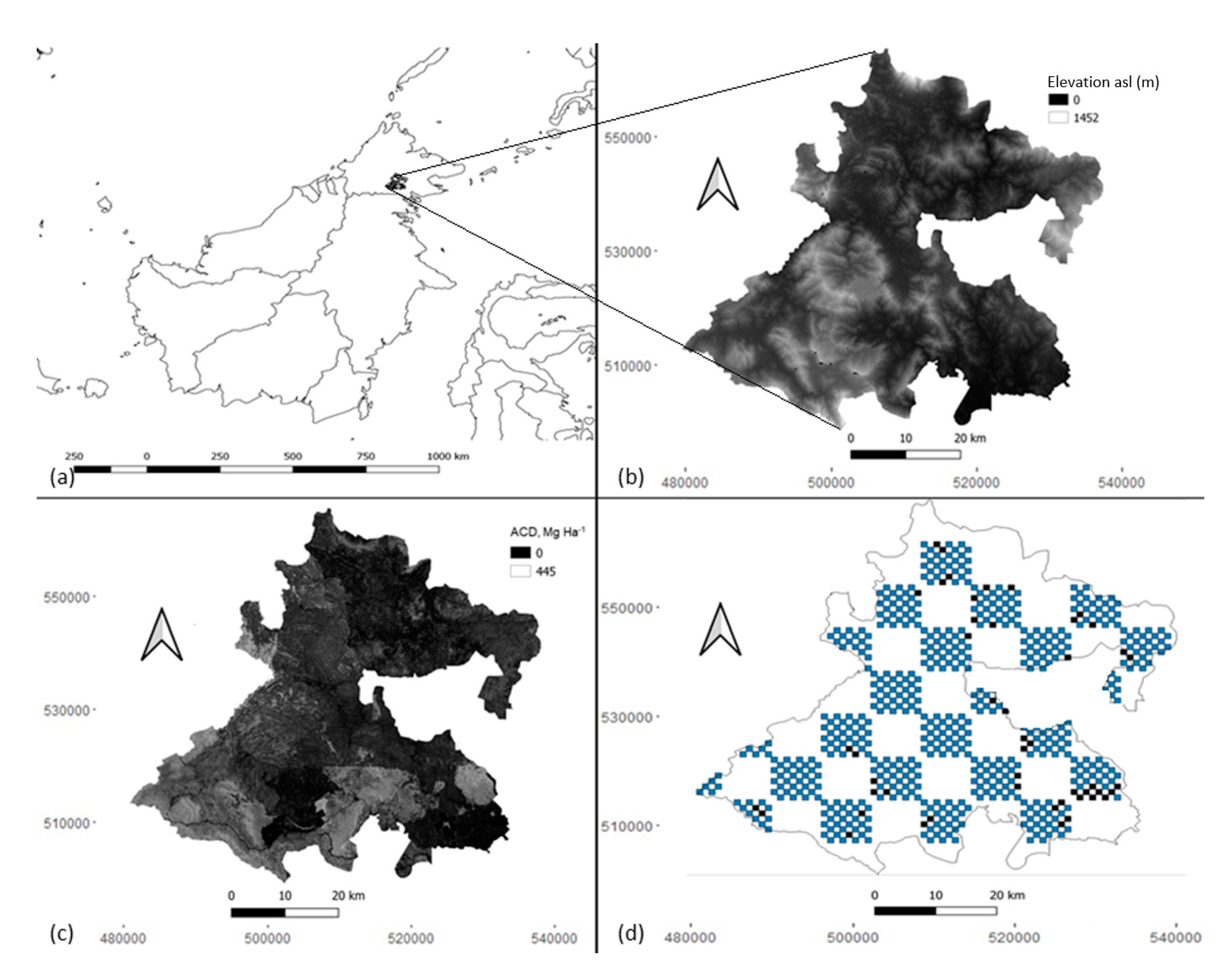
2.1. Study Area
2.2. Sampling Design and Survey Methods
2.3. Data Analysis
3. Results
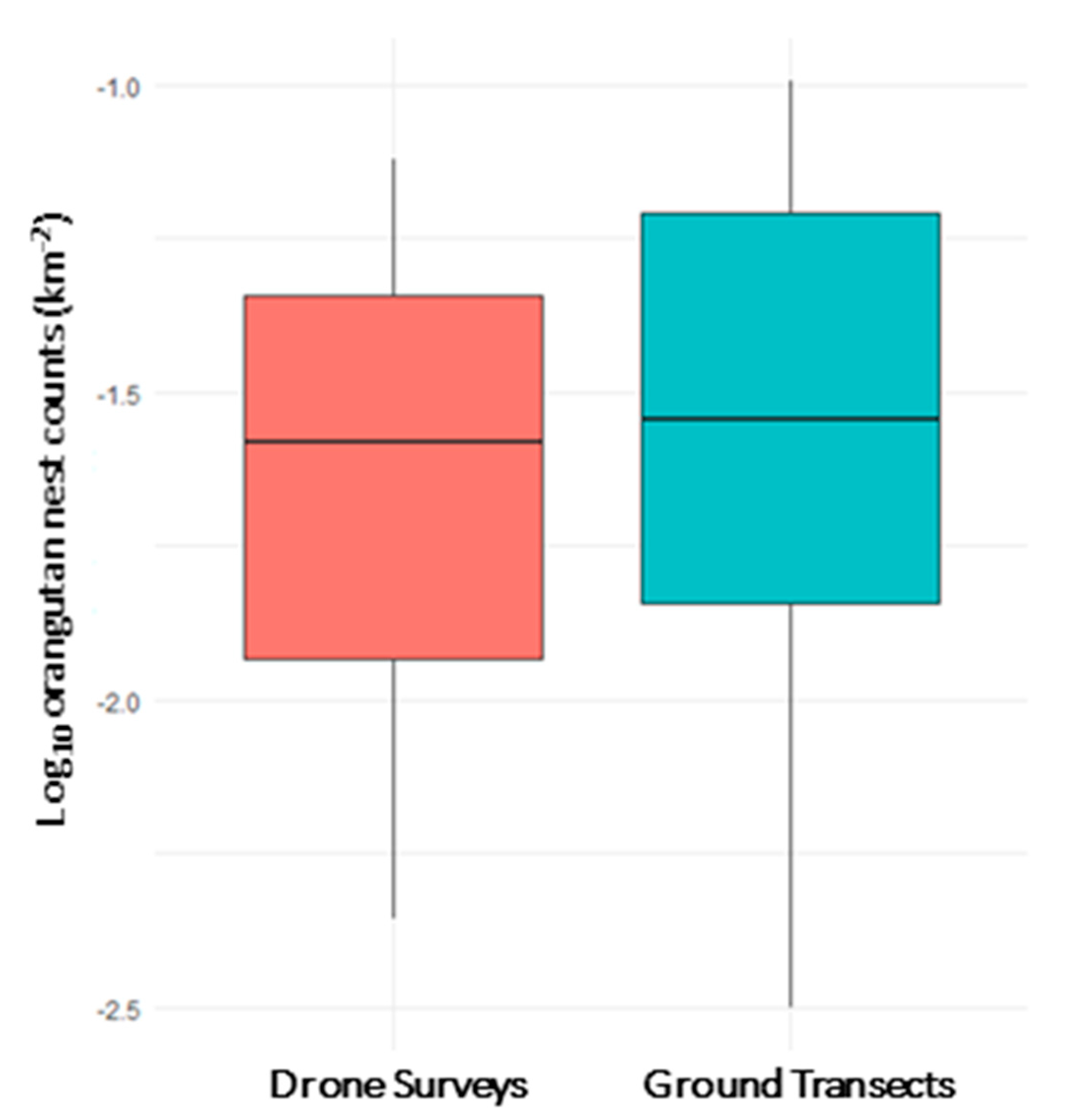
3.1. Orangutan Nest Density from Aerial and Ground Surveys
3.2. Effects of Survey Method on Orangutan Nest Counts
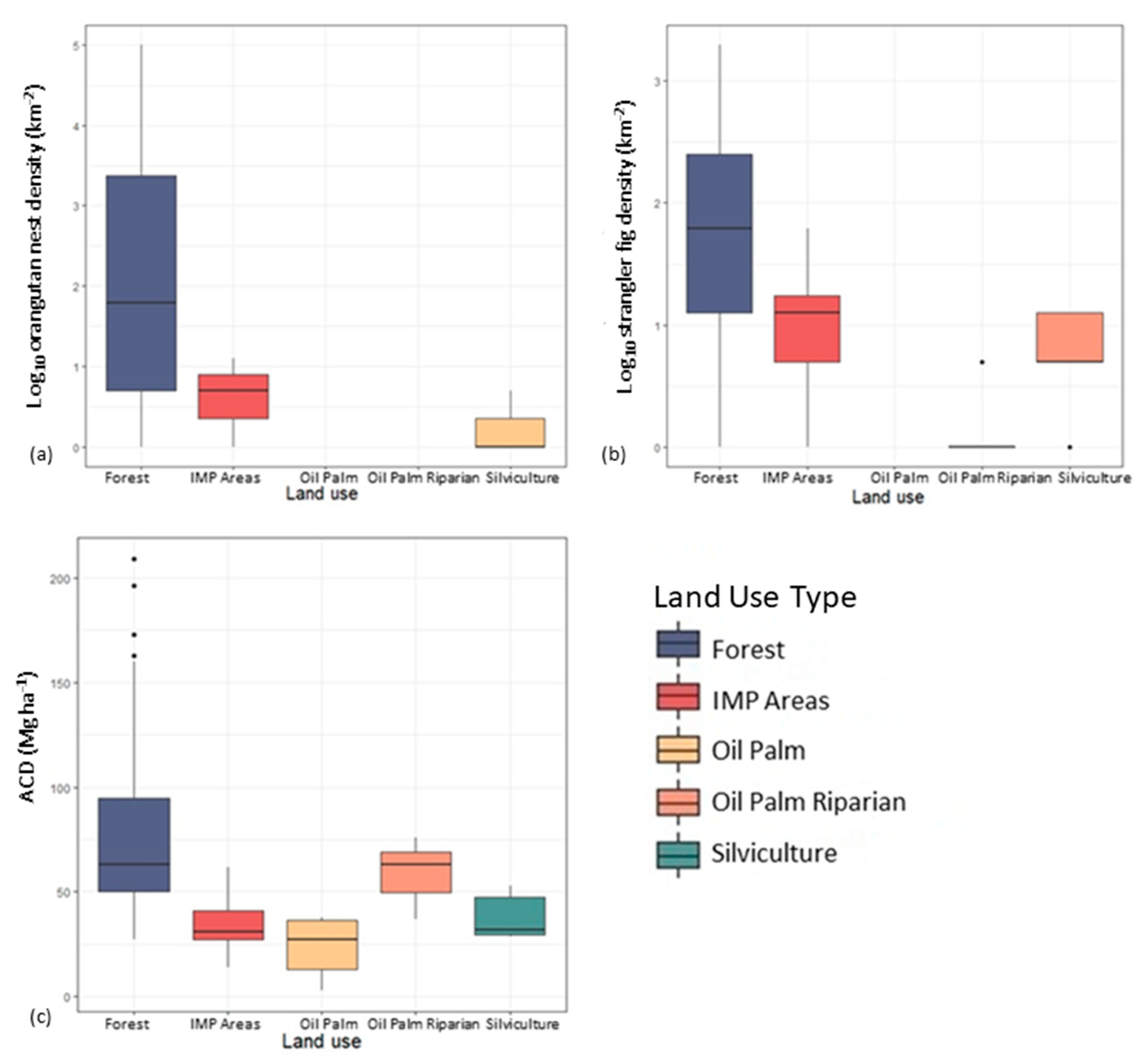
3.3. Influence of Land Use on Nest Counts, Strangler Fig Counts, and ACD in Aerial Surveys
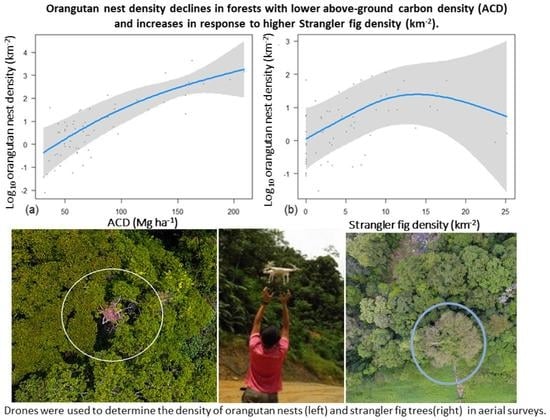
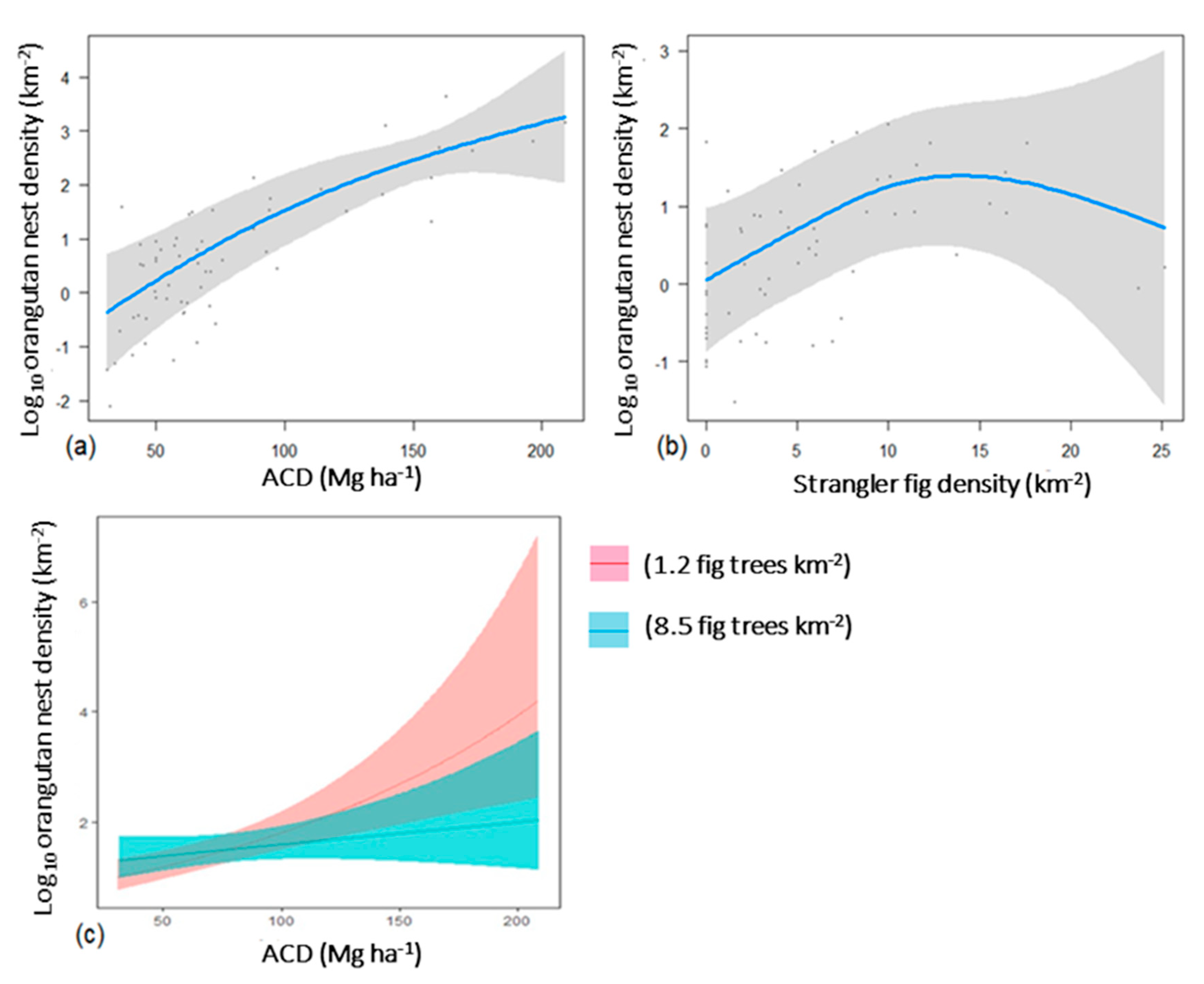
3.4. Effects of Forest Quality, Strangler Fig Density, and Elevation on Orangutan Nest Counts in Aerial Surveys
4. Discussion
4.1. Comparison of Survey Methods
4.2. Effect of Land Use on Orangutan Nest Counts, Strangler Fig Counts, and Above-Ground Carbon Density
4.3. Variation in Orangutan Nest Counts Across a Gradient of Forest Degradation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Giam, X. Global biodiversity loss from tropical deforestation. Proc. Natl. Acad. Sci. USA 2017, 114, 5775–5777. [Google Scholar] [CrossRef] [PubMed]
- Potapov, P.; Hansen, M.C.; Laestadius, L.; Turubanova, S.; Yaroshenko, A.; Thies, C.; Smith, W.; Zhuravleva, I.; Komarova, A.; Minnemeyer, S.; et al. The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 2017, 3, e1600821. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Wich, S.; Ancrenaz, M.; Meijaard, E.; Abram, N.; Banes, G.L.; Campbell-Smith, G.; D’Arcy, L.J.; Delgado, R.A.; Erman, A.; et al. Global Demand for Natural Resources Eliminated More Than 100,000 Bornean Orangutans. Curr. Biol. 2018, 28, 761–769.e5. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.; Knott, C.D.; Pamungkas, B.; Pasaribu, F.H.; Marshall, A.J. A survey of the orangutan (Pongo pygmaeus wurmbii) population in and around Gunung Palung National Park, West Kalimantan, Indonesia based on nest counts. Biol. Conserv. 2005, 121, 495–507. [Google Scholar] [CrossRef]
- Meijaard, E.; Sheil, D. The persistence and conservation of Borneo’s mammals in lowland rain forests managed for timber: Observations, overviews and opportunities. Ecol. Res. 2007, 23, 21–34. [Google Scholar] [CrossRef]
- Kamaruszaman, S.A.; Fadzly, N.; Abd-Mutalib, A.H.; Muslim, A.M.; Atmoko, S.S.U.; Mansor, M. Measuring Orangutan nest structure using Unmanned Aerial Vehicle (UAV) and ImageJ. bioRxiv 2018, 365338. [Google Scholar] [CrossRef]
- Buij, R.; Singleton, I.; Krakauer, E.; van Schaik, C.P. Rapid assessment of orangutan density. Biol. Conserv. 2003, 114, 103–113. [Google Scholar] [CrossRef]
- Wich, S.; Dellatore, D.; Houghton, M.; Ardi, R.; Koh, L.P. A preliminary assessment of using conservation drones for Sumatran orang-utan (Pongo abelii) distribution and density. J. Unmanned Veh. Syst. 2016, 4, 45–52. [Google Scholar] [CrossRef]
- Koh, L.P.; Wich, S. Dawn of drone ecology: Low-cost autonomous aerial vehicles for conservation. Trop. Conserv. Sci. 2012, 5, 121–132. [Google Scholar] [CrossRef]
- Wilson, H.B.; Meijaard, E.; Venter, O.; Ancrenaz, M.; Possingham, H.P. Conservation Strategies for Orangutans: Reintroduction versus Habitat Preservation and the Benefits of Sustainably Logged Forest. PLoS ONE 2014, 9, e102174. [Google Scholar] [CrossRef]
- Ancrenaz, M.; Sollmann, R.; Meijaard, E.; Hearn, A.J.; Ross, J.; Samejima, H.; Loken, B.; Cheyne, S.M.; Stark, D.J.; Gardner, P.C.; et al. Coming down from the trees: Is terrestrial activity in Bornean orangutans natural or disturbance driven? Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Hardus, M.E.; Lameira, A.R.; Menken, S.B.; Wich, S. Effects of logging on orangutan behavior. Biol. Conserv. 2012, 146, 177–187. [Google Scholar] [CrossRef]
- Ancrenaz, M.; Oram, F.; Ambu, L.; Lackman, I.; Ahmad, E.; Elahan, H.; Kler, H.; Abram, N.K.; Meijaard, E. Of Pongo, palms and perceptions: A multidisciplinary assessment of Bornean orang-utans Pongo pygmaeus in an oil palm context. Oryx 2014, 49, 465–472. [Google Scholar] [CrossRef]
- Husson, S.J.; Wich, S.A.; Marshall, A.J.; Dennis, R.D.; Ancrenaz, M.; Brassey, R. Orangutan distribution, density, abundance and impacts of disturbance. In Orangutans: Geographic Variation in Behavioral Ecology and Conservation; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Jucker, T.; Asner, G.P.; Dalponte, M.; Brodricki D, P.; Philipson, C.; Vaughn, N.R.; Teh, Y.A.; Brelsford, C.; Burslem, D.F.R.P.; Deere, N.J.; et al. Estimating aboveground carbon density and its uncertainty in Borneo’s structurally complex tropical forests using airborne laser scanning. Biogeosciences 2018, 15, 3811–3830. [Google Scholar] [CrossRef]
- Djojosudharmo, S.; van Schaik, C. Why are orang utans so rare in the highlands? Altitudinal changes in a Sumatran forest. Trop. Biodivers. 1992, 1, 11–22. [Google Scholar]
- Curran, L.M.; Trigg, S.; McDonald, A.K.; Astiani, D.; Hardiono, Y.M.; Siregar, P.; Caniago, I.; Kasischke, E. Lowland Forest Loss in Protected Areas of Indonesian Borneo. Science 2004, 303, 1000–1003. [Google Scholar] [CrossRef]
- Shanahan, M.; Compton, S.G. Vertical stratification of figs and fig-eaters in a Bornean low-land rain forest: How is the canopy different? In Plant Ecology; Springer: Berlin/Heidelberg, Germany, 2001; pp. 121–132. [Google Scholar]
- Janzen, D.H. Seed Predation by Animals. Annu. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Marshall, A.J.; Wrangham, R.W. Evolutionary Consequences of Fallback Foods. Int. J. Primatol. 2007, 28, 1219–1235. [Google Scholar] [CrossRef]
- Dew, J.L.; Boubli, J.P. Tropical fruits and frugivores: The search for strong interactors. In Tropical Fruits and Frugivores: The Search for Strong Interactors; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–260. [Google Scholar]
- Wich, S.; Buij, R.; van Schaik, C. Determinants of orangutan density in the dryland forests of the Leuser Ecosystem. Primates 2004, 45, 177–182. [Google Scholar] [CrossRef]
- Toevs, B. Processing of Metadata on Multimedia Using ExifTool: A Programming Approach in Python. In 2015 Annual Global Online Conference on Information and Computer Technology (GOCICT); IEEE: Piscataway, NJ, USA, 2016; pp. 26–30. [Google Scholar]
- Vavrek, M.J. Fossil: Palaeoecological and Palaeogeographical Analysis Tools. Available online: https://cran.r-project.org/web/packages/fossil/ (accessed on 13 April 2018).
- Aero, P. Ground Sample Distance (GSD) and How Does it Affect Your Drone Data? Available online: https://www.propelleraero.com/blog/ground-sample-distance-gsd-calculate-drone-data/ (accessed on 16 July 2018).
- Thomas, C.M.; Featherstone, W.E. Validation of Vincenty’s Formulas for the Geodesic Using a New Fourth-Order Extension of Kivioja’s Formula. J. Surv. Eng. 2005, 131, 20–26. [Google Scholar] [CrossRef]
- DJI. PHANTOM 4. Available online: https://dl.djicdn.com/downloads/Phantom_4_Advanced/20170609/Phantom_4_Advanced_Release_Notes_EN.pdf (accessed on 18 January 2019).
- Kluga, A.; Zelenkov, A.; Grab, E.; Belinska, V. Accuracy estimation of GPS receiver parameters with simulator in dynamic mode. Elektron. Elektrotech. 2009, 6, 9–14. [Google Scholar]
- Marshall, M.L.; David, A.; Miller, L. Package ‘Distance’. Gastroenterology 2020, 160, 362–377. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Osuri, A.M.; Machado, S.J.; Ratnam, J.; Sankaran, M.; Ayyappan, N.; Muthuramkumar, S.; Parthasarathy, N.; Pelissier, R.; Ramesh, B.R.; de Fries, R.S.; et al. Tree diversity and carbon storage cobenefits in tropical human-dominated landscapes. Conserv. Lett. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Wood, S.N. Low-Rank Scale-Invariant Tensor Product Smooths for Generalized Additive Mixed Models. Biometrics 2006, 62, 1025–1036. [Google Scholar] [CrossRef]
- Thorpe, K.S.; Crompton, R.S. Orangutans: Geographic Variation in Behavioral Ecology and Conservation, Orangutan Positional Behavior Interspecific; Oxford University Press: Oxford, UK, 2009. [Google Scholar] [CrossRef]
- Campbell-Smith, G.; Campbell-Smith, M.; Singleton, I.; Linkie, M. Apes in Space: Saving an Imperilled Orangutan Population in Sumatra. PLoS ONE 2011, 6, e17210. [Google Scholar] [CrossRef]
- Wearn, O.R. Mammalian Community Responses to a Gradient of Land Use Intensity on the Island of Borneo. Ph.D. Thesis, Imperial College London, London, UK, 2016. [Google Scholar]
- Seaman, D.J.I.; Bernard, H.; Ancrenaz, M.; Coomes, D.; Swinfield, T.; Milodowski, D.T. Densities of Bornean orang-utans (Pongo pygmaeus morio) in heavily degraded forest and oil palm plantations in Sabah, Borneo. Am. J. Primatol. 2019, 81, 1–12. [Google Scholar] [CrossRef]
- Pothasin, P.; Compton, S.G.; Wangpakapattanawong, P. Riparian Ficus Tree Communities: The Distribution and Abundance of Riparian Fig Trees in Northern Thailand. PLoS ONE 2014, 9, e108945. [Google Scholar] [CrossRef]
- Davies, A.B.; Oram, F.; Ancrenaz, M.; Asner, G.P. Combining behavioural and LiDAR data to reveal relationships between canopy structure and orangutan nest site selection in disturbed forests. Biol. Conserv. 2019, 232, 97–107. [Google Scholar] [CrossRef]
- Groves, C.P.; Bahir, M.M. Scientific Correspondence. Mar. Mammal. Sci. 2014, 30, 1279–1280. [Google Scholar] [CrossRef]
- Harrison, R.D.; Hamid, A.A.; Kenta, T.; LaFrankie, J.; Lee, H.-S.; Nagamasu, H.; Nakashizuka, T.; Palmiotto, P. The diversity of hemi-epiphytic figs (Ficus; Moraceae) in a Bornean lowland rain forest. Biol. J. Linn. Soc. 2003, 78, 439–455. [Google Scholar] [CrossRef]
- Utami, S.; Wich, S.; Sterck, E.H.M.; van Hooff, J.A.R.A.M. Food Competition Between Wild Orangutans in Large Fig Trees. Int. J. Primatol. 1997, 18, 909–927. [Google Scholar] [CrossRef]
- Marshall, A.J.; Ancrenaz, M.; Brearley, F.Q.; Fredriksson, G.M.; Ghaffar, N.; Heydon, M. The effects of forest phenology and floristics on populations of Bornean and Sumatran orangutans Are Sumatran forests better orangutan habitat than Bornean forests? In Orangutans: Geographic Variation in Behavioral Ecology and Conservation; Oxford University Press: Oxford, UK, 2009; pp. 97–118. [Google Scholar]
- Russon, A.E.; Wich, S.; Ancrenaz, M.; Kanamori, T.; Knott, C.D.; Kuze, N.; Morrogh-Bernard, H.C.; Pratje, P.; Ramlee, H.; Rodman, P.; et al. Geographic variation in orangutan diets. Orangutans 2008, 135–156. [Google Scholar] [CrossRef]
- Meijaard, E.; Albar, G.; Nardiyono-Rayadin, Y.; Ancrenaz, M.; Spehar, S. Unexpected Ecological Resilience in Bornean Orangutans and Implications for Pulp and Paper Plantation Management. PLoS ONE 2010, 5, e12813. [Google Scholar] [CrossRef] [PubMed]
- Ancrenaz, M.; Goossens, B.; Gimenez, O.; Sawang, A.; Lackman-Ancrenaz, I. Determination of ape distribution and population size using ground and aerial surveys: A case study with orang-utans in lower Kinabatangan, Sabah, Malaysia. Anim Conserv. 2004, 7, 375–385.46. [Google Scholar] [CrossRef]
- Philipson, C.D.; Cutler, M.E.; BrodrickiD, P.; Asner, G.P.; Boyd, D.S.; Costa, P.M.; Fiddes, J.; Foody, G.; van der Heijden, G.; Ledo, A.; et al. Active restoration accelerates the carbon recovery of human-modified tropical forests. Science 2020, 369, 838–841. [Google Scholar] [CrossRef]
- Dujon, A.M.; Schofield, G. Importance of machine learning for enhancing ecological studies using information-rich imagery. Endanger. Species Res. 2019, 39, 91–104. [Google Scholar] [CrossRef]
- Sabah Forestry Department. Proposed Land Use Map for UNDP-GEF Study Area; Sabah Forestry Department: Sandakan, Malaysia, 2012. [Google Scholar]
| Variables | edf | Ref.df | Chi.sq | p-Value |
|---|---|---|---|---|
| Fig Density | 2.230 | 2.687 | 10.428 | 0.012 * |
| Mean ACD | 1.603 | 1.864 | 21.999 | <0.001 *** |
| Mean Elevation | 1.000 | 1.000 | 1.365 | 0.243 |
| Fig Density * Mean ACD | 1.000 | 1.000 | 3.700 | 0.054 |
| Random Effect (Plot Location) | 12.806 | 15.000 | 165.475 | <2 × 10−16 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milne, S.; Martin, J.G.A.; Reynolds, G.; Vairappan, C.S.; Slade, E.M.; Brodie, J.F.; Wich, S.A.; Williamson, N.; Burslem, D.F.R.P. Drivers of Bornean Orangutan Distribution across a Multiple-Use Tropical Landscape. Remote Sens. 2021, 13, 458. https://doi.org/10.3390/rs13030458
Milne S, Martin JGA, Reynolds G, Vairappan CS, Slade EM, Brodie JF, Wich SA, Williamson N, Burslem DFRP. Drivers of Bornean Orangutan Distribution across a Multiple-Use Tropical Landscape. Remote Sensing. 2021; 13(3):458. https://doi.org/10.3390/rs13030458
Chicago/Turabian StyleMilne, Sol, Julien G. A. Martin, Glen Reynolds, Charles S. Vairappan, Eleanor M. Slade, Jedediah F. Brodie, Serge A. Wich, Nicola Williamson, and David F. R. P. Burslem. 2021. "Drivers of Bornean Orangutan Distribution across a Multiple-Use Tropical Landscape" Remote Sensing 13, no. 3: 458. https://doi.org/10.3390/rs13030458
APA StyleMilne, S., Martin, J. G. A., Reynolds, G., Vairappan, C. S., Slade, E. M., Brodie, J. F., Wich, S. A., Williamson, N., & Burslem, D. F. R. P. (2021). Drivers of Bornean Orangutan Distribution across a Multiple-Use Tropical Landscape. Remote Sensing, 13(3), 458. https://doi.org/10.3390/rs13030458