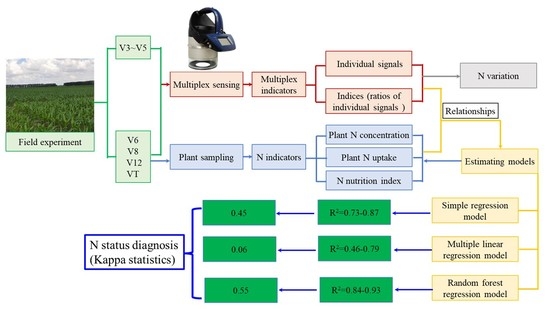
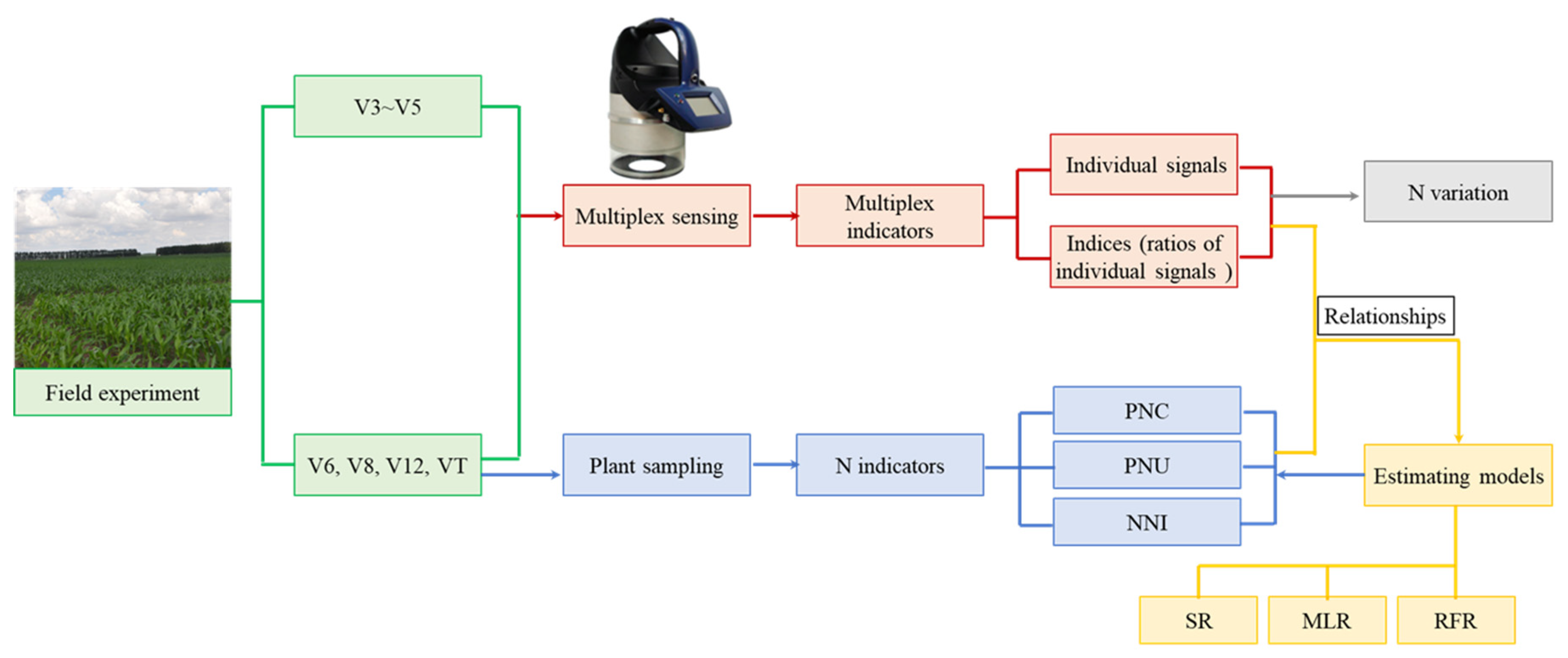
Canopy Fluorescence Sensing for In-Season Maize Nitrogen Status Diagnosis
Abstract
1. Introduction
2. Materials and Methods
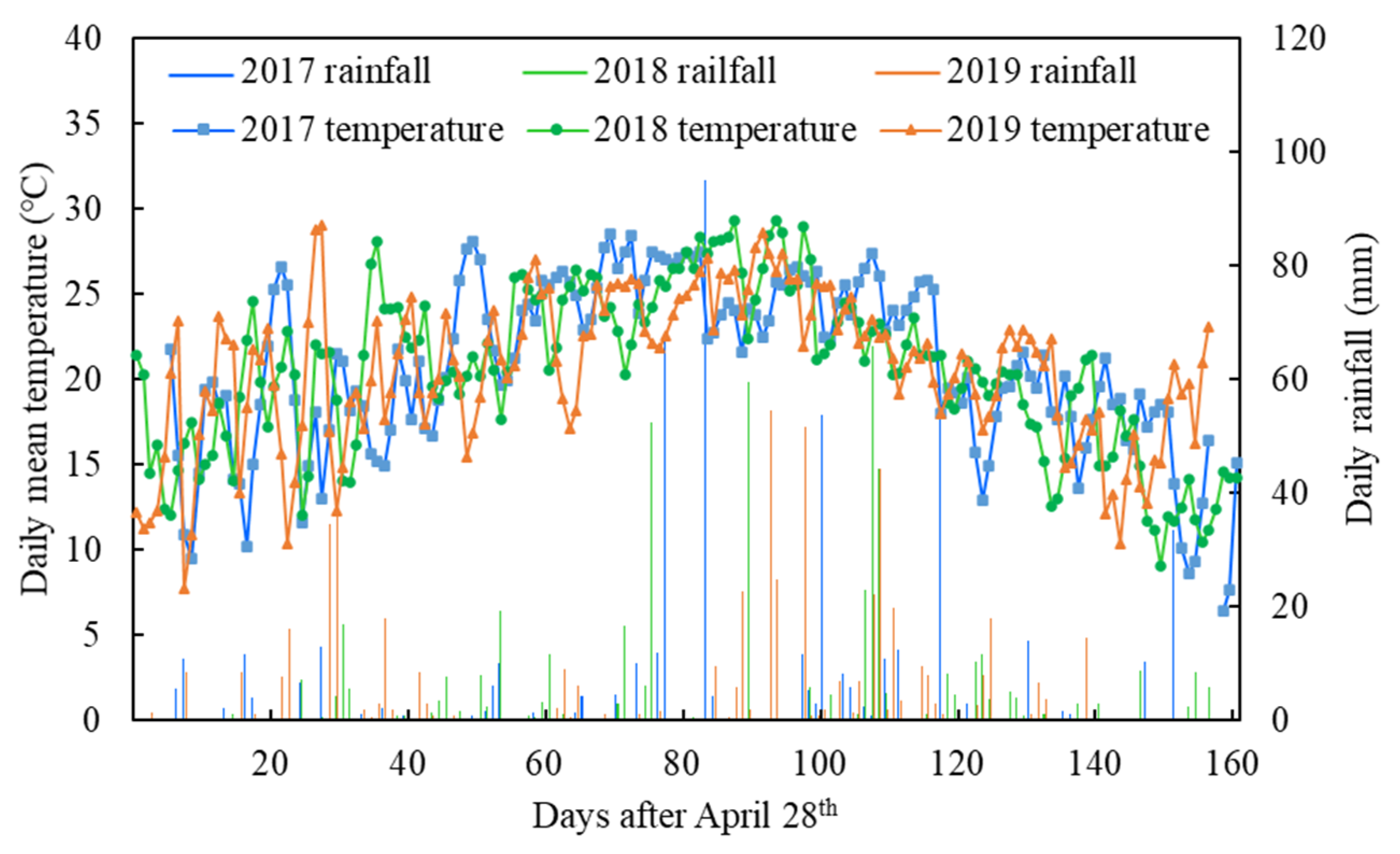
2.1. Experimental Design
2.2. Fluorescence Sensing
2.3. Sampling and Measurement
2.4. Regression Models to Estimate N Status Indicators
2.5. Statistical Analysis
3. Results
3.1. Differences in Fluorescence Parameters at Different N Rates
3.2. Simple Regression Analysis for Relationships between Raw Multiplex Indices and N Status Indicators
3.3. General Models for Estimating N status Indicators
3.3.1. Simple Regression Models Based on Normalized Multiplex Indices
3.3.2. Multiple Linear Regression and Random Forest Regression Models
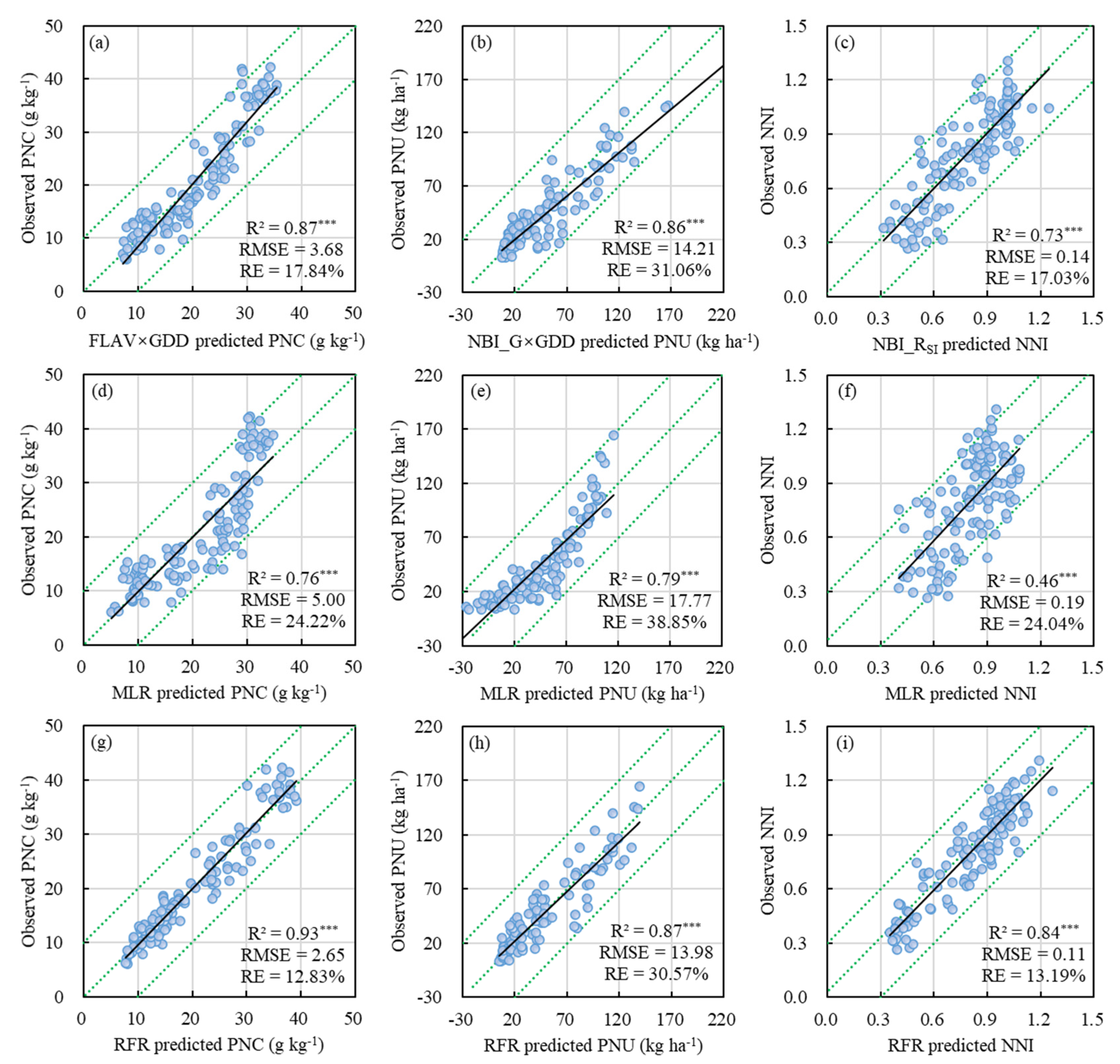
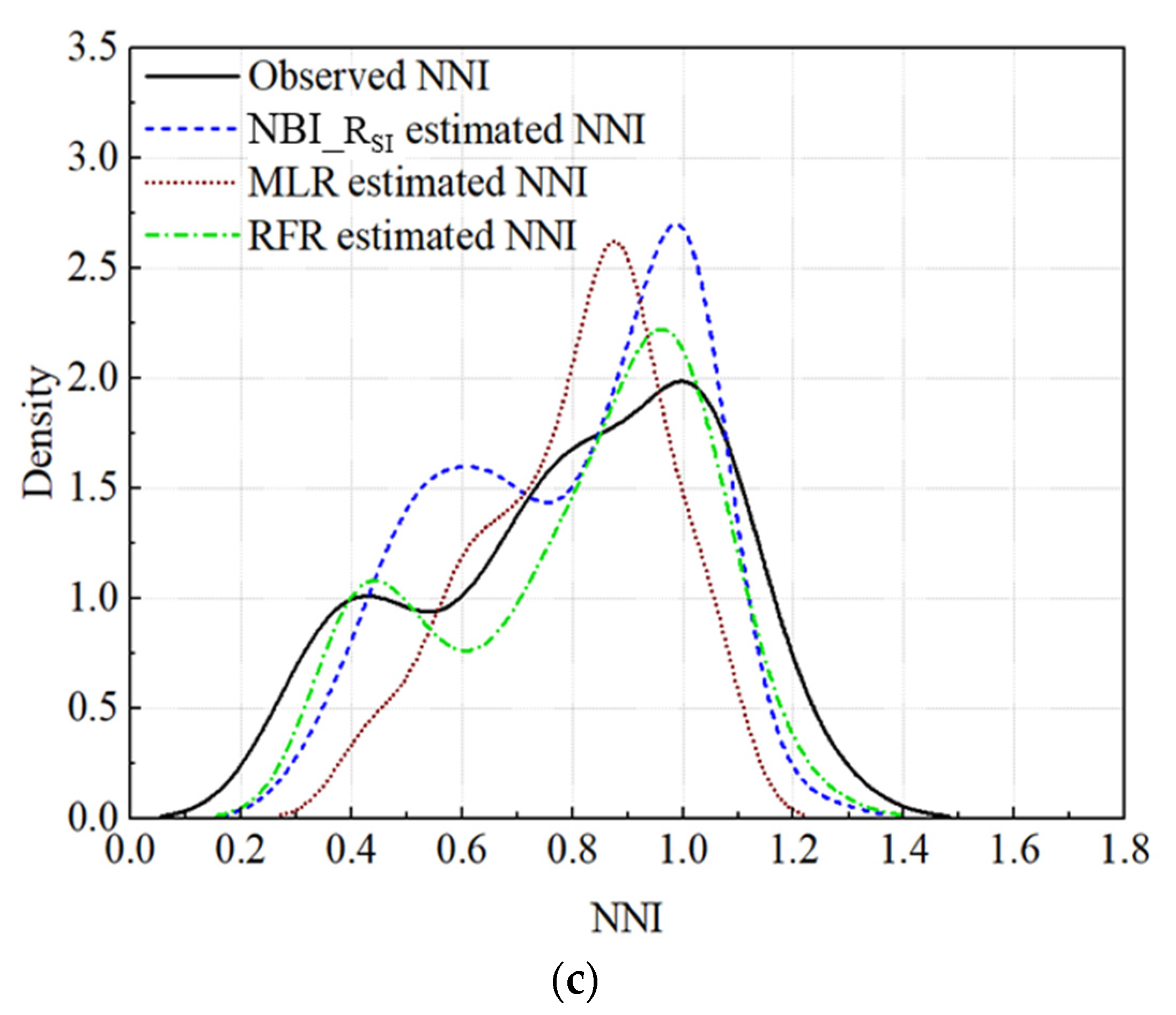
3.3.3. Validation of the Regression Models
4. Discussion
4.1. Detection of N Variability in Maize Using Fluorescence Parameters
4.2. Development of General Models to Estimate Maize N Status Indicators
4.2.1. Limitations for Developing General Models
4.2.2. Comparison of Different Regression Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kant, S.; Bi, Y.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.M.; Ulaganathan, K. Nitrogen nutrition, its regulation and biotechnological approaches to improve crop productivity. Am. J. Plant Sci. 2015, 6, 2745–2798. [Google Scholar] [CrossRef][Green Version]
- Jaynes, D.B.; Colvin, T.S.; Karlen, D.L.; Cambardella, C.A.; Meek, D.W. Nitrate loss in subsurface drainage as affected by nitrogen fertilizer rate. J. Environ. Qual. 2001, 30, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.T.; Rufty, T.W. Nitrogen and water resources commonly limit crop yield increases, not necessarily plant genetics. Glob. Food Sec. 2012, 1, 94–98. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Garnier, J.; Bouwman, L.; Velazquez, E.; Mueller, N.D.; Gerber, J.S. Nitrogen use in the global food system: Past trends and future trajectories of agronomic performance, pollution, trade, and dietary demand. Environ. Res. Lett. 2016, 11, 095007. [Google Scholar] [CrossRef]
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf nitrogen determination using non-destructive techniques—A review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Colaço, A.F.; Bramley, R.G.V. Do crop sensors promote improved nitrogen management in grain crops? Field Crops Res. 2018, 218, 126–140. [Google Scholar] [CrossRef]
- Berger, K.; Verrelst, J.; Féret, J.-B.; Wang, Z.; Wocher, M.; Strathmann, M.; Danner, M.; Mauser, W.; Hank, T. Crop nitrogen monitoring: Recent progress and principal developments in the context of imaging spectroscopy missions. Remote Sens. Environ. 2020, 242, 111758. [Google Scholar] [CrossRef]
- Karthikeyan, L.; Chawla, I.; Mishra, A.K. A review of remote sensing applications in agriculture for food security: Crop growth and yield, irrigation, and crop losses. J. Hydrol. 2020, 586, 124905. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with Crop Circle multispectral active canopy sensor. Field Crops Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Li, F.; Liu, B.; Yue, S.; Cheng, S.; Ustin, S.L.; Khosla, R. Active canopy sensing of winter wheat nitrogen status: An evaluation of two sensor systems. Comput. Electron. Agric. 2015, 112, 54–67. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, X.; Tan, C.; Yu, W.; Gnyp, M.L.; Lenz-Wiedemann, V.I.S.; Rascher, U.; et al. Satellite remote sensing-based in-season diagnosis of rice nitrogen status in Northeast China. Remote Sens. 2015, 7, 10646–10667. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving estimation of summer maize nitrogen status with red edge-based spectral vegetation indices. Field Crops Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
- Heege, H.J.; Reusch, S.; Thiessen, E. Prospects and results for optical systems for site-specific on-the-go control of nitrogen-top-dressing in Germany. Precis. Agric. 2008, 9, 115–131. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, G.; Pu, R.; Li, Z.; Li, H.; Xu, X.; Song, X.; Yang, X.; Zhao, C. An overview of crop nitrogen status assessment using hyperspectral remote sensing: Current status and perspectives. Eur. J. Agron. 2021, 124, 126241. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Longchamps, L.; Khosla, R. Early detection of nitrogen variability in maize using fluorescence. Agron. J. 2014, 106, 511–518. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Yuan, F.; Cao, Q.; Ye, H.; Lenz-Wiedemann, V.I.S.; Bareth, G. In-Season diagnosis of rice nitrogen status using proximal fluorescence canopy sensor at different growth stages. Remote Sens. 2019, 11, 1847. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Vegetation stress detection through chlorophyll a+b estimation and fluorescence effects on hyperspectral imagery. J. Environ. Qual. 2002, 31, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.K.; Middleton, E.M.; McMurtrey, J.E.; Corp, L.A.; Chappelle, E.W. Assessment of vegetation stress using reflectance or fluorescence measurements. J. Environ. Qual. 2007, 36, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tremblay, N.; Zhu, J. A first comparison of Multiplex® for the assessment of corn nitrogen status. J. Food Agric. Environ. 2012, 10, 1008–1016. [Google Scholar]
- Agati, G.; Foschi, L.; Grossi, N.; Guglielminetti, L.; Cerovic, Z.G.; Volterrani, M. Fluorescence-based versus reflectance proximal sensing of nitrogen content in Paspalum vaginatum and Zoysia matrella turfgrasses. Eur. J. Agron. 2013, 45, 39–51. [Google Scholar] [CrossRef]
- Li, J.W.; Zhang, J.X.; Zhao, Z.; Lei, X.D.; Xu, X.L.; Lu, X.X.; Weng, D.L.; Gao, Y.; Cao, L.K. Use of fluorescence-based sensors to determine the nitrogen status of paddy rice. J. Agric. Sci. 2013, 151, 862–871. [Google Scholar] [CrossRef]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; de Souza, R.; Thompson, R.B. Proximal optical sensors for nitrogen management of vegetable crops: A review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Proximal optical sensing of cucumber crop N status using chlorophyll fluorescence indices. Eur. J. Agron. 2016, 73, 83–97. [Google Scholar] [CrossRef]
- Cilia, C.; Panigada, C.; Rossini, M.; Meroni, M.; Busetto, L.; Amaducci, S.; Boschetti, M.; Picchi, V.; Colombo, R. Nitrogen status assessment for variable rate fertilization in maize through hyperspectral imagery. Remote Sens. 2014, 6, 6549–6565. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Y.; Huang, Y.; Zhao, Y.; Ren, N.; Fu, W.; Yue, S. Development of nitrogen fertilizer topdressing model for winter wheat based on critical nitrogen dilution curve. Int. J. Plant Prod. 2020, 14, 165–175. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, P.; Ji, R.; Min, J.; Shi, W.; Wang, D. Development of a model using the nitrogen nutrition index to estimate in-season rice nitrogen requirement. Field Crops Res. 2020, 245, 107664. [Google Scholar] [CrossRef]
- Bronson, K.F. Optimal internal nitrogen use efficiency for irrigated cotton in the southwestern United States. Agron. J. 2021, 113, 2821–2831. [Google Scholar] [CrossRef]
- Lu, J.; Miao, Y.; Shi, W.; Li, J.; Yuan, F. Evaluating different approaches to nondestructive nitrogen status diagnosis of rice using portable RapidSCAN active canopy sensor. Sci. Rep. 2017, 7, 14073. [Google Scholar] [CrossRef]
- Dong, R.; Miao, Y.; Wang, X.; Chen, Z.; Yuan, F. Improving maize nitrogen nutrition index prediction using leaf fluorescence sensor combined with environmental and management variables. Field Crops Res. 2021, 269, 108180. [Google Scholar] [CrossRef]
- Samborski, S.M.; Tremblay, N.; Fallon, E. Strategies to make use of plant sensors-based diagnostic information for nitrogen recommendations. Agron. J. 2009, 101, 800–816. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef]
- Padilla, F.M.; Farneselli, M.; Gianquinto, G.; Tei, F.; Thompson, R.B. Monitoring nitrogen status of vegetable crops and soils for optimal nitrogen management. Agric. Water Manag. 2020, 241, 106356. [Google Scholar] [CrossRef]
- Dong, R.; Miao, Y.; Wang, X.; Chen, Z.; Yuan, F.; Zhang, W.; Li, H. Estimating plant nitrogen concentration of maize using a leaf fluorescence sensor across growth stages. Remote Sens. 2020, 12, 1139. [Google Scholar] [CrossRef]
- Yao, X.; Huang, Y.; Shang, G.; Zhou, C.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y. Evaluation of six algorithms to monitor wheat leaf nitrogen concentration. Remote Sens. 2015, 7, 14939. [Google Scholar] [CrossRef]
- Pullanagari, R.R.; Kereszturi, G.; Yule, I.J. Mapping of macro and micro nutrients of mixed pastures using airborne AisaFENIX hyperspectral imagery. Isprs J. Photogramm. 2016, 117, 1–10. [Google Scholar] [CrossRef]
- Zhou, K.; Cheng, T.; Zhu, Y.; Cao, W.X.; Ustin, S.L.; Zheng, H.B.; Yao, X.; Tian, Y.C. Assessing the impact of spatial resolution on the estimation of leaf nitrogen concentration over the full season of paddy rice using near-surface imaging spectroscopy data. Front. Plant Sci. 2018, 9, 964. [Google Scholar] [CrossRef]
- Verrelst, J.; Camps-Valls, G.; Muñoz-Marí, J.; Rivera, J.P.; Veroustraete, F.; Clevers, J.G.P.W.; Moreno, J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties—A review. ISPRS J. Photogramm. 2015, 108, 273–290. [Google Scholar] [CrossRef]
- Zha, H.; Miao, Y.; Wang, T.; Li, Y.; Zhang, J.; Sun, W.; Feng, Z.; Kusnierek, K. Improving unmanned aerial vehicle remote sensing-based rice nitrogen nutrition index prediction with machine learning. Remote Sens. 2020, 12, 215. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Y.; Dong, R.; Zha, H.; Xia, T.; Chen, Z.; Kusnierek, K.; Mi, G.; Sun, H.; Li, M. Machine learning-based in-season nitrogen status diagnosis and side-dress nitrogen recommendation for corn. Eur. J. Agron. 2021, 123, 126193. [Google Scholar] [CrossRef]
- Abendroth, L.J.; Elmore, R.W.; Boyer, M.J.; Marley, S.K. Corn Growth and Development (Extension Publication PM 1009); Iowa State University Extension: Ames, IA, USA, 2011. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Li, W.; He, P.; Jin, J. Critical nitrogen curve and nitrogen nutrition index for spring maize in North-east China. J. Plant Nutr. 2012, 35, 1747–1761. [Google Scholar] [CrossRef]
- Abraham, A.; Pedregosa, F.; Eickenberg, M.; Gervais, P.; Mueller, A.; Kossaifi, J.; Gramfort, A.; Thirion, B.; Varoquaux, G. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform. 2014, 8, 14. [Google Scholar] [CrossRef]
- Silverman, B.W. Density Estimation for Statistics and Data Analysis, 1st ed.; Chapman and Hall/CRC: London, UK, 1986. [Google Scholar]
- Xia, T.; Miao, Y.; Wu, D.; Shao, H.; Khosla, R.; Mi, G. Active optical sensing of spring corn for in-season diagnosis of nitrogen status based on nitrogen nutrition index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar]
- Bausch, W.C.; Khosla, R. QuickBird satellite versus ground-based multi-spectral data for estimating nitrogen status of irrigated maize. Precis. Agric. 2010, 11, 274–290. [Google Scholar] [CrossRef]
- Agati, G.; Foschi, L.; Grossi, N.; Volterrani, M. In field non-invasive sensing of the nitrogen status in hybrid bermudagrass (Cynodon dactylon × C. transvaalensis Burtt Davy) by a fluorescence-based method. Europ. J. Agron. 2015, 63, 89–96. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Y.; Batchelor, W.D.; Dong, R.; Kusnierek, K. Evaluating model-based strategies for in-season nitrogen management of maize using weather data fusion. Agric. For. Meteorol. 2021, 308–309, 108564. [Google Scholar] [CrossRef]
- Bragazza, L.; Freeman, C. High nitrogen availability reduces polyphenol content in Sphagnum peat. Sci. Total Environ. 2007, 377, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, D.; Liu, D.; Geng, M.; Zhou, W.; Mi, W. Influence of nitrogen on the primary and secondary metabolism and synthesis of flavonoids in Chrysanthemum morifolium Ramat. J. Plant Nutr. 2010, 33, 240–254. [Google Scholar] [CrossRef]
- Hichem, H.; Mounir, D.; Naceur, E.A. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crop. Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
- Agati, G.; Cerovic, Z.G.; Pinelli, P.; Tattini, M. Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ. Exp. Bot. 2011, 73, 3–9. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Edwards, J. Maize Growth & Development; NSW Department of Primary Industries: Orange, NSW, Australia, 2009.
- Hank, T.B.; Berger, K.; Bach, H.; Clevers, J.G.P.W.; Gitelson, A.; Zarco-Tejada, P.; Mauser, W. Spaceborne imaging spectroscopy for sustainable agriculture: Contributions and challenges. Surv. Geophys. 2019, 40, 515–551. [Google Scholar] [CrossRef]
- Pinter, P.J.; Hatfield, J.L.; Schepers, J.S.; Barnes, E.M.; Moran, M.S.; Daughtry, C.S.T.; Upchurch, D.R. Remote sensing for crop management. Photogramm. Eng. Remote. Sens. 2003, 69, 647–664. [Google Scholar] [CrossRef]
- Andersen, R.A.; Kasperbauer, M.J. Effects of near-ultraviolet radiation and temperature on soluble phenols in Nicotiana tabacum. Phytochemistry 1971, 10, 1229–1232. [Google Scholar] [CrossRef]
- Barnes, P.W.; Searles, P.S.; Ballaré, C.L.; Ryel, R.J.; Caldwell, M.M. Noninvasive measurements of leaf epidermal transmittance of UV radiation using chlorophyll fluorescence: Field and laboratory studies. Physiol. Plant 2000, 109, 274–283. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, G.; Li, Z.; Li, H.; Li, Z.; Xu, X.; Song, X.; Zhang, Y.; Duan, D.; Zhao, C.; et al. Progress of hyperspectral data processing and modelling for cereal crop nitrogen monitoring. Comput. Electron. Agric. 2020, 172, 105321. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, X.; Wu, Y.; Zhu, Y.; Cao, Q.; Liu, X.; Cao, W. Combining texture, color, and vegetation indices from fixed-wing UAS imagery to estimate wheat growth parameters using multivariate regression methods. Comput. Electron. Agric. 2021, 185, 106138. [Google Scholar] [CrossRef]
- Palka, M.; Manschadi, A.M.; Koppensteiner, L.; Neubauer, T.; Fitzgerald, G.J. Evaluating the performance of the CCCI-CNI index for estimating N status of winter wheat. Europ. J. Agron. 2021, 130, 126346. [Google Scholar] [CrossRef]
- Ji, R.; Shi, W.; Wang, Y.; Zhang, H.; Min, J. Nondestructive estimation of bok choy nitrogen status with an active canopy sensor in comparison to a chlorophyll meter. Pedosphere 2020, 30, 769–777. [Google Scholar] [CrossRef]
- Debaeke, P.; Rouet, P.; Justes, E. Relationship between the normalized SPAD index and the nitrogen nutrition index: Application to durum wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Ziadi, N.; Brassard, M.; Bélanger, G.; Claessens, A.; Tremblay, N.; Cambouris, A.N.; Nolin, M.C.; Parent, L.E. Chlorophyll measurements and nitrogen nutrition index for the evaluation of corn nitrogen status. Agron. J. 2008, 100, 1264–1273. [Google Scholar] [CrossRef]
- Teal, R.K.; Tubana, B.; Girma, K.; Freeman, K.W.; Arnall, D.B.; Walsh, O.; Raun, W.R. In-season prediction of corn grain yield potential using normalized difference vegetation index. Agron. J. 2006, 98, 1488–1494. [Google Scholar] [CrossRef]
- Varvel, G.E.; Wilhelm, W.W.; Shanahan, J.F.; Schepers, J.S. An algorithm for corn nitrogen recommendations using a chlorophyll meter based sufficiency index. Agron. J. 2007, 99, 701–706. [Google Scholar] [CrossRef]
- Verrelst, J.; Muñoz, J.; Alonso, L.; Delegido, J.; Rivera, J.P.; Camps-Valls, G.; Moreno, J. Machine learning regression algorithms for biophysical parameter retrieval: Opportunities for sentinel-2 and -3. Remote Sens. Environ. 2012, 118, 127–139. [Google Scholar] [CrossRef]


| Multiplex Parameter | Description | Excitation | Formula |
|---|---|---|---|
| YF_UV | UV excited yellow fluorescence | UV | – |
| RF_UV | UV excited red fluorescence | UV | – |
| FRF_UV | UV excited far-red fluorescence | UV | – |
| YF_B | Blue excited yellow fluorescence | Blue | – |
| RF_B | Blue excited red fluorescence | Blue | – |
| FRF_B | Blue excited far-red fluorescence | Blue | – |
| YF_G | Green excited yellow fluorescence | Green | – |
| RF_G | Green excited red fluorescence | Green | – |
| FRF_G | Green excited far-red fluorescence | Green | – |
| RF_R | Red excited red fluorescence | Red | – |
| FRF_R | Red excited far-red fluorescence | Red | – |
| SFR_G | Green excited simple fluorescence ratio | Green | FRF_G/RF_G |
| SFR_R | Red excited simple fluorescence ratio | Red | FRF_R/RF_R |
| FLAV | Red and UV excited flavonols | Red and UV | Log (FRF_R/FRF_UV) |
| ANTH | Red and green excited anthocyanins | Red and Green | Log (FRF_R/FRF_G) |
| NBI_G | UV and green excited nitrogen balance index | UV and Green | FRF_UV/RF_G |
| NBI_R | UV and red excited nitrogen balance index | UV and Red | FRF_UV/RF_R |
| Experimental Year | V6 | V8 | V12 | VT |
|---|---|---|---|---|
| 2017 | 229.8 | 392.7 | – | 880.0 |
| 2019 | 271.1 | 406.8 | 605.1 | 808.1 |
| Multiplex Parameter | V3 (n = 54) | V4 (n = 54) | V5 (n = 54) | V6 (n = 108) | V8 (n = 108) | V12 (n = 54) | VT (n = 108) |
|---|---|---|---|---|---|---|---|
| YF_UV | ns | ns | ns | ns | ns | ns | ns |
| RF_UV | ns | * | ns | *** | *** | *** | *** |
| FRF_UV | * | * | ns | *** | *** | *** | *** |
| YF_B | ns | ns | ns | ns | ns | *** | ns |
| RF_B | ns | ns | ns | ns | ns | ns | ns |
| FRF_B | ns | ns | ns | ns | ns | ns | ns |
| YF_G | ns | ns | ns | ns | ns | *** | ** |
| RF_G | ns | ns | ns | ns | ns | ns | ns |
| FRF_G | * | ns | ns | *** | ns | * | ns |
| RF_R | ns | ns | ns | ns | ns | ns | ns |
| FRF_R | * | ns | ns | ** | ns | ns | ns |
| SFR_G | ** | ** | ns | *** | *** | *** | *** |
| SFR_R | ** | ** | ns | *** | *** | *** | *** |
| FLAV | ns | ns | *** | *** | *** | *** | *** |
| ANTH | ns | ns | ns | ns | *** | *** | *** |
| NBI_G | ** | *** | *** | *** | *** | *** | *** |
| NBI_R | ** | ** | *** | *** | *** | *** | *** |
| N Indicator | Year | SFR_G | SFR_R | FLAV | ANTH | NBI_G | NBI_R | SFR_G | SFR_R | FLAV | ANTH | NBI_G | NBI_R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V6 | V8 | ||||||||||||
| PNC (g kg−1) | 2017 | 0.31 *** | 0.38 *** | 0.28 *** | 0.14 * | 0.62 *** | 0.61 *** | 0.72 *** | 0.73 *** | 0.73 *** | 0.46 *** | 0.78 *** | 0.78 *** |
| 2019 | 0.48 *** | 0.53 *** | 0.36 *** | 0.15 * | 0.58 *** | 0.61 *** | 0.69 *** | 0.73 *** | 0.69 *** | 0.26 ** | 0.71 *** | 0.74 *** | |
| Across years | 0.34 *** | 0.36 *** | 0.25 *** | 0.05 * | 0.57 *** | 0.60 *** | 0.30 *** | 0.61 *** | 0.32 *** | 0.06 * | 0.28 *** | 0.38 *** | |
| PNU (kg ha−1) | 2017 | 0.43 *** | 0.41 *** | 0.24 ** | 0.22 ** | 0.48 *** | 0.40 *** | 0.59 *** | 0.64 *** | 0.78 *** | 0.49 *** | 0.80 *** | 0.81 *** |
| 2019 | 0.46 *** | 0.55 *** | 0.21 *** | 0.12 *** | 0.44 *** | 0.47 *** | 0.70 *** | 0.75 *** | 0.70 *** | 0.41 *** | 0.69 *** | 0.73 *** | |
| Across years | 0.12 ** | 0.13 ** | 0.31 *** | 0.38 *** | 0.21 *** | 0.39 *** | 0.62 *** | 0.64 *** | 0.64 *** | 0.41 *** | 0.67 *** | 0.73 *** | |
| NNI | 2017 | 0.31 *** | 0.38 *** | 0.28 *** | 0.14 * | 0.62 *** | 0.61 *** | 0.67 *** | 0.71 *** | 0.80 *** | 0.49 *** | 0.84 *** | 0.85 *** |
| 2019 | 0.48 *** | 0.53 *** | 0.36 *** | 0.15 * | 0.58 *** | 0.61 *** | 0.73 *** | 0.78 *** | 0.72 *** | 0.37 *** | 0.73 *** | 0.77 *** | |
| Across years | 0.34 *** | 0.36 *** | 0.25 *** | 0.05 * | 0.57 *** | 0.60 *** | 0.56 *** | 0.73 *** | 0.60 *** | 0.28 *** | 0.59 *** | 0.68 *** | |
| V12 | VT | ||||||||||||
| PNC (g kg−1) | 2017 | – | – | – | – | – | – | 0.57 *** | 0.53 *** | 0.71 *** | 0.67 *** | 0.70 *** | 0.72 *** |
| 2019 | 0.62 *** | 0.68 *** | 0.56 *** | 0.49 *** | 0.76 *** | 0.76 *** | 0.75 *** | 0.69 *** | 0.58 *** | 0.54 *** | 0.80 *** | 0.82 *** | |
| Across years | – | – | – | – | – | – | 0.66 *** | 0.61 *** | 0.63 *** | 0.45 *** | 0.74 *** | 0.75 *** | |
| PNU (kg ha−1) | 2017 | – | – | – | – | – | – | 0.65 *** | 0.59 *** | 0.80 *** | 0.68 *** | 0.82 *** | 0.82 *** |
| 2019 | 0.73 *** | 0.61 *** | 0.64 *** | 0.60 *** | 0.83 *** | 0.78 *** | 0.81 *** | 0.69 *** | 0.60 *** | 0.68 *** | 0.81 *** | 0.83 *** | |
| Across years | – | – | – | – | – | – | 0.73 *** | 0.65 *** | 0.68 *** | 0.53 *** | 0.80 *** | 0.80 *** | |
| NNI | 2017 | – | – | – | – | – | – | 0.66 *** | 0.61 *** | 0.80 *** | 0.71 *** | 0.81 *** | 0.82 *** |
| 2019 | 0.75 *** | 0.67 *** | 0.65 *** | 0.61*** | 0.86 *** | 0.82 *** | 0.82 *** | 0.70 *** | 0.61 *** | 0.68 *** | 0.83 *** | 0.84 *** | |
| Across years | – | – | – | – | – | – | 0.74 *** | 0.66 *** | 0.69 *** | 0.53 *** | 0.80 *** | 0.81 *** | |
| Raw Multiplex Index | PNC (g kg−1) | PNU (kg ha−1) | NNI | |||
|---|---|---|---|---|---|---|
| Model | R2 | Model | R2 | Model | R2 | |
| SFR_G | Q | ns | P | 0.31 *** | Q | 0.31 *** |
| SFR_R | Q | 0.24 *** | Q | 0.12 *** | Q | 0.26 *** |
| FLAV | E | 0.14 *** | P | 0.12 *** | Q | 0.30 *** |
| ANTH | E | 0.09 *** | E | 0.07 *** | Q | 0.14 *** |
| NBI_G | P | 0.10 *** | E | 0.20 *** | Q | 0.38 *** |
| NBI_R | P | 0.21 *** | E | 0.12 *** | Q | 0.40 *** |
| PNC (g kg−1) | PNU (kg ha−1) | NNI | ||||||
|---|---|---|---|---|---|---|---|---|
| Modified Multiplex Index | Model | R2 | Modified Multiplex Index | Model | R2 | Modified Multiplex Index | Model | R2 |
| SFR_G/GDD | Q | 0.83 *** | SFR_G×GDD | Q | 0.70 *** | SFR_GSI | Q | 0.46 *** |
| SFR_R/GDD | Q | 0.85 *** | SFR_R×GDD | Q | 0.66 *** | SFR_RSI | Q | 0.46 *** |
| FLAV×GDD | P | 0.83 *** | FLAV/GDD | P | 0.79 *** | FLAVSI | Q | 0.61 *** |
| ANTH×GDD | P | 0.84 *** | ANTH/GDD | P | 0.63 *** | ANTHSI | P | 0.38 *** |
| NBI_G/GDD | Q | 0.73 *** | NBI_G×GDD | Q | 0.84 *** | NBI_GSI | Q | 0.65 *** |
| NBI_R/GDD | Q | 0.79 *** | NBI_R×GDD | Q | 0.81 *** | NBI_RSI | Q | 0.68 *** |
| N Status Indicator | GDD | SFR_G | SFR_R | FLAV | ANTH | NBI_G | NBI_R | Constant | R2 |
|---|---|---|---|---|---|---|---|---|---|
| MLR | |||||||||
| PNC (g kg−1) | −0.0300 | 7.498 | −0.334 | −21.448 | 106.555 | −19.254 | 31.548 | −9.085 | 0.76 *** |
| PNU (kg ha−1) | 0.127 | 23.646 | −4.027 | −90.184 | −109.662 | −6.879 | 8.226 | 8.243 | 0.77 *** |
| NNI | 0.0000137 | 0.311 | −0.0670 | −0.811 | 2.045 | −0.432 | 0.831 | −0.320 | 0.41 *** |
| RFR | |||||||||
| PNC (g kg−1) | 0.770 | 0.0087 | 0.135 | 0.0106 | 0.00686 | 0.0322 | 0.0374 | – | 0.99 *** |
| PNU (kg ha−1) | 0.471 | 0.318 | 0.061 | 0.028 | 0.016 | 0.024 | 0.082 | – | 0.97 *** |
| NNI | 0.255 | 0.164 | 0.107 | 0.021 | 0.015 | 0.158 | 0.281 | – | 0.96 *** |
| PNC (g kg−1) | PNU (kg ha−1) | NNI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Modified Multiplex Index | R2 | RMSE | RE | Modified Multiplex Index | R2 | RMSE | RE | Modified Multiplex Index | R2 | RMSE | RE |
| SFR_G/GDD | 0.82 *** | 4.31 | 20.89% | SFR_G×GDD | 0.71 *** | 20.95 | 45.79% | SFR_GSI | 0.59 *** | 0.17 | 20.88% |
| SFR_R/GDD | 0.84 *** | 4.11 | 19.88% | SFR_R×GDD | 0.64 *** | 23.09 | 50.48% | SFR_RSI | 0.56 *** | 0.17 | 21.74% |
| FLAV×GDD | 0.87 *** | 3.68 | 17.84% | FLAV/GDD | 0.79 *** | 17.52 | 38.30% | FLAVSI | 0.68 *** | 0.15 | 18.60% |
| ANTH×GDD | 0.83 *** | 4.20 | 20.36% | ANTH/GDD | 0.62 *** | 23.80 | 52.03% | ANTHSI | 0.37 *** | 0.21 | 25.88% |
| NBI_G/GDD | 0.76 *** | 4.99 | 24.18% | NBI_G×GDD | 0.86 *** | 14.21 | 31.06% | NBI_GSI | 0.71 *** | 0.14 | 17.64% |
| NBI_R/GDD | 0.82 *** | 4.29 | 20.77% | NBI_R×GDD | 0.85 *** | 15.16 | 33.15% | NBI_RSI | 0.73 *** | 0.14 | 17.03% |
| NBI_RSI | MLR | RFR | |
|---|---|---|---|
| Areal agreement | 0.72 | 0.59 | 0.77 |
| Kappa statistics | 0.45 *** | 0.06 ns | 0.55 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, R.; Miao, Y.; Wang, X.; Yuan, F.; Kusnierek, K. Canopy Fluorescence Sensing for In-Season Maize Nitrogen Status Diagnosis. Remote Sens. 2021, 13, 5141. https://doi.org/10.3390/rs13245141
Dong R, Miao Y, Wang X, Yuan F, Kusnierek K. Canopy Fluorescence Sensing for In-Season Maize Nitrogen Status Diagnosis. Remote Sensing. 2021; 13(24):5141. https://doi.org/10.3390/rs13245141
Chicago/Turabian StyleDong, Rui, Yuxin Miao, Xinbing Wang, Fei Yuan, and Krzysztof Kusnierek. 2021. "Canopy Fluorescence Sensing for In-Season Maize Nitrogen Status Diagnosis" Remote Sensing 13, no. 24: 5141. https://doi.org/10.3390/rs13245141
APA StyleDong, R., Miao, Y., Wang, X., Yuan, F., & Kusnierek, K. (2021). Canopy Fluorescence Sensing for In-Season Maize Nitrogen Status Diagnosis. Remote Sensing, 13(24), 5141. https://doi.org/10.3390/rs13245141