Coral Reef Mapping with Remote Sensing and Machine Learning: A Nurture and Nature Analysis in Marine Protected Areas
Abstract
:1. Introduction
2. Materials and Methods
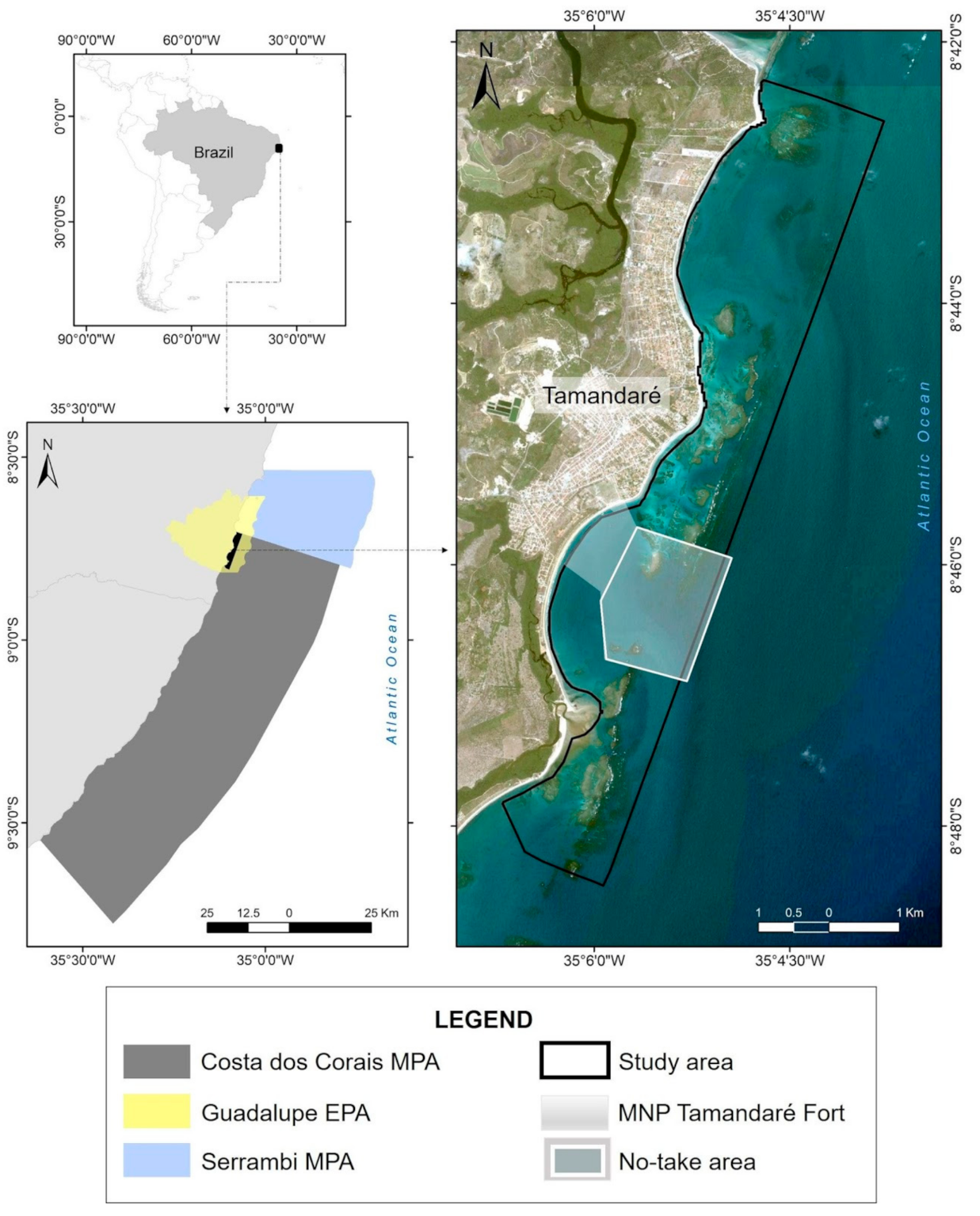
2.1. Study Area
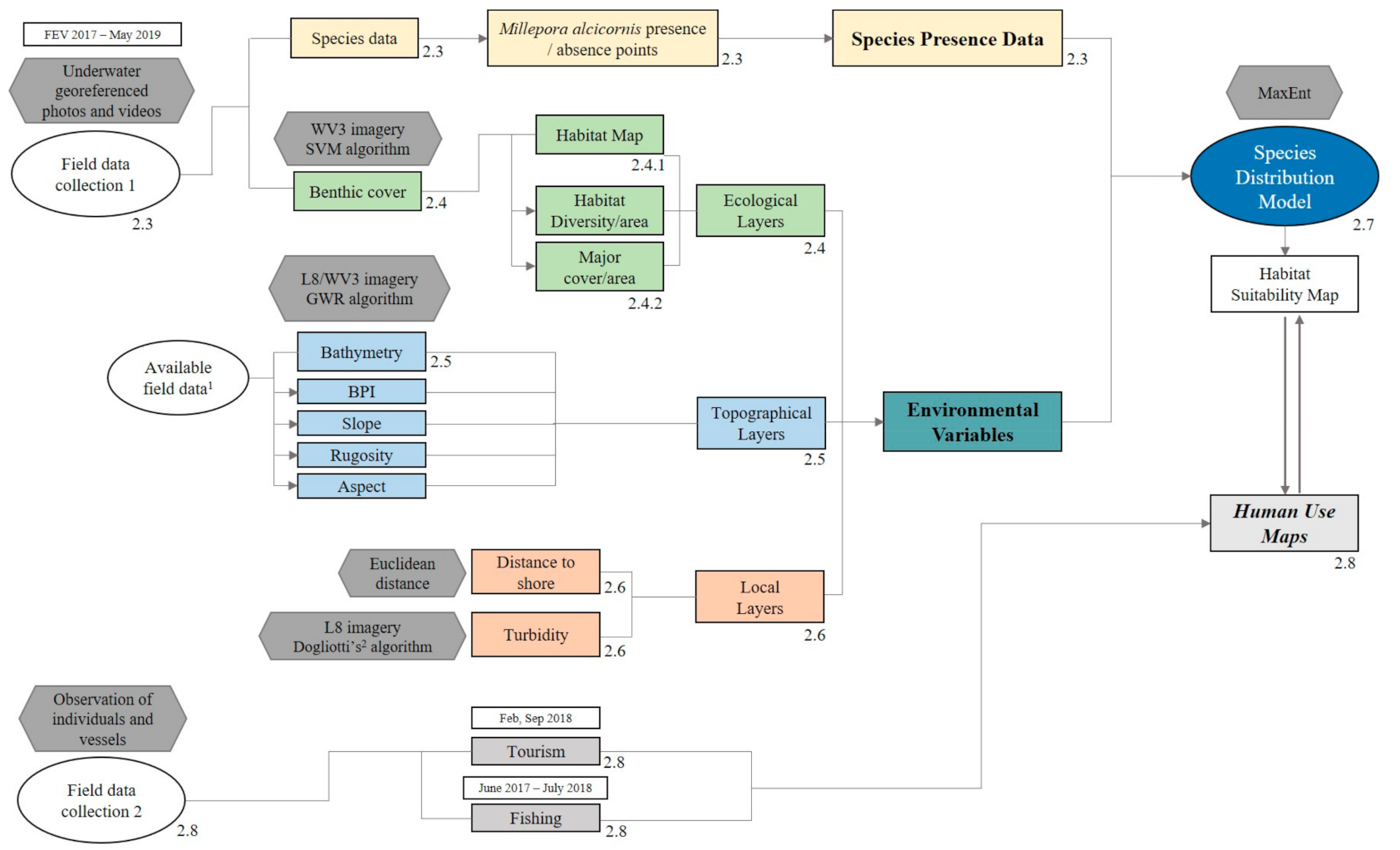
2.2. Methodological Steps—Overview
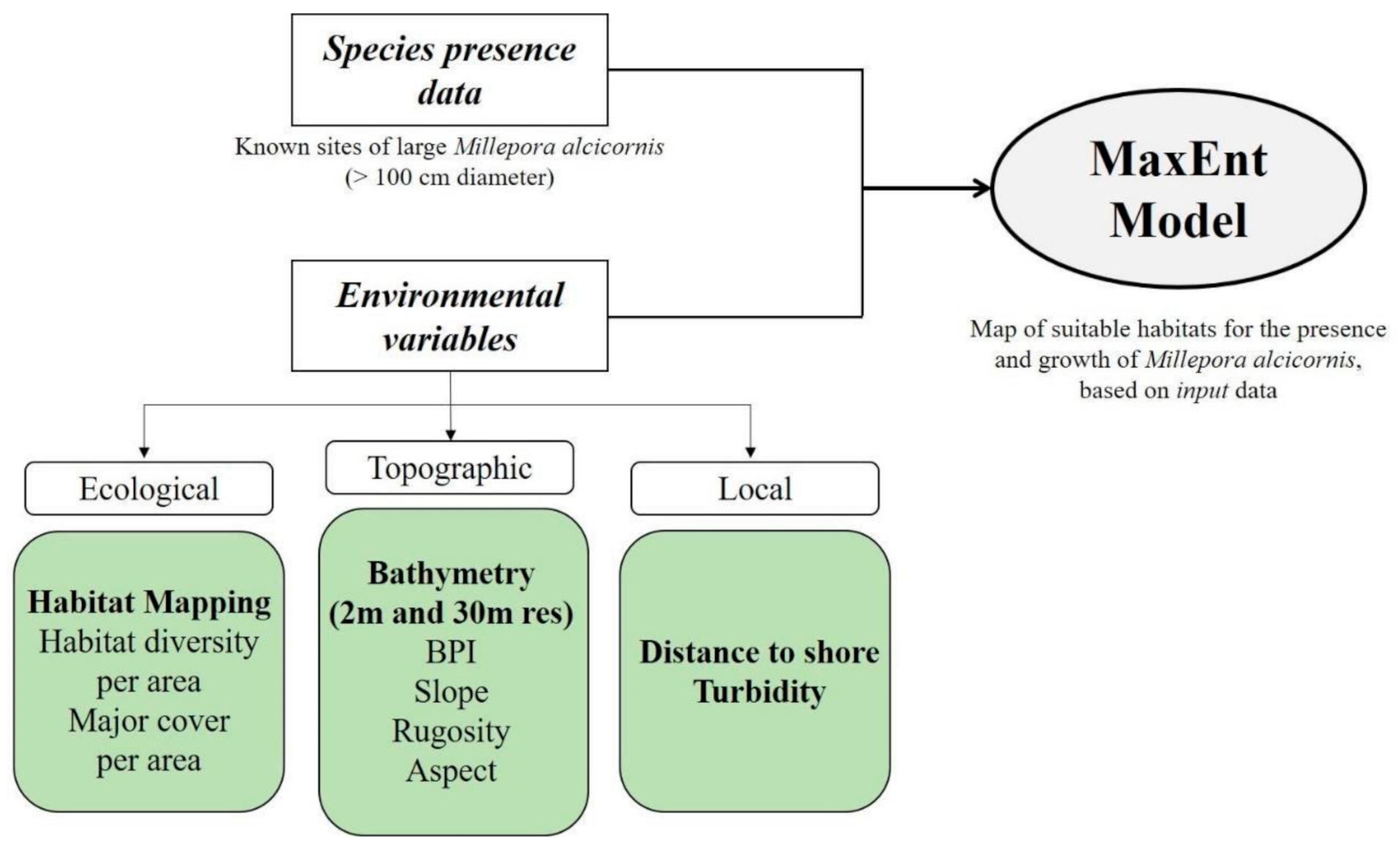
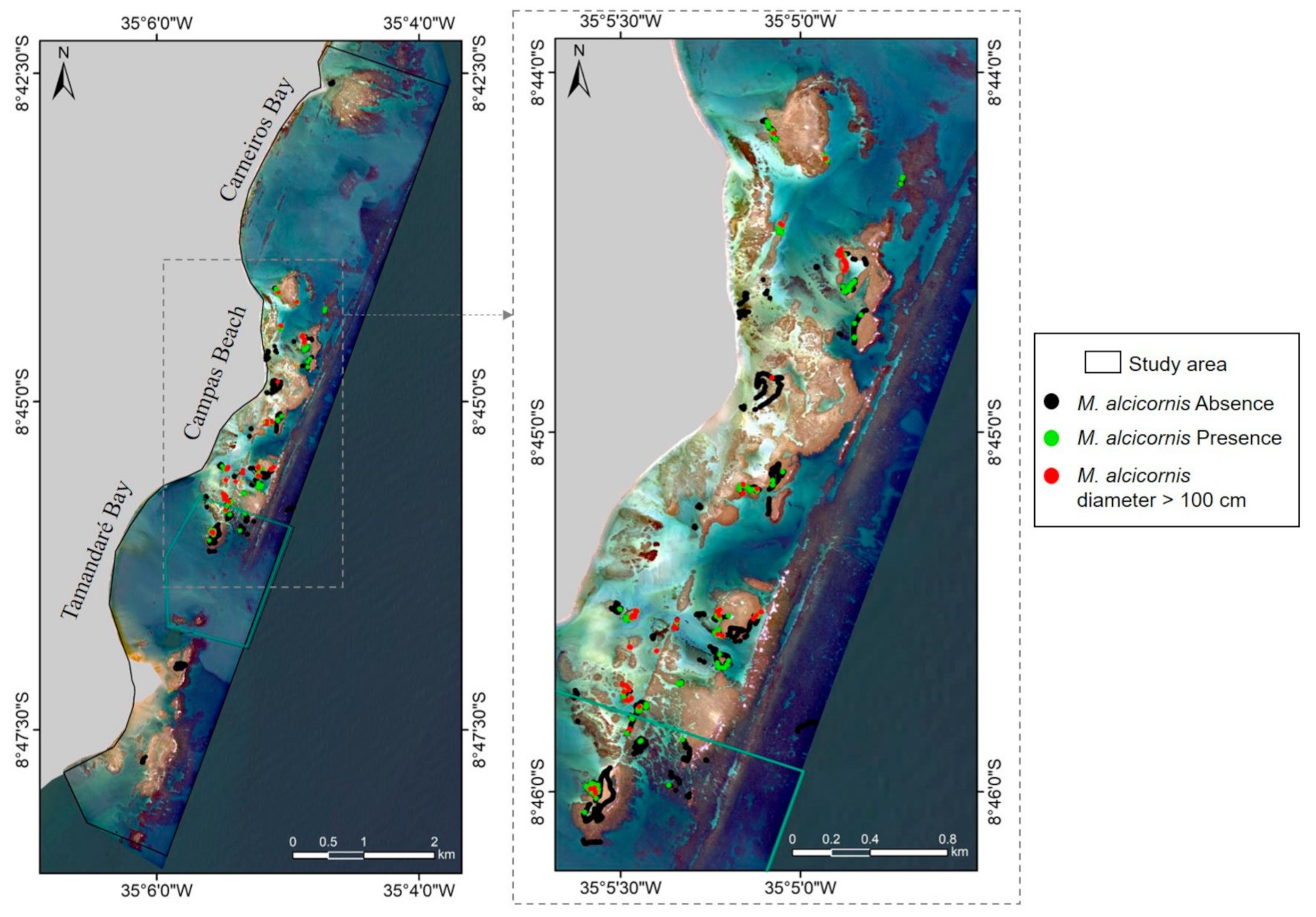
2.3. Species Presence Data
2.4. Environmental Variables—Ecological Layers
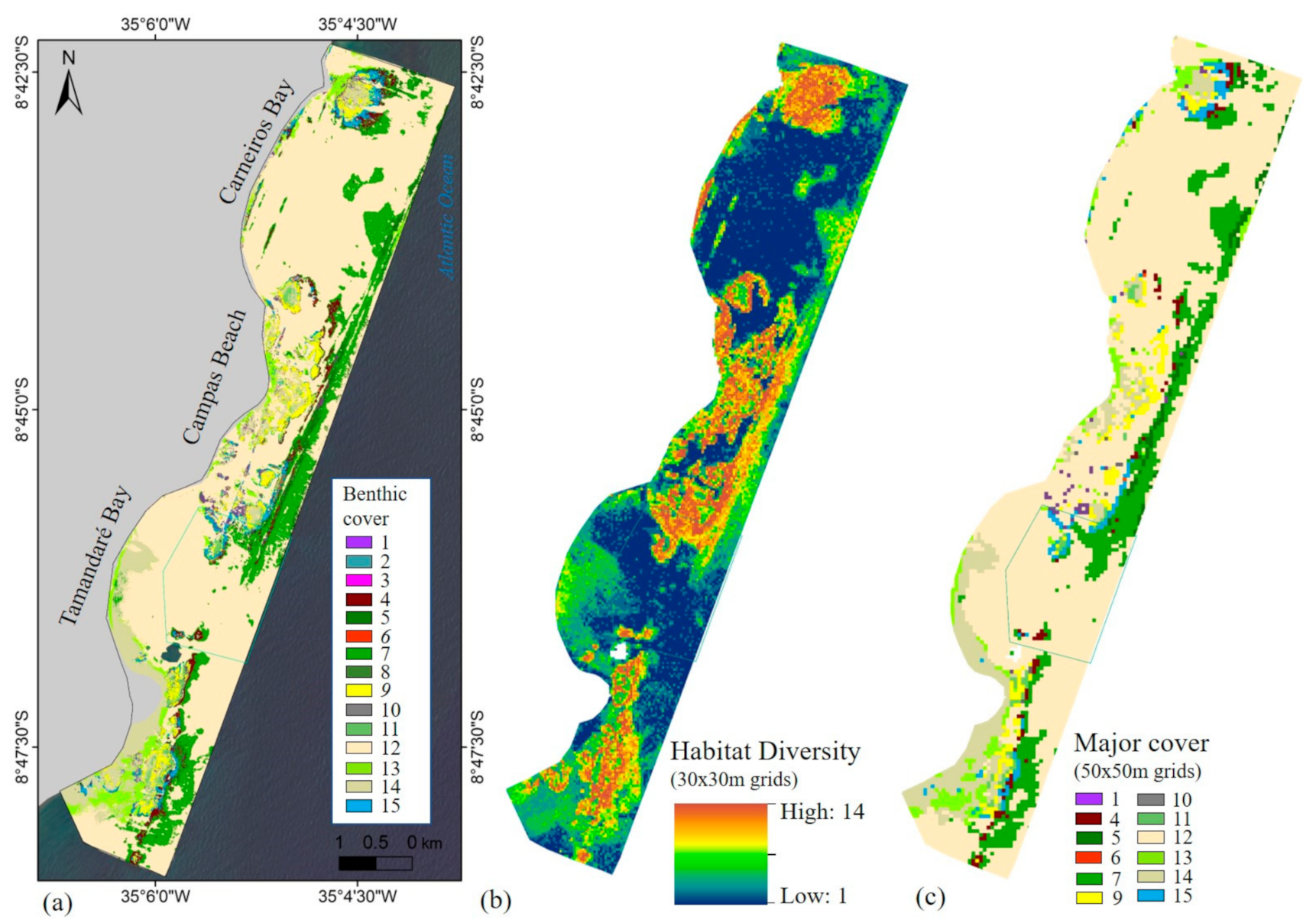
2.4.1. Habitat Mapping
2.4.2. Derivative Ecological Layers
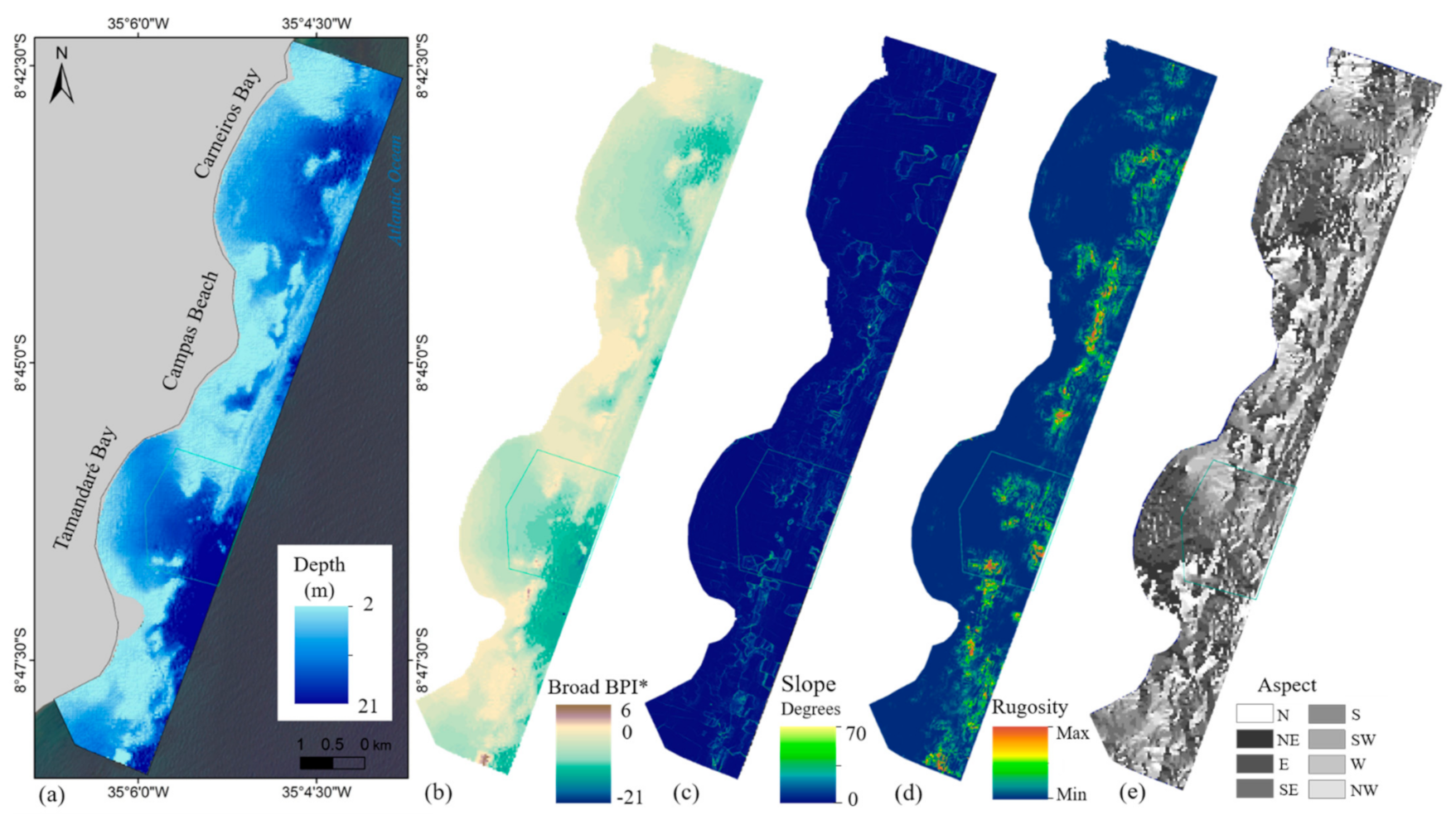
2.5. Environmental Variables—Topographical Layers
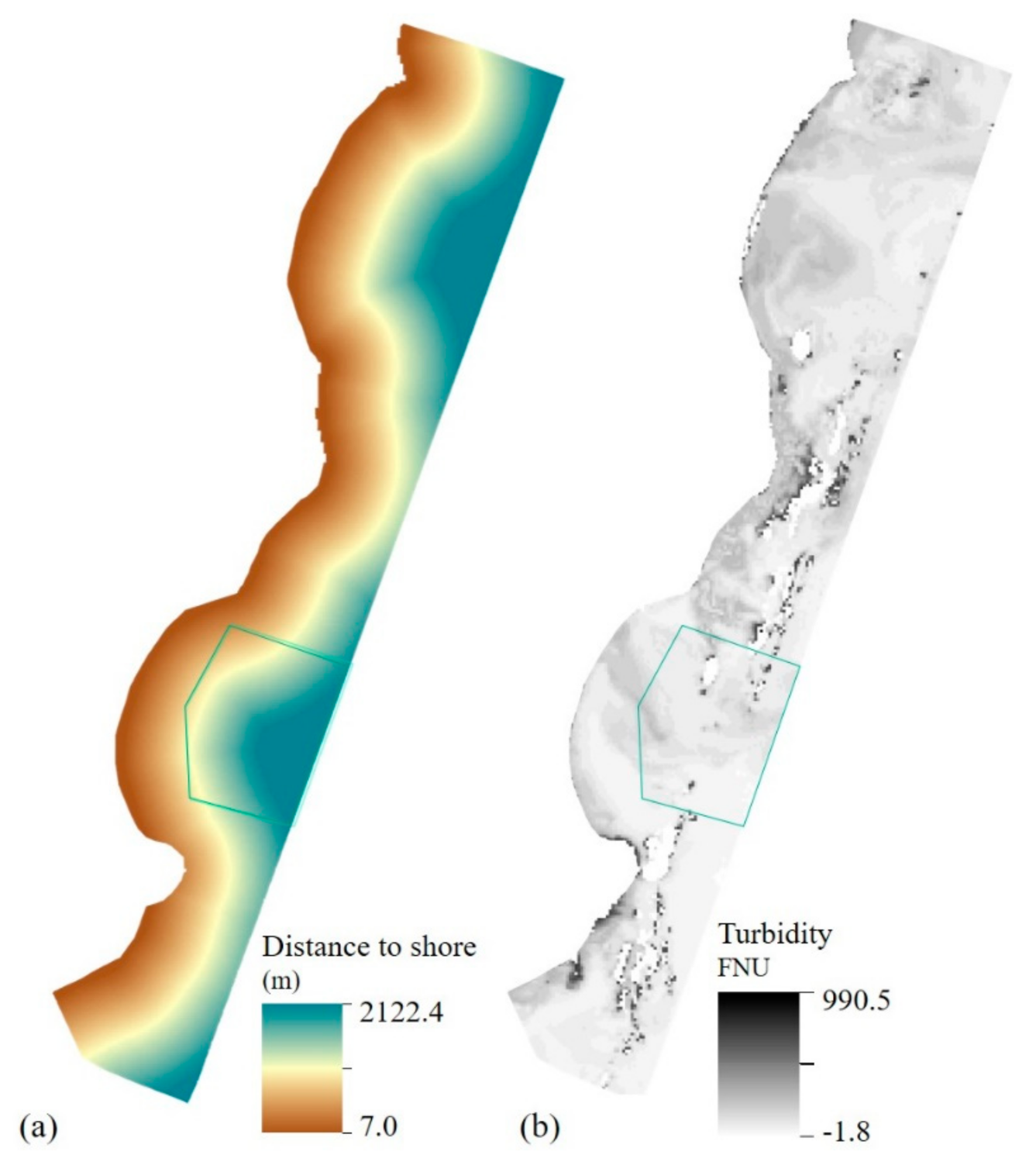
2.6. Environmental Variables—Local Layers
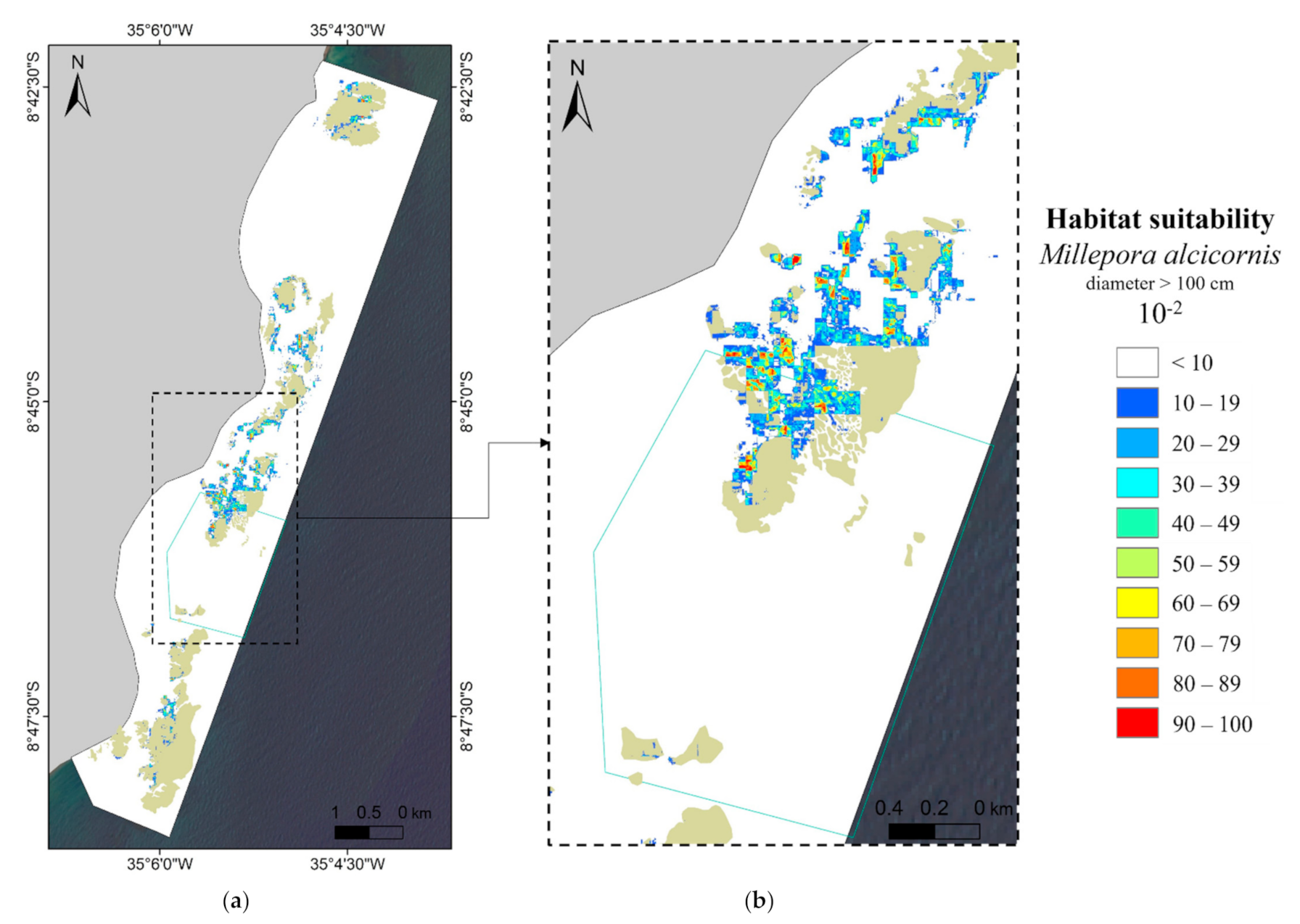
2.7. Modeling Suitable Habitats for Millepora alcicornis
2.8. Use Layers
3. Results
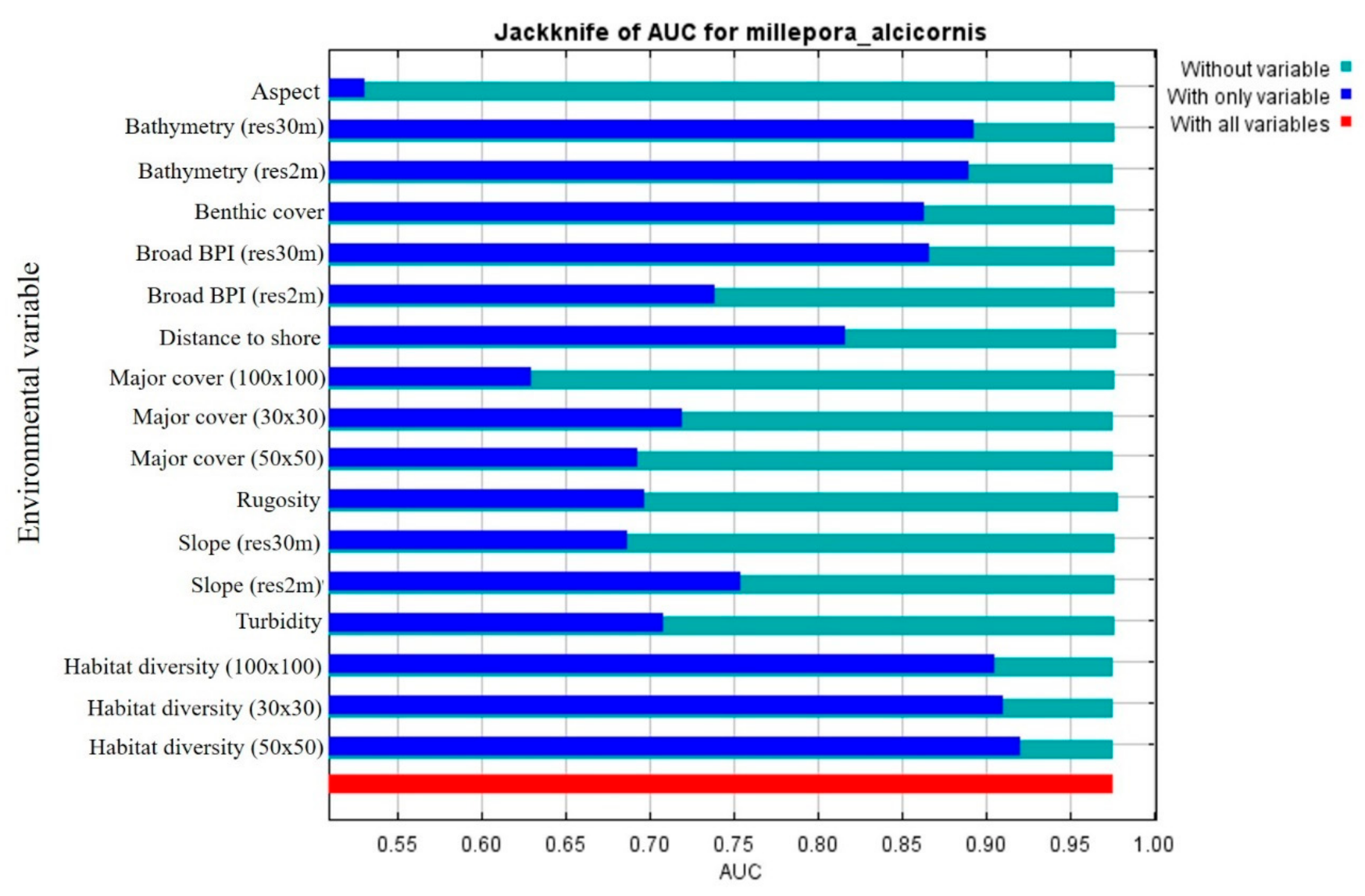
3.1. MaxEnt Input Parameters
3.1.1. Millepora alcicornis Presence Data
3.1.2. Ecological Layers
3.1.3. Topographical and Local Layers
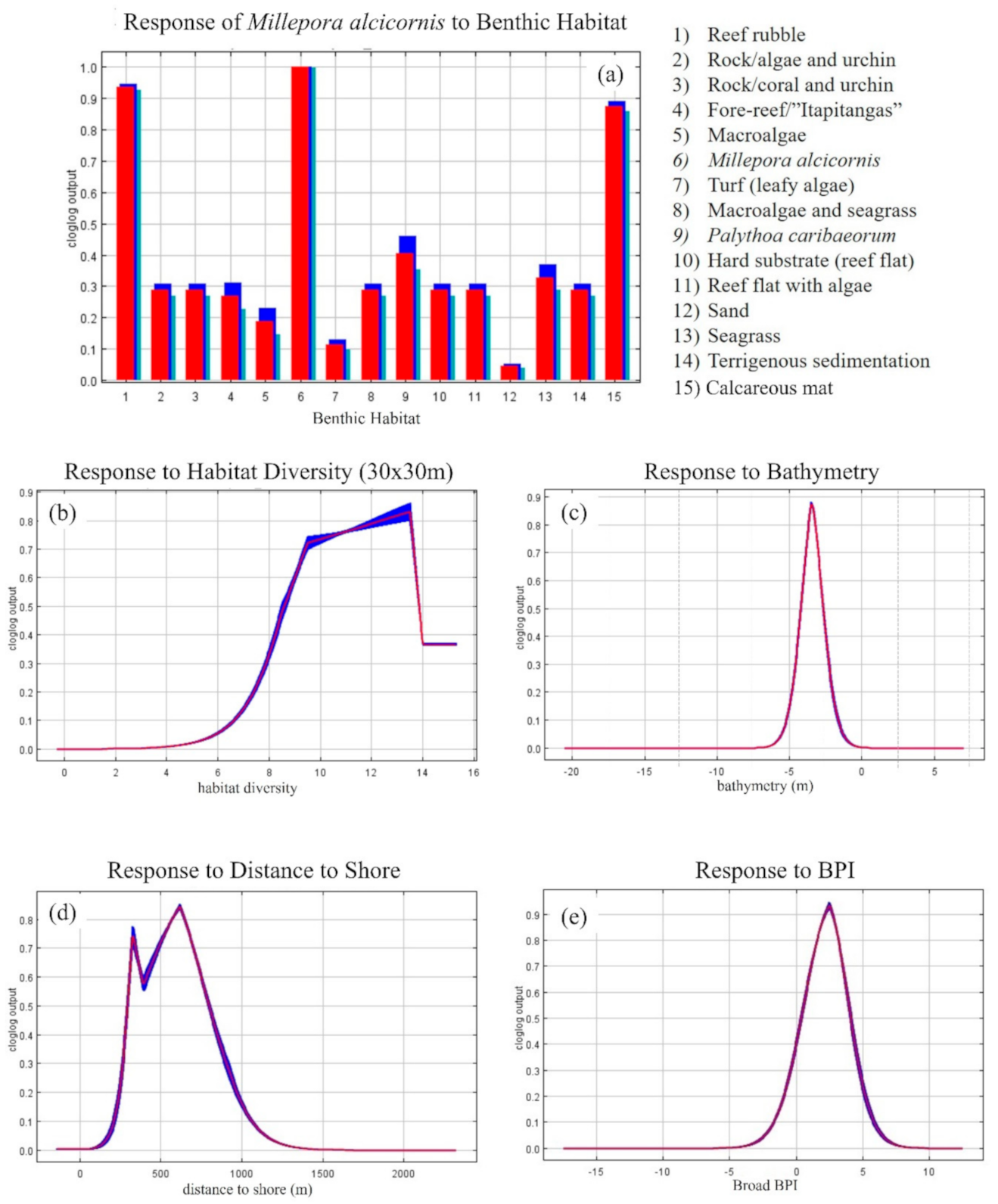
3.2. MaxEnt—Species Distribution Model’s Results
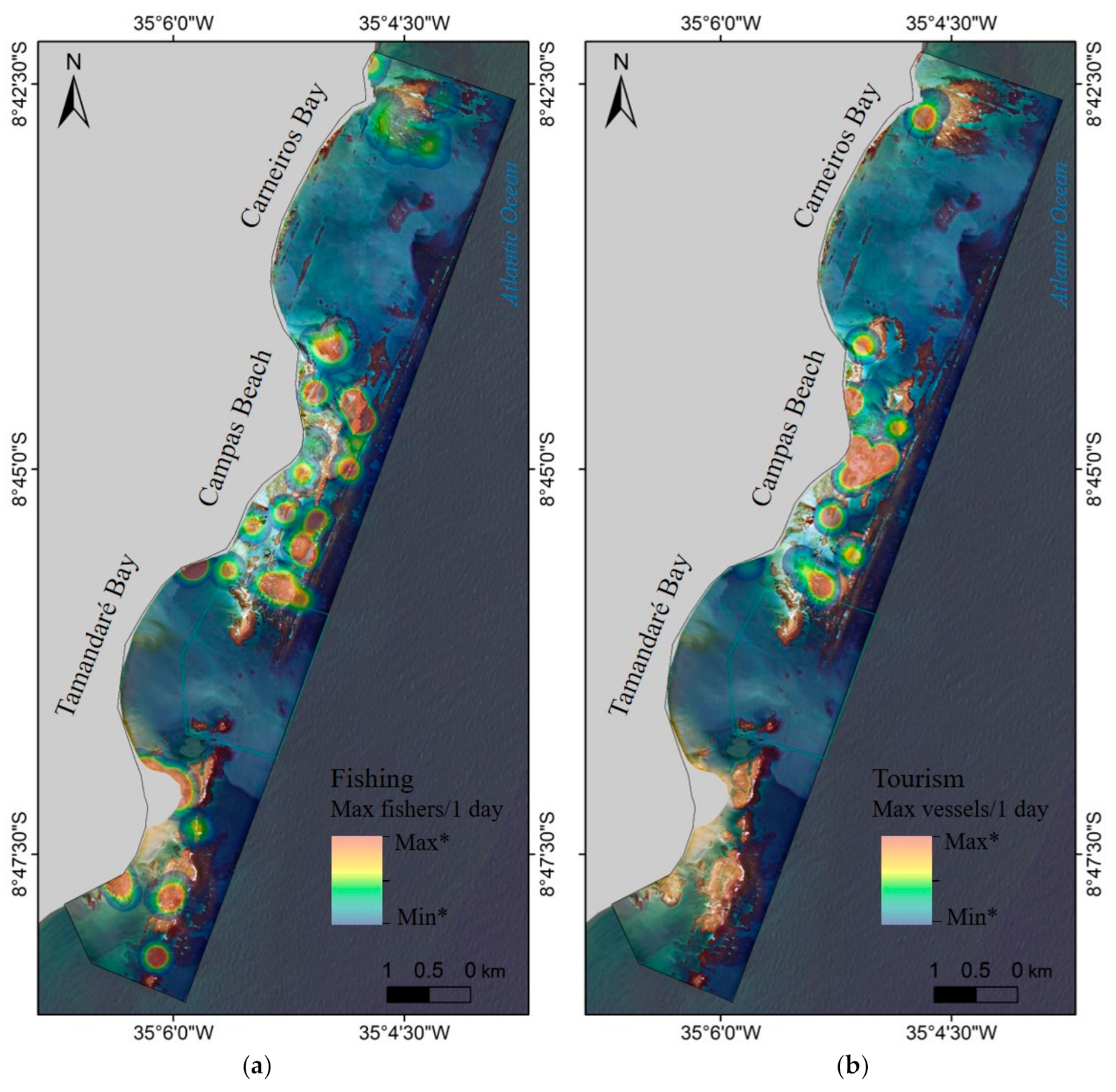
3.3. Human Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the Coral Reef Crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef]
- Pittman, S.J.; Brown, K.A. Multi-Scale Approach for Predicting Fish Species Distributions across Coral Reef Seascapes. PLoS ONE 2011, 6, e20583. [Google Scholar] [CrossRef]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A. Reefs at Risk Revisited; World Resources Institute: Washington, DC, USA, 2011. [Google Scholar]
- Wilkinson, C.; Salvat, B. Coastal Resource Degradation in the Tropics: Does the Tragedy of the Commons Apply for Coral Reefs, Mangrove Forests and Seagrass Beds. Mar. Pollut. Bull. 2012, 64, 1096–1105. [Google Scholar] [CrossRef]
- Roberts, C.M. Marine Biodiversity Hotspots and Conservation Priorities for Tropical Reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittman, S.J.; Christensen, J.D.; Caldow, C.; Menza, C.; Monaco, M.E. Predictive Mapping of Fish Species Richness across Shallow-Water Seascapes in the Caribbean. Ecol. Model. 2007, 204, 9–21. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [Green Version]
- Leão, Z.M.A.N.; Kikuchi, R.K.P.; Ferreira, B.P.; Neves, E.G.; Sovierzoski, H.H.; Oliveira, M.D.M.; Maida, M.; Correia, M.D.; Johnsson, R.; Leão, Z.M.A.N.; et al. Brazilian Coral Reefs in a Period of Global Change: A Synthesis. Braz. J. Oceanogr. 2016, 64, 97–116. [Google Scholar] [CrossRef]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B. Phase Shifts, Herbivory, and the Resilience of Coral Reefs to Climate Change. Curr. Biol. 2007, 17, 360–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sully, S.; Burkepile, D.E.; Donovan, M.K.; Hodgson, G.; van Woesik, R. A Global Analysis of Coral Bleaching over the Past Two Decades. Nat. Commun. 2019, 10, 1264. [Google Scholar] [CrossRef] [Green Version]
- Eakin, C.M.; Sweatman, H.P.A.; Brainard, R.E. The 2014–2017 Global-Scale Coral Bleaching Event: Insights and Impacts. Coral Reefs 2019, 38, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Duarte, G.A.S.; Villela, H.D.M.; Deocleciano, M.; Silva, D.; Barno, A.; Cardoso, P.M.; Vilela, C.L.S.; Rosado, P.; Messias, C.S.M.A.; Chacon, M.A.; et al. Heat Waves Are a Major Threat to Turbid Coral Reefs in Brazil. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Davenport, J.; Davenport, J.L. The Impact of Tourism and Personal Leisure Transport on Coastal Environments: A Review. Estuar. Coast. Shelf Sci. 2006, 67, 280–292. [Google Scholar] [CrossRef]
- Lai, S.; Loke, L.H.L.; Hilton, M.J.; Bouma, T.J.; Todd, P.A. The Effects of Urbanisation on Coastal Habitats and the Potential for Ecological Engineering: A Singapore Case Study. Ocean. Coast. Manag. 2015, 103, 78–85. [Google Scholar] [CrossRef]
- Agardy, T.; di Sciara, G.N.; Christie, P. Mind the Gap: Addressing the Shortcomings of Marine Protected Areas through Large Scale Marine Spatial Planning. Mar. Policy 2011, 35, 226–232. [Google Scholar] [CrossRef]
- Jameson, S.C.; Tupper, M.H.; Ridley, J.M. The Three Screen Doors: Can Marine “Protected” Areas Be Effective? Mar. Pollut. Bull. 2002, 44, 1177–1183. [Google Scholar] [CrossRef]
- Adam, T.; Burkepile, D.; Ruttenberg, B.; Paddack, M. Herbivory and the Resilience of Caribbean Coral Reefs: Knowledge Gaps and Implications for Management. Mar. Ecol. Prog. Ser. 2015, 520, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Bridge, T.; Beaman, R.; Done, T.; Webster, J. Predicting the Location and Spatial Extent of Submerged Coral Reef Habitat in the Great Barrier Reef World Heritage Area, Australia. PLoS ONE 2012, 7, e48203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledlie, M.H.; Graham, N.A.J.; Bythell, J.C.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Hardcastle, J. Phase Shifts and the Role of Herbivory in the Resilience of Coral Reefs. Coral Reefs 2007, 26, 641–653. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Nash, K.L. The Importance of Structural Complexity in Coral Reef Ecosystems. Coral Reefs 2013, 32, 315–326. [Google Scholar] [CrossRef]
- González-Rivero, M.; Harborne, A.R.; Herrera-Reveles, A.; Bozec, Y.-M.; Rogers, A.; Friedman, A.; Ganase, A.; Hoegh-Guldberg, O. Linking Fishes to Multiple Metrics of Coral Reef Structural Complexity Using Three-Dimensional Technology. Sci. Rep. 2017, 7, 13965. [Google Scholar] [CrossRef] [PubMed]
- Green, E.P.; Mumby, P.J.; Edwards, A.J.; Clark, C.D. A Review of Remote Sensing for the Assessment and Management of Tropical Coastal Resources. Coastal Manag. 1996, 24, 1–40. [Google Scholar] [CrossRef]
- Collin, A.; Hench, J.L. Towards Deeper Measurements of Tropical Reefscape Structure Using the WorldView-2 Spaceborne Sensor. Remote. Sens. 2012, 4, 1425–1447. [Google Scholar] [CrossRef] [Green Version]
- Reshitnyk, L.; Costa, M.; Robinson, C.; Dearden, P. Evaluation of WorldView-2 and Acoustic Remote Sensing for Mapping Benthic Habitats in Temperate Coastal Pacific Waters. Remote. Sens. Environ. 2014, 153, 7–23. [Google Scholar] [CrossRef]
- Da Silveira, C.B.L.; Strenzel, G.M.R.; Maida, M.; Araújo, T.C.M.; Ferreira, B.P. Multiresolution Satellite-Derived Bathymetry in Shallow Coral Reefs: Improving Linear Algorithms with Geographical Analysis. Coas 2020, 36, 1247–1265. [Google Scholar] [CrossRef]
- Lundblad, E.R.; Wright, D.J.; Miller, J.; Larkin, E.M.; Rinehart, R.; Naar, D.F.; Donahue, B.T.; Anderson, S.M.; Battista, T. A Benthic Terrain Classification Scheme for American Samoa. Mar. Geod. 2006, 29, 89–111. [Google Scholar] [CrossRef] [Green Version]
- Andréfouët, S.; Kramer, P.; Torres-Pulliza, D.; Joyce, K.E.; Hochberg, E.J.; Garza-Pérez, R.; Mumby, P.J.; Riegl, B.; Yamano, H.; White, W.H.; et al. Multi-Site Evaluation of IKONOS Data for Classification of Tropical Coral Reef Environments. Remote. Sens. Environ. 2003, 88, 128–143. [Google Scholar] [CrossRef]
- Eugenio, F.; Marcello, J.; Martin, J.; Rodríguez-Esparragón, D. Benthic Habitat Mapping Using Multispectral High-Resolution Imagery: Evaluation of Shallow Water Atmospheric Correction Techniques. Sensors 2017, 17, 2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirayath, V.; Instrella, R. Fluid Lensing and Machine Learning for Centimeter-Resolution Airborne Assessment of Coral Reefs in American Samoa. Remote. Sens. Environ. 2019, 235, 111475. [Google Scholar] [CrossRef]
- Hamylton, S.M.; Zhou, Z.; Wang, L. What Can Artificial Intelligence Offer Coral Reef Managers? Front. Mar. Sci. 2020, 7, 603829. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists: Statistical Explanation of MaxEnt. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Selig, E.R.; Turner, W.R.; Troëng, S.; Wallace, B.P.; Halpern, B.S.; Kaschner, K.; Lascelles, B.G.; Carpenter, K.E.; Mittermeier, R.A. Global Priorities for Marine Biodiversity Conservation. PLoS ONE 2014, 9, e82898. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Poulos, D.E.; Gallen, C.; Davis, T.; Booth, D.J.; Harasti, D. Distribution and Spatial Modelling of a Soft Coral Habitat in the Port Stephens–Great Lakes Marine Park: Implications for Management. Mar. Freshw. Res. 2016, 67, 256. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological Niche Models and Species Distribution Models in Marine Environments: A Literature Review and Spatial Analysis of Evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Ferreira, B.P.; Maida, M. Monitoramento dos Recifes de Coral do Brasil: Situação Atual e Perspectivas; MMA/SBF: Brasília, Brazil, 2006; ISBN 978-85-87166-86-9. [Google Scholar]
- ICMBio. Plano de Manejo Da APA Costa Dos Corais; ICMBio: Tamandaré, Brazil, 2012. [Google Scholar]
- Castro, C.B.; Pires, D.O. Brazilian Coral Reefs: What We Already Know and What Is Still Missing. Bull. Mar. Sci. 2001, 69, 15. [Google Scholar]
- Ferreira, B.P.; Messias, L.T.; Maida, M. The Environmental Municipal Councils as an Instrument in Coastal Integrated Management: The Área de Proteção Ambiental Costa Dos Corais (AL/PE) Experience. J. Coast. Res. 2006, 1003–1007. [Google Scholar]
- Fung, T.; Seymour, R.M.; Johnson, C.R. Alternative Stable States and Phase Shifts in Coral Reefs under Anthropogenic Stress. Ecology 2011, 92, 967–982. [Google Scholar] [CrossRef]
- Ferreira, B.P.; Maida, M.; Castro, C.B.; Pires, D.O.; Prates, A.P.L.; Marx, D. The Status of Coral Reefs of Brazil. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 27 June–2 July 2004; pp. 1011–1015. [Google Scholar]
- Cavalcante de Macêdo, E. Um Ensaio Sobre a Sedimentação e Suas Implicações Ecológicas Nos Recifes Costeiros Da Baía de Tamandaré/PE. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Pernambuco, Brazil, 2009. [Google Scholar]
- Maida, M.; Ferreira, B. Coral Reefs of Brazil: Overview and Field Guide. In Proceedings of the 8th International Coral Reef Symposium, Panama City, FL, USA, 24–29 June 1996; Volume 1, pp. 263–274. [Google Scholar]
- Leão, Z.M.A.N.; Kikuchi, R.K.P.; Testa, V. Corals and coral reefs of Brazil. In Latin American Coral Reefs; Cortés, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 9–52. ISBN 978-0-444-51388-5. [Google Scholar]
- Silveira, M.F. da Pesca Artesanal e Manejo: Uma Abordagem Temporal Comparativa em Tamandaré-PE. Available online: https://repositorio.ufpe.br/handle/123456789/31801 (accessed on 17 May 2021).
- Ferreira, B.; Maida, M.; Cava, F.C. Características e Perspectivas Para o Manejo Da Pesca Na APA Marinha Costa Dos Corais. In Proceedings of the Congresso Brasileiro de Unidades de Conservação, Campo Grande, Brazil; 2000; pp. 50–58. [Google Scholar]
- Coni, E.O.C.; Ferreira, C.M.; de Moura, R.L.; Meirelles, P.M.; Kaufman, L.; Francini-Filho, R.B. An Evaluation of the Use of Branching Fire-Corals (Millepora Spp.) as Refuge by Reef Fish in the Abrolhos Bank, Eastern Brazil. Env. Biol. Fish 2013, 96, 45–55. [Google Scholar] [CrossRef]
- Amaral, F.M.D.; Steiner, A.Q.; Broadhurst, M.K.; Cairns, S.D. An Overview of the Shallow-Water Calcified Hydroids from Brazil (Hydrozoa: Cnidaria), Including the Description of a New Species. Zootaxa 2008, 1930, 56–68. [Google Scholar] [CrossRef]
- Laborel, J. Madreporaires et Hydrocoralliares Recifaux Des Cotes Bresiliennes. Systematique, Ecologie. Repartition Verticale et Geographique. Results Sci. Camp. Calypso. 1969, 9, 171–229. [Google Scholar]
- Lewis, J.B. Biology and Ecology of the Hydrocoral Millepora on Coral Reefs. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 50, pp. 1–55. ISBN 978-0-12-026151-2. [Google Scholar]
- Garcia, T.; Matthews-Cascon, H.; Franklin-Júnior, W. Millepora Alcicornis (Cnidaria: Hydrozoa) as Substrate for Benthic Fauna. Braz. J. Oceanogr. Braz. J. Oceanogr. 2009, 57. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, B.P.; Gaspar, A.L.B.; Coxey, M.S.; Monteiro, A.C.G. Manual de Monitoramento Reef Check Brasil; Ministério do Meio Ambiente: Brasília, Brazil, 2018. [Google Scholar]
- Roelfsema, C. Integrating Field Data with High Spatial Resolution Multispectral Satellite Imagery for Calibration and Validation of Coral Reef Benthic Community Maps. J. Appl. Remote. Sens. 2010, 4, 043527. [Google Scholar] [CrossRef] [Green Version]
- Roelfsema, C.; Lyons, M.; Dunbabin, M.; Kovacs, E.M.; Phinn, S. Integrating Field Survey Data with Satellite Image Data to Improve Shallow Water Seagrass Maps: The Role of AUV and Snorkeller Surveys? Remote. Sens. Lett. 2015, 6, 135–144. [Google Scholar] [CrossRef]
- Rodríguez, L.; López, C.; Casado-Amezua, P.; Ruiz-Ramos, D.V.; Martínez, B.; Banaszak, A.; Tuya, F.; García-Fernández, A.; Hernández, M. Genetic Relationships of the Hydrocoral Millepora Alcicornis and Its Symbionts within and between Locations across the Atlantic. Coral Reefs 2019, 38, 255–268. [Google Scholar] [CrossRef]
- Wirtz, P.; Zilberberg, C. Fire! The Spread of the Caribbean Fire Coral Millepora Alcicornis in the Eastern Atlantic. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Edmunds, P.J. The Role of Colony Morphology and Substratum Inclination in the Success of Millepora Alcicornis on Shallow Coral Reefs. Coral Reefs 1999, 18, 133–140. [Google Scholar] [CrossRef]
- Oliveira, M.D.M.; Leão, Z.M.A.N.; Kikuchi, R.K.P. Cultivo de Millepora alcicornis como uma ferramenta para Restauração e Manejo dos Ecossistemas Recifais do Nordeste do Brasil. RGCI 2008, 8, 183–201. [Google Scholar] [CrossRef]
- Dalleau, M.; Andréfouët, S.; Wabnitz, C.C.C.; Payri, C.; Wantiez, L.; Pichon, M.; Friedman, K.; Vigliola, L.; Benzoni, F. Use of Habitats as Surrogates of Biodiversity for Efficient Coral Reef Conservation Planning in Pacific Ocean Islands. Conserv. Biol. 2010, 24, 541–552. [Google Scholar] [CrossRef]
- McManus, L.C.; Watson, J.R.; Vasconcelos, V.V.; Levin, S.A. Stability and Recovery of Coral-Algae Systems: The Importance of Recruitment Seasonality and Grazing Influence. Theor. Ecol. 2019, 12, 61–72. [Google Scholar] [CrossRef]
- Green, E.; Mumby, P.; Edwards, A.; Clark, C. Remote Sensing Handbook for Tropical Coastal Management; Green, E.P., Edwards, A.J., Eds.; Coastal management sourcebooks; Unesco Pub: Paris, France, 2000; ISBN 978-92-3-103736-8. [Google Scholar]
- Deidda, M.; Sanna, G. Pre-Processing of High Resolution Satellite Images for Sea Bottom Classification. Ital. J. Remote. Sens. Riv. Ital. Telerilevamento 2012, 83–95. [Google Scholar] [CrossRef]
- Richter, R.; Schläpfer, D. Geo-Atmospheric Processing of Airborne Imaging Spectrometry Data. Part 2: Atmospheric/Topographic Correction. Int. J. Remote. Sens. 2002, 23, 2631–2649. [Google Scholar] [CrossRef]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel Extensions (CPCe): A Visual Basic Program for the Determination of Coral and Substrate Coverage Using Random Point Count Methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Etnoyer, P.J.; Wagner, D.; Fowle, H.A.; Poti, M.; Kinlan, B.; Georgian, S.E.; Cordes, E.E. Models of Habitat Suitability, Size, and Age-Class Structure for the Deep-Sea Black Coral Leiopathes Glaberrima in the Gulf of Mexico. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2018, 150, 218–228. [Google Scholar] [CrossRef]
- Yamamoto, K.H.; Powell, R.L.; Anderson, S.; Sutton, P.C. Using LiDAR to Quantify Topographic and Bathymetric Details for Sea Turtle Nesting Beaches in Florida. Remote. Sens. Environ. 2012, 125, 125–133. [Google Scholar] [CrossRef]
- Secomandi, M.; Jones, E.; Terente, V.; Comrie, R.; Owen, M. Application of the Bathymetric Position Index Method (BPI) for the Purpose of Defining a Reference Seabed Level for Cable Burial. In Offshore Site Investigation Geotechnics 8th International Conference Proceedings; Society of Underwater Technology: London, England, 2017; pp. 904–913. ISBN 978-0-906940-57-0. [Google Scholar]
- Walbridge, S.; Slocum, N.; Pobuda, M.; Wright, D.J. Unified Geomorphological Analysis Workflows with Benthic Terrain Modeler. Geosciences 2018, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Sappington, J.M.; Longshore, K.M.; Thompson, D.B. Quantifying Landscape Ruggedness for Animal Habitat Analysis: A Case Study Using Bighorn Sheep in the Mojave Desert. J. Wildl. Manag. 2007, 71, 1419–1426. [Google Scholar] [CrossRef]
- Wilson, M.F.J.; O’Connell, B.; Brown, C.; Guinan, J.C.; Grehan, A.J. Multiscale Terrain Analysis of Multibeam Bathymetry Data for Habitat Mapping on the Continental Slope. Mar. Geod. 2007, 30, 3–35. [Google Scholar] [CrossRef] [Green Version]
- Henry, L.-A.; Davies, A.J.; Murray Roberts, J. Beta Diversity of Cold-Water Coral Reef Communities off Western Scotland. Coral Reefs 2010, 29, 427–436. [Google Scholar] [CrossRef]
- Henry, L.-A.; Moreno Navas, J.; Roberts, J.M. Multi-Scale Interactions between Local Hydrography, Seabed Topography, and Community Assembly on Cold-Water Coral Reefs. Biogeosciences 2013, 10, 2737–2746. [Google Scholar] [CrossRef] [Green Version]
- Nechad, B.; Ruddick, K.G.; Neukermans, G. Calibration and Validation of a Generic Multisensor Algorithm for Mapping of Turbidity in Coastal Waters. In Proceedings of the Remote Sensing of the Ocean, Sea Ice, and Large Water Regions, Berlin, Germany, 17 September 2009; Volume 7473, p. 74730H. [Google Scholar]
- Dogliotti, A.I.; Ruddick, K.G.; Nechad, B.; Doxaran, D.; Knaeps, E. A Single Algorithm to Retrieve Turbidity from Remotely-Sensed Data in All Coastal and Estuarine Waters. Remote. Sens. Environ. 2015, 156, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Vanhellemont, Q.; Ruddick, K. Acolite for Sentinel-2: Aquatic applications of MSI imagery. In Proceedings of the 2016 ESA Living Planet Symposium, Prague, Czech Republic, 9–13 May 2016. [Google Scholar]
- Vanhellemont, Q.; Ruddick, K. Atmospheric Correction of Metre-Scale Optical Satellite Data for Inland and Coastal Water Applications. Remote. Sens. Environ. 2018, 216, 586–597. [Google Scholar] [CrossRef]
- Couce, E.; Ridgwell, A.; Hendy, E.J. Environmental Controls on the Global Distribution of Shallow-Water Coral Reefs: Global Distribution Models of Shallow-Water Coral Reefs. J. Biogeogr. 2012, 39, 1508–1523. [Google Scholar] [CrossRef] [Green Version]
- Freeman, L.A.; Kleypas, J.A.; Miller, A.J. Coral Reef Habitat Response to Climate Change Scenarios. PLoS ONE 2013, 8, e82404. [Google Scholar] [CrossRef]
- Bargain, A.; Marchese, F.; Savini, A.; Taviani, M.; Fabri, M.-C. Santa Maria Di Leuca Province (Mediterranean Sea): Identification of Suitable Mounds for Cold-Water Coral Settlement Using Geomorphometric Proxies and Maxent Methods. Front. Mar. Sci. 2017, 4, 338. [Google Scholar] [CrossRef] [Green Version]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making Better Maxent Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Costa, B.; Kendall, M.S.; Parrish, F.A.; Rooney, J.; Boland, R.C.; Chow, M.; Lecky, J.; Montgomery, A.; Spalding, H. Identifying Suitable Locations for Mesophotic Hard Corals Offshore of Maui, Hawai‘i. PLoS ONE 2015, 10, e0130285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendall, M.S.; Monaco, M.E.; Buja, K.R.; Christensen, J.D.; Kruer, C.R.; Finkbeiner, M. Methods Used to Map the Benthic Habitats of Puerto Rico and the US Virgin Islands; NOAA Technical Memorandum 152; NOS NCCOS CCMA: Silver Springs, MD, USA, 2002. [Google Scholar]
- Feitosa, J.L.L.; Concentino, A.M.; Teixeira, S.F.; Ferreira, B.P. Food Resource Use by Two Territorial Damselfish (Pomacentridae: Stegastes) on South-Western Atlantic Algal-Dominated Reefs. J. Sea Res. 2012, 70, 42–49. [Google Scholar] [CrossRef]
- Tratalos, J.A.; Austin, T.J. Impacts of Recreational SCUBA Diving on Coral Communities of the Caribbean Island of Grand Cayman. Biol. Conserv. 2001, 102, 67–75. [Google Scholar] [CrossRef]
- Flynn, R.L.; Forrester, G.E. Boat Anchoring Contributes Substantially to Coral Reef Degradation in the British Virgin Islands. PeerJ 2019, 7, e7010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, J.R.M.B.; Oliveira, W.D.M.; Pereira, P.S.; de Camargo, J.M.R.; de Araújo, M.E. Substrate Zonation as a Function of Reef Morphology: A Case Study in Carneiros Beach, Pernambuco, Brazil. J. Coast. Res. 2018, 81, 1. [Google Scholar] [CrossRef]
- Roberts, C.M. Effects of Fishing on the Ecosystem Structure of Coral Reefs. Conserv. Biol. 1995, 9, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topor, Z.M.; Rasher, D.B.; Duffy, J.E.; Brandl, S.J. Marine Protected Areas Enhance Coral Reef Functioning by Promoting Fish Biodiversity. Conserv. Lett. 2019, 12, e12638. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Nugues, M.; Mwachireya, S. Fish and Sea Urchin Herbivory and Competition in Kenyan Coral Reef Lagoons: The Role of Reef Management. J. Exp. Mar. Biol. Ecol. 1994, 184, 237–254. [Google Scholar] [CrossRef]
- Graham, N.A.J.; McClanahan, T.R.; MacNeil, M.A.; Wilson, S.K.; Cinner, J.E.; Huchery, C.; Holmes, T.H. Human Disruption of Coral Reef Trophic Structure. Curr. Biol. 2017, 27, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedding, L.M.; Friedlander, A.M.; McGranaghan, M.; Yost, R.S.; Monaco, M.E. Using Bathymetric Lidar to Define Nearshore Benthic Habitat Complexity: Implications for Management of Reef Fish Assemblages in Hawaii. Remote. Sens. Environ. 2008, 112, 4159–4165. [Google Scholar] [CrossRef]
- Leão, Z.M.A.N.; Kikuchi, R.K.P. The Abrolhos Reefs of Brazil. In Coastal Marine Ecosystems of Latin America; Seeliger, U., Kjerfve, B., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2001; pp. 83–96. ISBN 978-3-662-04482-7. [Google Scholar]
- Zaneveld, J.R.; Burkepile, D.E.; Shantz, A.A.; Pritchard, C.E.; McMinds, R.; Payet, J.P.; Welsh, R.; Correa, A.M.S.; Lemoine, N.P.; Rosales, S.; et al. Overfishing and Nutrient Pollution Interact with Temperature to Disrupt Coral Reefs down to Microbial Scales. Nat. Commun. 2016, 7, 11833. [Google Scholar] [CrossRef]
- Dubé, C.E.; Mercière, A.; Vermeij, M.J.A.; Planes, S. Population Structure of the Hydrocoral Millepora Platyphylla in Habitats Experiencing Different Flow Regimes in Moorea, French Polynesia. PLoS ONE 2017, 12, e0173513. [Google Scholar] [CrossRef]
- Chaves, L.T.C.; Pereira, P.H.C.; Feitosa, J.L.L. Coral Reef Fish Association with Macroalgal Beds on a Tropical Reef System in North-Eastern Brazil. Mar. Freshw. Res. 2013, 64, 1101–1111. [Google Scholar] [CrossRef]
- Pawar, S.; Koo, M.S.; Kelley, C.; Ahmed, M.F.; Chaudhuri, S.; Sarkar, S. Conservation Assessment and Prioritization of Areas in Northeast India: Priorities for Amphibians and Reptiles. Biol. Conserv. 2007, 136, 346–361. [Google Scholar] [CrossRef]
- Carroll, C. Role of Climatic Niche Models in Focal-Species-Based Conservation Planning: Assessing Potential Effects of Climate Change on Northern Spotted Owl in the Pacific Northwest, USA. Biol. Conserv. 2010, 143, 1432–1437. [Google Scholar] [CrossRef]

| Satellite Sensor | WV3 |
|---|---|
| Scene | 104001002788CE00 |
| Map Projection | UTM |
| Datum | WGS84; Ellipsoid: WGS84 |
| Coverage | X: −8.80934577 to −8.68585772 Y: −35.11410824 to −35.06263346 |
| Sun azimuth | 94.20 |
| Sun elevation | 65.70 |
| Date acquired (DMY) | 23/02/2017 |
| Scene center time | 12:55:08 |
| Resolution (m/px) | 2 |
| Tide at acquisition | 1.1 m above spring tide |
| Class | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Total | UAC | Kappa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 35 | 0.77 | |
| 2 | 0 | 23 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0.92 | |
| 3 | 0 | 2 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0.91 | |
| 4 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 1.00 | |
| 5 | 0 | 0 | 0 | 0 | 21 | 1 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 28 | 0.75 | |
| 6 | 1 | 0 | 0 | 1 | 0 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 31 | 0.84 | |
| 7 | 0 | 0 | 0 | 5 | 0 | 1 | 25 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 0.76 | |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 1.00 | |
| 9 | 0 | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 27 | 3 | 2 | 0 | 0 | 0 | 0 | 37 | 0.73 | |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 26 | 1 | 0 | 0 | 0 | 0 | 27 | 0.96 | |
| 11 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 24 | 0 | 0 | 0 | 0 | 27 | 0.89 | |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 26 | 0 | 5 | 0 | 31 | 0.84 | |
| 13 | 0 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 27 | 3 | 1 | 37 | 0.73 | |
| 14 | 2 | 2 | 4 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 3 | 3 | 2 | 22 | 0 | 42 | 0.52 | |
| 15 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 24 | 30 | 0.80 | |
| Total points | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 450 | 0.00 | |
| PAC | 0.90 | 0.77 | 0.67 | 0.80 | 0.70 | 0.87 | 0.83 | 0.70 | 0.90 | 0.87 | 0.80 | 0.87 | 0.90 | 0.73 | 0.80 | 0.00 | 0.81 | |
| Kappa | 0.79 |
| Number | Major Habitat Classification | Benthic habitat | Main Cover (in Order of Contribution) | ||||
|---|---|---|---|---|---|---|---|
| 1 | Fragmented patchy reef | Reef Rubble | rubble (gravel) | turf algae | rock | Halimeda spp. | - |
| 2 | Coral reef and colonized hardbottom | Rock/algae and urchin | rock | turf algae | Echinometra lucunter | encrusting coralline algae | - |
| 3 | Coral reef and colonized hardbottom | Rock/coral and urchin | rock | coral (Porites astreoides) | Echinometra lucunter | Palythoa caribaeorum | - |
| 4 | Fore-reef | Itapitanga/Fore-reef | Palythoa caribaeorum | Rock | Caulerpa spp. | - | - |
| 5 | Submerged vegetation | Macroalgae | Sargassum spp. | Dictyota spp. | Caulerpa spp. | turf algae | - |
| 6 | Coral reef and colonized hardbottom | Millepora alcicornis | Millepora alcicornis | turf algae | - | - | - |
| 7 | Submerged vegetation | Turf algae (leafy) | turf algae | calcareous mat | Sargassum spp. | Halimeda spp. | rock |
| 8 | Submerged vegetation | Seagrass and macroalgae | Sargassum spp. | Seagrass (Halodule wrightii) | sand | - | - |
| 9 | Coral reef and colonized hardbottom | Palythoa caribaeorum | Palythoa caribaeorum | turf algae | - | - | - |
| 10 | Uncolonized hardbottom | Reef flat – (hard substratum) | rock | turf algae | Palythoa caribaeorum | - | - |
| 11 | Colonized hardbottom | Reef flat (algae) | rock | turf algae | filamentous algae | Halimeda spp. | - |
| 12 | Unconsolidated sediments | Sand | sand | gravel | - | - | - |
| 13 | Submerged vegetation | Seagrass | Seagrass (Halodule wrightii) | sand | gravel | - | - |
| 14 | Unknown 1 | Terrigenous sediment1 | - | - | - | - | - |
| 15 | Coral reef and colonized hardbottom | Calcareous mat | calcareous mat (multispecific articulated calcareous algae) | algae turf | rock | sand | M. alcicornis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silveira, C.B.L.; Strenzel, G.M.R.; Maida, M.; Gaspar, A.L.B.; Ferreira, B.P. Coral Reef Mapping with Remote Sensing and Machine Learning: A Nurture and Nature Analysis in Marine Protected Areas. Remote Sens. 2021, 13, 2907. https://doi.org/10.3390/rs13152907
da Silveira CBL, Strenzel GMR, Maida M, Gaspar ALB, Ferreira BP. Coral Reef Mapping with Remote Sensing and Machine Learning: A Nurture and Nature Analysis in Marine Protected Areas. Remote Sensing. 2021; 13(15):2907. https://doi.org/10.3390/rs13152907
Chicago/Turabian Styleda Silveira, Camila Brasil Louro, Gil Marcelo Reuss Strenzel, Mauro Maida, Ana Lídia Bertoldi Gaspar, and Beatrice Padovani Ferreira. 2021. "Coral Reef Mapping with Remote Sensing and Machine Learning: A Nurture and Nature Analysis in Marine Protected Areas" Remote Sensing 13, no. 15: 2907. https://doi.org/10.3390/rs13152907
APA Styleda Silveira, C. B. L., Strenzel, G. M. R., Maida, M., Gaspar, A. L. B., & Ferreira, B. P. (2021). Coral Reef Mapping with Remote Sensing and Machine Learning: A Nurture and Nature Analysis in Marine Protected Areas. Remote Sensing, 13(15), 2907. https://doi.org/10.3390/rs13152907






