Comparison of UAV and Boat Surveys for Detecting Changes in Breeding Population Dynamics of Sea Turtles
Abstract
1. Introduction
2. Materials and Methods
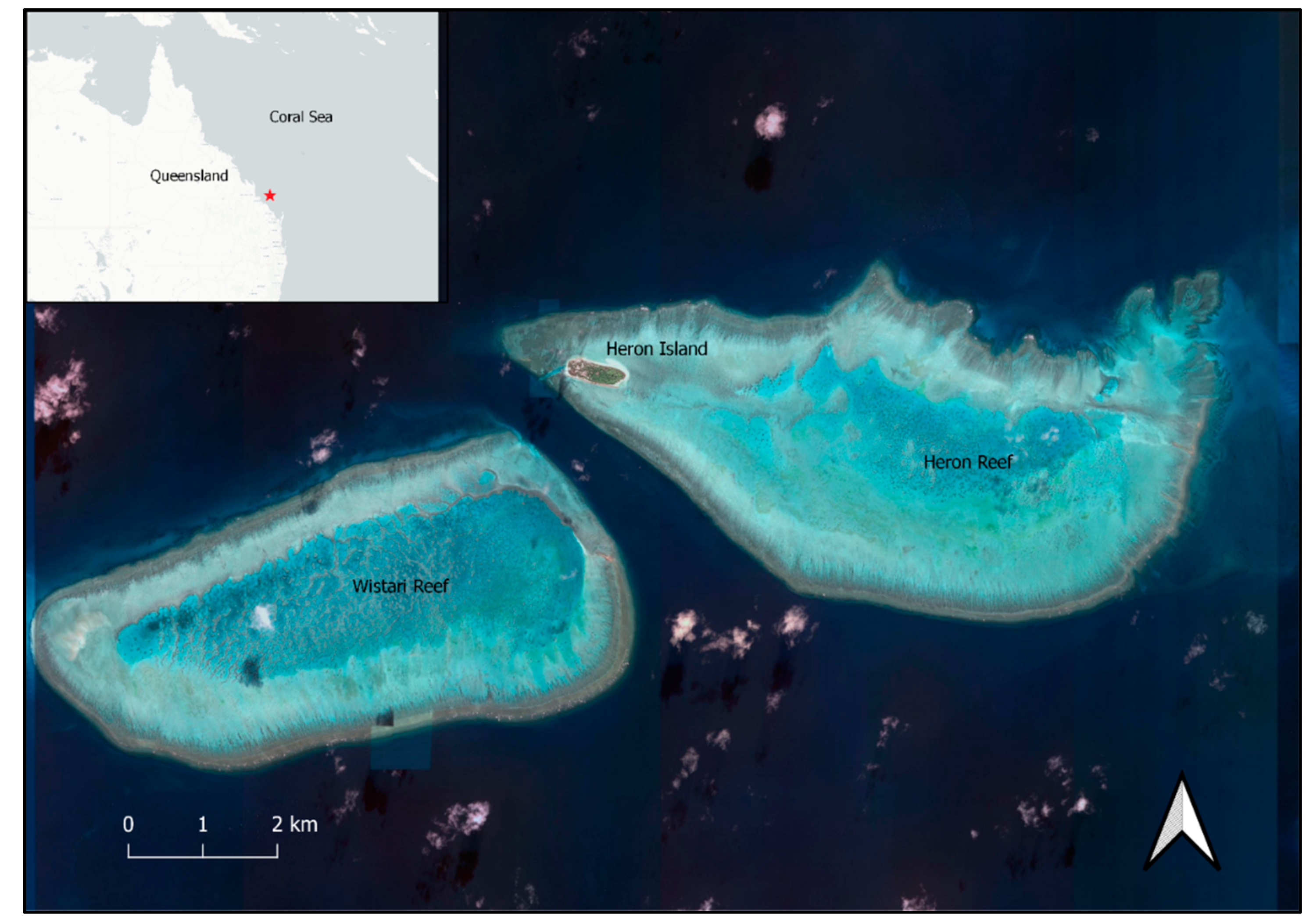
2.1. Study Area and Species
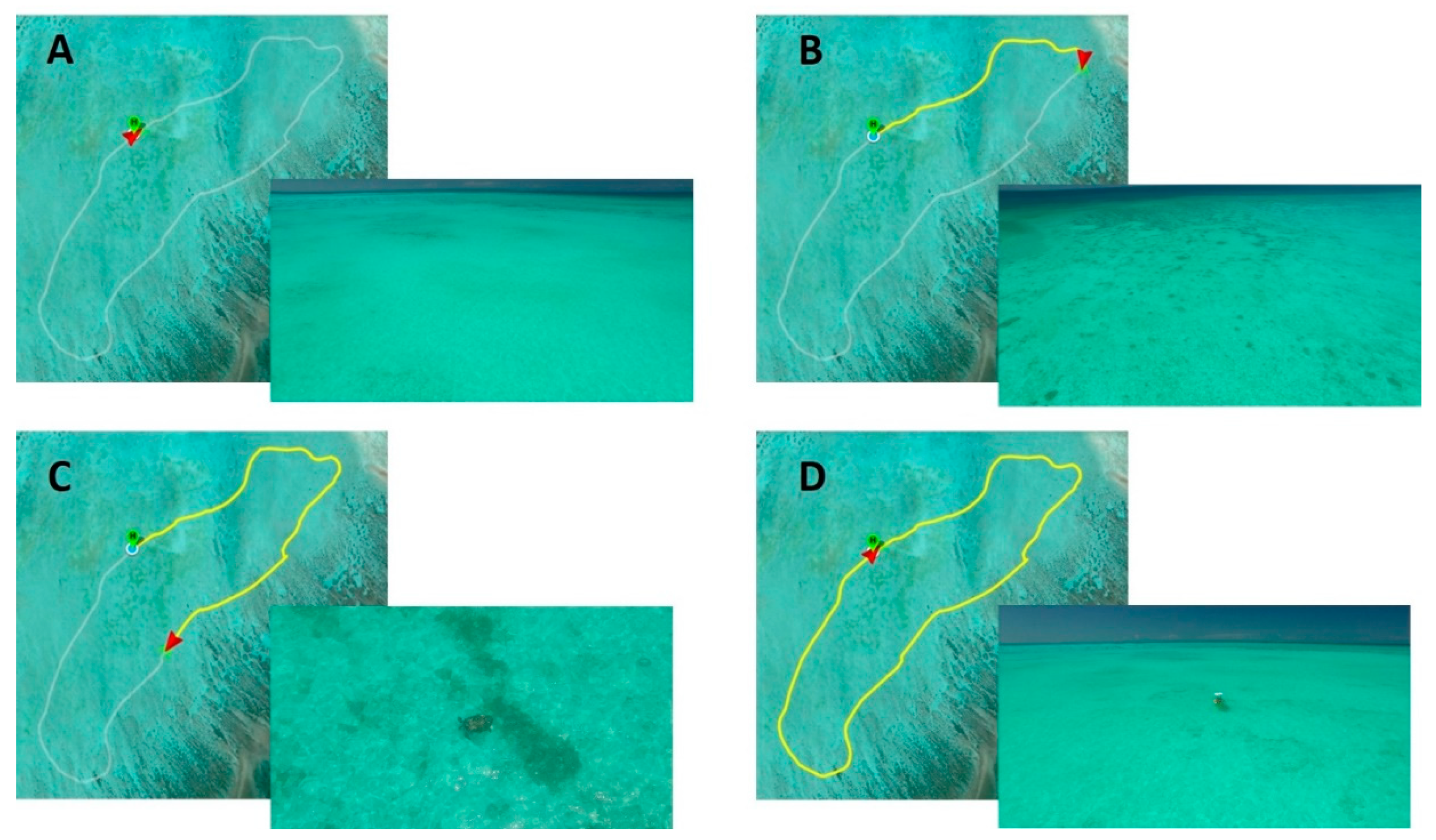
2.2. Survey Techniques
2.3. Survey Analysis
3. Results
Survey Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berglund, A. The operational sex ratio influences choosiness in a pipefish. Behav. Ecol. 1994, 5, 254–258. [Google Scholar] [CrossRef]
- Steifetten, Ø.; Dale, S. Viability of an endangered population of ortolan buntings: The effect of a skewed operational sex ratio. Biol. Conserv. 2006, 132, 88–97. [Google Scholar] [CrossRef]
- Hays, G.C.; Fossette, S.; Katselidis, K.A.; Schofield, G.; Gravenor, M.B. Breeding periodicity for male sea turtles, operational sex ratios, and implications in the face of climate change. Conserv. Biol. 2010, 24, 1636–1643. [Google Scholar] [CrossRef]
- Reina, R.D.; Mayor, P.A.; Spotila, J.R.; Piedra, R.; Paladino, F.V. Nesting ecology of the leatherback turtle, Dermochelys coriacea, at Parque Nacional Marino Las Baulas, Costa Rica: 1988–1989 to 1999–2000. Copeia 2002, 2002, 653–664. [Google Scholar] [CrossRef]
- Schofield, G.; Hobson, V.J.; Fossette, S.; Lilley, M.K.S.; Katselidis, K.A.; Hays, G.C. BIODIVERSITY RESEARCH: Fidelity to foraging sites, consistency of migration routes and habitat modulation of home range by sea turtles. Divers. Distrib. 2010, 16, 840–853. [Google Scholar] [CrossRef]
- Hays, G.C.; Schofield, G.; Mazaris, A.D. Different male versus female breeding periodicity helps mitigate offspring sex ratio skews in sea turtles. Front. Mar. Sci. 2014, 1, 1–9. [Google Scholar] [CrossRef]
- Jensen, M.P.; Allen, C.D.; Eguchi, T.; Bell, I.P.; LaCasella, E.L.; Hilton, W.A.; Hof, C.A.M.; Dutton, P.H. Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr. Biol. 2018, 28, 154–159.e4. [Google Scholar] [CrossRef]
- Booth, D.T.; Dunstan, A.; Bell, I.; Reina, R.D.; Tedeschi, J. Low male production at the world’s largest green turtle rookery. Mar. Ecol. Prog. Ser. 2020, 653, 181–190. [Google Scholar] [CrossRef]
- Laloe, J.-O.; Tedeschi, J.; Booth, D.T.; Bell, I.; Dunstan, A.; Reina, R.D.; Hays, G.C. Extreme rainfall events and cooling of sea turtle clutches: Implications in the face of climate warming. Ecol. Evol. 2020, 11, 560–565. [Google Scholar] [CrossRef]
- Godley, B.J.; Broderick, A.C.; Downie, J.R.; Glen, F.; Houghton, J.D.; Kirkwood, I.; Reece, S.; Hays, G.C. Thermal conditions in nests of loggerhead turtles: Further evidence suggesting female skewed sex ratios of hatchling production in the Mediterranean. J. Exp. Mar. Biol. Ecol. 2001, 263, 45–63. [Google Scholar] [CrossRef]
- Schofield, G.; Katselidis, K.A.; Lilley, M.K.S.; Reina, R.D.; Hays, G.C. Detecting elusive aspects of wildlife ecology using drones: New insights on the mating dynamics and operational sex ratios of sea turtles. Funct. Ecol. 2017, 31, 2310–2319. [Google Scholar] [CrossRef]
- Fuentes, M.; Hamann, M.; Limpus, C. Past, current and future thermal profiles of green turtle nesting grounds: Implications from climate change. J. Exp. Mar. Biol. Ecol. 2010, 383, 56–64. [Google Scholar] [CrossRef]
- Santidrián Tomillo, P.; Genovart, M.; Paladino, F.V.; Spotila, J.R.; Oro, D. Climate change overruns resilience conferred by temperature-dependent sex determination in sea turtles and threatens their survival. Glob. Chang. Biol. 2015, 21, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.; Godfrey, M.H.; Seminoff, J.A.; Arthur, K.; Barata, P.C.R.; Bjorndal, K.A.; Bolten, A.B.; Broderick, A.C.; Campbell, L.M.; Carreras, C.; et al. Global research priorities for sea turtles: Informing management and conservation in the 21st century. Endang. Species Res. 2010, 11, 245–269. [Google Scholar] [CrossRef]
- Eguchi, T.; Gerrodette, T.; Pitman, R.L.; Seminoff, J.A.; Dutton, P.H. At-sea density and abundance estimates of the olive ridley turtle Lepidochelys olivacea in the eastern tropical Pacific. Endang. Species Res. 2007, 3, 191–203. [Google Scholar] [CrossRef]
- Seminoff, J.A.; Eguchi, T.; Carretta, J.; Allen, C.D.; Prosperi, D.; Rangel, R.; Gilpatrick, J.W., Jr.; Forney, K.; Peckham, S.H. Loggerhead sea turtle abundance at a foraging hotspot in the eastern Pacific Ocean: Implications for at-sea conservation. Endang. Species Res. 2014, 24, 207–220. [Google Scholar] [CrossRef]
- Frick, M.G.; Slay, C.K.; Quinn, C.A.; Windham-Reid, A.; Duley, P.A.; Ryder, C.M.; Morse, L.J. Aerial observations of courtship behavior in loggerhead sea turtles (Caretta caretta) from Southeastern Georgia and Northeastern Florida. J. Herpetol. 2000, 34, 153–158. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Kelaher, B.P. The potential for unmanned aerial vehicles (UAVs) to conduct marine fauna surveys in place of manned aircraft. ICES J. Mar. Sci. 2017, 75, 1–8. [Google Scholar] [CrossRef]
- Rees, A.F.; Avens, L.; Ballorain, K.; Bevan, E.; Broderick, A.C.; Carthy, R.; Duclos, G.; Heithaus, M.R.; Johnston, D.; Mangel, J.C.; et al. The potential of unmanned aerial systems for sea turtle research and conservation: A review and future directions. Endang. Species Res. 2018, 35, 81–100. [Google Scholar] [CrossRef]
- Hone, J. On bias, precision and accuracy in wildlife aerial surveys. Wildl. Res. 2008, 35, 253–257. [Google Scholar] [CrossRef]
- Robinson, N.J.; Bigelow, W.; Cuffley, J.; Gary, M.; Hoefer, S.; Mills, S.; Smith, A.; Miguel Blanco, A. Validating the use of drones for monitoring the abundance and behaviour of juvenile green sea turtles in mangrove creeks in The Bahamas. Testudo 2020, 9, 24–35. [Google Scholar]
- Hodgson, J.C.; Baylis, S.M.; Mott, R.; Herrod, A.; Clarke, R.H. Precision wildlife monitoring using unmanned aerial vehicles. Sci. Rep. 2016, 6, 22574. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.P.; Wich, S.A. Dawn of drone ecology: Low-cost autonomous aerial vehicles for conservation. Trop. Conserv. Sci. 2012, 5, 121–132. [Google Scholar] [CrossRef]
- Angliss, R.P.; Ferguson, M.C.; Hall, P.; Helker, V.; Kennedy, A.; Sformo, T. Comparing manned to unmanned aerial surveys for cetacean monitoring in the Arctic: Methods and operational results. J. Unman. Veh. Syst. 2018, 6, 109–127. [Google Scholar] [CrossRef]
- Ferguson, M.C.; Angliss, R.P.; Kennedy, A.; Lynch, B.; Willoughby, A.; Helker, V.; Brower, A.A.; Clarke, J.T. Performance of manned and unmanned aerial surveys to collect visual data and imagery for estimating arctic cetacean density and associated uncertainty. J. Unman. Veh. Syst. 2018, 6, 128–154. [Google Scholar] [CrossRef]
- Johnston, D.W. Unoccupied aircraft systems in marine science and conservation. Ann. Rev. Mar. Sci. 2019, 11, 439–463. [Google Scholar] [CrossRef]
- McIntosh, R.R.; Holmberg, R.; Dann, P. Looking without landing—Using remote piloted aircraft to monitor fur seal populations without disturbance. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Goebel, M.E.; Perryman, W.L.; Hinke, J.T.; Krause, D.J.; Hann, N.A.; Gardner, S.; LeRoi, D.J. A small unmanned aerial system for estimating abundance and size of Antarctic predators. Polar Biol. 2015, 38, 619–630. [Google Scholar] [CrossRef]
- Fudala, K.; Bialik, R.J. Breeding colony dynamics of Southern elephant seals at Patelnia Point, King George Island, Antarctica. Remote Sens. 2020, 12, 2964. [Google Scholar] [CrossRef]
- Castellanos-Galindo, G.A.; Casella, E.; Mejía-Rentería, J.C.; Rovere, A. Habitat mapping of remote coasts: Evaluating the usefulness of lightweight unmanned aerial vehicles for conservation and monitoring. Biol. Conserv. 2019, 239, 108282. [Google Scholar] [CrossRef]
- Yaney-Keller, A.; Santidrián Tomillo, P.; Marshall, J.M.; Paladino, F.V. Using Unmanned Aerial Systems (UAS) to assay mangrove estuaries on the Pacific coast of Costa Rica. PLoS ONE 2019, 14, e0217310. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, J.A.; Desrochers, A.; Aubry, Y.; Pace, P.; Bird, D.M. A low-cost technique for radio-tracking wildlife using a small standard unmanned aerial vehicle. J. Unman. Veh. Syst. 2017, 5, 102–108. [Google Scholar] [CrossRef]
- Pirotta, V.; Smith, A.; Ostrowski, M.; Russell, D.; Jonsen, I.D.; Grech, A.; Harcourt, R. An economical custom-built drone for assessing whale health. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Durban, J.W.; Moore, M.J.; Chiang, G.; Hickmott, L.S.; Bocconcelli, A.; Howes, G.; Bahamonde, P.A.; Perryman, W.L.; LeRoi, D.J. Photogrammetry of blue whales with an unmanned hexacopter. Mar. Mamm. Sci. 2016, 32, 1510–1515. [Google Scholar] [CrossRef]
- Butcher, P.A.; Piddocke, T.P.; Colefax, A.P.; Hoade, B.; Peddemors, V.M.; Borg, L.; Cullis, B.R. Beach safety: Can drones provide a platform for sighting sharks? Wildl. Res. 2019, 46, 701–712. [Google Scholar] [CrossRef]
- Barnas, A.F.; Chabot, D.; Hodgson, A.J.; Johnston, D.W.; Bird, D.M.; Ellis-Felege, S.N. A standardized protocol for reporting methods when using drones for wildlife research. J. Unman. Veh. Syst. 2020, 8, 89–98. [Google Scholar] [CrossRef]
- Johnston, D.W.; Dale, J.; Murray, K.T.; Josephson, E.; Newton, E.; Wood, S. Comparing occupied and unoccupied aircraft surveys of wildlife populations: Assessing the gray seal (Halichoerus grypus) breeding colony on Muskeget Island, USA. J. Unman. Veh. Syst. 2017, 5, 178–191. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Mott, R.; Baylis, S.M.; Pham, T.T.; Wotherspoon, S.; Kilpatrick, A.D.; Raja Segaran, R.; Reid, I.; Terauds, A.; Koh, L.P. Drones count wildlife more accurately and precisely than humans. Meth. Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef]
- Sorrell, K.J.; Clarke, R.H.; Holmberg, R.; McIntosh, R.R. Remotely piloted aircraft improve precision of capture–mark–resight population estimates of Australian fur seals. Ecosphere 2019, 10, e02812. [Google Scholar] [CrossRef]
- Krause, D.J.; Hinke, J.T.; Goebel, M.E.; Perryman, W.L. Drones minimize Antarctic predator responses relative to ground survey methods: An appeal for context in policy advice. Front. Mar. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Schofield, G.; Esteban, N.; Katselidis, K.A.; Hays, G.C. Drones for research on sea turtles and other marine vertebrates—A review. Biol. Conserv. 2019, 238, 108214. [Google Scholar] [CrossRef]
- Read, T.C.; Wantiez, L.; Werry, J.M.; Farman, R.; Petro, G.; Limpus, C.J. Migrations of green turtles (Chelonia mydas) between nesting and foraging grounds across the Coral Sea. PLoS ONE 2014, 9, e100083. [Google Scholar] [CrossRef]
- Limpus, C.J. The green turtle, Chelonia mydas, in Queensland: Breeding males in the southern Great Barrier Reef. Wildl. Res. 1993, 20, 513–523. [Google Scholar] [CrossRef]
- Southwood, A.L.; Reina, R.D.; Jones, V.S.; Speakman, J.R.; Jones, D.R. Seasonal metabolism of juvenile green turtles (Chelonia mydas) at Heron Island, Australia. Can. J. Zool. 2006, 84, 125–135. [Google Scholar] [CrossRef]
- Southwood, A.L.; Reina, R.D.; Jones, V.S.; Jones, D.R. Seasonal diving patterns and body temperatures of juvenile green turtles at Heron Island, Australia. Can. J. Zool. 2003, 81, 1014–1024. [Google Scholar] [CrossRef]
- Amorocho, D.F.; Reina, R.D. Feeding ecology of the East Pacific green sea turtle, Chelonia mydas agassizii, at Gorgona National Park in Colombia. Endang. Species Res. 2007, 3, 43–51. [Google Scholar] [CrossRef]
- Amorocho, D.F.; Reina, R.D. Intake passage time, digesta composition, and digestibility in East Pacific green turtles (Chelonia mydas agassizii) at Gorgona National Park, Colombian Pacific. J. Exp. Mar. Biol. Ecol. 2008, 360, 117–124. [Google Scholar] [CrossRef]
- FitzSimmons, N.N.; Moritz, C.; Moor, S.S. Conservation and dynamics of microsatellite loci over 300 million years of marine turtle evolution. Mol. Biol. Evol. 1995, 12, 432–440. [Google Scholar] [CrossRef][Green Version]
- Schofield, G.; Scott, R.; Dimadi, A.; Fossette, S.; Katselidis, K.A.; Koutsoubas, D.; Lilley, M.K.S.; Pantis, J.D.; Karagouni, A.D.; Hays, G.C. Evidence-based marine protected area planning for a highly mobile endangered marine vertebrate. Biol. Conserv. 2013, 161, 101–109. [Google Scholar] [CrossRef]
- Jessop, T.S.; FitzSimmons, N.N.; Limpus, C.J.; Whittier, J.M. Interactions between behavior and plasma steroids within the scramble mating system of the promiscuous green turtle, Chelonia mydas. Horm. Behav. 1999, 36, 86–97. [Google Scholar] [CrossRef]
- Schofield, G.; Papafitsoros, K.; Haughey, R.; Katselidis, K. Aerial and underwater surveys reveal temporal variation in cleaning-station use by sea turtles at a temperate breeding area. Mar. Ecol. Prog. Ser. 2017, 575, 153–164. [Google Scholar] [CrossRef]
- Limpus, C.J.; Limpus, D.J.; Arthur, K.E.; Parmenter, C.J. Monitoring Green Turtle Population Dynamics in Shoalwater Bay: 2000–2004; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2005; p. 60.
- James, M.C.; Andrea Ottensmeyer, C.; Myers, R.A. Identification of high-use habitat and threats to leatherback sea turtles in northern waters: New directions for conservation. Ecol. Lett. 2005, 8, 195–201. [Google Scholar] [CrossRef]
- Plotkin, P.T.; Owens, D.W.; Byles, R.A.; Patterson, R. Departure of male olive ridley turtles Lepidochelys olivacea from a nearshore breeding ground. Herpetologica 1996, 52, 1–7. [Google Scholar]
- Godley, B.J.; Broderick, A.C.; Frauenstein, R.; Glen, F.; Hays, G.C. Reproductive seasonality and sexual dimorphism in green turtles. Mar. Ecol. Prog. Ser. 2002, 226, 125–133. [Google Scholar] [CrossRef]
- Fitzsimmons, N.N. Single paternity of clutches and sperm storage in the promiscuous green turtle (Chelonia mydas). Mol. Ecol. 1998, 7, 575–584. [Google Scholar] [CrossRef]
- Reina, R.D.; Abernathy, K.J.; Marshall, G.J.; Spotila, J.R. Respiratory frequency, dive behavior and social interactions of leatherback turtles, Dermochelys coriacea during the inter-nesting interval. J. Exp. Mar. Biol. Ecol. 2005, 316, 1–16. [Google Scholar] [CrossRef]
- Crim, J.L.; Spotila, L.D.; Spotila, J.R.; O’Connor, M.; Reina, R.D.; Williams, C.J.; Paladino, F.V. The leatherback turtle, Dermochelys coriacea, exhibits both polyandry and polygyny. Mol. Ecol. 2002, 11, 2097–2106. [Google Scholar] [CrossRef]
- Baker, J.R. The Evolution of Breeding Seasons; Oxford University Press: London, UK, 1938; pp. 161–177. [Google Scholar]
- Dunstan, A.; Robertson, K.; Fitzpatrick, R.; Pickford, J.; Meager, J. Use of unmanned aerial vehicles (UAVs) for mark-resight nesting population estimation of adult female green sea turtles at Raine Island. PLoS ONE 2020, 15, e0228524. [Google Scholar] [CrossRef]
- Fitzsimmons, N.N. Male Marine Turtles: Gene Flow, Philopatry and Mating Systems of the Green Turtle Chelonia mydas. Ph.D. Thesis, The University of Queensland, St Lucia, QL, Australia, August 1997. [Google Scholar]
- Bevan, E.; Wibbels, T.; Navarro, E.; Rosas, M.; Najera, B.M.Z.; Sarti, L.; Illescas, F.; Montana, J.; Peña, L.J.; Burchfield, P. Using unmanned aerial vehicle (UAV) technology for locating, identifying, and monitoring courtship and mating behavior in the green turtle (Chelonia mydas). Herpetol. Rev. 2016, 47, 27–32. [Google Scholar]
- Clarke, R. Understanding the drone epidemic. Comput. Law Sec. Rev. 2014, 30, 230–246. [Google Scholar] [CrossRef]
- Joyce, K.E.; Duce, S.; Leahy, S.M.; Leon, J.; Maier, S.W. Principles and practice of acquiring drone-based image data in marine environments. Mar. Freshw. Res. 2019, 70, 952–963. [Google Scholar] [CrossRef]
- Bevan, E.; Whiting, S.; Tucker, T.; Guinea, M.; Raith, A.; Douglas, R. Measuring behavioral responses of sea turtles, saltwater crocodiles, and crested terns to drone disturbance to define ethical operating thresholds. PLoS ONE 2018, 13, e0194460. [Google Scholar] [CrossRef] [PubMed]
- Raoult, V.; Colefax, A.P.; Allan, B.M.; Cagnazzi, D.; Castelblanco-Martínez, N.; Ierodiaconou, D.; Johnston, D.W.; Landeo-Yauri, S.; Lyons, M.; Pirotta, V.; et al. Operational protocols for the use of drones in marine animal research. Drones 2020, 4, 64. [Google Scholar] [CrossRef]
- Hodgson, A.; Kelly, N.; Peel, D. Unmanned Aerial Vehicles (UAVs) for Surveying Marine Fauna: A Dugong Case Study. PLoS ONE 2013, 8, e79556. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.C.; Fleishman, A.B.; Klein, D.J.; McKown, M.W.; Bézy, V.S.; Lohmann, K.J.; Johnston, D.W. A convolutional neural network for detecting sea turtles in drone imagery. Meth. Ecol. Evol. 2019, 10, 345–355. [Google Scholar] [CrossRef]
- Hensel, E.; Wenclawski, S.; Layman, C.A. Using a small, consumer-grade drone to identify and count marine megafauna in shallow habitats. Lat. Am. J. Aquatic. Res. 2018, 46, 1025–1033. [Google Scholar] [CrossRef]
- Barreto, J.; Cajaíba, L.; Teixeira, J.B.; Nascimento, L.; Giacomo, A.; Barcelos, N.; Fettermann, T.; Martins, A. Drone-monitoring: Improving the detectability of threatened marine megafauna. Drones 2021, 5, 14. [Google Scholar] [CrossRef]
- Schaub, J.; Hunt, B.P.V.; Pakhomov, E.A.; Holmes, K.; Lu, Y.; Quayle, L. Using unmanned aerial vehicles (UAVs) to measure jellyfish aggregations. Mar. Ecol. Prog. Ser. 2018, 591, 29–36. [Google Scholar] [CrossRef]
- Fust, P.; Loos, J. Development perspectives for the application of autonomous, unmanned aerial systems (UASs) in wildlife conservation. Biol. Conserv. 2020, 241, 108380. [Google Scholar] [CrossRef]

| Boat Surveys | Drone Surveys | |||||
|---|---|---|---|---|---|---|
| Month | Male Turtles | Female Turtles | Unknown Sex | Male Turtles | Female Turtles | Unknown Sex |
| Oct 2016 | 49 | 30 | 11 | 20 | 27 | 8 |
| Dec 2016 | 4 | 44 | 1 | 4 | 83 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaney-Keller, A.; San Martin, R.; Reina, R.D. Comparison of UAV and Boat Surveys for Detecting Changes in Breeding Population Dynamics of Sea Turtles. Remote Sens. 2021, 13, 2857. https://doi.org/10.3390/rs13152857
Yaney-Keller A, San Martin R, Reina RD. Comparison of UAV and Boat Surveys for Detecting Changes in Breeding Population Dynamics of Sea Turtles. Remote Sensing. 2021; 13(15):2857. https://doi.org/10.3390/rs13152857
Chicago/Turabian StyleYaney-Keller, Adam, Ricardo San Martin, and Richard D. Reina. 2021. "Comparison of UAV and Boat Surveys for Detecting Changes in Breeding Population Dynamics of Sea Turtles" Remote Sensing 13, no. 15: 2857. https://doi.org/10.3390/rs13152857
APA StyleYaney-Keller, A., San Martin, R., & Reina, R. D. (2021). Comparison of UAV and Boat Surveys for Detecting Changes in Breeding Population Dynamics of Sea Turtles. Remote Sensing, 13(15), 2857. https://doi.org/10.3390/rs13152857






