Habitat Suitability Modeling for the Feeding Ground of Immature Albacore in the Southern Indian Ocean Using Satellite-Derived Sea Surface Temperature and Chlorophyll Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Albacore Tuna Fishing Data
2.2. Standardization of Nominal Catch per Unit Effort Data
2.3. Moderate Resolution Imaging Spectroradiometer (MODIS)-Derived Remotely Sensed Data
2.4. Environmental Factor Selection for Model Building
2.5. Suitability Index of Environmental Variables and Standardized CPUE
2.6. Development of the HSI Model
2.7. Model Selection and Validation
3. Results
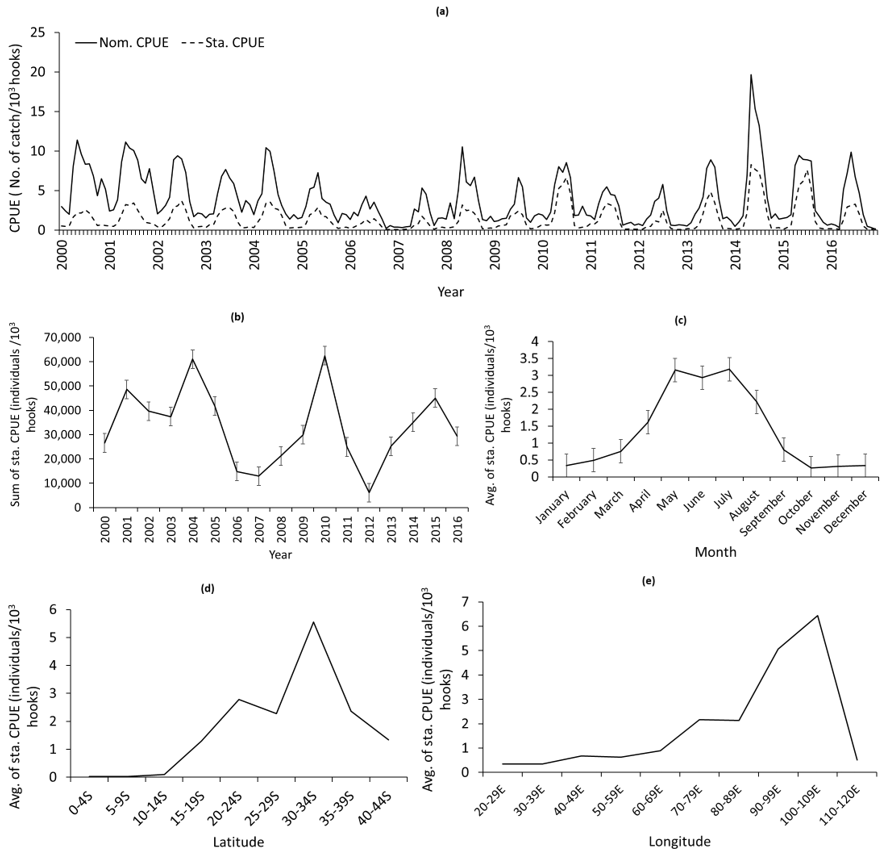
3.1. Spatiotemporal Variation of Standardized CPUE in the SIO
3.2. Variable Selection for Fitting into the Final Model
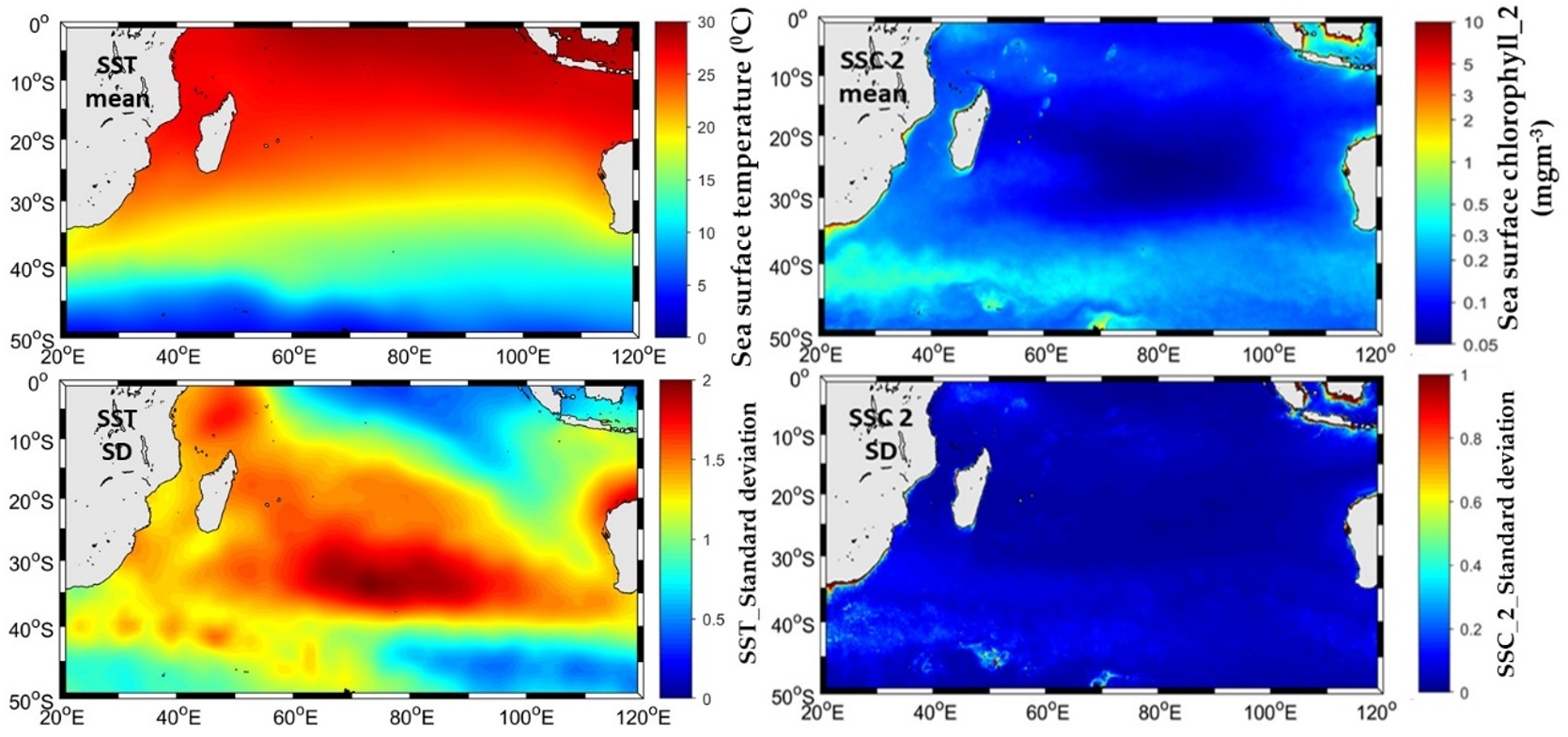
3.3. Variation in Selected Remote Sensing Environmental Variables in the SIO
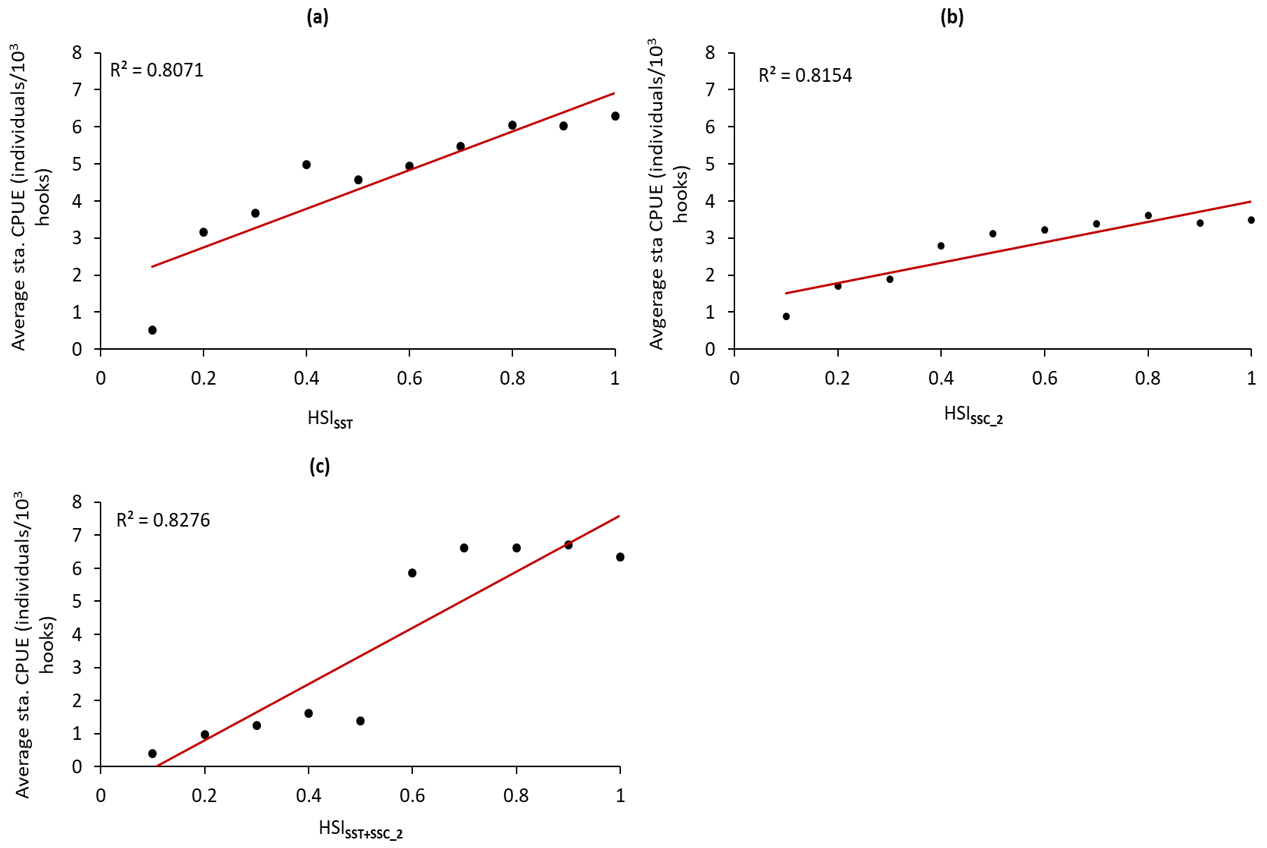
3.4. SI Curves of Selected Environmental Factors and Modeling the HSI of Albacore Tuna
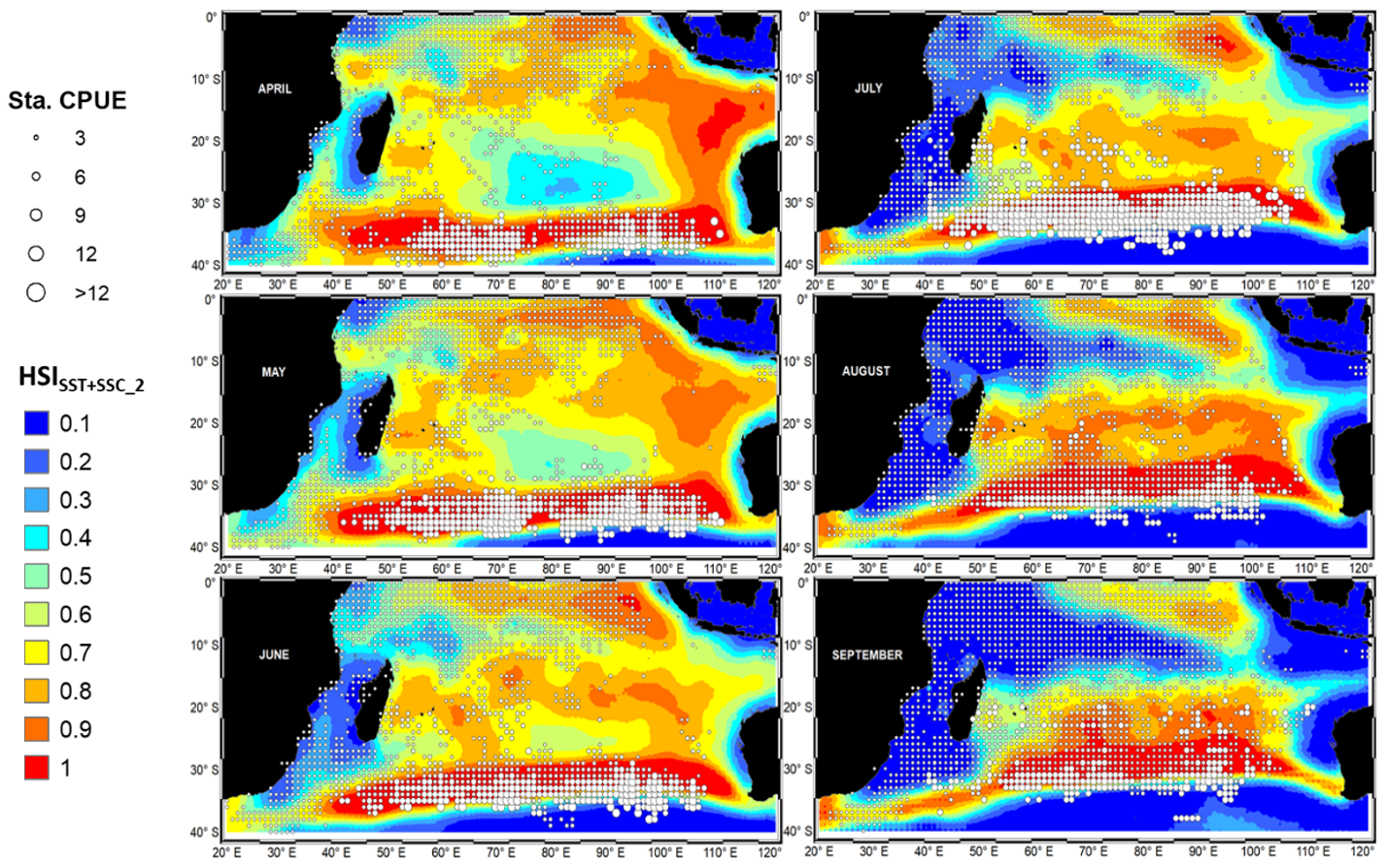
3.5. Validation of the HSI models and HSI prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirches, G.; Paperin, M.; Klein, H.; Brockmann, C.; Stelzer, K.; Kirches, G.; Paperin, M.; Klein, H.; Brockmann, C.; Stelzer, K. GRADHIST—A method for detection and analysis of oceanic fronts from remote sensing data. Remote Sens. Environ. 2016, 181, 264–280. [Google Scholar] [CrossRef]
- Ping, B.; Su, F.; Meng, Y.; Fang, S.; Du, Y. A model of sea surface temperature front detection based on a threshold interval. Acta Oceanol. Sin. 2014, 33, 65–71. [Google Scholar] [CrossRef]
- Diehl, S.F.; Budd, J.W.; Ullman, D.; Cayula, J.F. Geographic window sizes applied to remote sensing sea surface temperature front detection. J. Atmos. Ocean. Technol. 2002, 19, 1105–1113. [Google Scholar] [CrossRef]
- Lan, K.-W.; Kawamura, H.; Lee, M.-A.; Lu, H.-J.; Shimada, T.; Hosoda, K.; Sakaida, F. Relationship between albacore (Thunnus alalunga) fishing grounds in the Indian Ocean and the thermal environment revealed by cloud-free microwave sea surface temperature. Fish. Res. 2012, 113, 1–7. [Google Scholar] [CrossRef]
- Maul, G.A. Introduction to Satellite Oceanography; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1985; p. 3. [Google Scholar]
- Zhou, C.; He, P.; Xu, L.; Bach, P.; Wang, X.; Wan, R.; Tang, H.; Zhang, Y. The effects of mesoscale oceanographic structures and ambient conditions on the catch of albacore tuna in the South Pacific longline fishery. Fish. Oceanogr. 2020, 29, 238–251. [Google Scholar] [CrossRef]
- Chen, X.; Tian, S.; Chen, Y.; Liu, B. A modeling approach to identify optimal habitat and suitable fishing grounds for neon flying squid (Ommostrephes bartramii) in the northwest Pacific Ocean. Fish. Bull. 2010, 108, 1–14. [Google Scholar]
- Zainuddin, M.; Saitoh, S. Detection of potential fishing ground for albacore tuna using synoptic measurements of ocean color and thermal remote sensing in the northwestern North Pacific. Geophys. Res. Lett. 2004, 31, 31. [Google Scholar] [CrossRef]
- Zainuddin, M.; Saitoh, K.; Saitoh, S.-I. Albacore (Thunnus alalunga) fishing ground in relation to oceanographic conditions in the western North Pacific Ocean using remotely sensed satellite data. Fish. Oceanogr. 2008, 17, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Lan, K.-W.; Lee, M.-A.; Chou, C.-P.; Vayghan, A.H. Association between the interannual variation in the oceanic environment and catch rates of bigeye tuna (Thunnus obesus) in the Atlantic Ocean. Fish. Oceanogr. 2018, 27, 395–407. [Google Scholar] [CrossRef]
- Xu, Y.; Nieto, K.; Teo, S.L.; McClatchie, S.; Holmes, J. Influence of fronts on the spatial distribution of albacore tuna (Thunnus alalunga) in the Northeast Pacific over the past 30 years (1982–2011). Prog. Oceanogr. 2017, 150, 72–78. [Google Scholar] [CrossRef]
- Yen, K.-W.; Chen, C.-H. Research Gap Analysis of Remote Sensing Application in Fisheries: Prospects for Achieving the Sustainable Development Goals. Remote Sens. 2021, 13, 1013. [Google Scholar] [CrossRef]
- Lan, K.W.; Nishida, T.; Lee, M.A.; Lu, H.J.; Huang, H.W.; Chang, S.K.; Lan, Y.C. Influence of the marine environment varia-bility on the yellowfin tuna (Thunnus albacares) catch rate by the Taiwanese longline fishery in the Arabian sea, with special reference to the high catch in 2004. J. Mar. Sci. Technol. 2012, 20, 514–524. [Google Scholar]
- Majkowski, J. Global Fishery Resources of Tuna and Tuna-Like Species; FAO: Rome, Italy, 2007. [Google Scholar]
- Lehodey, P.; Senina, I.; Murtugudde, R. A spatial ecosystem and populations dynamics model (SEAPODYM)—Modeling of tuna and tuna-like populations. Prog. Oceanogr. 2008, 78, 304–318. [Google Scholar] [CrossRef]
- Klemas, V. Remote sensing of environmental indicators of potential fish aggregation: An overview. Baltica 2012, 25, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Gilman, E.; Weijerman, M.; Suuronen, P. Ecological data from observer programs underpin ecosystem-based fisheries management. ICES J. Mar. Sci. 2017, 74, 1481–1495. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.F.; Chen, I.C.; Tzeng, W.N. Spatial and temporal distribution patterns of bigeye tuna (Thunnus obesus) in the Indian Ocean. Zool. Stud. 2005, 44, 260. [Google Scholar]
- Zainuddin, M.; Farhum, A.; Safruddin, S.; Selamat, M.B.; Sudirman, S.; Nurdin, N.; Syamsuddin, M.; Ridwan, M.; Saitoh, S.-I. Detection of pelagic habitat hotspots for skipjack tuna in the Gulf of Bone-Flores Sea, southwestern Coral Triangle tuna, Indonesia. PLoS ONE 2017, 12, e0185601. [Google Scholar] [CrossRef] [Green Version]
- Lan, K.W.; Lee, M.A.; Lu, H.J.; Shieh, W.J.; Lin, W.K.; Kao, S.C. Ocean variations associated with fishing conditions for yel-lowfin tuna (Thunnus albacares) in the equatorial Atlantic Ocean. ICES J. Mar. Sci. 2011, 68, 1063–1071. [Google Scholar] [CrossRef]
- Mugo, R.; Saitoh, S.-I.; Nihira, A.; Kuroyama, T. Habitat characteristics of skipjack tuna (Katsuwonus pelamis) in the western North Pacific: A remote sensing perspective. Fish. Oceanogr. 2010, 19, 382–396. [Google Scholar] [CrossRef]
- Su, N.J.; Yeh, S.Z.; Sun, C.L.; Punt, A.E.; Chen, Y.; Wang, S.P. Standardizing catch and effort data of the Taiwanese distant-water longline fishery in the western and central Pacific Ocean for bigeye tuna, Thunnus obesus. Fish. Res. 2008, 90, 235–246. [Google Scholar] [CrossRef]
- Tian, S.; Chen, X.; Chen, Y.; Xu, L.; Dai, X. Standardizing CPUE of Ommastrephes bartramii for Chinese squid-jigging fishery in Northwest Pacific Ocean. Chin. J. Oceanol. Limnol. 2009, 27, 729–739. [Google Scholar] [CrossRef]
- Kumari, B.; Raman, M.; Mali, K. Locating tuna forage ground through satellite remote sensing. Int. J. Remote Sens. 2009, 30, 5977–5988. [Google Scholar] [CrossRef]
- Maunder, M.N.; Hinton, M.G.; Bigelow, K.A.; Langley, A.D. Developing indices of abundance using habitat data in a statistical framework. Bull. Mar. Sci. 2006, 79, 545–559. [Google Scholar]
- Maunder, M.N.; Thorson, J.T.; Xu, H.; Oliveros-Ramos, R.; Hoyle, S.D.; Tremblay-Boyer, L.; Lee, H.H.; Kai, M.; Chang, S.K.; Kitakado, T. The need for spatio-temporal modeling to determine catch-per-unit effort based indices of abundance and as-sociated composition data for inclusion in stock assessment models. Fish. Res. 2020, 229, 105594. [Google Scholar] [CrossRef]
- Vayghan, A.H.; Zarkami, R.; Sadeghi, R.; Fazli, H. Modeling habitat preferences of Caspian kutum, Rutilus frisii kutum (Kamensky, 1901) (Actinopterygii, Cypriniformes) in the Caspian Sea. Hydrobiologia 2016, 766, 103–119. [Google Scholar] [CrossRef]
- Vayghan, A.H.; Poorbagher, H.; Shahraiyni, H.T.; Fazli, H.; Saravi, H.N. Suitability indices and habitat suitability index model of Caspian kutum (Rutilus frisii kutum) in the southern Caspian Sea. Aquat. Ecol. 2013, 47, 441–451. [Google Scholar] [CrossRef]
- Vayghan, A.H.; Fazli, H.; Ghorbani, R.; Lee, M.-A.; Saravi, H.N. Temporal habitat suitability modeling of Caspian shad (Alosa spp.) in the southern Caspian Sea. J. Limnol. 2015, 75, 210–223. [Google Scholar] [CrossRef]
- Van der Lee, G.E.M.; Van der Molen, D.T.; Van den Boogaard, H.F.P.; Van der Klis, H. Uncertainty analysis ofa spatial habitat suitability model and implications for ecological management of water bodies. Landsc. Ecol. 2006, 21, 1019–1032. [Google Scholar] [CrossRef]
- Vayghan, A.; Lee, M.-A.; Weng, J.-S.; Mondal, S.; Lin, C.-T.; Wang, Y.-C. Multisatellite-Based Feeding Habitat Suitability Modeling of Albacore Tuna in the Southern Atlantic Ocean. Remote Sens. 2020, 12, 2515. [Google Scholar] [CrossRef]
- Lee, M.-A.; Weng, J.-S.; Lan, K.-W.; Vayghan, A.H.; Wang, Y.-C.; Chan, J.-W. Empirical habitat suitability model for immature albacore tuna in the North Pacific Ocean obtained using multisatellite remote sensing data. Int. J. Remote Sens. 2019, 41, 5819–5837. [Google Scholar] [CrossRef]
- Loukos, H.; Monfray, P.; Bopp, L.; Lehodey, P. Potential changes in skipjack tuna (Katsuwonus pelamis) habitat from a global warming scenario: Modelling approach and preliminary results. Fish. Oceanogr. 2003, 12, 474–482. [Google Scholar] [CrossRef]
- Lan, K.W.; Shimada, T.; Lee, M.A.; Su, N.J.; Chang, Y. Using remote-sensing environmental and fishery data to map potential yellowfin tuna habitats in the tropical Pacific Ocean. Remote Sens. 2017, 9, 444. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yu, W.; Chen, X.; Lei, L.; Chen, Y. Detection of potential fishing zones for neon flying squid based on remote-sensing data in the Northwest Pacific Ocean using an artificial neural network. Int. J. Remote Sens. 2015, 36, 3317–3330. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C.; Shao, Q.; Mulla, D.J. Remote sensing of sea surface temperature and chlorophyll-a: Implications for squid fisheries in the north-west Pacific Ocean. Int. J. Remote Sens. 2010, 31, 4515–4530. [Google Scholar] [CrossRef]
- Lan, K.-W.; Chang, Y.-J.; Wu, Y.-L. Influence of oceanographic and climatic variability on the catch rate of yellowfin tuna (Thunnus albacares) cohorts in the Indian Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 175, 104681. [Google Scholar] [CrossRef]
- Chen, I.C.; Lee, P.F.; Tzeng, W.N. Distribution of albacore (Thunnus alalunga) in the Indian Ocean and its relation to environmental factors. Fish. Oceanogr. 2005, 14, 71–80. [Google Scholar] [CrossRef]
- Arrizabalaga, S.; Li, Z. Factors influencing the assessment of albacore tuna resources in the Indian Ocean. Ccamlr Sci. 2018, 25, 107–120. [Google Scholar]
- Collette, B.B.; Nauen, C.E. FAO species catalogue. Scombrids of the world. An annotated and illustrated catalogue of tunas, mackerels, bonitos, and related species known to date. FAO Fish. Synop. 1983, 125, 137. [Google Scholar]
- Hoyle, S.D.; Langley, A.D.; Campbell, R.A. Recommended approaches for standardizing CPUE data from pelagic fisheries. In Proceedings of the Western and Central Pacific Fisheries Commission, Majuro, Marshall Islands, 6–14 August 2014; pp. 1–21. [Google Scholar] [CrossRef]
- Report of the 2013 Iccat North and South Atlantic Albacore Stock Assessment Meeting; Sukarrieta, Spain. 2013. Available online: https://www.iccat.int/Documents/Meetings/Docs/2013_ALB_ASSESS_REP_ENG.pdf (accessed on 20 March 2020).
- Baglin, R.E. Reproductive biology of western Atlantic bluefin tuna. Fish. Bull. 1982, 80, 121–134. [Google Scholar]
- Arrizabalaga, H.; López-Rodas, V.; de Zárate, V.O.; Costas, E.; Gonzaléz-Garcés, A. Study on the migrations and stock structure of albacore (Thunnus alalunga) from the Atlantic Ocean and the Mediterranean Sea based on conventional tag release-recapture experiences. Collect. Vol. Sci. Pap. ICCAT 2002, 54, 1479–1494. [Google Scholar]
- De Zárate, V.O.; Cort, J.L. Albacore (Thunnus alalunga, Bonnaterre) stock structure in the Atlantic Ocean, as inferred from distribution and migration patterns. In Proceedings of the ICCAT Tuna Symposium, Rio de Janeiro, Brazil, 15–22 November 1999; pp. 251–260. [Google Scholar]
- Sund, P.N.; Blackburn, M.; Williams, F. Tunas and their environment in the Pacific Ocean: A review. Oceanography. Mar. Biol. Ann. Rev. 1981, 19, 443–512. [Google Scholar]
- Ramos, A.G.; Santiago, J.; Sangra, P.; Canton, M. An application of satellite-derived sea surface temperature data to the skipjack (Katsuwonus pelamis, Linnaeus, 1758) and albacore tuna (Thunnus alalunga, Bonnaterre, 1788) fisheries in the north-east Atlantic. Int. J. Remote Sens. 1996, 17, 749–759. [Google Scholar] [CrossRef]
- Bakun, A. Patterns in the Ocean; California Sea Grant, in cooperation with Centro de Investigaciones Biologicas del Noroeste: La Paz, Mexico, 1998; p. 323. [Google Scholar]
- Olson, D.B.; Hitchcock, G.L.; Mariano, A.J.; Ashjian, C.J.; Peng, G.; Nero, R.W.; Podesta, G.P. Life on the edge: Marine life and fronts. Oceanography 1994, 7, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Polovina, J.J.; Howell, E.; Kobayashi, D.R.; Seki, M.P. The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog. Oceanogr. 2001, 49, 469–483. [Google Scholar] [CrossRef]
- Koga, S. On the stomach contents of tuna in the West Indian Ocean. Bull. Fac. Fish. 1958, 6, 85–92. [Google Scholar]
- Nishida, T.; Tanaka, M. General review of Indian Ocean albacore (Thunnus alalunga). In Proceedings of the 1st Session of the Working Party on Temperate Tuna, Shimizu, Japan, 2–5 August 2004; p. 8. [Google Scholar]
- Xu, L.; Tian, S.Q. A study of fisheries biology for albacore based on Chinese observer data. In Proceedings of the 3rd Session of the Working Party on Temperate Tuna, Busan, Korea, 20–22 September 2011; pp. 3–11. [Google Scholar]
- Chen, X.; Li, G.; Feng, B.; Tian, S. Habitat suitability index of Chub mackerel (Scomber japonicus) from July to September in the East China Sea. J. Oceanogr. 2009, 65, 93–102. [Google Scholar] [CrossRef]
- Lee, D.; Son, S.H.; Kang, C.-K. Spatio-Temporal Variability of the Habitat Suitability Index for the Todarodes pacificus (Japanese Common Squid) around South Korea. Remote Sens. 2019, 11, 2720. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Son, S.; Kim, W.; Park, J.M.; Joo, H.; Lee, S.H. Spatio-Temporal Variability of the Habitat Suitability Index for Chub Mackerel (Scomber Japonicus) in the East/Japan Sea and the South Sea of South Korea. Remote Sens. 2018, 10, 938. [Google Scholar] [CrossRef] [Green Version]
- Hess, G.R.; Bay, J.M. A Regional Assessment of Windbreak Habitat Suitability. Environ. Monit. Assess. 2000, 61, 239–256. [Google Scholar] [CrossRef]
- Lauver, C.L.; Busby, W.H.; Whistler, J.L. Testing a GIS Model of Habitat Suitability for a Declining Grassland Bird. Environ. Manag. 2002, 30, 88–97. [Google Scholar] [CrossRef]
- Hirzel, A.; Helfer, V.; Metral, F. Assessing habitat-suitability models with a virtual species. Ecol. Model. 2001, 145, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Muhling, B.A.; Brill, R.; Lamkin, J.T.; Roffer, M.A.; Lee, S.-K.; Liu, Y.; Muller-Karger, F. Projections of future habitat use by Atlantic bluefin tuna: Mechanistic vs. correlative distribution models. ICES J. Mar. Sci. 2016, 74, 698–716. [Google Scholar] [CrossRef]
- Nikolic, N.; Morandeau, G.; Hoarau, L.; West, W.; Arrizabalaga, H.; Hoyle, S.; Nicol, S.J.; Bourjea, J.; Puech, A.; Farley, J.H.; et al. Review of albacore tuna, Thunnus alalunga, biology, fisheries and management. Rev. Fish Biol. Fish. 2017, 27, 775–810. [Google Scholar] [CrossRef]
- Lee, M.-A.; Vayghan, A.H.; Liu, D.-C.; Yang, W.-C. Potential and prospective seasonal distribution of hotspot habitat of albacore tuna (Thunnus alalunga) in the South Indian Ocean using the satellite data. In Proceedings of the 2017 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017; pp. 5747–5750. [Google Scholar]
- Arrizabalaga, H.; Dufour, F.; Kell, L.; Merino, G.; Ibaibarriaga, L.; Chust, G.; Irigoien, X.; Santiago, J.; Murua, H.; Fraile, I.; et al. Global habitat preferences of commercially valuable tuna. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Lehodey, P.; Senina, I.; Dragon, A.-C.; Arrizabalaga, H. Spatially explicit estimates of stock size, structure and biomass of North Atlantic albacore tuna (Thunnus alalunga). Earth Syst. Sci. Data 2014, 6, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Chust, G.; Goikoetxea, N.; Ibaibarriaga, L.; Sagarminaga, Y.; Arregui, I.; Fontán, A.; Irigoien, X.; Arrizabalaga, H. Earlier migration and distribution changes of albacore in the Northeast Atlantic. Fish. Oceanogr. 2019, 28, 505–516. [Google Scholar] [CrossRef]
- Lehodey, P.; Senina, I.; Nicol, S.; Hampton, J. Modelling the impact of climate change on South Pacific albacore tuna. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 246–259. [Google Scholar] [CrossRef]
- Senina, I.N.; Lehodey, P.; Hampton, J.; Sibert, J. Quantitative modelling of the spatial dynamics of South Pacific and Atlantic albacore tuna populations. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 175, 104667. [Google Scholar] [CrossRef]




| Variables | Units | Data Source | Resolution |
|---|---|---|---|
| Sea Surface Temperature (SST) | °C | MODIS | 1° × 1° |
| Sea Surface Chlorophyll (SSC_0) | mg m−3 | MODIS | 1° × 1° |
| Sea Surface Chlorophyll 1-month lag (SSC_1) | mg m−3 | MODIS | 1° × 1° |
| Sea Surface Chlorophyll 2-month lag (SSC_2) | mg m−3 | MODIS | 1° × 1° |
| Sea Surface Chlorophyll 3-month lag (SSC_3) | mg m−3 | MODIS | 1° × 1° |
| Sea Surface Chlorophyll 4-month lag (SSC_4) | mg m−3 | MODIS | 1° × 1° |
| SST | SSC_0 | SSC_1 | SSC_2 | SSC_3 | SSC_4 | |
|---|---|---|---|---|---|---|
| SSC_0 | −0.079 * | |||||
| SSC_1 | −0.041 * | 0.877 ** | ||||
| SSC_2 | 0.049 * | 0.815 ** | 0.889 ** | |||
| SSC_3 | 0.105 * | 0.775 ** | 0.813 ** | 0.893 ** | ||
| SSC_4 | 0.123 * | 0.743 ** | 0.798 ** | 0.859 ** | 0.875 ** | |
| Sta. CPUE | −0.681 * | −0.152 * | −0.189 * | −0.224 * | −0.197 * | −0.166 * |
| Environmental Variables | AMM | GMM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α | β | Adjusted r2 | AIC | P(F) | α | β | Adjusted r2 | AIC | P(F) | |
| SST | 1.962 | 5.206 | 0.783 | 28.115 | <0.01 | 1.962 | 5.206 | 0.783 | 28.115 | <0.01 |
| SSC_2 | 1.318 | 2.812 | 0.819 | 13.568 | <0.01 | 1.318 | 2.812 | 0.819 | 13.568 | <0.01 |
| SST, SSC_2 | 0.534 | 1.221 | 0.839 | 1.432 | <0.01 | 4.394 | 3.104 | 0.178 | 42.046 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, S.; Vayghan, A.H.; Lee, M.-A.; Wang, Y.-C.; Semedi, B. Habitat Suitability Modeling for the Feeding Ground of Immature Albacore in the Southern Indian Ocean Using Satellite-Derived Sea Surface Temperature and Chlorophyll Data. Remote Sens. 2021, 13, 2669. https://doi.org/10.3390/rs13142669
Mondal S, Vayghan AH, Lee M-A, Wang Y-C, Semedi B. Habitat Suitability Modeling for the Feeding Ground of Immature Albacore in the Southern Indian Ocean Using Satellite-Derived Sea Surface Temperature and Chlorophyll Data. Remote Sensing. 2021; 13(14):2669. https://doi.org/10.3390/rs13142669
Chicago/Turabian StyleMondal, Sandipan, Ali Haghi Vayghan, Ming-An Lee, Yi-Chen Wang, and Bambang Semedi. 2021. "Habitat Suitability Modeling for the Feeding Ground of Immature Albacore in the Southern Indian Ocean Using Satellite-Derived Sea Surface Temperature and Chlorophyll Data" Remote Sensing 13, no. 14: 2669. https://doi.org/10.3390/rs13142669
APA StyleMondal, S., Vayghan, A. H., Lee, M.-A., Wang, Y.-C., & Semedi, B. (2021). Habitat Suitability Modeling for the Feeding Ground of Immature Albacore in the Southern Indian Ocean Using Satellite-Derived Sea Surface Temperature and Chlorophyll Data. Remote Sensing, 13(14), 2669. https://doi.org/10.3390/rs13142669








