1. Introduction
Global biodiversity is under major threat by a multitude of factors, such as nutrient loading, carbon dioxide enrichment, and invasive species; similar to these other threats, land-use and climate change pose considerable challenges [
1]. Climate change distresses both endotherms and ectotherms by affecting various population aspects including density, distribution shifts, phenology, morphology, and genetics [
2]. Many species are unable to adapt fast enough to the rapid rate of rising global average temperature. Although the impacts on endotherms is concerning, there have been relatively few studies examining the decline of less charismatic, but highly vulnerable and climatically sensitive ectothermic species [
3,
4,
5,
6,
7]. This is especially concerning for reptiles, such as turtles, because these species lack the dispersal ability of other taxa such as birds, mammals, and many fishes [
8] and population declines are expected to be expedited [
5]. Additionally, ectotherm physiology plays a strong role where it is influenced by thermal conditions, which in turn are often highly affected by the local scale physical characteristics of a habitat [
9]. In a changing climate, thermal heterogeneity is crucial for providing both sunny, warm patches and shady, cool patches to move in or out of as needed. However, maintaining optimal thermal physiology can be difficult in thermally variable environments, and consistent sub-optimal conditions can reduce population viability and directly impact dispersal and population persistence [
10,
11].
Terrapene carolina carolina has been shown to have a thermal body temperature range of 20 °C–25 °C [
12,
13,
14] or 27 °C–31 °C [
15]. They are often found in less optimal temperatures within closed, forested habitat; however, open habitats tend to peak frequently above 35 °C especially during the active season [
16]. When interacting with temperatures above 35 °C, turtles often seek to lower their body temperature and avoid their lethal upper limit of 43 °C [
17]. Forests may be thermally suboptimal; however, they provide refuge from excessive temperatures, reduced desiccation risk, and access to resources [
16]. Rising temperatures will make it more challenging for
T. c. carolina to behaviorally regulate body temperature and will likely require increased movement to find optimal thermal environments. As with this species, other reptiles will face similar issues with warming climate, such as overheating and will require greater fine-scale heterogeneity.
There are many protected areas across the United States; however, many of these areas are fragmented by the large network of roads and human-dominated land cover, which make it difficult for species to relocate. This reduces connectivity and negatively impacts reptilian populations by reducing gene flow and dispersal. Efforts made to increase connectivity among protected areas can enhance species resilience to factors such as climate change, especially with the expected potential shifts in distribution ranges [
18]. These climatic changes will likely cause species to shift their range, change their population dynamics, adapt to new climates or become extirpated from local sites. It is imperative to understand how they will respond to these changes across multiple scales [
2,
8,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28]. It is expected that changes will be concentrated in areas with the largest temperature changes such as higher latitudes and altitudes with fewer changes in other areas [
2]. Therefore, it is important to examine expected changes from unsuitable to suitable, or vice versa, across a species distribution range and manage the areas that vary in climate extremes. In other words, there is a need to translate large scale climate changes to local scale habitat alterations to better manage for vulnerable species. Species that live in areas with large changes from current or historical temperature regimes may have increased vulnerability to declines or extirpation.
Species distribution models have become commonplace to predict potential changes from current conditions to varying climate scenarios [
3,
29]. These models have been used to infer species distributions or habitat suitability in past, current, and future scenarios for speciation, refinement of protected areas for different species, prediction of invasive species distributions, and impacts of climate change on wildlife [
21,
23,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48]. One major drawback to climatic prediction modeling is that it may solely rely on the correlation between abiotic factors and species presence creating a “bioclimatic envelope” [
6] and selected parameters often include sources of uncertainty [
49,
50,
51,
52]. However, advances in species distribution modeling have helped to reduce prediction uncertainties by using robust statistical modelling, which can create forecasts at different scales [
53,
54,
55]. Although these models cannot directly predict species occurrence in the future, we can predict changes in suitability over time using direct connections of occurrence data to habitat and direct habitat alterations linked to climate change. Species dispersal plays a strong role in distributions, which should be included in models to aid identification of future suitable habitat. Predicting suitable habitat is meaningless if the organism is unable to disperse to the location. Therefore, efforts to assess current and projected land use and climate changes are critical to conserve diverse wildlife communities [
56].
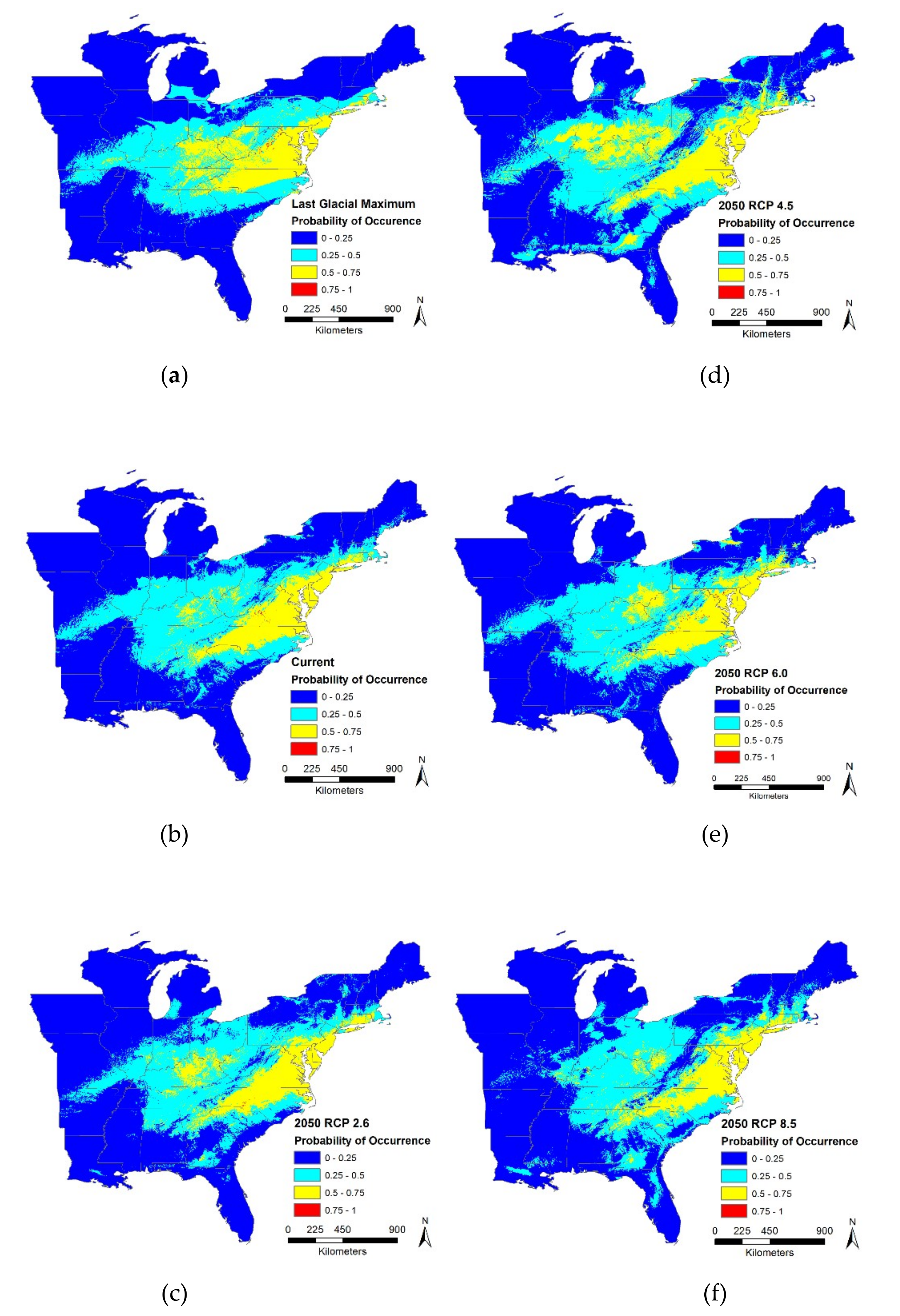
In the present study, (a) we used climate suitability models (CSMs) to estimate impact scenarios on the geographic extent of
T. c. carolina, a relatively narrow range, low dispersal, and vulnerable species, (b) examined changes in suitable climatic habitat between model time periods, and (c) identified dispersal capabilities within a changing climate context. We modeled three time periods (Last Glacial Maximum or LGM, current, and future 2050) using MaxEnt models for habitat suitability. Our study incorporated other studies’ methodologies to build our MaxEnt models and evaluate change over time [
6,
57,
58]; however, our approach to evaluate change over time with current thermal usage and least cost pathways are novel. Our main objectives were to model climatic habitat suitability and changes over time while assessing the main climatic variables influencing suitable habitat regions. We hypothesized that there would be an increase in suitable habitat in the northern portion of the range as a result of increased warming and precipitation. Therefore, we predicted that there would be a northward shift in distribution. We predicted that habitat suitability would increase over time with greater gains for higher greenhouse gas concentration scenarios. We hypothesized that mean temperature of warmest quarter and precipitation of driest quarter would drive
T. c. carolina responses as a result of increased vulnerability to very hot and dry conditions.
4. Discussion
MaxEnt modeling is a common method for modeling future changes in habitat suitability. Our prediction that this species would have expanding climatic habitat northward was supported with both our MaxEnt and alternative distribution models. Other studies have shown northern expansion for several taxa, such as
Ambrosia artemisiifolia [
75], Lepidoterans [
76],
Dolichophis caspius [
6], and several mesopredators:
Lontra canadensis,
Mephitis,
Canis latrans [
77]. For our study, these changes are based on temperature and precipitation, which are important to reptilian movements. Overall, with warming climate, reptiles will face similar problems such as overheating and will require greater fine-scale heterogeneity which includes more shaded areas provided by more vegetation [
78]. Forest canopy cover can provide critical structural complexity in its underlying microclimates [
79] and facilitate fine-scale thermoregulation; however, local movements are limited by other physical barriers such as streams, roads, and human-modified landscapes. Therefore, habitat fragmentation may lead to the isolation of dispersing individuals within habitats that become suboptimal in terms of temperature and precipitation [
80]. Utilizing land surface temperature maps can help identify critical areas of thermal limits for different species and land managers can manage the habitat to increase or decrease temperatures (e.g., manage amount of canopy cover). As with many problems that species face, there will be winners and losers as in Popescu et al. (2013) [
81], which found that reptiles show mixed responses to climate change across species. Our study suggests that
T. c. carolina may benefit from climate change at a landscape scale with increased suitable habitat in the future, assuming that habitat is detectable and accessible, which will be dependent on local scale characteristics.
Our results for LGM, current and future regional models supported mean temperature of driest quarter as the most influential bioclimatic variable driving the model, except for RCP 2.6 scenario where mean temperature of warmest quarter was the most influential. This likely occurred because precipitation patterns may change more drastically at higher temperatures, i.e., RCP 2.6 to RCP 8.5 scenarios. Many climate models for North America have shown an increase in precipitation over time [
82]. This may occur from warmer winter and spring temperatures that lead to more snowmelt and rain-on-snow events that create severe flooding [
83]. We found that greatest change occurred between LGM and current conditions; however, the temporal difference between these two scenarios is much greater than between current and future scenarios. Therefore, we caution making too many conclusions about the change from past to present. Global climate changes are occurring at an unprecedented rate and many of these changes are alterations in temperature and precipitation. Surprisingly, we saw little changes among the four future scenarios. Although our results suggest that
T. c. carolina will have the greatest response to these changes within the driest and warmest quarters of the year, as we predicted based on physiological constraints. We found small percent contributions from precipitation of the wettest and warmest quarters (< 5.7%) across all models. This suggests high vulnerability during the driest and warmest temperatures of the year and declines may be expedited if these quarters become longer or temporally shift. Surprisingly at the local scale, we found an opposite trend for the current model where precipitation of wettest and driest quarters mattered the most and mean temperature was less important. This suggests that currently, temperature is vital for regional suitability, while precipitation matters locally. However, when we examined future models, mean temperature was most important, but varied which quarter mattered the most. At the local scale,
T. c. carolina utilize a wide range of habitats for thermoregulation; however, when their body temperatures are too high, they seek cover in flooded or wet areas [
84], spending days in these areas especially during high temperatures and droughts [
85]. Therefore, precipitation and where water is located during the driest quarter would result in greater movement displacement. Physiologically, this makes sense because water stress exacerbates temperature regulation issues, therefore shaded and floodplain areas will become disproportionately more important during the driest season for these vulnerable species. Other studies have utilized different sets of these bioclimatic predictor variables and found that other variables were most important. For example, the most influential variable for
D. caspius was mean temperature of coldest quarter [
6]; for
Pseudopus apodus it was temperature seasonality and annual precipitation [
86]. For several coral snake species, they were most affected by annual mean temperature, precipitation of wettest month, and precipitation of warmest quarter [
87]. Since each study used a different set of bioclimatic factors, it is difficult to effectively compare variable importance. However, each species will be affected differently based on their ecological requirements, physiology, and location of their range. This underscores the need to translate regional climate change effects to the local scale context.
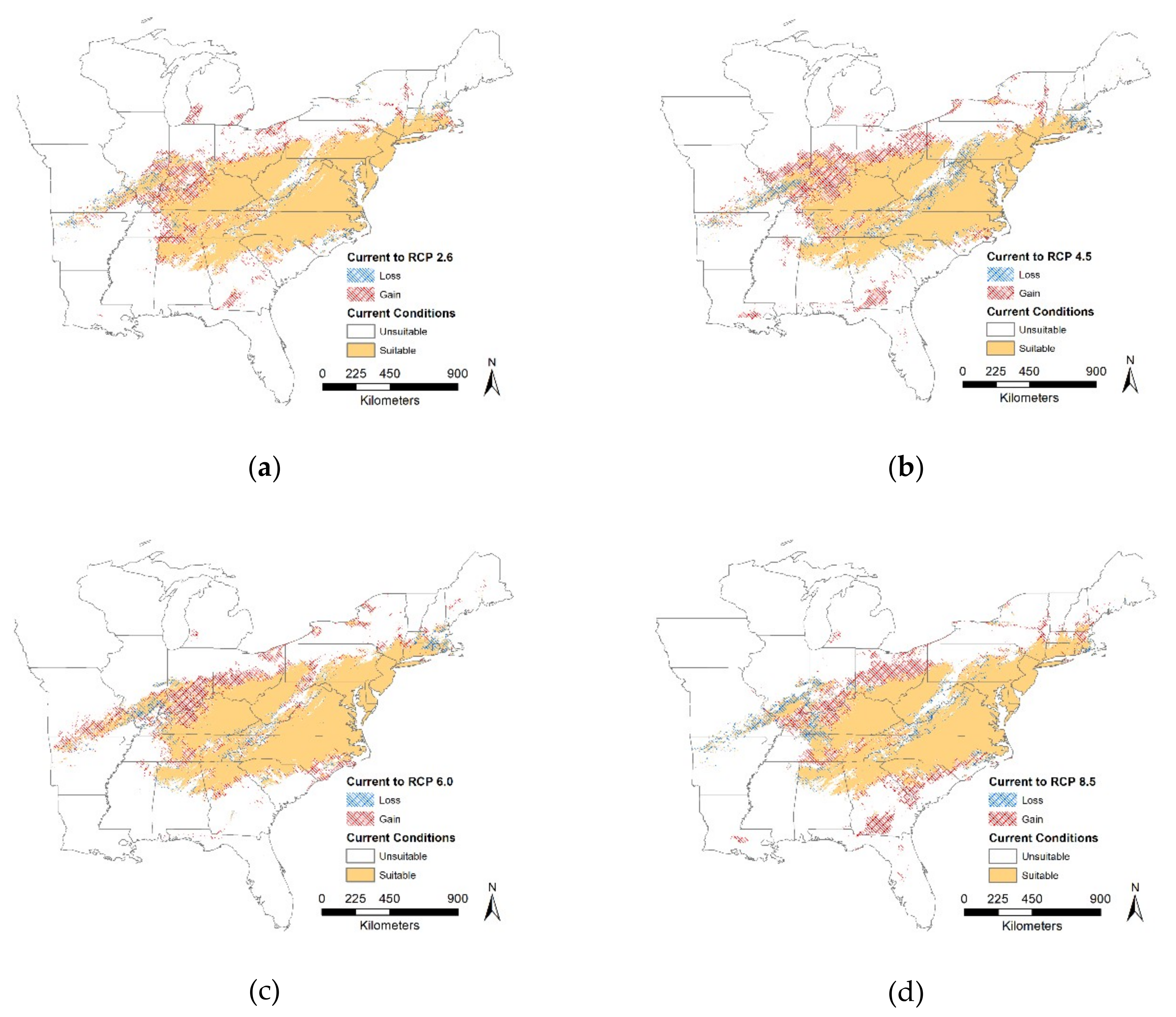
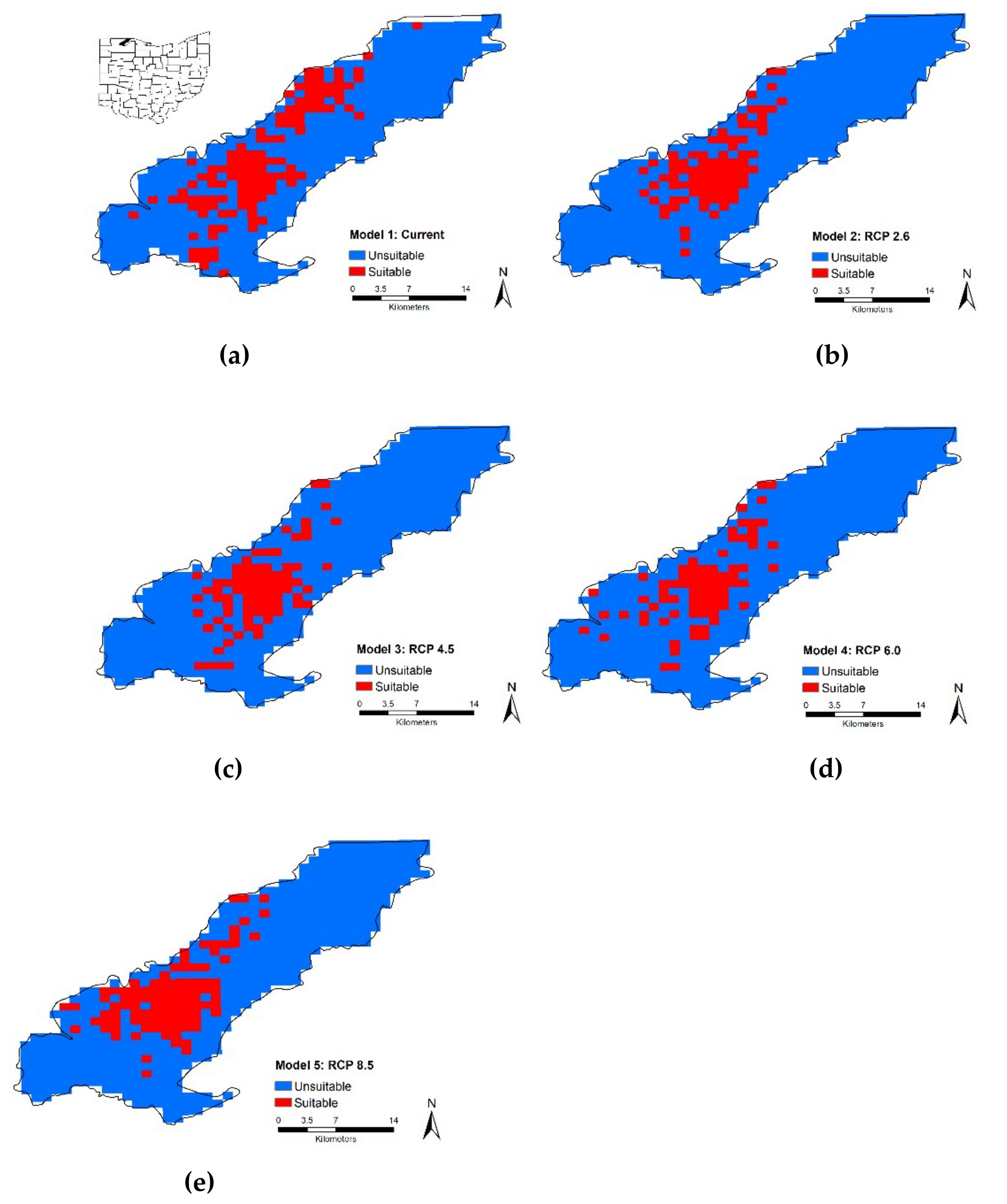
Under current conditions, our MaxEnt model predicted that
T. c. carolina has a concentrated distribution within the middle of the eastern United States which is consistent with known range distributions. We expected that all future scenarios at a regional scale would result in the gain of suitable habitat and our results supported our hypothesis. We found that the proportion of suitable or unsuitable habitat showed moderate changes, i.e., 19% to 27%, it is critical to note, though, that where these changes occur will have large impacts on this species. Most of the changes, both gains and losses, occur on the distribution edge and relatively few changes within the center of the distribution. Although identifying these large-scale changes can help pinpoint critical priority areas for conservation efforts, we need finer-scale data to provide recommendations for on the ground management. In the future, suitable habitat may become inaccessible and isolated, therefore these models should distinguish between suitable and accessible locations [
88]. Identifying these areas, requires downscaling from regional to local scale maps. Therefore, we compared local scale models across current and future scenarios. We found that suitable habitat decreases over time and is limited (i.e., ~15%). For our case study, percentage of forest was the driving factor for all models except Model 2 which suggests that the availability of shade will drive individual responses. Depending on which emission scenario is used will vary which quarter (i.e., wettest, driest, or warmest) will drive turtle responses. Once again highlighting the need for local scale climatic datasets, especially when modeling future suitable habitat. However, in all cases, suitable habitat is limited and fragmented, which makes dispersal challenging. Warming climate may make forest habitat more suitable; however, it will also make open areas less suitable and costly to cross. This suggests that canopy cover is important for dispersal, although NDVI is a good model for canopy cover, it cannot substitute for measuring canopy cover at a fine scale. Our results, though, support the need to incorporate land cover or other remote sensed data with climate factors, as percentage of habitat and NDVI contribute to habitat suitability. We recognize that projecting changes into the future is challenging and deriving models with more models requires a lot of assumptions. Therefore, a mixture of different techniques that address both changes in spatial and temporal scale are needed to tackle this challenge. From a climatic perspective,
T. c. carolina may not need to move, especially at the northern part of the distribution, therefore other biological processes (i.e., increased disturbance, fragmentation, competition, predation) will drive local scale movements.
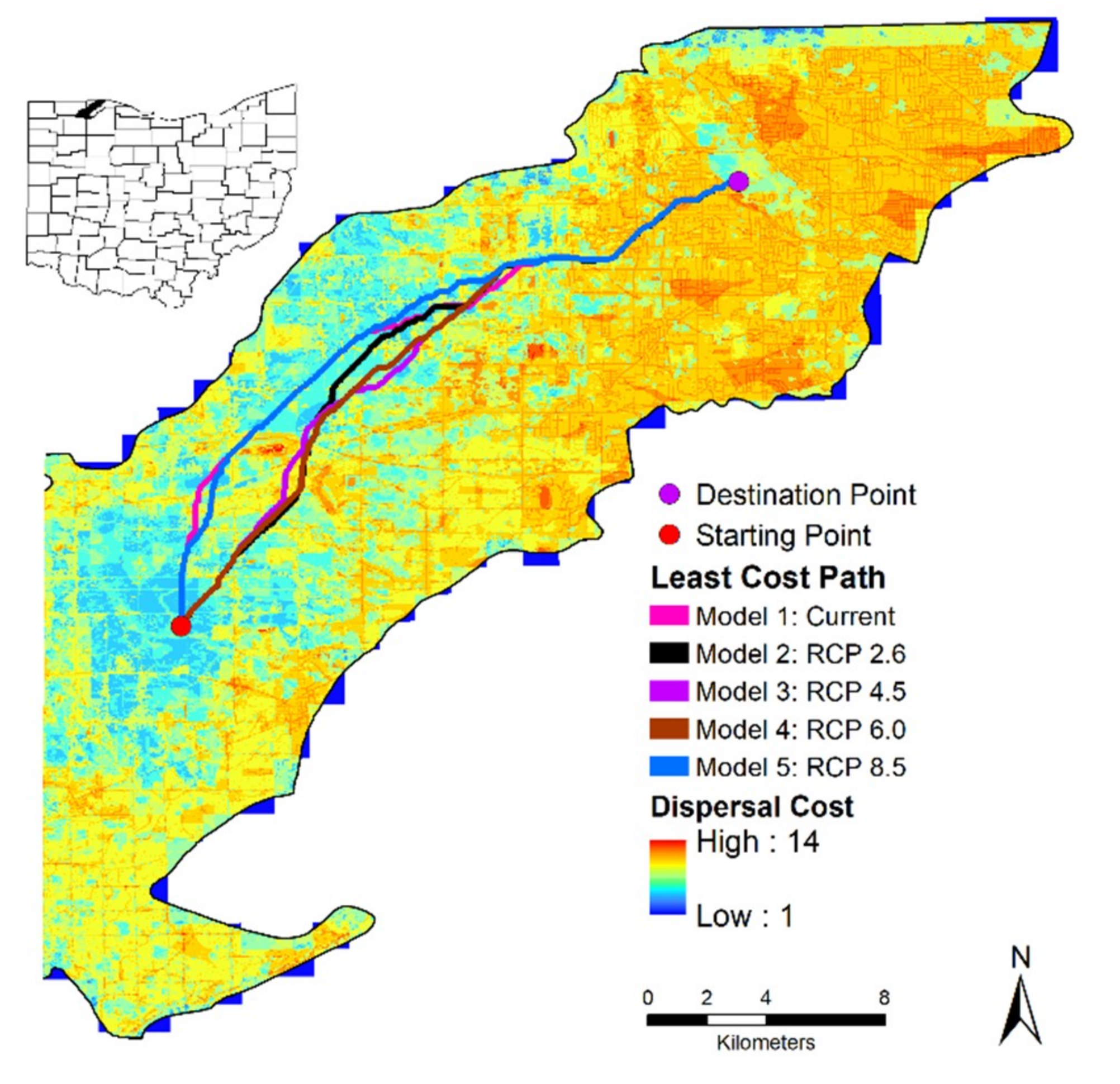
We examined this problem using both our CSMs and finer-scale environmental data in Oak Openings Region to evaluate whether individuals could feasibly disperse despite climatic changes. Based on field data an individual could feasibly disperse to a new area within its lifetime to accommodate a changing landscape. However, this assumes that there are relatively few to no barriers and constant movement each day towards potentially new suitable habitat, as well as an ability to detect these new habitats. For example, in Oak Openings Region, we found that individuals would need to travel a total of 22.95 to 23.84 km to get from one protected area to another (an additional 2 to 3 km from the straight-line distance) using only physical factors on the landscape. It would take individuals approximately 5.5 months to 9.5 years based on our minimum to maximum dispersal scenarios and seasonal activity period to travel this distance. Incorporating climate suitability produced a northern route and different scenarios did not vary much. We did find that the end portion of all models were the same and we suggest that local managers focus on protecting this route for potential dispersal. Although this distance ranges from 23% to 24% of the minimum dispersal distance scenario, it is unlikely that most of the individuals will take the least costly pathway, always following a straight-line path. Individuals would have to carefully traverse a multitude of suboptimal habitat including roads, rivers, crops, and developed areas. Additionally, individuals would have to traverse a variety of thermal gradients that may become too intolerable in the future. Individuals would be able to travel the required distance; however, when considering other structural features, many individuals would likely perish trying to disperse such large distances.
T. c. carolina can persist in developed areas and can traverse croplands and flooded areas; however, they are susceptible to road mortality. Deaths on roads often change the population demographic and cause skewed adult sex ratios [
89]. Additionally, this species is more susceptible to road mortality than other turtle species because they tend to close their shell when threatened and remain closed for longer periods of time [
90]. Increased road traffic will increase the probability of mortality and assuming no further habitat changes, will lead to a threshold effect that causes widespread local extinction [
91]. Although we found that there will be more suitable habitat in the future, it may not do any good with continued habitat fragmentation. We found that this species will face many difficult barriers and potentially have increased mortality when dispersing to future suitable habitat even with expanding suitable habitat, it may not be accessible.
Although
T. c. carolina may not be as heavily impacted by climatic changes in terms of suitable area at the landscape-scale, it is important to provide a model for local scale context.
T. c. carolina will remain under threat from a variety of sources, e.g., habitat destruction, invasive species, environmental pollution, disease, unsustainable use [
5]. Therefore, models should incorporate other biologically relevant variables (e.g., roads, land cover, elevation, streams/lakes, etc.) to represent the synergistic effects of multiple threats. Such a multifaceted approach is critical because the response of local populations will be dependent on regional weather patterns and local structural characteristics. In addition, climate change interacts with the landscape by altering the configuration and composition of land cover. For an example, lakes are sensitive indicators of climate alterations such as fluctuating water levels [
92] and timing changes in ice formation and thawing [
93]. As with many other studies, land-use patterns are often a larger driving factor that influences populations more so than climate alone [
94,
95]. Our climatic habitat models illustrate that climatic change may be beneficial for this species; however, there are local scale challenges when other factors are examined that will affect how the species responds to these changes.