Remote Sensing and Argo Float Observations Reveal Physical Processes Initiating a Winter-Spring Phytoplankton Bloom South of the Kuroshio Current Near Shikoku
Abstract
1. Introduction
2. Materials and Methods
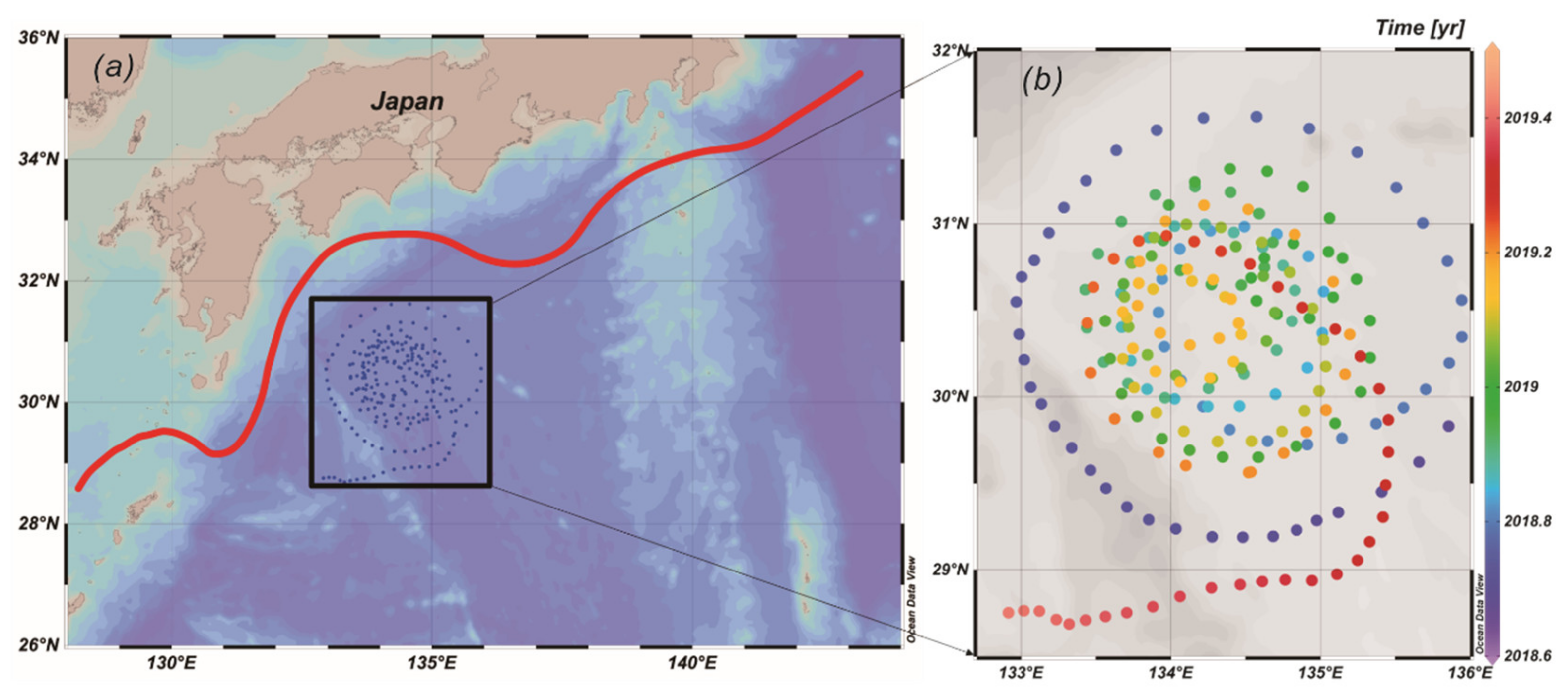
2.1. BIO-Argo Float
2.2. Satellite Observations
2.3. Model Design and Numerical Products
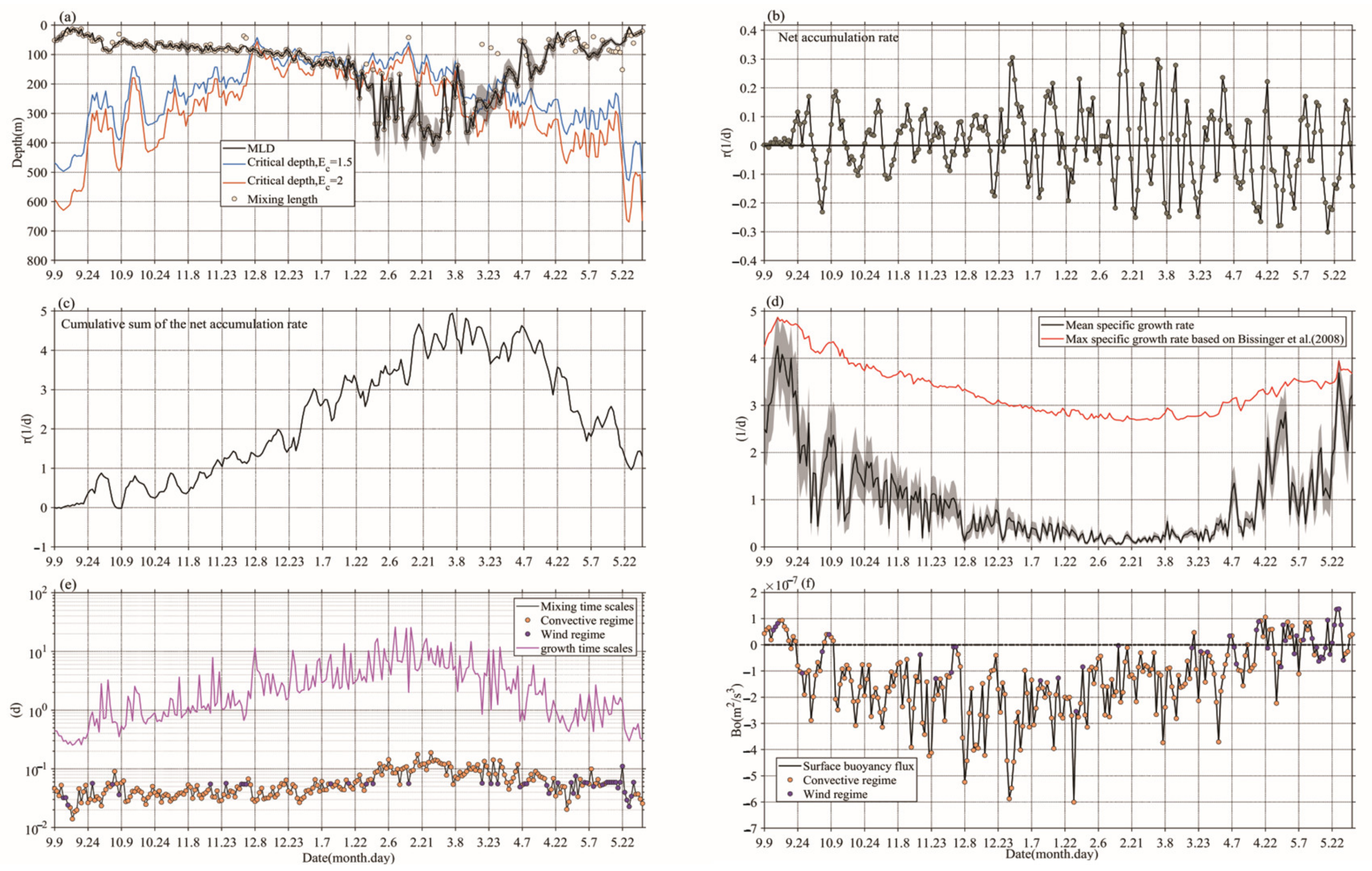
2.4. Mixed Layer Depth
2.5. Turbulence Regimes, Mixing Depth and Mixing Time Scales
2.6. Phytoplankton Specific Growth Rate
2.7. Net Accumulation Rate of Phytoplankton
3. Results
3.1. Distribution of Chl-a Concentration
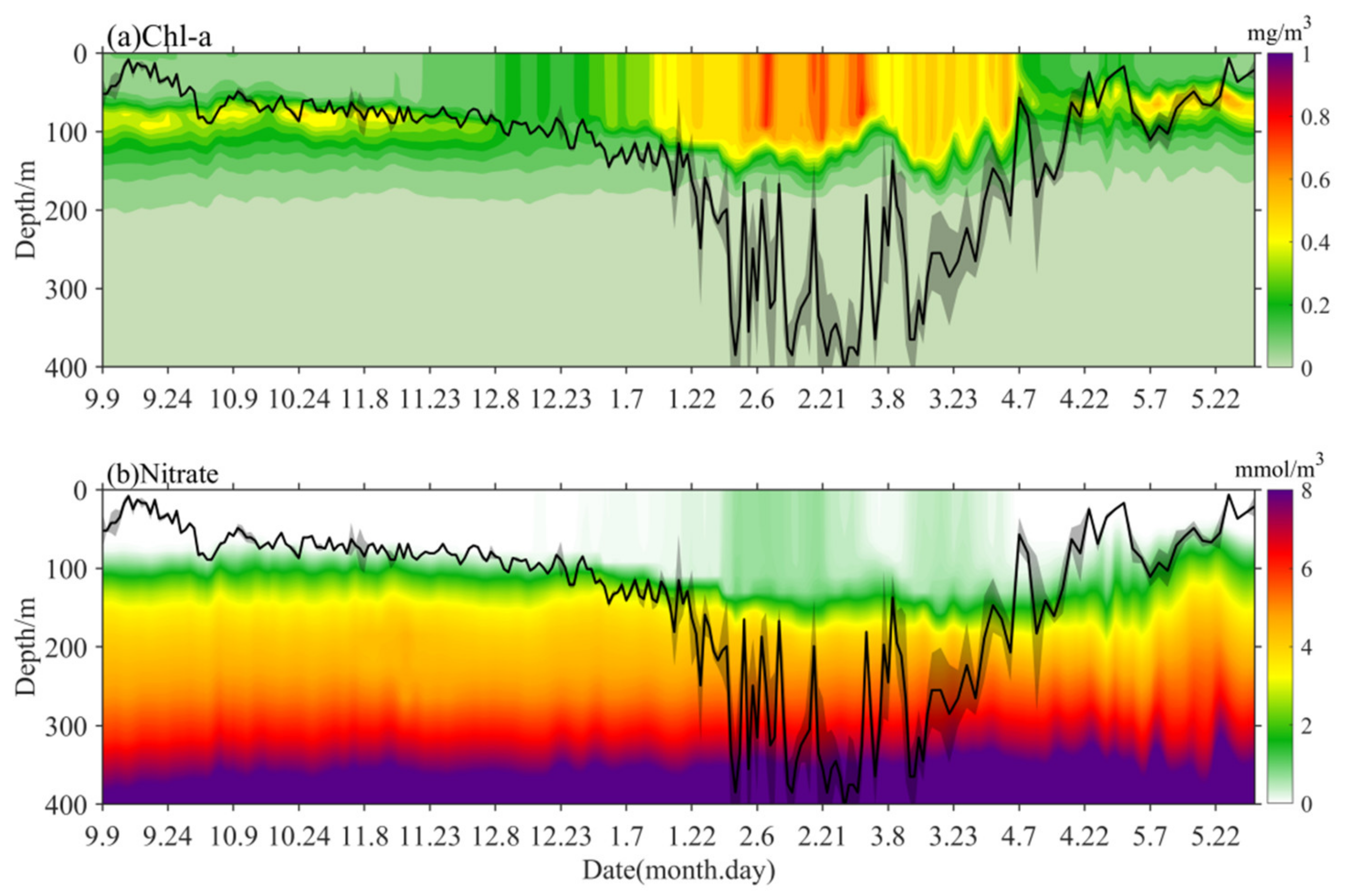
3.2. Distribution of Ocean Conditions during Winter-Spring Blooms
4. Discussion
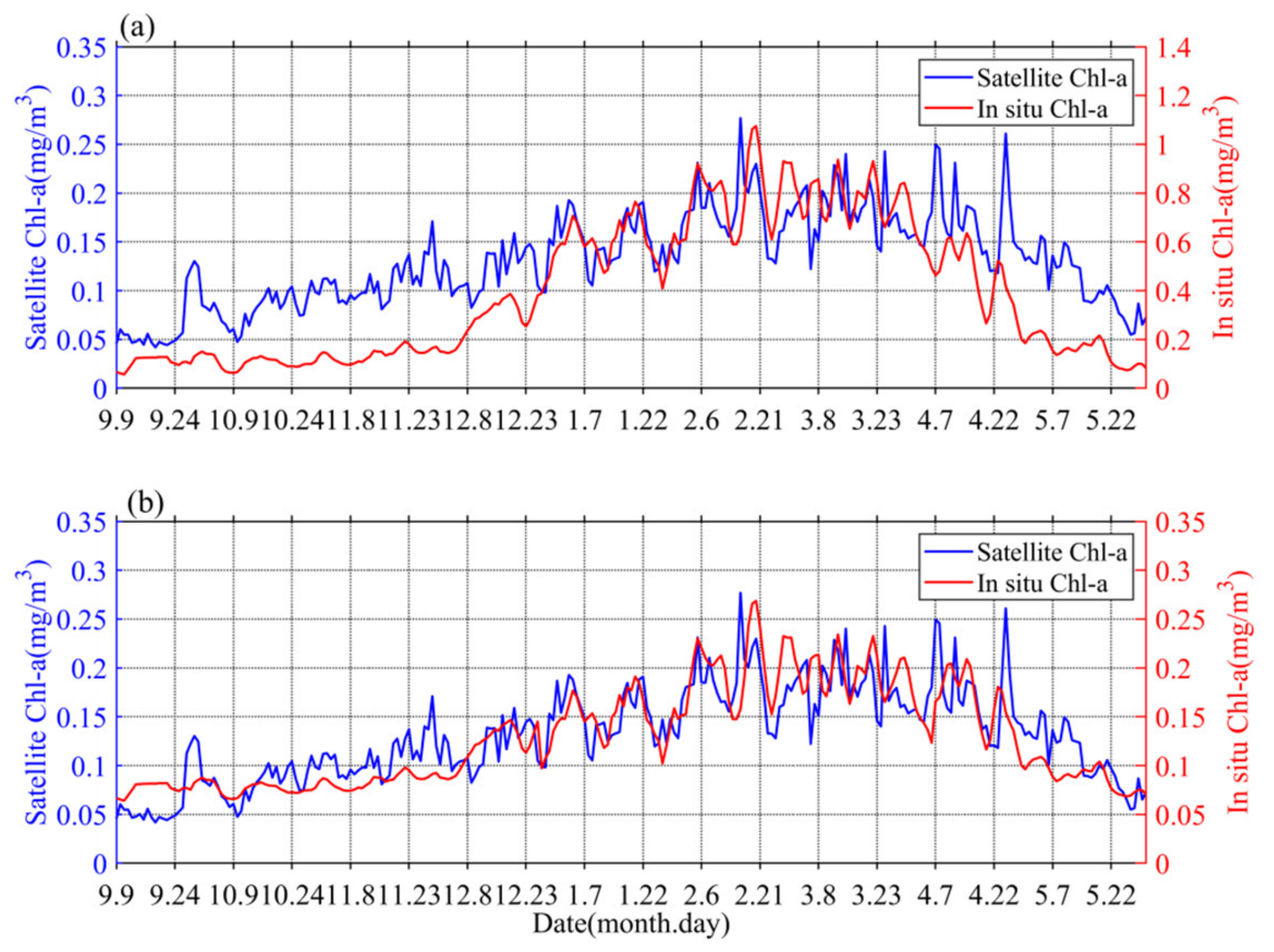
4.1. The Comparison of Different Spatial Scale Data
4.2. The Exclusion of the CDH and CTH for Bloom Initiation
4.3. The Winter-Spring Bloom Development Revealed by DRH
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, Z.; Mao, Z.; Jamet, C.; Linderman, M.A.; Wang, Y.; Chen, X. Seasonal Cycles of Phytoplankton Expressed by Sine Equations Using the Daily Climatology from Satellite-Retrieved Chlorophyll-a Concentration (1997–2019) Over Global Ocean. Remote Sens. 2020, 12, 2662. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E. Beam attenuation and chlorophyll concentration as alternative optical indices of phytoplankton biomass. J. Mar. Res. 2006, 64, 431–451. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.; Siegel, D.A.; Shea, D.M. Carbon-based ocean productivity and phytoplankton physiology from space. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Kodama, T.; Wagawa, T.; Ohshimo, S.; Morimoto, H.; Iguchi, N.; Fukudome, K.-I.; Goto, T.; Takahashi, M.; Yasuda, T. Improvement in recruitment of Japanese sardine with delays of the spring phytoplankton bloom in the Sea of Japan. Fish. Oceanogr. 2018, 27, 289–301. [Google Scholar] [CrossRef]
- Omand, M.M.; D’Asaro, E.A.; Lee, C.M.; Perry, M.J.; Briggs, N.; Cetinić, I.; Mahadevan, A. Eddy-driven subduction exports particulate organic carbon from the spring bloom. Science 2015, 348, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Koeller, P.; Fuentes-Yaco, C.; Platt, T.; Sathyendranath, S.; Richards, A.; Ouellet, P.; Orr, D.; Skúladóttir, U.; Wieland, K.; Savard, L.; et al. Basin-scale coherence in phenology of shrimps and phytoplankton in the North Atlantic Ocean. Science 2009, 324, 791–793. [Google Scholar] [CrossRef]
- Lehahn, Y.; D’Ovidio, F.; Koren, I. A satellite-based Lagrangian view on phytoplankton dynamics. Annu. Rev. Mar. Sci. 2018, 10, 99–119. [Google Scholar] [CrossRef]
- Riley, G. The relationship of vertical turbulence and spring diatom flowering. J. Mar. Res. 1942, 5, 67–87. [Google Scholar]
- Sverdrup, H.U. On conditions for the vernal blooming of phytoplankton. ICES J. Mar. Sci. 1953, 18, 287–295. [Google Scholar] [CrossRef]
- Taylor, J.R.; Ferrari, R. Shutdown of turbulent convection as a new criterion for the onset of spring phytoplankton blooms. Limnol. Oceanogr. 2011, 56, 2293–2307. [Google Scholar] [CrossRef]
- Behrenfeld, M.J. Abandoning Sverdrup’s critical depth hypothesis on phytoplankton blooms. Ecology 2010, 91, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Behrenfeld, M.J.; Boss, E.S. Resurrecting the ecological underpinnings of ocean plankton blooms. Annu. Rev. Mar. Sci. 2014, 6, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Behrenfeld, M.J.; Doney, S.C.; Lima, I.; Boss, E.S.; Siegel, D.A. Annual cycles of ecological disturbance and recovery underlying the subarctic Atlantic spring plankton bloom. Glob. Biogeochem. Cycles 2013, 27, 526–540. [Google Scholar] [CrossRef]
- Goes, J.I.; Gomes, H.D.R.; Limsakul, A.; Balch, W.M.; Saino, T. El Niño related interannual variations in biological production in the North Pacific as evidenced by satellite and ship data. Prog. Oceanogr. 2001, 49, 211–225. [Google Scholar] [CrossRef]
- Limsakul, A.; Saino, T.; Midorikawa, T.; Goes, J.I. Temporal variations in lower trophic level biological environments in the northwestern North Pacific Subtropical Gyre from 1950 to 1997. Prog. Oceanogr. 2001, 49, 129–149. [Google Scholar] [CrossRef]
- Guo, M.; Chai, F.; Xiu, P.; Li, S.; Rao, S. Impacts of mesoscale eddies in the South China Sea on biogeochemical cycles. Ocean Dyn. 2015, 65, 1335–1352. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, X.; Wu, Q.-S.; Huang, D. The Kuroshio nutrient stream and its temporal variation in the East China Sea. J. Geophys. Res. Space Phys. 2012, 117. [Google Scholar] [CrossRef]
- Welschmeyer, N.A. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- Garver, S.A.; Siegel, D.A. Inherent optical property inversion of ocean color spectra and its biogeochemical interpretation: 1. Time series from the Sargasso Sea. J. Geophys. Res. Space Phys. 1997, 102, 18607–18625. [Google Scholar] [CrossRef]
- Maritorena, S.; Siegel, D.A. Consistent merging of satellite ocean color data sets using a bio-optical model. Remote Sens. Environ. 2005, 94, 429–440. [Google Scholar] [CrossRef]
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll-a algorithms for oligotrophic oceans: A novel approach based on three-band reflectance difference. J. Geophys. Res. Space Phys. 2012, 117. [Google Scholar] [CrossRef]
- Antoine, D.; Morel, A. Oceanic primary production: 1. Adaptation of a spectral lightphotosynthesis model in view of application to satellite chlorophyll observations. Glob. Biogeochem. Cycles 1996, 10, 43–55. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Maritorena, S.; Mitchell, B.G.; Siegel, D.A.; Carder, K.L.; Garver, S.A.; Kahru, M.; McClain, C. Ocean color chlorophyll algorithms for SeaWiFS. J. Geophys. Res. Space Phys. 1998, 103, 24937–24953. [Google Scholar] [CrossRef]
- Gohin, F.; Druon, J.N.; Lampert, L. A five channel chlorophyll concentration algorithm applied to SeaWiFS data processed by SeaDAS in coastal waters. Int. J. Remote Sens. 2002, 23, 1639–1661. [Google Scholar] [CrossRef]
- Morel, A.; Huot, Y.; Gentili, B.; Werdell, P.J.; Hooker, S.B.; Franz, B.A. Examining the consistency of products derived from various ocean color sensors in open ocean (Case 1) waters in the perspective of a multi-sensor approach. Remote Sens. Environ. 2007, 111, 69–88. [Google Scholar] [CrossRef]
- Fanton d’Andon, O.H.; Antoine, D.; Mangin, A.; Maritorena, S.; Durand, D.; Pradhan, Y.; Lavender, S.; Morel, A.; Demaria, J.; Barrot, G. Ocean colour sensors characterisation and expected error estimates of ocean colour merged products from GlobColour. In Proceedings of the Ocean Optics, Barga, Italy, 6–10 October 2008. [Google Scholar]
- Garnesson, P.; Mangin, A.; Bretagnon, M. CMEMS-OC-QUID-009-30-32-33-37-81-82-83-85-86-98. 2020. Available online: http://marine.copernicus.eu/documents/QUID/CMEMS-OC-QUID-009-030-032-033-037-081-082-083-085-086-098.pdf (accessed on 24 June 2020).
- Rochford, P.A.; Kara, A.B.; Wallcraft, A.J.; Arnone, R.A. Importance of solar subsurface heating in ocean general circulation models. J. Geophys. Res. Space Phys. 2001, 106, 30923–30938. [Google Scholar] [CrossRef]
- Behringer, D.W.; Xue, Y. Evaluation of the global ocean data assimilation system at NCEP: The Pacific Ocean. In Proceedings of the Eighth Symposium on Integrated Observing and Assimilation Systems for Atmosphere, Oceans, and Land Surface, AMS 84th Annual Meeting, Seattle, WA, USA, 10–15 January 2004. [Google Scholar]
- Damerell, G.M.; Heywood, K.J.; Thompson, A.F.; Binetti, U.; Kaiser, J. The vertical structure of upper ocean variability at the Porcupine Abyssal Plain during 2012–2013. J. Geophys. Res. Oceans 2016, 121, 3075–3089. [Google Scholar] [CrossRef]
- Rumyantseva, A.; Henson, S.A.; Martin, A.; Thompson, A.F.; Damerell, G.M.; Kaiser, J.; Heywood, K.J. Phytoplankton spring bloom initiation: The impact of atmospheric forcing and light in the temperate North Atlantic Ocean. Prog. Oceanogr. 2019, 178, 102202. [Google Scholar] [CrossRef]
- Monin, A.S.; Obukhov, A.M. Basic laws of turbulent mixing in the surface layer of the atmosphere. Contrib. Geophys. Inst. Acad. Sci. USSR 1954, 151, e187. [Google Scholar]
- Thorpe, S.A. The Turbulent Ocean; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Franks, P.J. Has Sverdrup’s critical depth hypothesis been tested? Mixed layers vs. turbulent layers. ICES J. Mar. Sci. 2014, 72, 1897–1907. [Google Scholar] [CrossRef]
- Enriquez, R.M.; Taylor, J.R. Numerical simulations of the competition between wind-driven mixing and surface heating in triggering spring phytoplankton blooms. ICES J. Mar. Sci. 2015, 72, 1926–1941. [Google Scholar] [CrossRef]
- Deardorff, J.W. Numerical investigation of neutral and unstable planetary boundary layers. J. Atmos. Sci. 1972, 29, 91–115. [Google Scholar] [CrossRef]
- Bissinger, J.E.; Montagnes, D.J.S.; Harples, J.; Atkinson, D. Predicting marine phytoplankton maximum growth rates from temperature: Improving on the Eppley curve using quantile regression. Limnol. Oceanogr. 2008, 53, 487–493. [Google Scholar] [CrossRef]
- Nagai, T.; Durán, G.S.; Otero, D.A.; Mori, Y.; Yoshie, N.; Ohgi, K.; Hasegawa, D.; Nishina, A.; Kobari, T. How the Kuroshio Current delivers nutrients to sunlit layers on the continental shelves with aid of near-inertial waves and turbulence. Geophys. Res. Lett. 2019, 46, 6726–6735. [Google Scholar] [CrossRef]
- Evans, G.T.; Parslow, J.S. A model of annual plankton cycles. Biol. Oceanogr. 1985, 3, 327–347. [Google Scholar] [CrossRef]
- Siegel, D.A.; Doney, S.C.; Yoder, J.A. The North Atlantic spring phytoplankton bloom and Sverdrup’s critical depth hypothesis. Science 2002, 296, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Wells, M.L.; Chen, S.; Lin, S.; Chai, F. Enhanced winter carbon export observed by BGC-Argo in the Northwest Pacific Ocean. Geophys. Res. Lett. 2020, 47, e2020GL089847. [Google Scholar] [CrossRef]
- Yoshie, N.; Yamanaka, Y.; Kishi, M.J.; Saito, H. One dimensional ecosystem model simulation of the effects of vertical dilution by the winter mixing on the spring diatom bloom. J. Oceanogr. 2003, 59, 563–571. [Google Scholar] [CrossRef]
- Boss, E.; Behrenfeld, M. In situ evaluation of the initiation of the North Atlantic phytoplankton bloom. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Gaube, P.; Chelton, D.B.; Samelson, R.M.; Schlax, M.G.; O’Neill, L.W. Satellite observations of mesoscale eddy-induced Ekman pumping. J. Phys. Oceanogr. 2015, 45, 104–132. [Google Scholar] [CrossRef]
- McGillicuddy, D.J.J.; Anderson, L.A.; Bates, N.R.; Bibby, T.; Buesseler, K.O.; Carlson, C.A.; Davis, C.S.; Ewart, C.; Falkowski, P.G.; Goldthwait, S.A.; et al. Eddy/wind interactions stimulate extraordinary mid-ocean plankton blooms. Science 2007, 316, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Cassianides, A.; Martinez, E.; Maes, C.; Carton, X.; Gorgues, T. Monitoring the Influence of the Mesoscale Ocean Dynamics on Phytoplanktonic Plumes around the Marquesas Islands Using Multi-Satellite Missions. Remote Sens. 2020, 12, 2520. [Google Scholar] [CrossRef]


Pubsher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Chen, F.; Zhang, S.; Pan, J.; Devlin, A.T.; Ning, H.; Zeng, W. Remote Sensing and Argo Float Observations Reveal Physical Processes Initiating a Winter-Spring Phytoplankton Bloom South of the Kuroshio Current Near Shikoku. Remote Sens. 2020, 12, 4065. https://doi.org/10.3390/rs12244065
Wang T, Chen F, Zhang S, Pan J, Devlin AT, Ning H, Zeng W. Remote Sensing and Argo Float Observations Reveal Physical Processes Initiating a Winter-Spring Phytoplankton Bloom South of the Kuroshio Current Near Shikoku. Remote Sensing. 2020; 12(24):4065. https://doi.org/10.3390/rs12244065
Chicago/Turabian StyleWang, Tongyu, Fajin Chen, Shuwen Zhang, Jiayi Pan, Adam Thomas Devlin, Hao Ning, and Weiqiang Zeng. 2020. "Remote Sensing and Argo Float Observations Reveal Physical Processes Initiating a Winter-Spring Phytoplankton Bloom South of the Kuroshio Current Near Shikoku" Remote Sensing 12, no. 24: 4065. https://doi.org/10.3390/rs12244065
APA StyleWang, T., Chen, F., Zhang, S., Pan, J., Devlin, A. T., Ning, H., & Zeng, W. (2020). Remote Sensing and Argo Float Observations Reveal Physical Processes Initiating a Winter-Spring Phytoplankton Bloom South of the Kuroshio Current Near Shikoku. Remote Sensing, 12(24), 4065. https://doi.org/10.3390/rs12244065





