Comparison of Field and Laboratory Wet Soil Spectra in the Vis-NIR Range for Soil Organic Carbon Prediction in the Absence of Laboratory Dry Measurements
Abstract
1. Introduction
2. Materials and Methods
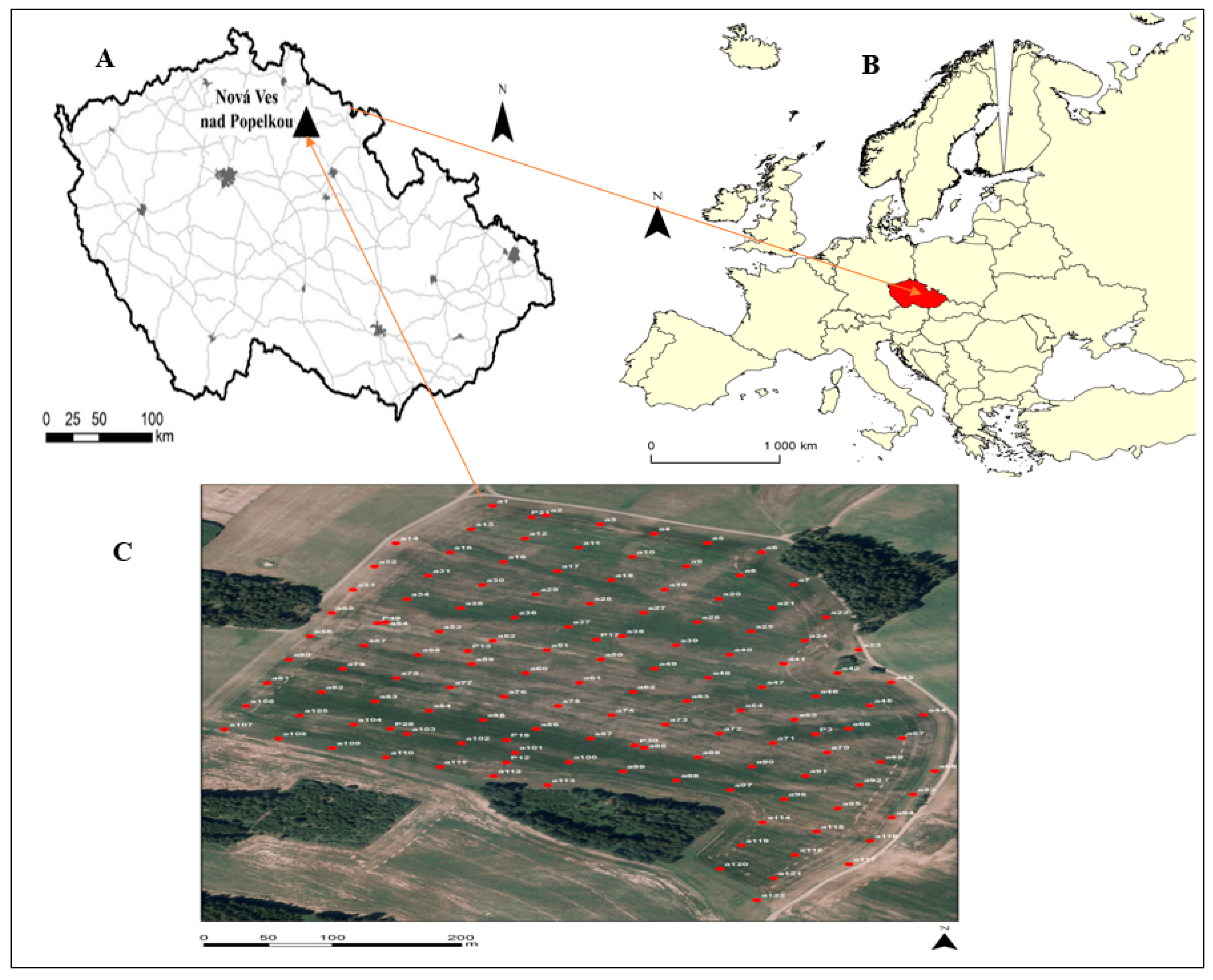
2.1. Study Area
2.2. Soil Sampling and Spectral Measurement
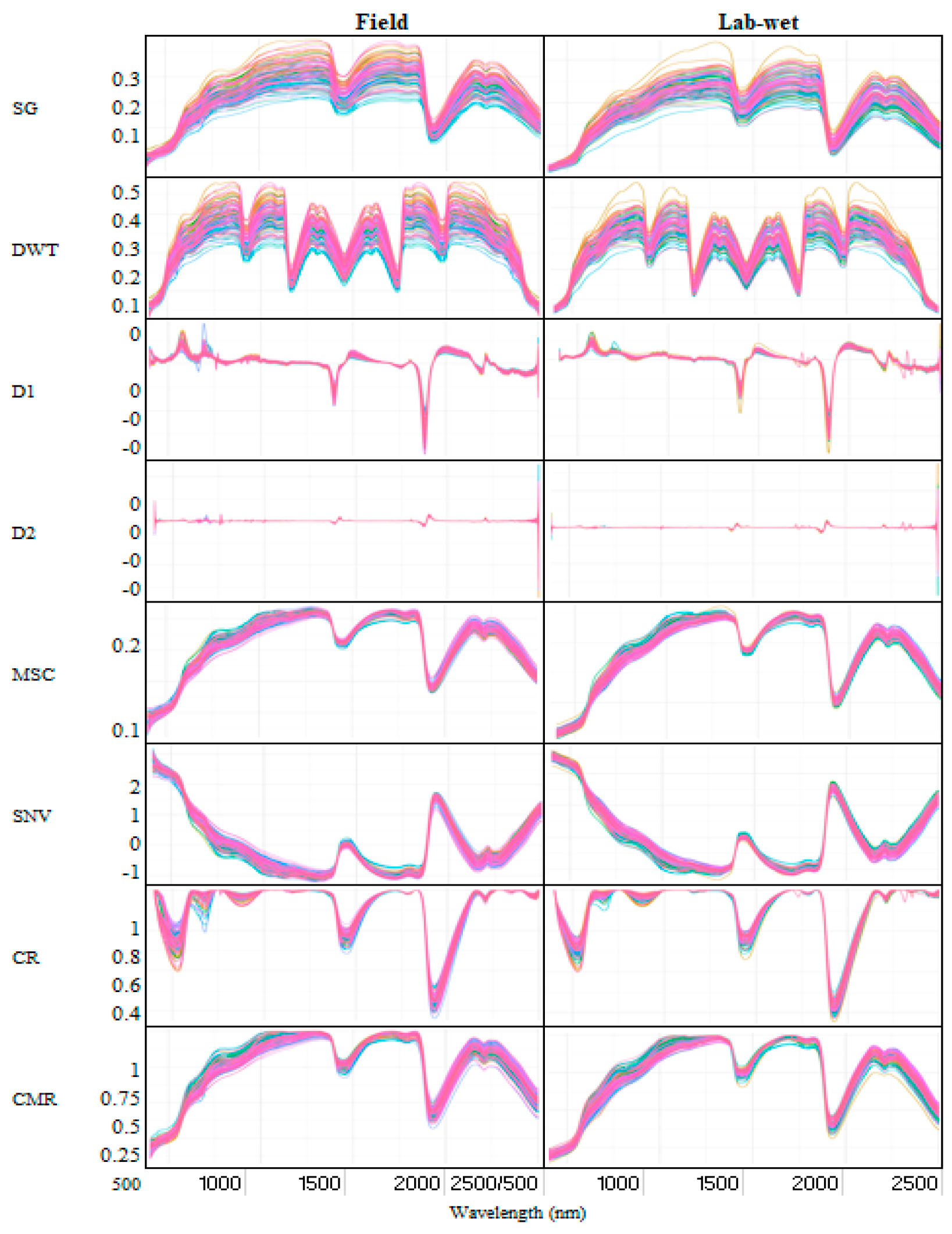
2.3. Spectra Pretreatment and Prediction Model Development
3. Results
3.1. SOC Descriptive Statistics
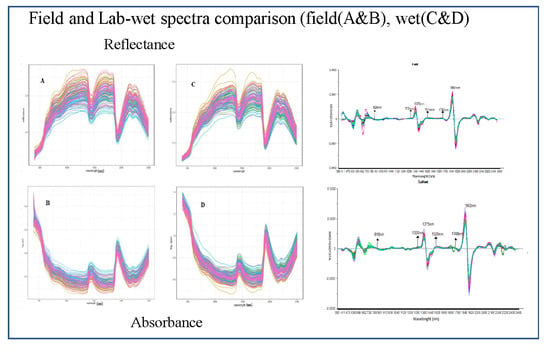
3.2. Basic Comparison of Field And Lab-Wet Spectra
3.3. Detailed Comparison of Field and Lab-Wet Transformed Spectra
3.4. Comparing Field and Lab-Wet Spectra Predictive Capabilities without OSC
3.5. Comparing Field and Lab-Wet Spectra Predictive Capabilities with OSC Approach
4. Discussion
4.1. Comparison of Field and Lab-Wet Spectra
4.2. Spectra Pretreatment and Prediction Models
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scharlemann, J.P.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; De Courcelles, V.D.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Bouma, J.; McBratney, A. Framing soils as an actor when dealing with wicked environmental problems. Geoderma 2013, 200, 130–139. [Google Scholar] [CrossRef]
- Vargas-Rojas, R.; Cuevas-Corona, R.; Yigini, Y.; Tong, Y.; Bazza, Z.; Wiese, L. Unlocking the potential of soil organic carbon: A feasible way forward. In International Yearbook of Soil Law and Policy; Springer: Cham, Switzerland, 2018; pp. 373–395. [Google Scholar]
- Hutengs, C.; Ludwig, B.; Jung, A.; Eisele, A.; Vohland, M. Comparison of portable and bench-top spectrometers for mid-infrared diffuse reflectance measurements of soils. Sensors 2018, 18, 993. [Google Scholar] [CrossRef] [PubMed]
- Nocita, M.; Stevens, A.; Van Wesemael, B.; Aitkenhead, M.; Bachmann, M.; Barthès, B.; Ben Dor, E.; Brown, D.J.; Clairotte, M.; Csorba, A.; et al. Soil Spectroscopy: An alternative to wet chemistry for soil monitoring. Adv. Agron. 2015, 132, 139–159. [Google Scholar]
- Stevens, A.; Nocita, M.; Toth, G.L.; Montanarella, L.; Van Wesemael, B. Prediction of soil organic carbon at the European Scale by sisible and near InfraRed reflectance spectroscopy. PLoS ONE 2013, 8, e66409. [Google Scholar] [CrossRef]
- Vohland, M.; Besold, J.; Hill, J.; Fründ, H.C. Comparing different multivariate calibration methods for the determination of soil organic carbon pools with visible to near infrared spectroscopy. Geoderma 2011, 166, 198–205. [Google Scholar] [CrossRef]
- Xie, H.; Yang, X.M.; Drury, C.F.; Yang, J.Y.; Zhang, X.D. Predicting soil organic carbon and total nitrogen using mid and near-infrared spectra for Brookston clay loam soil in Southwestern Ontario, Canada. Can. J. Soil Sci. 2011, 91, 53–63. [Google Scholar] [CrossRef]
- Ji, W.; Li, S.; Chen, S.; Shi, Z.; Viscarra-Rossel, R.; Mouazen, A.M. Prediction of soil attributes using the Chinese soil spectral library and standardized spectra recorded at field conditions. Soil Tillage Res. 2016, 155, 492–500. [Google Scholar] [CrossRef]
- Udelhoven, T.; Emmerling, C.; Jarmer, T. Quantitative analysis of soil chemical properties with diffuse reflectance spectrometry and partial least-square regression: A feasibility study. Plant Soil 2003, 251, 319–329. [Google Scholar] [CrossRef]
- Stevens, A.; Van Wesemael, B.; Bartholomeus, H.; Rosillon, D.; Tychon, B.; Ben-Dor, E. Laboratory, field and airborne spectroscopy for monitoring organic carbon content in agricultural soils. Geoderma 2008, 144, 395–404. [Google Scholar] [CrossRef]
- Viscarra-Rossel, R.; Behrens, T.; Ben-Dor, E.; Brown, D.; Demattê, J.; Shepherd, K.; Shi, Z.; Stenberg, B.; Stevens, A.; Adamchuk, V.; et al. A global spectral library to characterize the world’s soil. Earth Sci. Rev. 2016, 155, 198–230. [Google Scholar] [CrossRef]
- Christy, C. Real-time measurement of soil attributes using on-the-go near infrared reflectance spectroscopy. Comput. Electron. Agric. 2008, 61, 10–19. [Google Scholar] [CrossRef]
- Nocita, M.; Kooistra, L.; Bachmann, M.; Müller, A.; Powell, M.; Weel, S. Predictions of soil surface and topsoil organic carbon content through the use of laboratory and field spectroscopy in the Albany Thicket Biome of Eastern Cape Province of South Africa. Geoderma 2011, 167, 295–302. [Google Scholar] [CrossRef]
- Stevens, A.; Udelhoven, T.; Denis, A.; Tychon, B.; Lioy, R.; Hoffmann, L.; Van Wesemael, B. Measuring soil organic carbon in croplands at regional scale using airborne imaging spectroscopy. Geoderma 2010, 158, 32–45. [Google Scholar] [CrossRef]
- Sparks, D.L. Soil Physical Chemistry; CRC Press: Boca Raton, FL, USA, 1998 7 July. [Google Scholar]
- Nocita, M.; Stevens, A.; Noon, C.; Van Wesemael, B. Prediction of soil organic carbon for different levels of soil moisture using Vis-NIR spectroscopy. Geoderma 2013, 199, 37–42. [Google Scholar] [CrossRef]
- Wijewardane, N.K.; Ge, Y.; Morgan, C.L.S. Prediction of soil organic and inorganic carbon at different moisture contents with dry ground VNIR: A comparative study of different approaches. Eur. J. Soil Sci. 2016, 67, 605–615. [Google Scholar] [CrossRef]
- Rienzi, E.A.; Mijatovic, B.; Mueller, T.G.; Matocha, C.J.; Sikora, F.J.; Castrignanò, A. Prediction of soil organic carbon under varying moisture levels using reflectance spectroscopy. Soil Sci. Soc. Am. J. 2014, 78, 958–967. [Google Scholar] [CrossRef]
- Barnard, R.L.; Blazewicz, S.J.; Firestone, M.K. Rewetting of soil: Revisiting the origin of soil CO2 emissions. Soil Biol. Biochem. 2020, 147, 107819. [Google Scholar] [CrossRef]
- Bailey, V.; Pries, C.E.H.; Lajtha, K. What do we know about soil carbon destabilization? Environ. Res. Lett. 2019, 14, 083004. [Google Scholar] [CrossRef]
- Birch, H.F. The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 1958, 10, 9–31. [Google Scholar] [CrossRef]
- Wold, S.; Antti, H.; Lindgren, F.; Öhman, J. Orthogonal signal correction of near-infrared spectra. Chemom. Intell. Lab. Syst. 1998, 44, 175–185. [Google Scholar] [CrossRef]
- Liu, S.; Shen, H.; Chen, S.; Zhao, X.; Biswas, A.; Jia, X.; Shi, Z.; Fang, J. Estimating forest soil organic carbon content using vis-NIR spectroscopy: Implications for large-scale soil carbon spectroscopic assessment. Geoderma 2019, 348, 37–44. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Schmidt, K.; Behrens, T.; Friedrich, K.; Scholten, T. A method to generate soilscapes from soil maps. J. Plant Nutr. Soil Sci. 2009, 173, 163–172. [Google Scholar] [CrossRef]
- Shi, T.; Wang, J.; Chen, Y.; Wu, G. Improving the prediction of arsenic contents in agricultural soils by combining the reflectance spectroscopy of soils and rice plants. Int. J. Appl. Earth Obs. Geoinf. 2016, 52, 95–103. [Google Scholar] [CrossRef]
- Murray, I. Aspects of interpretation of NIR spectra. In Analytical Application of Spectroscopy; Creaser, C.S., Davies, A.M.C., Eds.; Royal Society of Chemistry: London, UK, 1988; pp. 9–21. [Google Scholar]
- Breunig, M.M.; Kriegel, H.P.; Ng, R.T.; Sander, J. LOF: Identifying density-based local outliers. In Proceedings of the 2000 ACM SIGMOD International Conference on Management of Data, Dallas, TX, USA, 16 May 2000; pp. 93–104. [Google Scholar]
- Wehrens, R.; Mevik, B.H. The pls package: Principal component and partial least squares regression in R. J. Stat. Softw. 2007, 18. [Google Scholar] [CrossRef]
- Renka, R.J. Algorithm 751: TRIPACK: A constrained two-dimensional Delaunay triangulation package. ACM Trans. Math. Softw. 1996, 22, 1–8. [Google Scholar] [CrossRef]
- Aldrich, E. A package of functions for computing wavelet filters, wavelet transforms and multi-resolution Analyses. 2013. Available online: http://cran.rproject.org/web/packages/wavelets/wavelets.pdf (accessed on 21 September 2012).
- Duckworth, J. Mathematical data pre-processing. Near Infrared Spectrosc. Agric. 2004, 44, 113–132. [Google Scholar]
- Vitorello, I.; Galvão, L.S. Spectral properties of geologic materials in the 400-to 2500 nm range: Review for applications to mineral exploration and lithologic mapping. Photo Interprétat. 1996, 34, 77–99. [Google Scholar]
- Demattê, J.A.; Campos, R.C.; Alves, M.C.; Fiorio, P.R.; Nanni, M.R. Visible–NIR reflectance: A new approach on soil evaluation. Geoderma 2004, 121, 95–112. [Google Scholar] [CrossRef]
- Islam, K.; Singh, B.; McBratney, A. Simultaneous estimation of several soil properties by ultra-violet, visible, and near-infrared reflectance spectroscopy. Soil Res. 2003, 41, 1101–1114. [Google Scholar] [CrossRef]
- Fystro, G. The prediction of C and N content and their potential mineralisation in heterogeneous soil samples using Vis–NIR spectroscopy and comparative methods. Plant Soil 2002, 246, 139–149. [Google Scholar] [CrossRef]
- Madeira Netto, J.S. Spectral reflectance properties of soils. Photo Interprétat. 1996, 34, 59–76. [Google Scholar]
- Howari, F.M.; Goodell, P.C.; Miyamoto, S. Spectral properties of salt crusts formed on saline soils. J. Environ. Qual. 2002, 31, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Minasny, B.; McBratney, A.; Bellon-Maurel, V.; Roger, J.-M.; Gobrecht, A.; Ferrand, L.; Joalland, S. Removing the effect of soil moisture from NIR diffuse reflectance spectra for the prediction of soil organic carbon. Geoderma 2011, 167, 118–124. [Google Scholar] [CrossRef]
- Bogrekci, I.; Lee, W.S. Effects of soil moisture content on absorbance spectra of sandy soils in sensing phosphorus concentrations using UV-VIS-NIR spectroscopy. Trans. ASABE 2006, 49, 1175–1180. [Google Scholar] [CrossRef]
- Mouazen, A.; Karoui, R.; De Baerdemaeker, J.; Ramon, H. Characterization of soil water content using measured visible and near infrared spectra. Soil Sci. Soc. Am. J. 2006, 70, 1295–1302. [Google Scholar] [CrossRef]
- Reeves, J.; Mccarty, G.; Mimmo, T. The potential of diffuse reflectance spectroscopy for the determination of carbon inventories in soils. Environ. Pollut. 2002, 116, S277–S284. [Google Scholar] [CrossRef]
- Wentzell, P.D.; Montoto, L.V. Comparison of principal components regression and partial least squares regression through generic simulations of complex mixtures. Chemom. Intell. Lab. Syst. 2003, 65, 257–279. [Google Scholar] [CrossRef]
- Baumgardner, M.F.; Silva, L.F.; Biehl, L.L.; Stoner, E.R. Reflectance properties of soils. Adv. Agron. 1986, 38, 1–44. [Google Scholar] [CrossRef]
- Viscarra-Rossel, R.; McBratney, A. Laboratory evaluation of a proximal sensing technique for simultaneous measurement of soil clay and water content. Geoderma 1998, 85, 19–39. [Google Scholar] [CrossRef]
- Mouazen, A.; De Baerdemaeker, J.; Ramon, H. Towards development of on-line soil moisture content sensor using a fibre-type NIR spectrophotometer. Soil Tillage Res. 2005, 80, 171–183. [Google Scholar] [CrossRef]
- Clark, C.C.; Clark, L.; Clark, L. “Anting” behavior by common grackles and European starlings. Wilson Bull. 1990, 102, 167–169. [Google Scholar]
- Bishop, C.W. Expansion of Moisture Monitoring Network at the Subsurface Disposal Area of the Radioactive Waste Management Complex; INEL-94/0144; Lockheed Idaho Technologies Company: Idaho Falls, ID, USA, 1994. [Google Scholar]
- Knadel, M.; Deng, F.; Alinejadian, A.; De Jonge, L.W.; Moldrup, P.; Greve, M. The effects of moisture conditions-from wet to hyper dry-on visible near-infrared spectra of Danish reference soils. Soil Sci. Soc. Am. J. 2014, 78, 422–433. [Google Scholar] [CrossRef]
- Haubrock, S.; Chabrillat, S.; Lemmnitz, C.; Kaufmann, H. Surface soil moisture quantification models from reflectance data under field conditions. Int. J. Remote. Sens. 2008, 29, 3–29. [Google Scholar] [CrossRef]
- Dwivedi, D.; Riley, W.; Torn, M.; Spycher, N.; Maggi, F.; Tang, J. Mineral properties, microbes, transport, and plant-input profiles control vertical distribution and age of soil carbon stocks. Soil Biol. Biochem. 2017, 107, 244–259. [Google Scholar] [CrossRef]
- Price, J.C. How unique are spectral signatures? Remote. Sens. Environ. 1994, 49, 181–186. [Google Scholar] [CrossRef]
- Adar, S.; Shkolnisky, Y.; Ben-Dor, E. Change detection of soils under small-scale laboratory conditions using imaging spectroscopy sensors. Geoderma 2014, 216, 19–29. [Google Scholar] [CrossRef]
- Poulin, B.A.; Ryan, J.N.; Aiken, G.R. Effects of iron on optical properties of dissolved organic matter. Environ. Sci. Technol. 2014, 48, 10098–10106. [Google Scholar] [CrossRef]
- Maleki, M.R.; Mouazen, A.; Ramon, H.; De Baerdemaeker, J. Multiplicative scatter correction during on-line measurement with near infrared spectroscopy. Biosyst. Eng. 2007, 96, 427–433. [Google Scholar] [CrossRef]
- Pelliccia, D. Instruments & data tools, two scatter correction techniques for NIR spectroscopy. 2019. Available online: https://www.idtools.com.au/two-scatter-correction-techniques-nir-spectroscopy-python/ (accessed on 21 July 2018).
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Martens, H.; Jensen, S.A.; Geladi, P. Multivariate linearity transformation for near-infrared reflectance spectrometry. In Proceedings of the Nordic Symposium on Applied Statistics, Stavanger, Norway, 12–14 June 1983; Stokkand Forlag Publishers: Stavanger, Norway, 1983; pp. 205–234. [Google Scholar]
- West, J.B.; Bowen, G.J.; Dawson, T.E.; Tu, K.P. Isoscapes: Understanding Movement, Pattern, and Process on Earth Through Isotope Mapping; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Shi, T.; Chen, Y.; Liu, Y.; Wu, G. Visible and near-infrared reflectance spectroscopy—An alternative for monitoring soil contamination by heavy metals. J. Hazard. Mater. 2014, 265, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Morón, A.; Cozzolino, D. Application of near infrared reflectance spectroscopy for the analysis of organic C, total N and pH in soils of Uruguay. J. Near Infrared Spectrosc. 2002, 10, 215–221. [Google Scholar] [CrossRef]
- Mouazen, A.; Maleki, M.; De Baerdemaeker, J.; Ramon, H. On-line measurement of some selected soil properties using a VIS–NIR sensor. Soil Tillage Res. 2007, 93, 13–27. [Google Scholar] [CrossRef]
- Hobbs, J.; Braverman, A.; Cressie, N.; Granat, R.; Gunson, M. Simulation-based uncertainty quantification for estimating atmospheric CO2 from satellite data. SIAM/ASA J. Uncertain. Quantif. 2017, 5, 956–985. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Žižala, D.; Saberioon, M.; Boruvka, L. Soil organic carbon and texture retrieving and mapping using proximal, airborne and sentinel-2 spectral imaging. Remote Sens. Environ. 2018, 218, 89–103. [Google Scholar] [CrossRef]


| Property | Mean | Median | SD | SV | Kurtosis | Skewness | Range | Min | Max | CV(%) |
|---|---|---|---|---|---|---|---|---|---|---|
| SOC content (%) (n = 130) | 1.44 | 1.44 | 0.33 | 0.11 | 2.41 | 0.57 | 2.33 | 0.60 | 2.93 | 23.00 |
| SD: standard deviation, CV: coefficient of variation, n: number of samples, SV: sample variance | ||||||||||
| Pre- | Field_PLSR | |||||
| Treatment | VIS | NIR | VIS-NIR | |||
| Methods | R2cv | RMSEPcv | R2cv | RMSEPcv | R2cv | RMSEPcv |
| Raw | 0.36 | 0.27 | 0.33 | 0.28 | 0.36 | 0.27 |
| SG | 0.35 | 0.27 | 0.33 | 0.28 | 0.35 | 0.27 |
| DWT | 0.36 | 0.27 | 0.33 | 0.28 | 0.35 | 0.27 |
| D1 | 0.36 | 0.27 | 0.3 | 0.28 | 0.3 | 0.28 |
| D2 | 0.21 | 0.3 | 0.17 | 0.31 | 0.19 | 0.3 |
| MSC | 0.42 | 0.26 | 0.27 | 0.29 | 0.3 | 0.28 |
| SNV | 0.42 | 0.26 | 0.26 | 0.29 | 0.28 | 0.29 |
| LOG | 0.42 | 0.26 | 0.36 | 0.27 | 0.4 | 0.26 |
| CR | 0.28 | 0.29 | 0.2 | 0.3 | 0.22 | 0.3 |
| CMR | 0.4 | 0.26 | 0.26 | 0.29 | 0.29 | 0.29 |
| Pre- | Field_SVMR | |||||
| Treatment | VIS | NIR | VIS-NIR | |||
| Methods | R2cv | RMSEPcv | R2cv | RMSEPcv | R2cv | RMSEPcv |
| Raw | 0.35 | 0.27 | 0.27 | 0.29 | 0.3 | 0.29 |
| SG | 0.33 | 0.27 | 0.27 | 0.29 | 0.31 | 0.28 |
| DWT | 0.37 | 0.27 | 0.28 | 0.29 | 0.32 | 0.28 |
| D1 | 0.33 | 0.29 | 0.24 | 0.32 | 0.26 | 0.31 |
| D2 | 0.07 | 0.45 | 0.22 | 0.31 | 0.25 | 0.3 |
| MSC | 0.42 | 0.26 | 0.19 | 0.32 | 0.19 | 0.32 |
| SNV | 0.41 | 0.26 | 0.13 | 0.35 | 0.18 | 0.33 |
| LOG | 0.47 | 0.24 | 0.31 | 0.28 | 0.37 | 0.27 |
| CR | 0.24 | 0.29 | 0.23 | 0.29 | 0.26 | 0.29 |
| CMR | 0.36 | 0.27 | 0.17 | 0.32 | 0.19 | 0.32 |
| Pre- | Lab-wet_PLSR | |||||
| Treatment | VIS | NIR | VIS-NIR | |||
| Methods | R2cv | RMSEPcv | R2cv | RMSEPcv | R2cv | RMSEPcv |
| Raw | 0.32 | 0.28 | 0.24 | 0.29 | 0.26 | 0.29 |
| SG | 0.33 | 0.28 | 0.24 | 0.29 | 0.27 | 0.29 |
| DWT | 0.33 | 0.28 | 0.23 | 0.29 | 0.26 | 0.29 |
| D1 | 0.29 | 0.28 | 0.21 | 0.31 | 0.26 | 0.29 |
| D2 | 0.08 | 0.33 | 0.20 | 0.30 | 0.22 | 0.30 |
| MSC | 0.41 | 0.26 | 0.23 | 0.30 | 0.27 | 0.29 |
| SNV | 0.41 | 0.26 | 0.22 | 0.30 | 0.26 | 0.29 |
| LOG | 0.39 | 0.26 | 0.26 | 0.29 | 0.34 | 0.27 |
| CR | 0.34 | 0.27 | 0.17 | 0.31 | 0.29 | 0.29 |
| CMR | 0.37 | 0.27 | 0.21 | 0.30 | 0.27 | 0.29 |
| Pre- | Lab-wet_SVMR | |||||
| Treatment | VIS | NIR | VIS-NIR | |||
| Methods | R2cv | RMSEPcv | R2cv | RMSEPcv | R2cv | RMSEPcv |
| Raw | 0.33 | 0.28 | 0.31 | 0.28 | 0.39 | 0.26 |
| SG | 0.32 | 0.28 | 0.30 | 0.28 | 0.39 | 0.27 |
| DWT | 0.33 | 0.27 | 0.31 | 0.28 | 0.39 | 0.26 |
| D1 | 0.29 | 0.28 | 0.14 | 0.44 | 0.29 | 0.32 |
| D2 | 0.07 | 0.33 | 0.09 | 0.50 | 0.11 | 0.44 |
| MSC | 0.41 | 0.26 | 0.30 | 0.30 | 0.40 | 0.27 |
| SNV | 0.41 | 0.26 | 0.30 | 0.29 | 0.42 | 0.26 |
| LOG | 0.39 | 0.26 | 0.32 | 0.28 | 0.44 | 0.25 |
| CR | 0.34 | 0.27 | 0.19 | 0.30 | 0.34 | 0.27 |
| CMR | 0.38 | 0.27 | 0.27 | 0.31 | 0.40 | 0.27 |
| Dataset | Modelling Method | VIS | NIR | VIS-NIR | |||
|---|---|---|---|---|---|---|---|
| R2cv | RMSEPcv | R2cv | RMSEPcv | R2cv | RMSEPcv | ||
| Field | PLSR | 0.42 | 0.27 | 0.51 | 0.25 | 0.52 | 0.25 |
| PCR | 0.45 | 0.27 | 0.49 | 0.25 | 0.49 | 0.25 | |
| Lab-wet | PLSR | 0.45 | 0.26 | 0.54 | 0.24 | 0.55 | 0.24 |
| PCR | 0.45 | 0.26 | 0.42 | 0.27 | 0.43 | 0.27 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biney, J.K.M.; Borůvka, L.; Chapman Agyeman, P.; Němeček, K.; Klement, A. Comparison of Field and Laboratory Wet Soil Spectra in the Vis-NIR Range for Soil Organic Carbon Prediction in the Absence of Laboratory Dry Measurements. Remote Sens. 2020, 12, 3082. https://doi.org/10.3390/rs12183082
Biney JKM, Borůvka L, Chapman Agyeman P, Němeček K, Klement A. Comparison of Field and Laboratory Wet Soil Spectra in the Vis-NIR Range for Soil Organic Carbon Prediction in the Absence of Laboratory Dry Measurements. Remote Sensing. 2020; 12(18):3082. https://doi.org/10.3390/rs12183082
Chicago/Turabian StyleBiney, James Kobina Mensah, Luboš Borůvka, Prince Chapman Agyeman, Karel Němeček, and Aleš Klement. 2020. "Comparison of Field and Laboratory Wet Soil Spectra in the Vis-NIR Range for Soil Organic Carbon Prediction in the Absence of Laboratory Dry Measurements" Remote Sensing 12, no. 18: 3082. https://doi.org/10.3390/rs12183082
APA StyleBiney, J. K. M., Borůvka, L., Chapman Agyeman, P., Němeček, K., & Klement, A. (2020). Comparison of Field and Laboratory Wet Soil Spectra in the Vis-NIR Range for Soil Organic Carbon Prediction in the Absence of Laboratory Dry Measurements. Remote Sensing, 12(18), 3082. https://doi.org/10.3390/rs12183082