Thermography as a Tool to Assess Inter-Cultivar Variability in Garlic Performance along Variations of Soil Water Availability
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Weather Conditions
2.2. Plant Material, Experimental Design, and Watering Treatment
2.3. Crop Water Stress Index (CWSI), Bulb Biomass, and Bulb Diameter Measurement
2.4. Statistical Analysis
3. Results and Discussion
3.1. Models’ Verification and Variable Reliability as Bulb Biomass Predictors
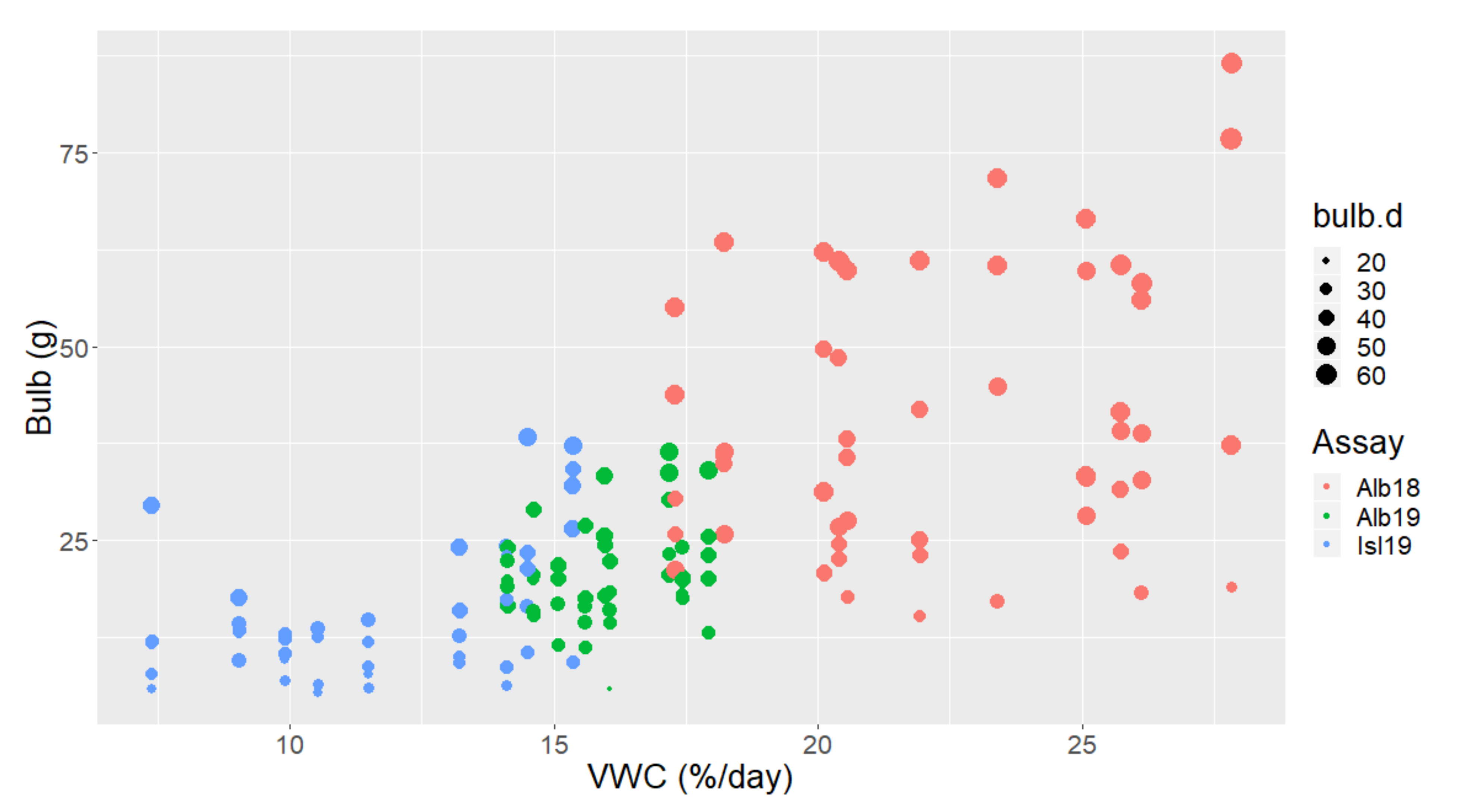
3.2. Inter-Cultivar Variability Analysis on the Sensitivity of Bulb Production to VWCs and CWSI Gradients
3.3. Climatic Conditions and Bulb Biomass Production Cross Experimental Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, W.K. Closing the gap between actual and potential yield of rainfed wheat. The impacts of environment, management and cultivar. Field Crop. Res. 2010, 116, 14–22. [Google Scholar] [CrossRef]
- De Vita, P.; Mastrangelo, A.M.; Matteu, L.; Mazzucotelli, E.; Virzì, N.; Palumbo, M.; Storto, M.L.; Rizza, F.; Cattivelli, L. Genetic improvement effects on yield stability in durum wheat genotypes grown in Italy. Field Crop. Res. 2010, 119, 68–77. [Google Scholar] [CrossRef]
- Del Moral, L.F.G.; Rharrabti, Y.; Villegas, D.; Royo, C. Evaluation of Grain Yield and Its Components in Durum Wheat under Mediterranean Conditions. Agron. J. 2003, 95, 266–274. [Google Scholar] [CrossRef]
- Batisani, N. Climate variability, yield instability and global recession: The multi-stressor to food security in Botswana. Clim. Dev. 2012, 4, 129–140. [Google Scholar] [CrossRef]
- Di Falco, S.; Chavas, J.-P. Crop genetic diversity, farm productivity and the management of environmental risk in rainfed agriculture. Eur. Rev. Agric. Econ. 2006, 33, 289–314. [Google Scholar] [CrossRef]
- Di Falco, S.; Chavas, J.-P. Rainfall Shocks, Resilience, and the Effects of Crop Biodiversity on Agroecosystem Productivity. Land Econ. 2008, 84, 83–96. [Google Scholar] [CrossRef]
- De Young, C.; Soto, D.; Bahri, T.; Brown, D. Building resilience for adaptation to climate change in the fisheries and aquaculture sector. Build. Resil. Adapt. Clim. Chang. Agric. Sect. 2012, 23, 103. [Google Scholar]
- Heinemann, J.A.; Massaro, M.; Coray, D.S.; Agapito-Tenfen, S.Z.; Wen, J.D. Sustainability and innovation in staple crop production in the US Midwest. Int. J. Agric. Sustain. 2013, 12, 71–88. [Google Scholar] [CrossRef]
- Pereira, A. Plant Abiotic Stress Challenges from the Changing Environment. Front. Plant Sci. 2016, 7, 2013–2015. [Google Scholar] [CrossRef]
- Picasso, V.; Casler, M.D.; Undersander, D. Resilience, Stability, and Productivity of Alfalfa Cultivars in Rainfed Regions of North America. Crop. Sci. 2019, 59, 800–810. [Google Scholar] [CrossRef]
- Scheben, A.; Yuan, Y.; Edwards, D. Advances in genomics for adapting crops to climate change. Curr. Plant Boil. 2016, 6, 2–10. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for Yield Potential and Stress Adaptation in Cereals. Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Araus, J.L.; Li, J.-S.; Parry, M.A.J.; Wang, J. Phenotyping and other breeding approaches for a New Green Revolution. J. Integr. Plant Boil. 2014, 56, 422–424. [Google Scholar] [CrossRef]
- Rosenqvist, E.; Großkinsky, D.K.; Ottosen, C.-O.; Van De Zedde, R. The Phenotyping Dilemma—The Challenges of a Diversified Phenotyping Community. Front. Plant Sci. 2019, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating High-Throughput Phenotyping into Genetic Gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Da Silva, J.M.; Pinheiro, C.; Barón, M.; Mylona, P.; Centritto, M.; Haworth, M.; Loreto, F.; Uzilday, B.; Turkan, I.; et al. Opportunities and Limitations of Crop Phenotyping in Southern European Countries. Front. Plant Sci. 2019, 10, 1125. [Google Scholar] [CrossRef]
- FAO. Coping with Water Scarcity: An Action Framework for Agriculture and Food Security; Food and Agriculture Organization: Rome, Italy, 2008. [Google Scholar]
- Deery, D.M.; Rebetzke, G.J.; Jimenez-Berni, J.A.; James, R.A.; Condon, A.G.; Bovill, W.D.; Hutchinson, P.; Scarrow, J.; Davy, R.; Furbank, R.T. Methodology for High-Throughput Field Phenotyping of Canopy Temperature Using Airborne Thermography. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Lin, B. Resilience in Agriculture through Crop Diversification: Adaptive Management for Environmental Change. Bioscience 2011, 61, 183–193. [Google Scholar] [CrossRef]
- Shelef, O.; Weisberg, P.J.; Provenza, F.D. The Value of Native Plants and Local Production in an Era of Global Agriculture. Front. Plant Sci. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Araus, J.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Singh, A.; Ganapathysubramanian, B.; Singh, A.K.; Sarkar, S. Machine Learning for High-Throughput Stress Phenotyping in Plants. Trends Plant Sci. 2016, 21, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant–environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G.; Stoll, M.; Santos, T.; De Sousa, C.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Idso, S.; Jackson, R.; Pinter, P.; Reginato, R.; Hatfield, J. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: A review. J. Exp. Bot. 2012, 63, 4671–4712. [Google Scholar] [CrossRef]
- Biju, S.; Fuentes, S.; Gupta, D. The use of infrared thermal imaging as a non-destructive screening tool for identifying drought-tolerant lentil genotypes. Plant Physiol. Biochem. 2018, 127, 11–24. [Google Scholar] [CrossRef]
- Bellvert, J.; Marsal, J.; Girona, J.; Zarco-Tejada, P.J. Seasonal evolution of crop water stress index in grapevine varieties determined with high-resolution remote sensing thermal imagery. Irrig. Sci. 2014, 33, 81–93. [Google Scholar] [CrossRef]
- Colaizzi, P.D.; Barnes, E.; Clarke, T.R.; Choi, C.Y.; Waller, P. Estimating Soil Moisture Under Low Frequency Surface Irrigation Using Crop Water Stress Index. J. Irrig. Drain. Eng. 2003, 129, 27–35. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Yactayo, W.; Rens, L.R.; Rolando, J.; Palacios, S.; De Mendiburu, F.; Mares, V.; Barreda, C.; Loayza, H.; Monneveux, P.; et al. Defining biological thresholds associated to plant water status for monitoring water restriction effects: Stomatal conductance and photosynthesis recovery as key indicators in potato. Agric. Water Manag. 2016, 177, 369–378. [Google Scholar] [CrossRef]
- FAOSTAT Database; Retrieved 15 February 2020 from FAO; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; Available online: http://faostat3.fao.org/home/E (accessed on 15 February 2020).
- Etoh, T.; Simon, P.W. Diversity, Fertility and Seed Porduction of Garlic. In Allium Crop Science: Recent Advances; Rabinowitch, H., Currah, L., Eds.; CABI Publishing: Wallingford, UK; New York, NY, USA, 2002; pp. 101–117. [Google Scholar]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M. Importance of functional foods in the Mediterranean diet. Public Heal. Nutr. 2006, 9, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Martínez-Romero, A.; Leite, K.N.; Tarjuelo, J.; De Juan, J.; López-Urrea, R. Combination of typical meteorological year with regulated deficit irrigation to improve the profitability of garlic growing in central spain. Agric. Water Manag. 2013, 130, 154–167. [Google Scholar] [CrossRef]
- Cortés, C.F.; de Santa Olalla, F.M.; López-Urrea, R. Production of garlic (Allium sativum L.) under controlled deficit irrigation in a semi-arid climate. Agric. Water Manag. 2003, 59, 155–167. [Google Scholar] [CrossRef]
- Wu, C.; Wang, M.; Cheng, Z.; Meng, H. Response of garlic (Allium sativum L.) bolting and bulbing to temperature and photoperiod treatments. Boil. Open 2016, 5, 507–518. [Google Scholar] [CrossRef]
- Bolle, H.-J. Climate, Climate Variability, and Impacts in the Mediterranean Area: An Overview. In Mediterranean Climate; Bolle, H.-J., Ed.; Springer: Berlin/Heidelberg Germany, 2003; pp. 5–86. [Google Scholar]
- Luterbacher, J.; Xoplaki, E.; Casty, C.; Wanner, H.; Pauling, A.; Küttel, M.; Rutishauser, T.; Brönnimann, S.; Fischer, E.; Fleitmann, D.; et al. Chapter 1 Mediterranean climate variability over the last centuries: A review. In The Mediterranean Climate: An Overview of the Main Characteristics and Issues; Lionello, P., Malanotte-Rizzoli, P., Boscolo, R., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2006; Volume 4, pp. 27–148. [Google Scholar]
- Fader, M.; Shi, S.; Von Bloh, W.; Bondeau, A.; Cramer, W. Mediterranean irrigation under climate change: More efficient irrigation needed to compensate for increases in irrigation water requirements. Hydrol. Earth Syst. Sci. 2016, 20, 953–973. [Google Scholar] [CrossRef]
- Malek, Ž.; Verburg, P.H. Adaptation of land management in the Mediterranean under scenarios of irrigation water use and availability. Mitig. Adapt. Strat. Glob. Chang. 2017, 23, 821–837. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Shafir, I.L.; Zemah, H.; Barzilay, A.; Rabinowitch, H. Environmental Control of Garlic Growth and Florogenesis. J. Am. Soc. Hortic. Sci. 2004, 129, 144–151. [Google Scholar] [CrossRef]
- Zheng, S.; Kamenetsky, R.; Féréol, L.; Barandiaran, X.; Rabinowitch, H.; Chovelon, V.; Kik, C. Garlic breeding system innovations. Med. Aromat. Plant Sci. Biotechnol. 2007, 1, 6–15. [Google Scholar]
- Egea, L.A.; Mérida-García, R.; Kilian, A.; Hernández, P.; Dorado, G. Assessment of Genetic Diversity and Structure of Large Garlic (Allium sativum) Germplasm Bank, by Diversity Arrays Technology “Genotyping-by-Sequencing” Platform (DArTseq). Front. Genet. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Barboza, K.; Salinas, M.C.; Acuña, C.V.; Bannoud, F.; Beretta, V.; García-Lampasona, S.; Burba, J.L.; Galmarini, C.R.; Cavagnaro, P. Assessment of genetic diversity and population structure in a garlic (Allium sativum L.) germplasm collection varying in bulb content of pyruvate, phenolics, and solids. Sci. Hortic. 2020, 261, 108900. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of Garlic Cultivars for Polyphenolic Content and Antioxidant Properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Shen, D.; Oiu, Y.; Song, J.-P. Diversity evaluation of morphological traits and allicin content in garlic (Allium sativum L.) from China. Euphytica 2014, 198, 243–254. [Google Scholar] [CrossRef]
- Hsiao, J.; Yun, K.; Moon, K.H.; Kim, S.-H. A process-based model for leaf development and growth in hardneck garlic (Allium sativum). Ann. Bot. 2019, 124, 1143–1160. [Google Scholar] [CrossRef]
- Nackley, L.L.; Jeong, J.H.; Oki, L.R.; Kim, S.-H. Photosynthetic Acclimation, Biomass Allocation, and Water Use Efficiency of Garlic in Response to Carbon Dioxide Enrichment and Nitrogen Fertilization. J. Am. Soc. Hortic. Sci. 2016, 141, 373–380. [Google Scholar] [CrossRef]
- Badran, A.E. Comparative Analysis of Some Garlic Varieties under Drought Stress Conditions. J. Agric. Sci. 2015, 7, 271. [Google Scholar] [CrossRef][Green Version]
- Statista. Available online: https://www.statista.com/statistics/803595/global-demand-for-natural-organic-environmental-friendly-cosmetics/ (accessed on 24 July 2020).
- Sánchez-Virosta, A.; Sánchez-Gómez, D. Inter-cultivar variability in the functional and biomass response of garlic (Allium sativum L.) to water availability. Sci. Hortic. 2019, 252, 243–251. [Google Scholar] [CrossRef]
- Jones, H.G. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ. 1999, 22, 1043–1055. [Google Scholar] [CrossRef]
- Lopez-Bellido, F.; Muñoz-Romero, V.; Fernandez-Garcia, P.; Lopez-Bellido, L.; Lopez-Bellido, R. New phenological growth stages of garlic (Allium sativum). Ann. Appl. Boil. 2016, 169, 423–439. [Google Scholar] [CrossRef]
- Gençoğlan, C.; Gençoğlan, S. Determination relationship between crop water stress index (CWSI) and yield of Comice pear (Pyrus communis L.). Mediterr. Agric. Sci. 2018, 31, 275–281. [Google Scholar] [CrossRef][Green Version]
- Helman, D.; Lensky, I.M.; Bonfil, D.J. Early prediction of wheat grain yield production from root-zone soil water content at heading using Crop RS-Met. Field Crop. Res. 2019, 232, 11–23. [Google Scholar] [CrossRef]
- Kirnak, H.; Irik, H.; Unlukara, A. Potential use of crop water stress index (CWSI) in irrigation scheduling of drip-irrigated seed pumpkin plants with different irrigation levels. Sci. Hortic. 2019, 256, 108608. [Google Scholar] [CrossRef]
- Schillinger, W.F.; Schofstoll, S.E.; Alldredge, J.R. Available water and wheat grain yield relations in a Mediterranean climate. Field Crop. Res. 2008, 109, 45–49. [Google Scholar] [CrossRef]
- Sánchez-Virosta, A.; Léllis, B.; Pardo, J.; Martínez-Romero, A.; Sánchez-Gómez, D.; Domínguez, A. Functional response of garlic to optimized regulated deficit irrigation (ORDI) across crop stages and years: Is physiological performance impaired at the most sensitive stages to water deficit? Agric. Water Manag. 2020, 228, 105886. [Google Scholar] [CrossRef]
- Albert, C.H.; Grassein, F.; Schurr, F.M.; Vieilledent, G.; Violle, C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 2011, 13, 217–225. [Google Scholar] [CrossRef]
- Ali, H.; Reineking, B.; Münkemüller, T. Effects of plant functional traits on soil stability: Intraspecific variability matters. Plant Soil 2016, 411, 359–375. [Google Scholar] [CrossRef]
- Irujo, G.A.P.; Izquierdo, N.G.; Covi, M.; Nolasco, S.M.; Quiroz, F.; Aguirrezábal, L.A.N. Variability in sunflower oil quality for biodiesel production: A simulation study. Biomass Bioenergy 2009, 33, 459–468. [Google Scholar] [CrossRef]
- Crespo-Herrera, L.A.; Crossa, J.; Huerta-Espino, J.; Autrique, E.; Mondal, S.; Velu, G.; Vargas, M.; Braun, H.J.; Singh, R.P. Genetic Yield Gains in CIMMYT’s International Elite Spring Wheat Yield Trials By Modeling The Genotype × Environment Interaction. Crop. Sci. 2017, 57, 789–801. [Google Scholar] [CrossRef]
- Padhi, J.; Misra, R.K.; Payero, J. Use of infrared thermography to detect water deficit response in an irrigated cotton crop.. 2009, 1–10. In Proceedings of the International Conference on Food Security and Environmental Sustainability (FSES 2009), Kharagpur, India, 17–19 December 2009; pp. 1–10. [Google Scholar]
- Taghvaeian, S.; Chávez, J.L.; Hansen, N.C. Infrared Thermometry to Estimate Crop Water Stress Index and Water Use of Irrigated Maize in Northeastern Colorado. Remote Sens. 2012, 4, 3619–3637. [Google Scholar] [CrossRef]
- Bellvert, J.; Zarco-Tejada, P.J.; Girona, J.; Fereres, E. Mapping crop water stress index in a ‘Pinot-noir’ vineyard: Comparing ground measurements with thermal remote sensing imagery from an unmanned aerial vehicle. Precis. Agric. 2013, 15, 361–376. [Google Scholar] [CrossRef]
- Grant, O.M.; Ochagavia, H.; Baluja, J.; Santamaria, M.P.D.; Tardáguila, J. Thermal imaging to detect spatial and temporal variation in the water status of grapevine (Vitis vinifera L.). J. Hortic. Sci. Biotechnol. 2016, 91, 43–54. [Google Scholar] [CrossRef]
- Testi, L.; Goldhamer, D.A.; Iniesta, F.; Salinas, M. Crop water stress index is a sensitive water stress indicator in pistachio trees. Irrig. Sci. 2008, 26, 395–405. [Google Scholar] [CrossRef]
- Beloni, T.; Santos, P.M.; Rovadoscki, G.A.; Balachowski, J.; Volaire, F. Large variability in drought survival among Urochloa spp. cultivars. Grass Forage Sci. 2018, 73, 947–957. [Google Scholar] [CrossRef]
- Bota, J.; Flexas, J.; Medrano, H. Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Ann. Appl. Boil. 2001, 138, 353–361. [Google Scholar] [CrossRef]
- Shrestha, R.; Turner, N.C.; Siddique, K.H.M.; Turner, D.W. Physiological and seed yield responses to water deficits among lentil genotypes from diverse origins. Aust. J. Agric. Res. 2006, 57, 903–915. [Google Scholar] [CrossRef]
- Agam, N.; Cohen, Y.; Alchanatis, V.; Ben-Gal, A. How sensitive is the CWSI to changes in solar radiation? Int. J. Remote Sens. 2013, 34, 6109–6120. [Google Scholar] [CrossRef]
- McCann, I.R.; Stark, J.C.; King, B.A. Evaluation and interpretation of the crop water stress index for well-watered potatoes. Am. J. Potato Res. 1992, 69, 831–841. [Google Scholar] [CrossRef]
- Ortiz-Bustos, C.M.; Pérez-Bueno, M.L.; Barón, M.; Molinero-Ruiz, L. Use of Blue-Green Fluorescence and Thermal Imaging in the Early Detection of Sunflower Infection by the Root Parasitic Weed Orobanche cumana Wallr. Front. Plant Sci. 2017, 8, 833. [Google Scholar] [CrossRef]
- Sezen, S.M.; Yazar, A.; Daşgan, Y.; Yücel, S.; Akyildiz, A.; Tekin, S.; Akhoundnejad, Y.; Akyıldız, A. Evaluation of crop water stress index (CWSI) for red pepper with drip and furrow irrigation under varying irrigation regimes. Agric. Water Manag. 2014, 143, 59–70. [Google Scholar] [CrossRef]
- Erdem, Y.; Arin, L.; Erdem, T.; Polat, S.; Devecí, M.; Okursoy, H.; Gültaş, H.T. Crop water stress index for assessing irrigation scheduling of drip irrigated broccoli (Brassica oleracea L. var. italica). Agric. Water Manag. 2010, 98, 148–156. [Google Scholar] [CrossRef]
- Sánchez-Virosta, A.; Sadras, V.O.; Sánchez-Gómez, D. Inter-cultivar and inter-year variation of functional traits and phenotypic plasticity in response to water availability in garlic. Sci. Hortic. under review.
- Campbell, B.T.; Jones, M.A. Assessment of genotype × environment interactions for yield and fiber quality in cotton performance trials. Euphytica 2005, 144, 69–78. [Google Scholar] [CrossRef]
- Sadras, V.; Lake, L.; Leonforte, A.; McMurray, L.; Paull, J. Screening field pea for adaptation to water and heat stress: Associations between yield, crop growth rate and seed abortion. Field Crop. Res. 2013, 150, 63–73. [Google Scholar] [CrossRef]
- Bänziger, M.; Cooper, M. Breeding for low input conditions and consequences for participatory plant breeding examples from tropical maize and wheat. Euphytica 2001, 122, 503–519. [Google Scholar] [CrossRef]
- Kovar, M.; Černý, I.; Ernst, D. Analysis of relations between crop temperature indices and yield of different sunflower hybrids foliar treated by biopreparations. Agriculture 2016, 62, 28–40. [Google Scholar] [CrossRef][Green Version]
- Zia, S.; Romano, G.; Spreer, W.; Sanchez, C.; Cairns, J.E.; Araus, J.L.; Müller, J. Infrared Thermal Imaging as a Rapid Tool for Identifying Water-Stress Tolerant Maize Genotypes of Different Phenology. J. Agron. Crop. Sci. 2012, 199, 75–84. [Google Scholar] [CrossRef]
- Calderini, D.F.; Slafer, G.A. Has yield stability changed with genetic improvement of wheat yield? Euphytica 1999, 107, 51–59. [Google Scholar] [CrossRef]
- Paul, K.; Pauk, J.; Deák, Z.; Sass, L.; Vass, I. Contrasting response of biomass and grain yield to severe drought in Cappelle Desprez and Plainsman V wheat cultivars. PeerJ 2016, 4, e1708. [Google Scholar] [CrossRef][Green Version]
- Lopes, M.S.; Araus, J.L.; Van Heerden, P.D.R.; Foyer, C.H. Enhancing drought tolerance in C4 crops. J. Exp. Bot. 2011, 62, 3135–3153. [Google Scholar] [CrossRef]
- European Commission—DG JRC. Crop Monitoring in Europe—26 August 2019; Mars Bulletin Volume 27 No. 8; Bulletins & Publications/MARS Unit—MARS 2019; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission—DG JRC. Mars Crop Monitoring in Europe—27 August 2018; Bulletin Volume 26 No. 8; Bulletins & Publications/MARS Unit—MARS 2018; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Campoy, J.; Campos, I.; Plaza, C.; Calera, M.; Jiménez, N.; Bodas, V.; Calera, A. Water use efficiency and light use efficiency in garlic using a remote sensing-based approach. Agric. Water Manag. 2019, 219, 40–48. [Google Scholar] [CrossRef]
- Tchórzewska, D.; Bocianowski, J.; Najda, A.; Dabrowska, A.; Winiarczyk, K. Effect of environment fluctuations on biomass and allicin level in Allium sativum (cv. Harnas, Arkus) and Allium ampeloprasum var. ampeloprasum (GHG-L). J. Appl. Bot. Food Qual. 2017, 90, 106–114. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jeong, J.H.; Nackley, L.L. Photosynthetic and Transpiration Responses to Light, CO2, Temperature, and Leaf Senescence in Garlic: Analysis and Modeling. J. Am. Soc. Hortic. Sci. 2013, 138, 149–156. [Google Scholar] [CrossRef]
- Lafitte, R.; Courtois, B. Interpreting Cultivar × Environment Interactions for Yield in Upland Rice. Crop. Sci. 2002, 42, 1409–1420. [Google Scholar] [CrossRef]
- Pennypacker, B.W.; Risius, M.L. Environmental Sensitivity of Soybean Cultivar Response to Sclerotinia sclerotiorum. Phytopathology 1999, 89, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Subira, J.; Alvaro, F.; Del Moral, L.F.G.; Royo, C. Breeding effects on the cultivar × environment interaction of durum wheat yield. Eur. J. Agron. 2015, 68, 78–88. [Google Scholar] [CrossRef]
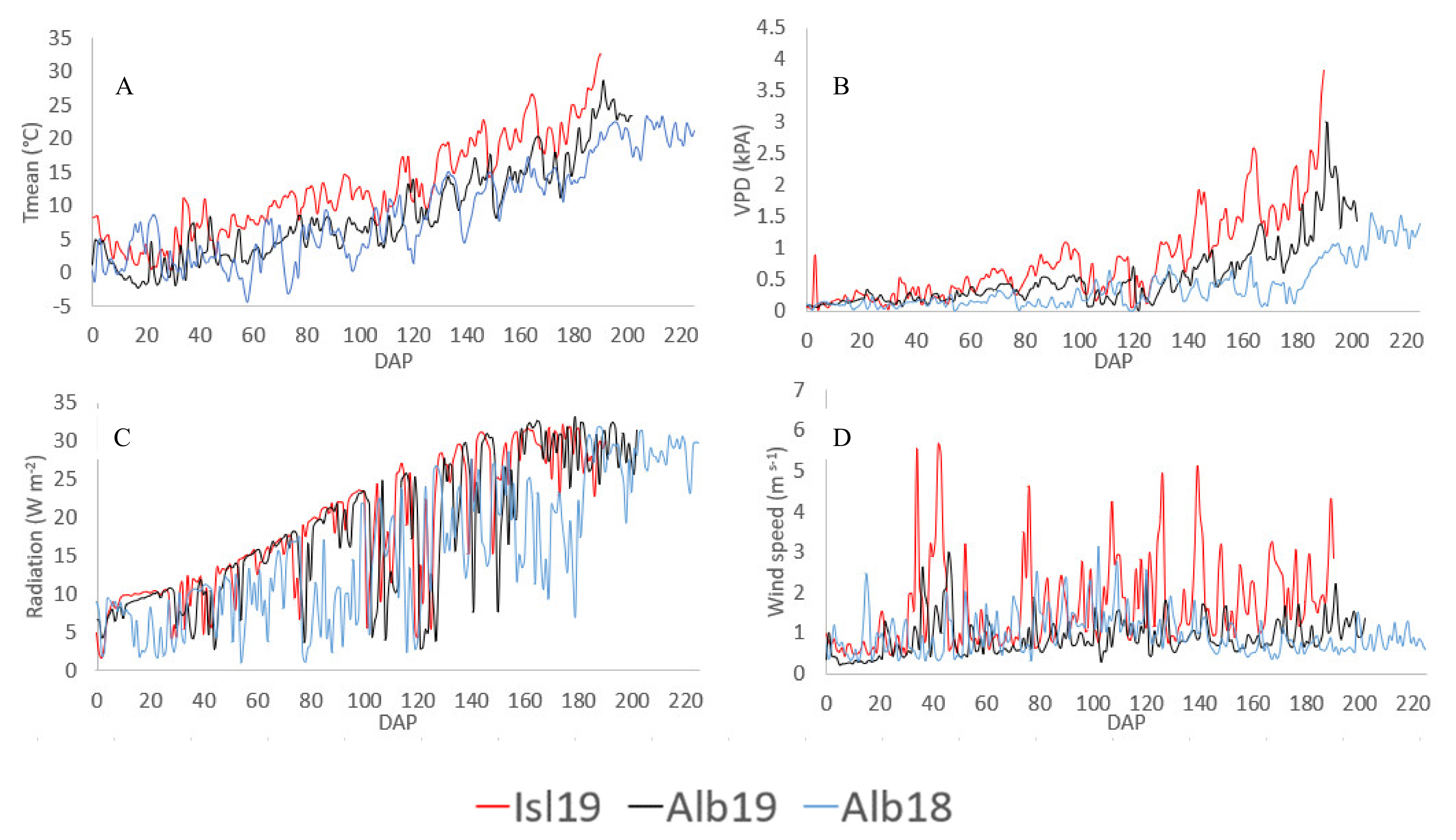
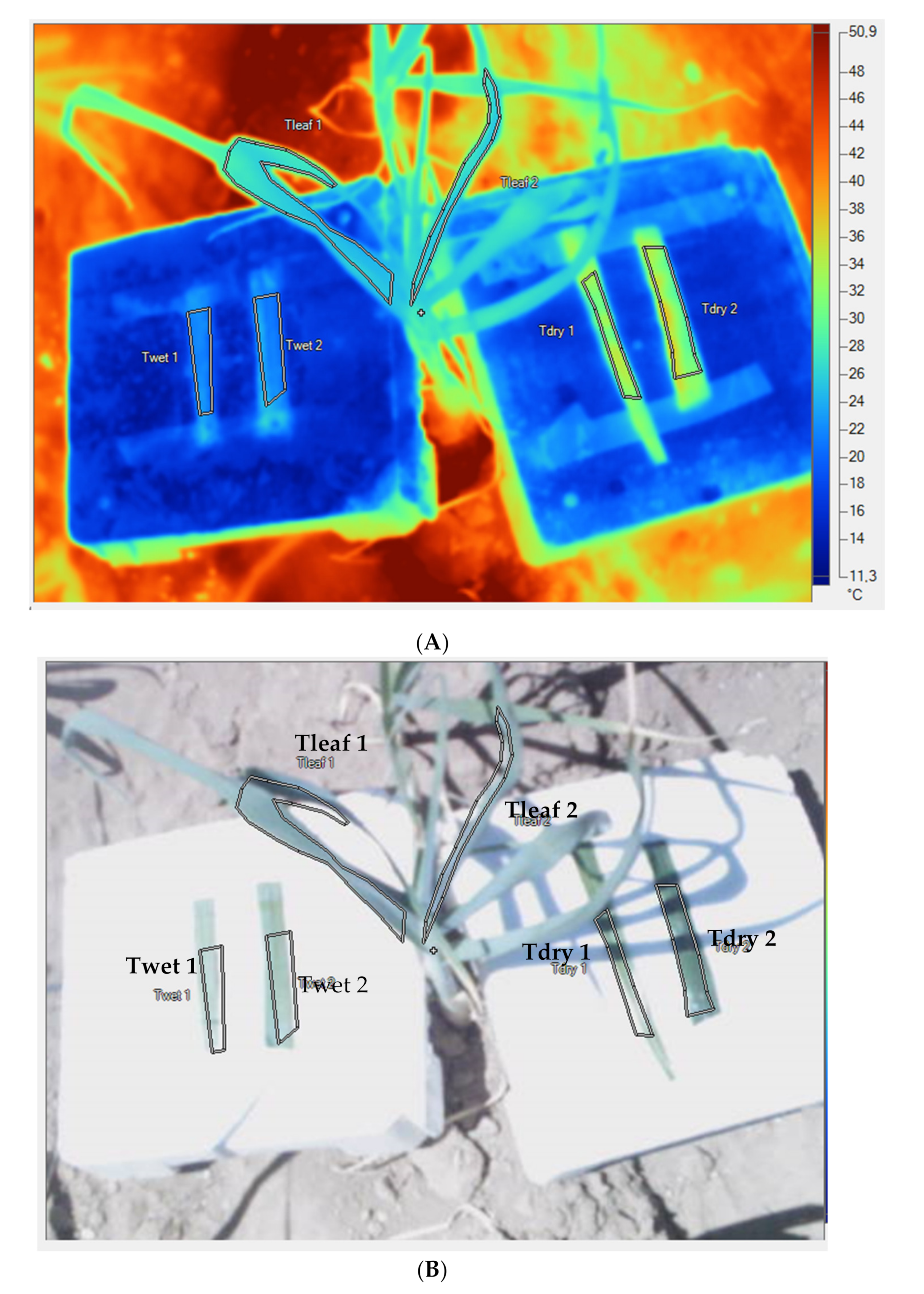
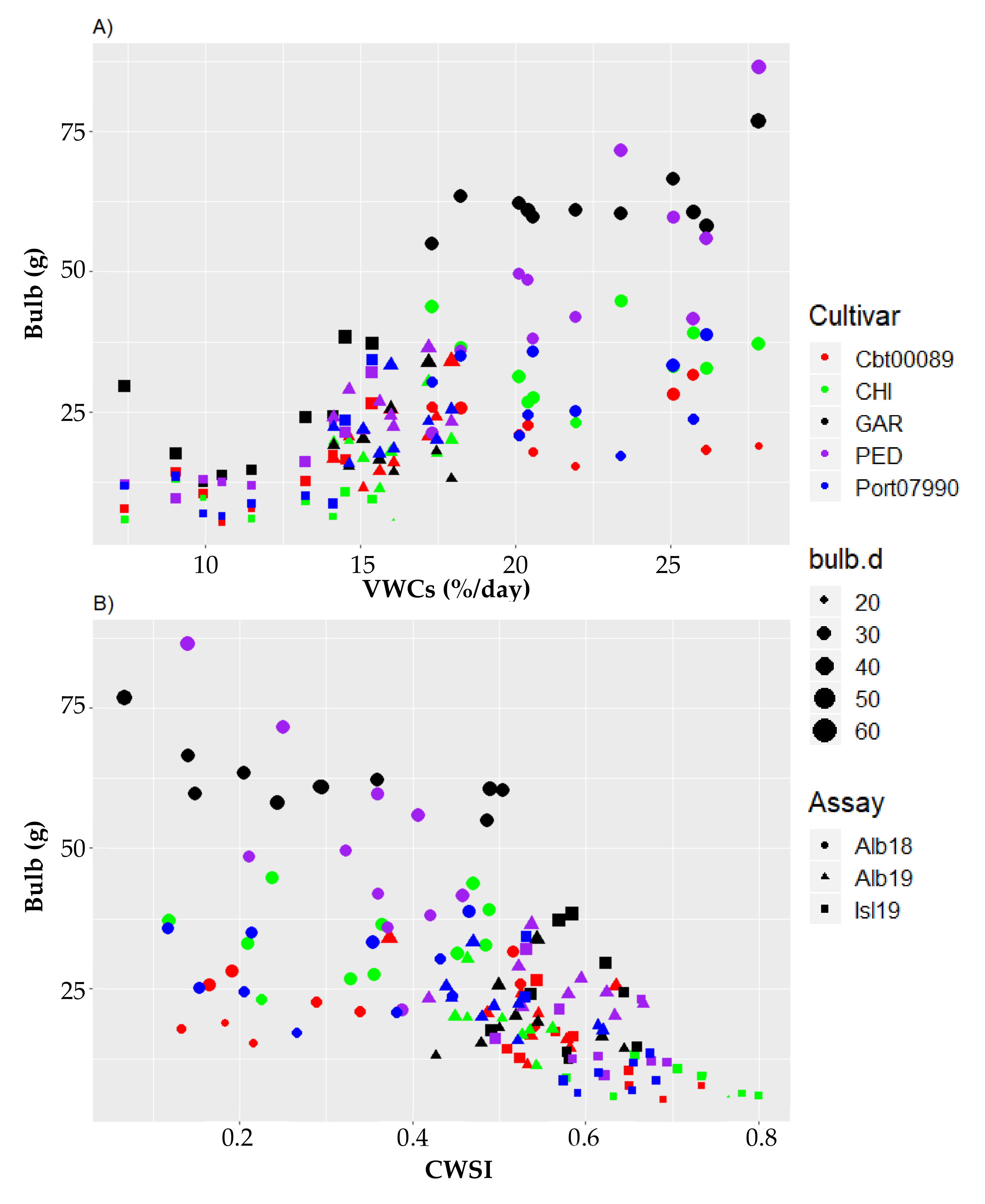
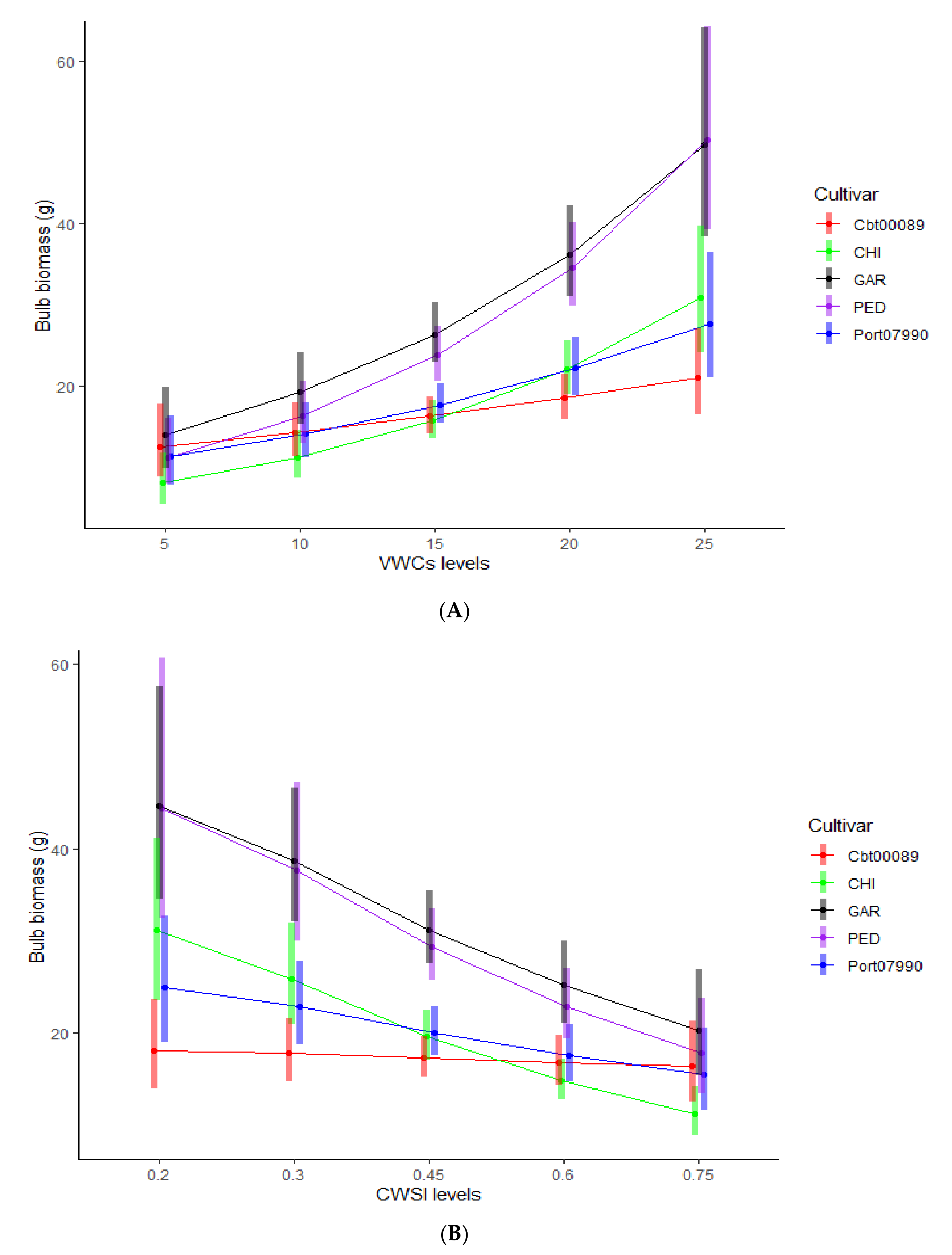
| Cultivar | Traditional Cultivation Area | December to March Historical Rainfall (mm) | April to July Historical Rainfall (mm) | December to July Min-Max Historical Temperature (°C) |
|---|---|---|---|---|
| “Gardacho” (GAR) * | Commercial | N/A | N/A | N/A |
| “Pedroñeras” (PED) ** | “Las Pedroñeras” (Spain) | 154 | 137 | 0.5–32.3 |
| “Chinchón” (CHI) *** | “Chinchón” (Spain) | 171 | 134 | 0.5–26.6 |
| Cbt00089(DRYII) *** | Vilaflor (Spain) | 284 | 44 | 5.5–22.9 |
| Port07990(RAIN) *** | Viana do Castelo (Portugal) | 633 | 245 | 6.6–23.6 |
| Assay | Plot | Target VWCs (%) | VWCs (% Day−1) | Assay | Plot | Target VWC (%) | VWCs (% Day−1) |
|---|---|---|---|---|---|---|---|
| Alb18 | P1 | X >25 | 27.82 | Alb19 | P15 | 17> X >15 | 16.06 |
| Alb18 | P2 | X >25 | 26.13 | Alb19 | P16 | 17> X >15 | 15.96 |
| Alb18 | P3 | X >25 | 25.73 | Alb19 | P17 | 17> X >15 | 15.60 |
| Alb18 | P4 | X >25 | 25.09 | Isl19 | P18 | 17> X >15 | 15.36 |
| Alb18 | P5 | 25> X >20 | 23.39 | Alb19 | P19 | 17> X >15 | 15.07 |
| Alb18 | P6 | 25> X >20 | 21.93 | Alb19 | P20 | 15> X >12 | 14.61 |
| Alb18 | P7 | 25> X >20 | 20.56 | Isl19 | P21 | 15> X >12 | 14.50 |
| Alb18 | P8 | 25> X >20 | 20.38 | Alb19 | P22 | 15> X >12 | 14.13 |
| Alb18 | P9 | 25> X >20 | 20.11 | Isl19 | P23 | 15> X >12 | 14.11 |
| Alb18 | P10 | 20> X >17 | 18.23 | Isl19 | P24 | 15> X >12 | 13.21 |
| Alb19 | P11 | 20> X >17 | 17.93 | Isl19 | P25 | X < 12 | 11.48 |
| Alb19 | P12 | 20> X >17 | 17.44 | Isl19 | P26 | X < 12 | 10.53 |
| Alb18 | P13 | 20> X >17 | 17.29 | Isl19 | P27 | X < 12 | 9.92 |
| Alb19 | P14 | 20> X >17 | 17.18 | Isl19 | P28 | X < 12 | 9.04 |
| Isl19 | P29 | X < 12 | 7.39 |
| Model |
|---|
| Models 1: Simple linear models with one continuous predictor |
| Model 1VWC: LnBulb (g) = α + β1VWC + ε |
| Model 1CWSI: LnBulb (g) = α + β1CWSI + ε |
| Models 2: Multiple linear models with one continuous predictor and the categorical factor Cultivar |
| Model 2VWC: LnBulb (g) = α + β1VWC + βjCultivar + ε |
| Model 2CWSI: LnBulb (g) = α + β1CWSI + βjCultivar + ε |
| Models 3: Multiple linear models with one continuous predictor (VWCs or CWSI) and the interaction of one predictor with the categorical factor Cultivar |
| Model 3VWC: LnBulb (g) = α + β1VWC + βjCultivar + βiCultivar:VWC + ε |
| Model 3CWSI: LnBulb (g) = α + β1CWSI + βjCultivar + βiCultivar:CWSI + ε |
| Models 4: Multiple linear models with two continuous predictors (VWCs and CWSI) and the interaction of one predictor with the categorical factor Cultivar |
| Model4Cult*VWC: LnBulb (g) = α + β1VWC + β2CWSI + βjCultivar + βiCultivar:VWC + ε |
| Model4Cult*CWSI: LnBulb (g) = α + β1VWC + β2CWSI + βjCultivar + βjCultivar:CWSI + ε |
| Models 5: Multiple linear mixed models with two continuous predictors (VWCs and CWSI) and the interaction of one predictor with the factor Cultivar and the random factor Assay |
| Model5VWC: LnBulb (g) = α + β1VWC + β2CWSI + βjCultivar + βiCultivar:VWC + υAssay + ε |
| Model5CWSI: LnBulb (g) = α + β1VWC + β2CWSI + βjCultivar + βjCultivar:CWSI + υAssay + ε |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Continuous Predictor (Cp) | R2adj | AIC | β1 | βCultivar | βCultivar * Cp | β2 |
| VWCs | ||||||
| Model 1VWC | 0.47 | 176.5 | F(1/141) = 126.57 *** | |||
| Model 2VWC | 0.62 | 134.3 | F(1/137) = 174.92 *** | F(4/137) = 14.34 *** | ||
| Model 3VWC | 0.63 | 132.0 | F(1/133) = 182.26 *** | F(4/133) = 15.02 *** | F(4/133) = 2.48 * | |
| CWSI | ||||||
| Model 1CWSI | 0.43 | 185.7 | F(1/141) = 109.92 *** | |||
| Model 2CWSI | 0.56 | 153.2 | F(1/137) = 141.77 *** | F(4/137) = 11.21 *** | ||
| Model 3CWSI | 0.59 | 149.4 | F(1/133) = 149.42 *** | F(4/133) = 11.82 *** | F(4/133) = 2.85 * | |
| VWCs + CWSI | ||||||
| Model4Cult * VWC | 0.68 | 115.0 | F(1/132) = 206.69 *** | F(4/132) = 17.04 *** | F(4/132) = 2.48 * | F(1/132) = 20.16 *** |
| Model4Cult * CWSI | 0.68 | 112.8 | F(1/132) = 209.76 *** | F(4/132) = 16.11 *** | F(4/132) = 3.01 * | F(1/132) = 25.19 *** |
| VWCs + CWSI + Assay | R2C + | |||||
| Model5Cult * VWC | 0.68 | 170.8 | F(1/130) = 116.19 *** | F(4/130) = 16.00 *** | F(4/130) = 2.51 * | F(1/130) = 23.25 *** |
| Model5Cult * CWSI | 0.68 | 141.3 | F(1/130) = 142.76 *** | F(4/130) = 16.19 *** | F(4/130) = 3.02 * | F(1/130) = 24.21 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Virosta, Á.; Sánchez-Gómez, D. Thermography as a Tool to Assess Inter-Cultivar Variability in Garlic Performance along Variations of Soil Water Availability. Remote Sens. 2020, 12, 2990. https://doi.org/10.3390/rs12182990
Sánchez-Virosta Á, Sánchez-Gómez D. Thermography as a Tool to Assess Inter-Cultivar Variability in Garlic Performance along Variations of Soil Water Availability. Remote Sensing. 2020; 12(18):2990. https://doi.org/10.3390/rs12182990
Chicago/Turabian StyleSánchez-Virosta, Álvaro, and David Sánchez-Gómez. 2020. "Thermography as a Tool to Assess Inter-Cultivar Variability in Garlic Performance along Variations of Soil Water Availability" Remote Sensing 12, no. 18: 2990. https://doi.org/10.3390/rs12182990
APA StyleSánchez-Virosta, Á., & Sánchez-Gómez, D. (2020). Thermography as a Tool to Assess Inter-Cultivar Variability in Garlic Performance along Variations of Soil Water Availability. Remote Sensing, 12(18), 2990. https://doi.org/10.3390/rs12182990






